Abstract
Purpose
GVAX pancreas, granulocyte-macrophage colony-stimulating factor–secreting allogeneic pancreatic tumor cells, induces T-cell immunity to cancer antigens, including mesothelin. GVAX is administered with low-dose cyclophosphamide (Cy) to inhibit regulatory T cells. CRS-207, live-attenuated Listeria monocytogenes–expressing mesothelin, induces innate and adaptive immunity. On the basis of preclinical synergy, we tested prime/boost vaccination with GVAX and CRS-207 in pancreatic adenocarcinoma.
Patients and Methods
Previously treated patients with metastatic pancreatic adenocarcinoma were randomly assigned at a ratio of 2:1 to two doses of Cy/GVAX followed by four doses of CRS-207 (arm A) or six doses of Cy/GVAX (arm B) every 3 weeks. Stable patients were offered additional courses. The primary end point was overall survival (OS) between arms. Secondary end points were safety and clinical response.
Results
A total of 90 patients were treated (arm A, n = 61; arm B, n = 29); 97% had received prior chemotherapy; 51% had received ≥ two regimens for metastatic disease. Mean number of doses (± standard deviation) administered in arms A and B were 5.5 ± 4.5 and 3.7 ± 2.2, respectively. The most frequent grade 3 to 4 related toxicities were transient fevers, lymphopenia, elevated liver enzymes, and fatigue. OS was 6.1 months in arm A versus 3.9 months in arm B (hazard ratio [HR], 0.59; P = .02). In a prespecified per-protocol analysis of patients who received at least three doses (two doses of Cy/GVAX plus one of CRS-207 or three of Cy/GVAX), OS was 9.7 versus 4.6 months (arm A v B; HR, 0.53; P = .02). Enhanced mesothelin-specific CD8 T-cell responses were associated with longer OS, regardless of treatment arm.
Conclusion
Heterologous prime/boost with Cy/GVAX and CRS-207 extended survival for patients with pancreatic cancer, with minimal toxicity.
INTRODUCTION
There is increasing evidence that immunotherapy can be effective in patients with solid tumors. Sipuleucel-T (Provenge; Dendreon, Seattle, WA) is the first US Food and Drug Administration–approved immunotherapeutic for prostate cancer.1 Ipilimumab (Yervoy; Bristol-Myers Squibb, New York, NY), an antagonist antibody to cytotoxic T-lymphocyte antigen-4, is approved for melanoma.2 Promising results in multiple tumor types have been observed with an agent that inhibits the anti–programmed death-1 (PD-1) receptor.3 Evidence also is emerging for activity of immunotherapy in pancreatic ductal adenocarcinoma (PDA).4–6
GVAX and CRS-207 are cancer vaccines that have been evaluated in PDA. GVAX is composed of two irradiated, granulocyte-macrophage colony-stimulating factor (GM-CSF) –secreting allogeneic PDA cell lines administered 24 hours after treatment with low-dose cyclophosphamide (Cy) to inhibit regulatory T cells.7 GVAX induces T cells against a broad array of PDA antigens, and mesothelin-specific T-cell responses have been shown to correlate with survival.5,8 Mesothelin is a tumor-associated antigen overexpressed in most PDAs. In a prior study, patients with previously treated advanced PDA who received Cy/GVAX had better induction of mesothelin-specific CD8+ T cells than those treated with GVAX alone. Median survival was 4.3 and 2.3 months, respectively.7 CRS-207 is recombinant live-attenuated, double-deleted Listeria monocytogenes (LADD Lm), engineered to secrete mesothelin into the cytosol of infected antigen presentation cells, which subsequently gets processed and presented in the context of major histocompatibility complex molecules.9,10 In PDA mouse models, a heterologous prime/boost using GVAX and LADD Lm–expressing mesothelin demonstrated synergistic activity in both antigen-specific T-cell induction and antitumor activity (Appendix Fig A1, online only). In the CRS-207 phase I study, patients with PDA who received GVAX before entering the study (n = 3) lived a median of 17 months, compared with 5 months for those who did not receive prior GVAX (n = 4).10 Following up on these observations, a phase II randomized, multicenter study was conducted comparing Cy/GVAX followed by CRS-207 with Cy/GVAX alone in patients with metastatic PDA.
PATIENTS AND METHODS
Patients
Eligible patients had histologically or cytologically proven metastatic PDA and had received at least one prior therapy regimen, were age ≥ 18 years, had an Eastern Cooperative Oncology Group performance status of 0 or 1, had an anticipated life expectancy > 12 weeks, and had adequate organ function. Exclusion criteria included brain metastases; allergy to both penicillin and sulfa; artificial implants that could not be easily removed (biliary stents and mediports were allowed); cirrhosis; radiographic ascites or pleural effusions; thromboembolic disease in the prior 2 months; autoimmune disease; immunocompromised state; HIV, hepatitis B, or hepatitis C; receipt of systemic steroids within 28 days; > 3 g per day of acetaminophen; and inability to avoid contact with individuals at risk for listeriosis.
Study Design
This multicenter, randomized, phase II trial was conducted at 10 US centers. Patients were randomly assigned at a ratio of 2:1 at each clinical site in two treatment arms. Arm A was assigned to receive two doses of Cy/GVAX and four doses of CRS-207. Arm B was assigned to receive six doses of Cy/GVAX. The primary objective of the study was to compare overall survival (OS) in the two arms, with secondary objectives to assess safety and clinical responses. The study was reviewed by local institutional review boards, biosafety committees, the US Food and Drug Administration, and the National Institutes of Health Recombinant DNA Advisory Committee. The trial was conducted according to the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference of Harmonisation. Interim data were reviewed by an independent data monitoring committee. All data presented are as of September 10, 2013, with the exception of the survival analyses, which include data through October 9, 2013. Data from rollover patients were censored as of the first date of rollover treatment (April 29, 2013).
Treatment
One treatment course was 20 weeks, consisting of six vaccine doses. Treatments were administered at 3-week intervals. Cy (200 mg/m2; Baxter, Deerfield, IL) was delivered intravenously 1 day before each GVAX treatment. GVAX consisted of two irradiated, allogeneic, GM-CSF–secreting PDA cell lines (Panc 6.03 and Panc 10.05, at 2.5 × 108 cells each; Johns Hopkins University, Baltimore, MD), combined and administered as six intradermal injections.5 CRS-207, at a dose of 1 × 109 colony-forming units (Aduro BioTech, Berkeley, CA), was delivered by 2-hour intravenous infusion followed by a 4-hour observation period. Oral antibiotics were initiated 7 days after the final CRS-207 of each course. After a 4-week rest, clinically stable patients were offered additional courses.
Assessments
Before each treatment, patients were assessed by physical examination, Eastern Cooperative Oncology Group status, complete blood counts, chemistries, and carbohydrate antigen 19-9 (CA19-9) levels. Blood counts and chemistries were also performed the day after CRS-207 infusion. Imaging was performed at baseline, week 10, and week 20. Tumor response was determined by investigator assessment according to RECIST (version 1.1). Patients with progressive disease who were clinically stable were allowed to continue on study. OS and progression-free survival (PFS) were calculated from the first dose of Cy until the date of death and date of disease progression or death, respectively.
Immunologic Assessments
Mesothelin-specific CD8+ T cells were detected in CD8+ peripheral-blood lymphocytes (PBLs) isolated from cryopreserved peripheral-blood mononuclear cells by interferon gamma enzyme-linked immunosorbent spot assay as previously described.4,5,7,8,10 Peripheral-blood mononuclear cells were isolated within 6 hours of blood draw by qualified laboratory specialists and stored frozen at −140°C until being analyzed. TAP-deficient T2 cells expressing patient-matched HLA class I molecules pulsed with 2 μg/mL of peptides were used as antigen-presenting cells. Each mesothelin peptide was assessed in three replicates of 1 × 105 CD8+ PBLs per well. Standard deviations between replicates were typically < 10%. Background reactivity, measured against T2 antigen-presenting cells pulsed with negative control peptides derived from irrelevant melanoma and renal cell carcinoma antigens, was subtracted from experimental values. The CEF pool of cytomegalovirus, Epstein-Barr virus, and influenza peptides was used as a positive control. Validation studies have demonstrated that the detection limit is 1:100,000 CD8+ PBLs. Data are presented as the number of mesothelin peptide–specific interferon gamma spot-forming cells per 1 × 105 CD8+ PBLs for each patient analyzed. For each mesothelin peptide, T-cell responses were considered enhanced when post-treatment T-cell levels were > five per 1 × 105 CD8+ PBLs and had increased by at least two-fold compared with levels measured at baseline. For each patient, the size of the enhanced post-treatment mesothelin-specific CD8+ T-cell repertoire was defined as the percentage of mesothelin peptides for which an enhanced response was detected.
Statistical Analysis
The primary efficacy analysis was a comparison of OS for Cy/GVAX and CRS-207 with that for Cy/GVAX alone using the log-rank test, with a one-sided type I error rate of 0.15. Primary comparisons were conducted for all randomly assigned patients who received at least one dose of Cy (full analysis set). Secondary analysis was performed in all patients who received at least three doses (per-protocol set; at least two doses of Cy/GVAX and one dose of CRS-207 in arm A or three doses of Cy/GVAX in arm B).
A total of 90 patients enrolled during an 18-month period and observed for 24 months would provide approximately 80% power to detect a benefit for OS in arm A, assuming the true median survival times for arms A and B were 8.1 and 5 months (hazard ratio [HR] for death, 0.62). Power was computed for a two-stage group sequential design with a single interim analysis after 41 deaths and a final analysis after 70 deaths. To preserve an overall one-sided type I error rate of 0.15, the gamma cumulative error alpha spending function (gamma, −4) was used.11 To stop the study at the interim analysis in favor of arm A, a one-sided P value < .0263 was required. If the study was not stopped at the interim analysis, a one-sided P value < .1461 at the final analysis was required.
Treatment differences in baseline characteristics and tumor marker kinetics were compared using the t test for continuous variables and Pearson's χ2 test for categorical variables. Mesothelin-specific CD8 T-cell responses were compared using the Wilcoxon rank sum test. Standard survival analysis methods were used to estimate OS (Kaplan Meier), with 95% CIs for median OS (Brookmeyer and Crowley) and 1-year survival (large-sample normal approximation, using Greenwood's formula for SE). The Cox proportional hazards model and Wald statistics were used to estimate HRs and CIs. The best overall response rate across all courses was compared using Pearson's χ2 test, and exact 95% Clopper-Pearson CIs were constructed for point estimates. All tests were two sided, except for the log-rank test, which was conducted as one sided in favor of arm A.
RESULTS
Patients and Treatment
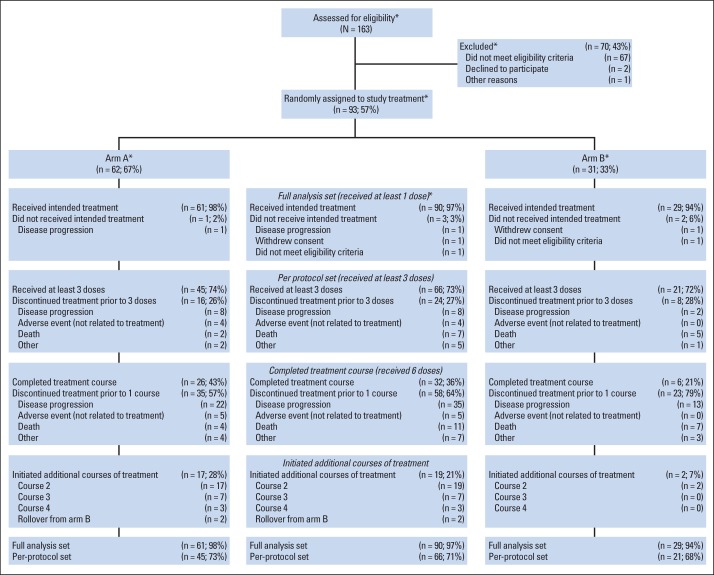
Between September 2011 and November 2012, 93 patients were enrolled and randomly assigned. Three patients (arm A, n = 1; arm B, n = 2) did not receive treatment (Fig 1). The full analysis set included 61 patients in arm A and 29 patients in arm B. The per-protocol analysis set included 45 patients in arm A and 21 patients in arm B. Demographic and baseline disease characteristics are listed in Table 1. Ninety-seven percent of patients had received prior chemotherapy, with 32% having received one and 51% having received ≥ two prior regimens for metastatic disease. The average number of vaccinations administered was 5.5 (range, one to 19) in arm A and 3.7 (range, one to 11) in arm B.
Fig 1.
CONSORT diagram. Percentages are based on number of participants treated in each arm and overall, except where indicated with asterisk (*).
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | Cy/GVAX Plus CRS-207 (n = 61) |
Cy/GVAX (n = 29) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .9135 | ||||
| Median | 63 | 67 | |||
| Range | 45-87 | 46-80 | |||
| Sex | .3782 | ||||
| Male | 34 | 56 | 19 | 66 | |
| Female | 27 | 44 | 10 | 34 | |
| ECOG PS | .627 | ||||
| 0 | 39 | 64 | 17 | 59 | |
| 1 | 22 | 36 | 12 | 41 | |
| Site of metastasis | .5066 | ||||
| Liver | 39 | 64 | 22 | 76 | |
| Lung only | 14 | 23 | 4 | 14 | |
| Other | 8 | 13 | 3 | 10 | |
| Disease status at study entry | .9102 | ||||
| Stable disease | 12 | 20 | 6 | 21 | |
| Progressive disease | 49 | 80 | 23 | 79 | |
| Any prior chemotherapy | N/A | ||||
| Yes | 59 | 97 | 28 | 97 | |
| No* | 2 | 3 | 1 | 3 | |
| No. of prior chemotherapy treatments for metastatic disease | .7005 | ||||
| 0 | 11 | 18 | 4 | 14 | |
| 1 | 18 | 30 | 11 | 38 | |
| ≥ 2 | 32 | 52 | 14 | 48 | |
| Previous surgical resection | .7383 | ||||
| Yes | 23 | 38 | 12 | 41 | |
| No | 38 | 62 | 17 | 59 | |
Abbreviations: Cy, cyclophosphamide; ECOG PS, Eastern Cooperative Oncology Group performance status; N/A, not applicable.
Declined chemotherapy.
Efficacy
Survival.
A planned interim analysis was performed on study data through January 2, 2013. The study was determined by the data monitoring committee to meet the prespecified criteria for early stopping for efficacy and recommended that the study be stopped and patients in arm B offered arm A treatment, at which time the study was considered to be completed for the primary end point analysis. The interim OS analysis for the full analysis set was based on 42 deaths among 88 patients (47.7%; arm A, 25 [41.7%] of 60; arm B, 17 [60.7%] of 28). The mean duration of follow-up was 3.4 months (minimum, 0.1; maximum, 8.3 months). The median OS was 6.0 months (95% CI, 4.2 to 8.2) in arm A, as compared with 3.4 months in arm B (95% CI, 2.1 to 4.6; HR for death, 0.45; 95% CI, 0.24 to 0.85; one-sided P = .0057; Appendix Fig A2A, online only). The interim OS analysis for the per-protocol analysis set was based on 25 deaths among 56 patients (44.6%). The median OS was 7.3 months (95% CI, 5.3 to 8.3) in arm A, as compared with 4.6 months in arm B (95% CI, 3.3 to 7.2; HR for death, 0.39; 95% CI, 0.16 to 0.91; one-sided P = .0238; Appendix Fig A2B, online only). An intent-to-treat analysis consisting of all randomly assigned patients was also conducted, which did not affect the outcome.
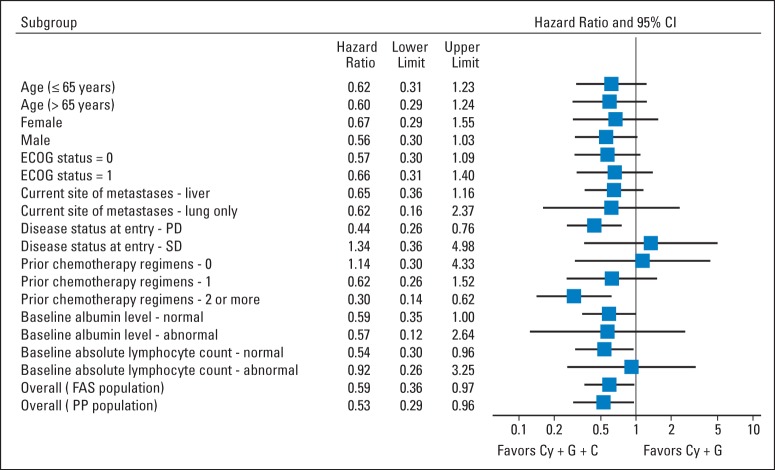
Two of five eligible arm B patients had crossed over to arm A treatment as of April 29, 2013. Subsequent OS analyses were conducted, with the rollover patients censored as of this date. Sensitivity analyses, where survival after the rollover was attributed to arm B, confirmed that censorship of these patients did not affect the results. Updated OS analyses as of October 9, 2013, on the full analysis set were based on 75 deaths among 90 patients (83.3%; arm A, 50 [82%] of 61; arm B, 25 [86%] of 29). The mean duration of follow-up was 6.6 months (minimum, 0.8; maximum, 20.2 months). The median OS was 6.1 months (95% CI, 4.4 to 9.2) in arm A, as compared with 3.9 months (95% CI, 2.8 to 4.9) in arm B (HR for death, 0.59; 95% CI, 0.36 to 0.97; one-sided P = .02; Fig 2A). The 1-year survival probability for arm A was 24% (95% CI, 14% to 36%), as compared with 12% (95% CI, 3% to 29%) for arm B (Appendix Table A1, online only). The updated OS analysis for the per-protocol analysis set was based on 51 deaths among 66 patients (77%; arm A, 34 [76%] of 45; arm B, 17 [81%] of 21). The median OS was 9.7 months (95% CI, 6.1 to 10.5) in Arm A as compared with 4.6 months in Arm B (95% CI, 3.8 to 8.5) (hazard ratio for death, 0.53; 95% CI, 0.29 to 0.96; P = .02, one-sided) (Fig 2B). Median OS in subgroup analyses for second line patients in the full analysis set was 7.7 months (95% CI, 3.8 to 10.3) in arm A, as compared with 3.8 months (95% CI, 1.5 to 12.5) in arm B (HR for death, 0.62; 95% CI, 0.26 to 1.52; one-sided P = .15); for patients receiving ≥ third-line therapy, it was 5.7 months (95% CI, 3.4 to 9.7), as compared with 3.7 months (95% CI, 1.2 to 4.2) in arm B (HR for death, 0.30; 95% CI, 0.14 to 0.62; one-sided P < .001; Appendix Table A1, online only). The OS effect of arm A treatment was seen in all subgroups except for patients with baseline stable disease or low lymphocyte counts (Fig 3).
Fig 2.
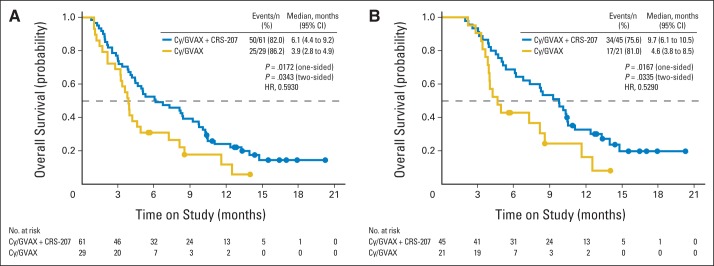
Kaplan-Meier estimates of overall survival (OS) according to treatment group. (A) OS for full analysis set (patients receiving ≥ one dose of cyclophosphamide [Cy]); median OS was 6.1 months in group receiving Cy/GVAX followed by CRS-207 and 3.9 months in group receiving Cy/GVAX alone. (B) OS for per-protocol analysis set (patients receiving ≥ three doses [≥ two doses of Cy/GVAX and one dose of CRS-207 in arm A or three doses of Cy/GVAX in arm B]); median OS was 9.7 months in group receiving Cy/GVAX followed by CRS-207 and 4.6 months in group receiving Cy/GVAX alone. Solid circles represent censored survival time for alive patients. HR, hazard ratio.
Fig 3.
Forest plot of treatment effect on overall survival in subgroup analyses. Horizontal lines represent 95% CIs. Position of each square represents point estimate of hazard ratio for treatment effect. Cy, cyclophosphamide; ECOG, Eastern Cooperative Oncology Group; FAS, full analysis set; PD, progressive disease; PP, per-protocol analysis set; SD, stable disease.
Response to therapy.
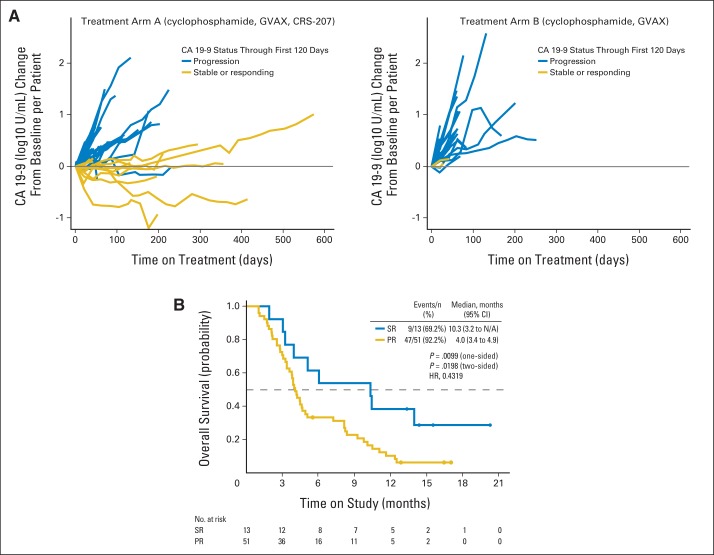
The response to therapy is summarized in Appendix Table A2 (online only). The rate of stable disease was 31% (95% CI, 20 to 44) in arm A and 24% (95% CI, 10 to 44) in arm B (two-sided P = .49). Stabilization or reduction in CA19-9 was seen in 27% of patients (11 of 41) in arm A and 9% of patients (two of 23) in arm B (two-sided P = .08; Fig 4A). The median OS in patients with a stable or better CA19-9 response was 10.3 months (95% CI, 3.2 to not applicable), as compared with 4.0 months (95% CI, 3.4 to 4.9) in patients with CA19-9 progression (HR for death, 0.43; two-sided P = .02; Fig 4B). There was no difference in PFS (data not shown).
Fig 4.
Carbohydrate antigen 19-9 (CA 19-9) responses. (A) CA 19-9 kinetics; in patients with abnormal baseline tumor marker CA 19-9 (> 37 U/mL), the following definitions to distinguish stable or responding (SR) from progression (PR) were used1: SR if < 50% increase for all assessments through 120 days,2 and PR if ≥ 50% increase in first 120 days for any assessment. (B) Overall survival based on CA 19-9 responses. HR, hazard ratio; N/A, not applicable.
Adverse Events
The most frequent adverse events for the first course of treatment in the Cy/GVAX and CRS-207 treatment arm were injection site reactions (erythema, 77%; induration, 71%; pain, 62%; pruritis, 71%), nausea (53%), vomiting (43%), chills (67%), fever (62%), and fatigue (53%). Systemic events resolved within 24 hours. Clinically significant related grade 3 to 4 adverse events are summarized in Table 2. Grade 3 to 4 laboratory changes after CRS-207 included lymphopenia (44%) and transaminitis (5%).
Table 2.
Grade ≥ 3 Clinically Significant Related Adverse Events
| Adverse Event | Cy/GVAX Plus CRS-207 (n = 61) |
Cy/GVAX (n = 29) |
Total (N = 90) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Hematologic | ||||||
| Anemia | 0 | 0.0 | 1 | 3.4 | 1 | 1.1 |
| Lymphopenia | 5 | 8.2* | 1 | 3.4 | 6 | 6.7 |
| Neutropenia | 1 | 1.6 | 0 | 0.0 | 1 | 1.1 |
| Nonhematologic | ||||||
| ALT increased | 1 | 1.6 | 0 | 0.0 | 1 | 1.1 |
| AST increased | 3 | 4.9 | 0 | 0.0 | 3 | 3.3 |
| Hyperglycemia | 1 | 1.6 | 0 | 0.0 | 1 | 1.1 |
| Hyponatremia | 1 | 1.6 | 0 | 0.0 | 1 | 1.1 |
| Hypophosphatemia | 2 | 3.3 | 0 | 0.0 | 2 | 2.2 |
| Myalgia | 1 | 1.6 | 0 | 0.0 | 1 | 1.1 |
| Hypotension | 2 | 3.3 | 0 | 0.0 | 2 | 2.2 |
| Abdominal pain | 1 | 1.6 | 1 | 3.4 | 2 | 2.2 |
| Diarrhea | 1 | 1.6 | 0 | 0.0 | 1 | 1.1 |
| Chills | 2 | 3.3 | 0 | 0.0 | 2 | 2.2 |
| Fatigue | 3 | 4.9 | 0 | 0.0 | 3 | 3.3 |
| Pyrexia | 3 | 4.9 | 0 | 0.0 | 3 | 3.3 |
| Vaccination site exfoliation | 1 | 1.6 | 0 | 0.0 | 1 | 1.1 |
| Vaccination site pain | 1 | 1.6 | 1 | 3.4 | 2 | 2.2 |
Two patients with grade 4 lymphopenia; these were the only treatment-related grade > 3 adverse events.
Immune Responses
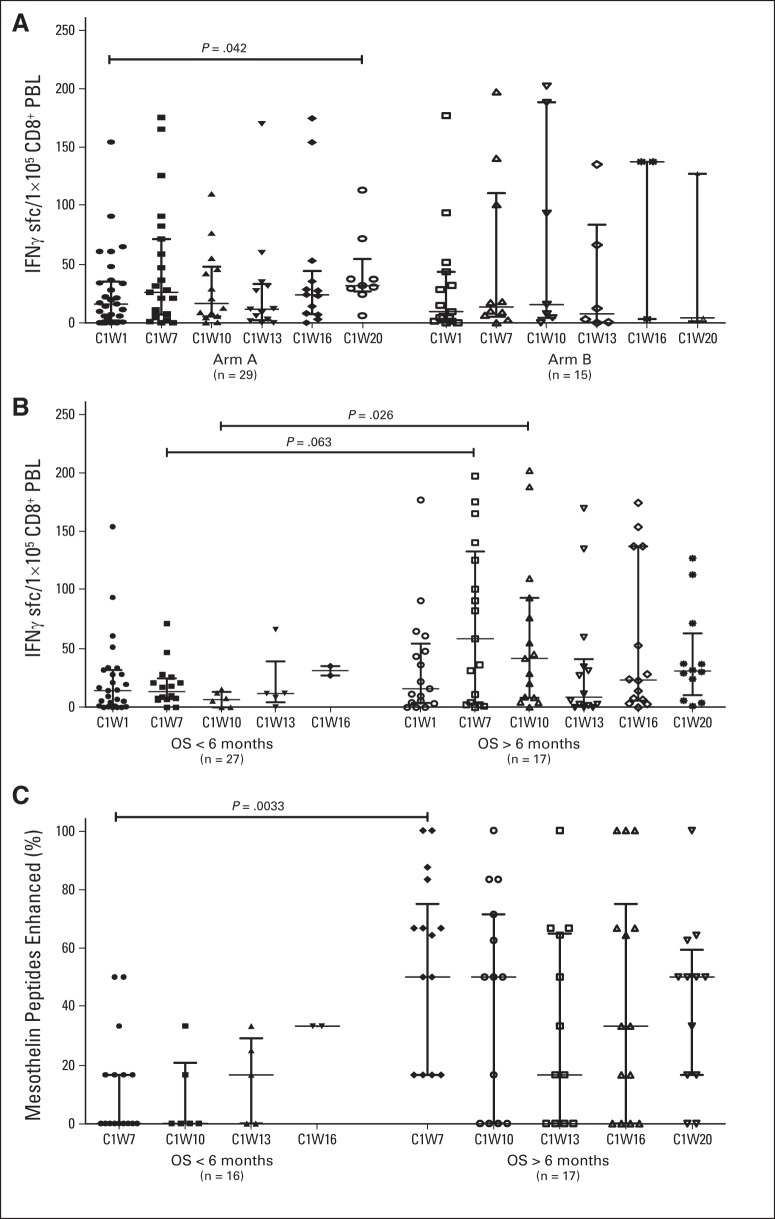
Mesothelin-specific CD8 T-cell levels were evaluated from 44 patients (arm A, n = 29; arm B, n = 15) who expressed HLA-A1, -A2, and/or -A3 alleles. An increase (P = .042) in mesothelin-specific CD8 T cells over baseline was observed at week 20 (one treatment course) in arm A only. No differences were observed between arms in mesothelin-specific CD8 T-cell levels (Fig 5A). The quantity (Fig 5B) and breadth (Fig 5C) of mesothelin-specific CD8 T-cell immunity after two Cy/GVAX administrations in both arms were associated with longer OS.
Fig 5.
Mesothelin-specific T-cell responses. Mesothelin-specific CD8 T cells were quantified in peripheral blood lymphocytes (PBLs) using interferon gamma enzyme-linked immunosorbent spot assays at baseline (C1W1), 3 weeks after two treatments with cyclophosphamide (Cy)/GVAX (C1W7), and 3 weeks after each subsequent dose of either CRS-207 (arm A) or Cy/GVAX (arm B; C1W10, C1W13, C1W16, and C1W20) in 44 HLA-A1–, HLA-A2–, and/or HLA-A3–positive patients who received ≥ one treatment of Cy/GVAX. CD8 T-cell responses to mesothelin peptides were considered enhanced when post-treatment T-cell levels were > five per 1 × 105 CD8+ PBLs and increased by ≥ two-fold compared with baseline levels. Cumulative numbers of T cells measured against each HLA-matched mesothelin peptide are reported for each sample. (A) Mesothelin-specific CD8 T-cell levels per 1 × 105 CD8+ PBLs in patients separated by treatment arm. (B) Mesothelin-specific CD8 T-cell levels per 1 × 105 CD8+ PBLs in patients separated by overall survival (OS) > or < 6 months. (C) Size of enhanced post-treatment mesothelin-specific CD8 T-cell repertoire in patients separated by OS > or < 6 months.
DISCUSSION
This randomized study demonstrated that Cy/GVAX followed by CRS-207 significantly improved OS as compared with Cy/GVAX alone in patients with metastatic PDA. This 56% improvement (2.2 months) is significant in a disease where effective first-line therapy—gemcitabine plus nab-paclitaxel—showed a 27% improvement over gemcitabine alone (8.5 v 6.7 months).12 The stable disease rate of 31%, 1-year survival rate of 24%, and changes in CA19-9 levels that correlated with survival for the combination arm are encouraging. The extended survival for the patients in the per-protocol analyses suggests maximal benefit for immunotherapy in PDA depends on proper patient selection and indicates CRS-207 plays an important role in the treatment.
The survival benefit was evident despite no effect on PFS. Of note, sipuleucel-T and ipilimumab were approved based on OS benefits despite no PFS differences.1,2 A study evaluated tumor growth rates in prostate cancer in chemotherapy trials and a vaccine trial using PROSTVAC (pox virus, prostate-specific antigen based). For PROSTVAC, there was deceleration of tumor growth rate, which showed no impact in PFS but improved OS.13–15 Explanations could include changes in the equilibrium between tumor growth and immune surveillance, control of micrometastases, improved responses to subsequent therapies, and cytokine-mediated beneficial effects on host immunity and cachexia. PFS has limitations as a measure of activity in a combination where administration of the boosting component begins 6 weeks into the treatment course.
Induction of mesothelin-specific CD8 T cells was associated with longer OS. Enhancement of responses after two doses of Cy/GVAX may be a prerequisite for improved outcomes with CRS-207 boosting (Fig 5C). Interestingly, an initial drop in responses was observed in patients after the first two doses of CRS-207, which later rebounded. One possible explanation is that CRS-207 induces T cells to leave the periphery and enter tissues. This is likely aided by stimulatory cytokines released in response to CRS-207.10 The epitopes used in these assays were previously defined based on responses induced by GVAX, but it is expected based on preclinical data that the repertoire induced by CRS-207 could differ. Although this specific assay is a tool to assess immune response, additional studies designed to elucidate the full spectra of the boosting effects of CRS-207 should address alterations in T-cell trafficking or the tumor microenvironment, epitope spreading, CD4 T-cell responses, and innate immunity.
In conclusion, this is the first study to our knowledge to demonstrate a survival advantage using immunotherapy in PDA. A follow-up study in previously treated PDA has been opened to compare this combination with chemotherapy and explore CRS-207 alone. A study combining the heterologous prime/boost strategy with anti–PD-1 is under development. On the basis of the observed survival and favorable safety profile, Cy/GVAX and CRS-207 are being further explored as a treatment for PDA.
Supplementary Material
Acknowledgment
We thank all the patients who participated in this study and their supporting families. We also thank the referring physicians and the supporting staff at all the participating clinical sites; Drew Pardoll, Md, PhD, for critical scientific input; Brad Phillips and David Browning (Medelis, Nashville, TN) for project management and data collection; and Sandy Ferber and Allan Rosen (Array Biostatistics, Chicago, IL) and Seth Zuckerman (Cytel, Cambridge, MA) for biostatistics support. We also thank members of the independent data monitoring committee (Eileen O'Reilly, MD, Yu Shyr, PhD, and Karolina Palucka, MD, PhD) for expert review of the data and recommendations on conduct of the study.
Glossary Terms
- HLA (human leukocyte antigen):
the human major histocompatibility complex, which is expressed as two sets of highly polymorphic cell surface molecules, termed HLA class I and HLA class II. HLA class I molecules are expressed on all nucleated cells and are encoded by diverse alleles of the HLA-A, HLA-B, or HLA-C genes (eg, HLA-A1 [HLA molecule encoded by the A1 allele of the HLA-A gene] and HLA-B7 [HLA molecule encoded by the B7 allele of the HLA-B gene]). HLA class I molecules bind peptides derived from cellular proteins upon processing. Cytotoxic T lymphocytes, expressing the CD8 coreceptor, recognize cell-bound peptides in association with HLA class I molecules on target cells.
- epitope:
region within an antigen that has the potential to give rise to an antibody response. With respect to protein antigens, epitopes may be defined on the basis of primary, secondary, or tertiary structure of the molecule and, consequently, maybe exposed or hidden within the molecule.
- antigen:
a substance that promotes, or is the target of, an immune response.
- GM-CSF (granulocyte-macrophage colony-stimulating factor):
a growth factor that stimulates the production of white blood cells. Normally used in cancer therapy and bone marrow transplantation, GM-CSF augments WBC production, decreasing the risk of infection. In vaccine therapy, it is an effective vaccine adjuvant administered to activate endogenous dendritic cells, the most effective antigen-presenting cells of the immune system.
- cytokines:
cell communication molecules that are secreted in response to external stimuli.
- antigen-presenting cells (APCs):
cells of the immune system that play a major role in adaptive immunity. APCs are responsible for binding and processing antigens for presentation to T lymphocytes and producing signals that lead to lymphocyte proliferation and differentiation. Dendritic cells and macrophages are examples of APCs.
Appendix
Table A1.
Survival Summary
| Therapy Setting | Full Analysis Set |
Per-Protocol Set |
||
|---|---|---|---|---|
| Cy/GVAX Plus CRS-207 | Cy/GVAX | Cy/GVAX Plus CRS-207 | Cy/GVAX | |
| All patients, No. | 61 | 29 | 45 | 21 |
| Median survival, months | 6.1 | 3.9 | 9.7 | 4.6 |
| HR | 0.59 | 0.53 | ||
| One-sided log-rank P | .0172 | .0167 | ||
| Probable survival > 12 months, % | 24 | 12 | 33 | 16 |
| Difference | 12 | 16 | ||
| 95% CI | −5 to 30 | −7 to 40 | ||
| Second line | 18 | 11 | 13 | 8 |
| Median survival, months | 7.7 | 3.8 | 9.8 | 7.2 |
| HR | 0.62 | 0.51 | ||
| One-sided log-rank P | .1468 | .1148 | ||
| Probable survival > 12 months, % | 22 | 30 | 31 | 42 |
| Difference | −8 | −11 | ||
| 95% CI | −45 to 29 | −58 to 36 | ||
| ≥ Third line | 32 | 14 | 24 | 10 |
| Median survival, months | 5.7 | 3.7 | 8.3 | 4.0 |
| HR | 0.30 | 0.22 | ||
| One-sided log-rank P | < .001 | < .001 | ||
| % Probable survival > 12 months | 21 | 0 | 29 | 0 |
| Difference | 21 | 29 | ||
| 95% CI | N/A | N/A | ||
Abbreviations: Cy, cyclophosphamide; HR, hazard ratio; N/A, not applicable.
Table A2.
Best Overall Response Rate
| Response | Cy/GVAX Plus CRS-207 (n = 61) |
Cy/GVAX (n = 29) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| CR | 0 | 0 | 0 | 0 |
| PR | 0 | 0 | 0 | 0 |
| SD | 19 | 31 | 7 | 24 |
| PD | 31 | 51 | 15 | 52 |
| NE | 2 | 3 | 1 | 3 |
| Missing* | 9 | 15 | 6 | 21 |
Abbreviations: CR, complete response; Cy, cyclophosphamide; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Clinical progression before week-10 response assessment.
Fig A1.
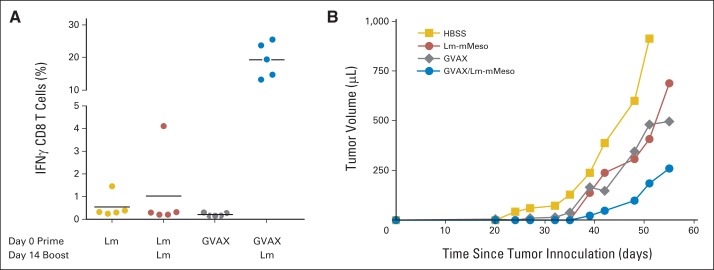
Heterologous prime/boost of GVAX and Listeria-based vaccines induces robust antigen-specific T-cell immunity and delays tumor growth in experimental mouse pancreatic tumor model. (A) AH1-specific T-cell responses at peak of response in BALB/c mice. On day 0, BALB/c mice (n = 5) were immunized with either 5 × 106 colony-forming units (CFUs) of Lm-AH1 (Lm) intravenously (IV) or 1 × 106 cells of GVAX subcutaneously (SC). On day 14, specific groups were boosted IV with 5 × 106 CFUs of Lm. Seven days after prime and 5 days after boost, spleens from mice were obtained, and interferon gamma (IFN-γ) AH1-specific immune responses were assessed by intracellular cytokine staining. (B) Therapeutic antitumor efficacy of combination of GVAX and Lm in C57BL/6 mice. On day 0, C57BL/6 mice (n = 10) were implanted SC with 1 × 106 Panc02 cells. On day 3, GVAX animals were pretreated with cyclophosphamide 100 mg/kg and PC-61 50 μg. On day 4, mice were vaccinated either SC with 6 × 106 cells of GVAX (3 × 106 irradiated Panc02 cells plus 3 × 106 irradiated B78H1 cells) or IV with 5 × 106 CFUs of Lm-mouse mesothelin (Lm-mMeso). Fourteen days after vaccination, indicated groups were boosted IV with 5 × 106 CFUs of Lm-mMeso. Mice were monitored over time, and tumor growth was measured twice per week. HBSS, Hank's balanced salt solution.
Fig A2.
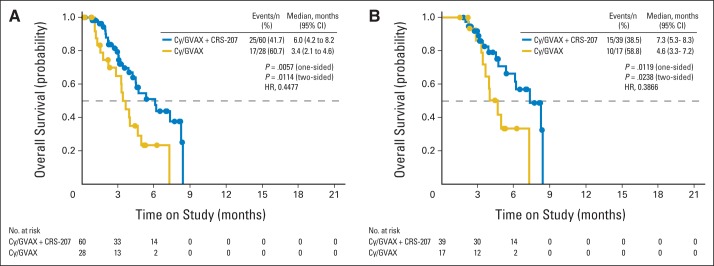
Kaplan-Meier estimates of overall survival (OS) at interim analyses according to treatment group. (A) OS for full analysis set (patients receiving ≥ one dose of cyclophosphamide [Cy]); median OS was 6.0 months in group receiving Cy/GVAX followed by CRS-207 and 3.4 months in group receiving Cy/GVAX alone. (B) OS for per-protocol analysis set (patients receiving ≥ three doses [≥ two doses of Cy/GVAX and one dose of CRS-207 in arm A or three doses of Cy/GVAX in arm B]); median OS was 7.3 months in group receiving Cy/GVAX followed by CRS-207 and 4.6 months in group receiving Cy/GVAX alone. Solid circles represent censored survival time for alive patients.
Footnotes
See accompanying editorial on page 1315; listen to the podcast by Dr Kaufman at www.jco.org/podcasts
Processed as a Rapid Communication manuscript.
Supported by Aduro BioTech, National Institutes of Health Grant No. 5K23 CA163672-02, and Washington University Institute of Clinical and Translational Sciences Grants No. UL1 TR000448 and KL2 TR000450 from the National Center for Advancing Translational Sciences.
Presented in part at the American Society of Clinical Oncology Gastrointestinal Cancers Symposium, San Francisco, CA, January 16-18, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical trial information: NCT01417000.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Dung T. Le, Jennifer N. Uram, Aimee Luck Murphy, Thomas Dubensky Jr, Dirk G. Brockstedt, Elizabeth M. Jaffee
Provision of study materials or patients: Dung T. Le, Andrea Wang-Gillam, Vincent Picozzi, Tim F. Greten, Todd Crocenzi, Gregory Springett, Michael Morse, Herbert Zeh, Deirdre Cohen, Robert L. Fine, Daniel A. Laheru, Justin Skoble
Collection and assembly of data: Dung T. Le, Tim F. Greten, Michael Morse, Beth Onners, Jennifer N. Uram, Daniel A. Laheru, Eric R. Lutz, Sara Solt, Aimee Luck Murphy, John Grous
Data analysis and interpretation: Dung T. Le, Andrea Wang-Gillam, Vincent Picozzi, Todd Crocenzi, Gregory Springett, Herbert Zeh, Deirdre Cohen, Robert L. Fine, Beth Onners, Daniel A. Laheru, Eric R. Lutz, Sara Solt, Aimee Luck Murphy, Justin Skoble, Ed Lemmens, John Grous, Thomas Dubensky Jr, Dirk G. Brockstedt, Elizabeth M. Jaffee
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Dung T. Le
Research Funding: Aduro BioTech, Bristol-Myers Squibb, Merck
Andrea Wang-Gillam
Consulting or Advisory Role: Merrimack
Vincent Picozzi
Stock or Other Ownership: Amgen, Johnson & Johnson
Research Funding: Theranostics Health, Aduro BioTech, OncoMed, Clovis Oncology, FibroGen, Immunomedics
Tim F. Greten
No relationship to disclose
Todd Crocenzi
No relationship to disclose
Gregory Springett
Travel, Accommodations, Expenses: Halozyme
Michael Morse
Stock or Other Ownership: Phytochem
Honoraria: Genetech, Bristol-Myers Squibb, Amgen, Novartis, Bayer, Onyx Pharmaceuticals, Prometheus, sanofi-aventis, Genomic Health
Speakers' Bureau: Genentech, Novartis, Genomic Health
Research Funding: Aduro BioTech, Bristol-Myers Squibb, Onyx Pharmaceuticals, AlphaVax, Precision Therapeutics, Newlink Genetics
Herbert Zeh
Research Funding: Aduro BioTech
Deirdre Cohen
Consulting or Advisory Role: Merrimack Pharmaceuticals
Speakers' Bureau: Bayer, Genentech
Robert L. Fine
No relationship to disclose
Beth Onners
No relationship to disclose
Jennifer N. Uram
No relationship to disclose
Daniel A. Laheru
No relationship to disclose
Eric R. Lutz
Research Funding: Aduro BioTech
Sara Solt
No relationship to disclose
Aimee Luck Murphy
Employment: Aduro BioTech
Stock or Other Ownership: Aduro BioTech
Justin Skoble
Employment: Aduro BioTech
Stock or Other Ownership: Aduro BioTech
Patents, Royalties, Other Intellectual Property: Patents related to Aduro BioTech vaccine technology
Ed Lemmens
Employment: Aduro BioTech
Stock or Other Ownership: Aduro BioTech
Patents, Royalties, Other Intellectual Property: Patents related to Aduro BioTech vaccine technology (attenuation and expression technology for Listeria vaccines)
Travel, Accommodations, Expenses: Aduro BioTech
John Grous
Consulting or Advisory Role: Aduro BioTech
Thomas Dubensky Jr
Employment: Aduro BioTech
Leadership: Aduro BioTech
Stock or Other Ownership: Aduro BioTech
Patents, Royalties, Other Intellectual Property: Several issued and pending patents related to vaccine platforms described in this report, on which I am named inventor; patents are held by Aduro BioTech, my employer (Inst)
Dirk G. Brockstedt
Employment: Aduro BioTech
Leadership: Aduro BioTech
Stock or Other Ownership: Aduro BioTech
Patents, Royalties, Other Intellectual Property: Patents related to Aduro BioTech vaccine technology (attenuation and expression technology for Listeria vaccines)
Elizabeth M. Jaffee
Consulting or Advisory Role: MedImmune
Research Funding: Aduro BioTech
Patents, Royalties, Other Intellectual Property: Through licensing agreement between Aduro BioTech and Johns Hopkins University, has potential to receive royalties from GVAX vaccine
REFERENCES
- 1.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma: A phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: A pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brockstedt DG, Giedlin MA, Leong ML, et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: Phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang IK, Shih WJ, De Cani JS. Group sequential designs using a family of type I error probability spending functions. Stat Med. 1990;9:1439–1445. doi: 10.1002/sim.4780091207. [DOI] [PubMed] [Google Scholar]
- 12.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nabpaclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: The growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–917. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanda MG, Smith DC, Charles LG, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 15.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.