Abstract
Purpose
Trials in castration-resistant prostate cancer (CRPC) need new clinical end points that are valid surrogates for survival. We evaluated circulating tumor cell (CTC) enumeration as a surrogate outcome measure.
Patients and Methods
Examining CTCs alone and in combination with other biomarkers as a surrogate for overall survival was a secondary objective of COU-AA-301, a multinational, randomized, double-blind phase III trial of abiraterone acetate plus prednisone versus prednisone alone in patients with metastatic CRPC previously treated with docetaxel. The biomarkers were measured at baseline and 4, 8, and 12 weeks, with 12 weeks being the primary measure of interest. The Prentice criteria were applied to test candidate biomarkers as surrogates for overall survival at the individual-patient level.
Results
A biomarker panel using CTC count and lactate dehydrogenase (LDH) level was shown to satisfy the four Prentice criteria for individual-level surrogacy. Twelve-week surrogate biomarker data were available for 711 patients. The abiraterone acetate plus prednisone and prednisone-alone groups demonstrated a significant survival difference (P = .034); surrogate distribution at 12 weeks differed by treatment (P < .001); the discriminatory power of the surrogate to predict mortality was high (weighted c-index, 0.81); and adding the surrogate to the model eliminated the treatment effect on survival. Overall, 2-year survival of patients with CTCs < 5 (low risk) versus patients with CTCs ≥ 5 cells/7.5 mL of blood and LDH > 250 U/L (high risk) at 12 weeks was 46% and 2%, respectively.
Conclusion
A biomarker panel containing CTC number and LDH level was shown to be a surrogate for survival at the individual-patient level in this trial of abiraterone acetate plus prednisone versus prednisone alone for patients with metastatic CRPC. Additional trials are ongoing to validate the findings.
INTRODUCTION
The recent progress in prostate cancer therapeutics is unprecedented. In a 3-year period, five different therapies were proven to prolong life in patients with progressive castration-resistant disease (CRPC).1–7 The results give new hope to those in need of effective treatment, but at the same time, the availability of more life-prolonging treatments makes it more difficult to demonstrate a survival benefit for future new drugs. Future trials designed with a primary end point of survival will have to be larger, longer running, and more costly, with a higher risk of failure. Urgently needed are reproducible and reliable post-treatment outcome measures that are surrogates for survival that can be used to guide patient management and facilitate regulatory approval. Such surrogates would make new drugs available to patients more rapidly and significantly reduce drug development timelines and costs.
Shedding of tumor cells into the circulation is a necessary (but not sufficient) step for the formation of metastases,8 and multiple assays and devices are now available to detect, isolate, enumerate, and characterize circulating tumor cells (CTCs),9 but only one, CellSearch (Janssen Diagnostics, Raritan, NJ), is US Food and Drug Administration cleared10,11 “as an aid in the monitoring of patients” based on trials in metastatic breast cancer, metastatic colorectal cancer, and metastatic CRPC (mCRPC). Trials demonstrated that the number of CTCs measured during the course of treatment, reported as unfavorable (≥ 5 cells/7.5 mL of blood) versus favorable (≤ 4 cells/7.5 mL), is prognostic and predictive of overall survival.12–14
One mechanism contributing to CRPC progression is upregulation of the androgen biosynthetic machinery that leads to an increase in intratumoral androgens.15,16 Abiraterone acetate is a prodrug of abiraterone, which is a selective CYP450 17A1 inhibitor that reduces androgen production in the testes, adrenal glands, and tumor tissues17 and lowers serum testosterone levels to the 1-ng/dL range.18 A concern in the development of this and other androgen-modulating agents has been that post-therapy prostate-specific antigen (PSA) declines may not reflect a favorable effect on tumor growth.19–22 To address this, CTC enumeration using CellSearch was explored as a secondary end point in two phase II trials of abiraterone acetate in patients with mCRPC experiencing progression after chemotherapy. Both trials showed significant and durable declines in PSA and favorable changes in CTC count.23,24 A separate analysis showed that each of the following was strongly prognostic for survival pre- and post-treatment: a biomarker panel containing CTC count alone, a panel containing lactate dehydrogenase (LDH) alone, and a panel containing the combination of CTC count and LDH level. All were stronger than PSA.22
On the basis of those results, CTC enumeration was included as an outcome measure in the abiraterone acetate phase III registration trial (COU-AA-301) in patients with mCRPC previously treated with docetaxel; the primary end point was overall survival. The aim was to identify a biomarker or biomarker panel using the Prentice25 criteria that could serve as an efficacy-response surrogate for overall survival, to be confirmed in future trials. The biomarker aspects of the trial design were reviewed by the US Food and Drug Administration Centers for Devices and Radiological Health and Drug Evaluation Research.
PATIENTS AND METHODS
Study Design and Patients
The trial was conducted at 147 sites in 13 countries in North America, Europe, and Australia. CTC samples were not collected in Australia for logistic reasons. Details of the methodology, patient population, and treatment have been reported previously, along with interim and final study results.2,3 Patients were stratified by the four baseline factors listed in Table 1 (ie, Eastern Cooperative Oncology Group status, worst pain level, number of prior chemotherapy regimens, and type of disease progression) and then randomly assigned at a ratio of 2:1 to receive abiraterone acetate 1,000 mg daily or matched placebo; both groups received prednisone 10 mg daily. Treatment was continued until disease progression based on PSA determinations, imaging, and/or clinical criteria or until unacceptable toxicity. The review boards at all participating institutions approved the study, which was conducted according to the principles set forth in the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonisation. All patients provided written informed consent to participate. CTC numbers were measured using CellSearch. One of the secondary objectives was to explore CTC number as a potential surrogate for survival.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | All Randomly Assigned Patients (N = 1,195) |
Patients With Biomarker Data Available at Week 12 (n = 711) |
||||||
|---|---|---|---|---|---|---|---|---|
| AA Plus Prednisone (n = 797) |
Prednisone Alone (n = 398) |
AA Plus Prednisone (n = 484) |
Prednisone Alone (n = 227) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||
| Median | 69 | 69 | 70 | 69 | ||||
| Range | 42 to 95 | 39 to 90 | 42 to 95 | 45 to 90 | ||||
| Baseline ECOG status | ||||||||
| 0 to 1 | 715 | 90 | 353 | 89 | 453 | 94 | 208 | 92 |
| 2 | 82 | 10 | 45 | 11 | 31 | 6 | 19 | 8 |
| Level of worst pain at entry* | ||||||||
| 0 to 3 | 426 | 54 | 219 | 56 | 278 | 57 | 134 | 59 |
| 4 to 10 | 359 | 46 | 170 | 44 | 206 | 43 | 93 | 41 |
| No. of prior chemotherapy regimens | ||||||||
| 1 | 557 | 70 | 275 | 69 | 356 | 74 | 161 | 71 |
| 2 | 240 | 30 | 123 | 31 | 128 | 26 | 66 | 29 |
| Type of disease progression at baseline | ||||||||
| PSA only | 238 | 30 | 125 | 31 | 145 | 30 | 78 | 34 |
| Radiographic ± PSA | 559 | 70 | 273 | 69 | 339 | 70 | 149 | 66 |
| Extent of disease at baseline | n = 225 | |||||||
| Bone | 710 | 89 | 358 | 91 | 429 | 89 | 197 | 88 |
| Nodal | 361 | 45 | 164 | 42 | 231 | 48 | 87 | 39 |
| Visceral (liver and/or lung) | 173 | 22 | 65 | 16 | 88 | 18 | 28 | 12 |
| Baseline CTC count, cells/7.5 mL† | n = 595 | n = 300 | n = 457 | n = 217 | ||||
| 0 to 4 | 292 | 49 | 134 | 45 | 243 | 53 | 110 | 51 |
| ≥ 5 | 303 | 51 | 166 | 55 | 214 | 47 | 107 | 49 |
| Median | 5 | 6 | 4 | 4 | ||||
| Range | 0 to 100.1 | 0 to 100.1 | 0 to 100.1 | 0 to 100.1 | ||||
| Baseline LDH, U/L | n = 783 | n = 386 | n = 480 | n = 222 | ||||
| > 250 | 302 | 39 | 168 | 44 | 144 | 30 | 72 | 32 |
| ≤ 250 | 481 | 61 | 218 | 55 | 336 | 70 | 150 | 68 |
| Median | 223 | 238 | 211 | 222 | ||||
| Range | 84 to 3,373 | 123 to 5,125 | 84 to 3,373 | 124 to 1,246 | ||||
| Baseline PSA, ng/mL | n = 790 | n = 393 | n = 483 | n = 226 | ||||
| Median | 129 | 138 | 117 | 109 | ||||
| Range | 0.40 to 9,253 | 0.60 to 10,110 | 0.40 to 9,253 | 3.8 to 10,114 | ||||
| Baseline hemoglobin, g/dL | n = 779 | n = 389 | n = 476 | n = 225 | ||||
| Median | 12.0 | 12.0 | 12.0 | 12.0 | ||||
| Range | 7.3 to 16.1 | 7.2 to 16.5 | 7.3 to 15.2 | 8.3 to 16.5 | ||||
| Baseline alkaline phosphatase, U/L | n = 790 | n = 92 | n = 483 | n = 226 | ||||
| Median | 134 | 134 | 114 | 112 | ||||
| Range | 33 to 4,896 | 20 to 4,617 | 33 to 2,056 | 20 to 4,617 | ||||
| Baseline albumin, g/dL | n = 790 | n = 392 | n = 483 | n = 226 | ||||
| Median | 4.1 | 4.1 | 4.0 | 4.1 | ||||
| Range | 2.5 to 5.0 | 2.9 to 5.1 | 2.9 to 4.9 | 3.1 to 4.9 | ||||
NOTE. Trial eligibility criteria required PSA progression per Prostate Cancer Working Group 2 criteria,21 hemoglobin ≥ 9 g/dL, and albumin ≥ 3 g/dL; there were no prespecified criteria for LDH or alkaline phosphatase.
Abbreviations: AA, abiraterone acetate; CTC, circulating tumor cell; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PSA, prostate-specific antigen.
Brief Pain Inventory–Short Form question 3.
CTC count > 100 cells/7.5 mL was entered as 100.1 in database. Australian patients did not contribute CTC enumeration data.
Biomarker Panel
Factors measured at monthly intervals post-treatment were considered, with the addition of PSA, which was measured only every 12 weeks to maintain study blinding. The factors were CTC, PSA, LDH, hemoglobin, albumin, and alkaline phosphatase levels, based on inclusion in published nomograms for this population.26,27 Cut points for each variable were based on the upper or lower limits of normal: LDH, 250 U/L; hemoglobin, 12 g/dL; albumin, 4 g/dL; and alkaline phosphatase, 130 U/L. For CTC number, the US Food and Drug Administration–approved cutoff values for favorable (≤ 4 cells/7.5 mL) and unfavorable counts (≥ 5 cells/7.5 mL) were used, and for PSA, 30% and 50% decreases from baseline to week 12 were used, respectively. Each biomarker panel tested included CTCs, in accordance with the secondary objective of the trial, analyzed in one of three ways: fixed time point (eg, absolute CTC count at 12 weeks), difference from baseline (eg, CTC count at 12 weeks minus baseline CTC count), or relative difference from baseline (eg, percent change in CTC count from baseline to 12 weeks).
Surrogacy Analyses
The Prentice25 criteria were applied to assess the surrogate at the individual-patient level (Table 2). Prentice criterion one was assessed using a stratified log-rank test, criterion two using the score test from the proportional odds model, and criterion three using the likelihood ratio test from the stratified Cox model. The inverse-probability weighted c-index was used to scan for possible CTC-based surrogate biomarker combinations and to provide a quantitative measure of Prentice criterion three.28 The test to determine if Prentice criterion four was satisfied is described in the Data Supplement. The test is based on the proportional hazards model, and a test of proportionality based on the Schoenfeld residuals was applied.29 If the proportional hazards assumption was rejected, a non–model-based approach was used to evaluate Prentice criterion four. For more details on testing the proportional hazards assumption, see the Data Supplement.
Table 2.
Prentice25 Criteria for Individual Patient–Level Surrogacy
| Criterion | Description | Assessment |
|---|---|---|
| 1 | Treatment must have significant effect on clinical end point (ie, survival) | Treatment effect on survival was assessed using stratified log-rank test |
| 2 | Treatment must have significant effect on proposed biomarker | Treatment effect on surrogate was assessed using stratified score statistic from proportional odds regression model |
| 3 | Biomarker must have significant impact on clinical end point | Weighted c-index was used to quantify discriminatory power of surrogate with respect to survival; heuristically, for any two patients, c-index measures probability that patient classified as lower risk by surrogate also has longer survival time; values range between 0.5 and 1.0, with value 0.5 indicating that surrogate provides no information on survival time ranking |
| 4 | Full effect of treatment on clinical end point must be captured by biomarker | To show that after accounting for surrogate, treatment had no residual effect on survival, test for conditional independence was undertaken between survival model that included both patient surrogate and treatment assignment and survival model based solely on surrogate; Data Supplement provides details and results of this test |
To test the sensitivity of the surrogacy analysis to the exclusion of patients with missing 12-week biomarker data, surrogacy was reassessed by imputing the latest postbaseline biomarker data recorded ≤ 12 weeks from the start of treatment as the surrogate value for each patient. Thus, if a patient had marker values at weeks 4 and 8, but was missing a week-12 value for a marker, we used the week-8 value as the surrogate.
RESULTS
In this phase III trial, 1,542 patients were assessed for eligibility, 1,195 were enrolled, 1,091 survived for at least 12 weeks, and 899 had postbaseline CTC and LDH data and were observed for at least 12 weeks. Of the 296 patients who did not have CTC or LDH data recorded at week 12, 86 died or were withdrawn from the study before week 12. Thirty-four of the 86 patients were randomly assigned to receive prednisone alone, and 52 were randomly assigned to receive abiraterone acetate plus prednisone. The final analysis included a total of 711 patients with both CTC and LDH data recorded at week 12 (Fig 1). The baseline demographics and 12-week marker values for these patients are listed in Tables 1 and 3, respectively.
Fig 1.
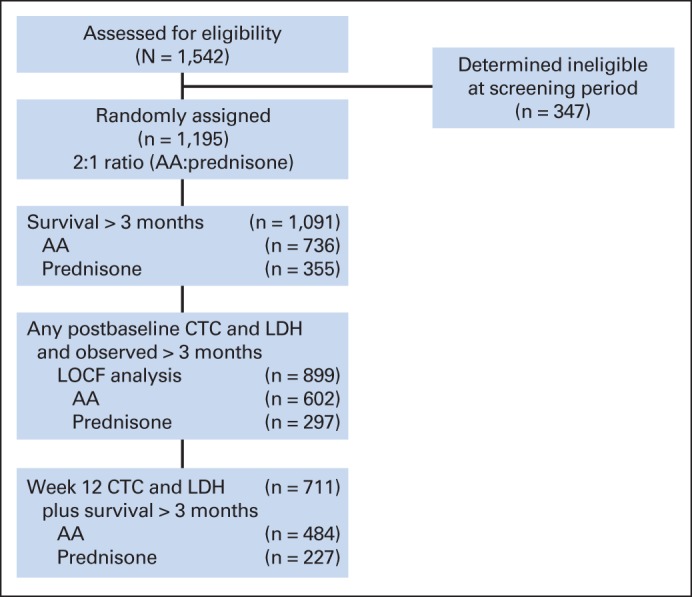
CONSORT diagram. AA, abiraterone acetate plus prednisone group; CTC, circulating tumor cell; LDH, lactate dehydrogenase; LOCF, last observation carried forward.
Table 3.
Descriptive Statistics for Markers at 12 Weeks
| Marker | All Randomly Assigned Patients (N = 1,195) |
Patients With Biomarker Data Available at Week 12 (n = 711) |
||
|---|---|---|---|---|
| AA Plus Prednisone (n = 797) | Prednisone Alone (n = 398) | AA Plus Prednisone (n = 484) | Prednisone Alone (n = 227) | |
| CTCs, cells/7.5 mL* | n = 489 | n = 232 | n = 484 | n = 227 |
| Median | 1 | 6 | 1 | 6 |
| Range | 0 to 100.1 | 0 to 100.1 | 0 to 100.1 | 0 to 100.1 |
| LDH, U/L | n = 656 | n = 300 | n = 476 | n = 225 |
| Median | 212 | 246 | 212 | 239 |
| Range | 87 to 4,895 | 133 to 3,563 | 87 to 4,895 | 133 to 2,093 |
| PSA, ng/mL | n = 647 | n = 294 | n = 464 | n = 221 |
| Median | 70 | 209 | 7.9 | 197.9 |
| Range | 0.1 to 8,582 | 0.7 to 8,985 | 0.1 to 8,582 | 0.7 to 8,985 |
| Hemoglobin, g/dL | n = 650 | n = 295 | n = 473 | n = 222 |
| Median | 12.4 | 12.0 | 12.4 | 12.2 |
| Range | 5.7 to 16.3 | 7.0 to 16.6 | 5.7 to 16.3 | 7.0 to 16.6 |
| Phosphatase, U/L | n = 646 | n = 287 | n = 471 | n = 221 |
| Median | 135 | 154 | 133 | 144 |
| Range | 27 to 2,499 | 22 to 3,632 | 27 to 2,499 | 22 to 3,632 |
| Albumin, g/dL | n = 664 | n = 301 | n = 486 | n = 226 |
| Median | 4.1 | 4.1 | 4.1 | 4.1 |
| Range | 2.6 to 5.3 | 2.8 to 5.7 | 2.6 to 5.3 | 2.8 to 5.0 |
Abbreviations: AA, abiraterone acetate; CTC, circulating tumor cell; LDH, lactate dehydrogenase; PSA, prostate-specific antigen.
CTC count > 100 cells/7.5 mL was entered as 100.1 in database. Australian patients did not contribute CTC enumeration data.
The Kaplan-Meier estimates of overall survival by treatment group (Data Supplement) showed a statistically significant (P = .035) and clinically meaningful survival difference between the abiraterone acetate plus prednisone and prednisone-alone groups (17.7 v 15.1 months; hazard ratio, 0.80; 95% CI, 0.65 to 0.98), which mirrored the previously reported survival benefit shown in the overall intent-to-treat population.2,3 This finding satisfied Prentice25 criterion one and provided the framework for evaluating a surrogate end point for survival.
A landmark analysis at 12 weeks was used to explore the discriminatory power of CTC count alone or CTC-containing biomarker combinations. For a two-biomarker combination, the categorization of risk groups was 0, 1, or 2, representing the number of markers above the upper limit or below the lower limit of normal, as appropriate. The results listed in Table 4 indicate that CTC count alone provided the strongest discrimination between risk groups, followed by CTC count in combination with LDH level and LDH level alone. However, for the CTC count–alone and LDH level–alone biomarkers, the proportional hazards assumption was not satisfied (global test of proportionality P = .04), and Prentice25 criterion four was not attained using a non–model-based evaluation (Data Supplement). As a result, we proceeded to construct a biomarker panel with the CTC plus LDH combination biomarker. For completeness, Table 4 summarizes the discriminatory power of all single- and two-factor combinations. The P values were based on a bootstrap test comparing the weighted c-indices of each biomarker combination with the CTC plus LDH combination. As shown, non–CTC-based combinations had significantly smaller c-indices than the CTC plus LDH combination.
Table 4.
Weighted C-Indices As Measure of Concordance Between Survival and Week-12 Biomarkers Alone or in Combination (n = 711)
| Marker Combination* | Weighted C-Index† | SE | P‡ |
|---|---|---|---|
| CTC | 0.82 | 0.02 | |
| CTC absolute change§‖ | 0.73 | 0.03 | |
| CTC relative change§¶ | 0.73 | 0.03 | |
| CTC plus LDH | 0.80 | 0.02 | |
| LDH | 0.78 | 0.02 | .075 |
| PSA50§ | 0.73 | 0.03 | .008 |
| PSA30§ | 0.71 | 0.02 | .002 |
| HGB | 0.71 | 0.03 | .002 |
| ALK | 0.72 | 0.03 | .026 |
| ALB | 0.71 | 0.03 | < .001 |
| CTC plus PSA50 | 0.77 | 0.02 | .314 |
| CTC plus PSA30 | 0.76 | 0.02 | .035 |
| CTC plus HGB | 0.75 | 0.02 | .149 |
| CTC plus ALK | 0.75 | 0.03 | .096 |
| CTC plus ALB | 0.76 | 0.02 | .333 |
| LDH plus PSA50 | 0.76 | 0.02 | .002 |
| LDH plus PSA30 | 0.74 | 0.02 | .001 |
| LDH plus HGB | 0.75 | 0.02 | < .001 |
| LDH plus ALK | 0.75 | 0.02 | .001 |
| LDH plus ALB | 0.76 | 0.02 | .002 |
| PSA50 plus HGB | 0.75 | 0.02 | .031 |
| PSA50 plus ALK | 0.75 | 0.02 | .024 |
| PSA50 plus ALB | 0.75 | 0.02 | .005 |
| HGB plus ALK | 0.72 | 0.03 | .006 |
| HGB plus ALB | 0.73 | 0.02 | < .001 |
| ALK plus ALB | 0.74 | 0.02 | .002 |
Abbreviations: ALB, albumin; ALK, alkaline phosphatase; CTC, circulating tumor cell; HGB, hemoglobin; LDH, lactate dehydrogenase; PSA, prostate-specific antigen; PSA30, 30% decrease in PSA from baseline to week 12; PSA50, 50% decrease in PSA from baseline to week 12.
For two-biomarker combination, categorization of risk groups was 0, 1, and 2, which represented number of markers above or below the upper or lower limit of normal.
Weighted c-index evaluated concordance between risk group score at 12 weeks and survival time.
P value was based on bootstrap test comparing weighted c-indices of biomarker combinations with CTC plus LDH biomarker combination; significant P value (< .05) was indication that CTC plus LDH combination had higher c-index.
Most panels were tested using only absolute values (first option listed under Patients and Methods, Biomarker Panel). Exceptions were CTC absolute change (second option), CTC relative change (third option), and CTC plus PSA50 (PSA50 was dichotomous variable based on relative difference from baseline).
Absolute change of CTC biomarker from baseline to week 12; threshold chosen for absolute change was 0.50, derived from regression tree analysis.
Relative change of CTC biomarker from baseline to week 12; threshold chosen for relative change was 0.15, derived from regression tree analysis.
The four Prentice25 criteria were satisfied using a constructed CTC plus LDH biomarker, categorized as: low (CTCs ≤ 4; any LDH), intermediate (CTCs ≥ 5; LDH ≤ 250), and high risk (CTCs ≥ 5 cells/7.5 mL of blood; LDH > 250 U/L). The dichotomization of CTCs (≥ 5 v ≤ 4 cells/7.5 mL of blood) and LDH level (abnormal [> 250] v normal [≤ 250 U/L]) was consistent with previous work.22
With the three risk groups defined by the CTC plus LDH biomarker, the prednisone-alone group had a higher percentage of high-risk, poor-prognosis patients and a lower percentage of low-risk, better-prognosis patients than the abiraterone acetate plus prednisone group (Table 5) at 12 weeks. The treatment effect on the surrogate, using a stratified score statistic from the proportional odds regression model, was statistically significant (P < .001), indicating that the surrogate distribution differed by treatment, satisfying Prentice25 criterion two.
Table 5.
Frequency of Surrogate Risk Group Categories at 12 Weeks and Survival Probability Estimates
| Surrogate Category | AA Plus Prednisone (n = 484) |
Prednisone Alone (n = 227) |
All Patients (n = 711) |
Survival Probability |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Year |
2 Years |
|||||||||
| No. | % | No. | % | No. | % | % | 95% CI | % | 95% CI | |
| High risk (CTCs ≥ 5 cells/7.5 mL; LDH > 250 U/L) | 71 | 15 | 74 | 33 | 145 | 20 | 0.25 | 0.19 to 0.33 | 0.02 | 0.00 to 0.11 |
| Intermediate risk (CTCs ≥ 5 cells/7.5 mL; LDH ≤ 250 U/L) | 72 | 15 | 44 | 19 | 116 | 16 | 0.51 | 0.42 to 0.61 | 0.10 | 0.03 to 0.29 |
| Low risk (CTC < 5 cells/7.5 mL) | 341 | 70 | 109 | 48 | 450 | 63 | 0.82 | 0.79 to 0.86 | 0.46 | 0.39 to 0.54 |
NOTE. Prentice25 criterion two is satisfied by AA Plus Prednisone and Prednisone Alone columns, which show higher frequency of the favorable (low risk) category in patients treated with AA plus prednisone (ie, that surrogate measure reflected treatment effect of AA plus prednisone).
Abbreviations: AA, abiraterone acetate; CTC, circulating tumor cell; LDH, lactate dehydrogenase.
Figure 2A shows the Kaplan-Meier estimates of survival for the three surrogate risk categories based on the 12-week CTC and LDH values. Median overall survival for the high-, intermediate-, and low-risk groups was, respectively: 8.71 (95% CI, 7.8 to 9.63), 12.02 (95% CI, 1.68 to 15.31), and 22.18 months (95% CI, 2.83 to upper limit not reached). The three surrogate groups separated patient risk, and the result of the stratified log-rank test for the surrogate effect on survival was statistically significant (P < .001). The weighted c-index for the three surrogate risk categories was 0.81 (SE, 0.02), a high value that provides strong evidence that the surrogate was able to discriminate survival time, satisfying Prentice25 criterion three. Table 5 lists the 1- and 2-year survival probabilities, respectively, by risk group: 82% and 46% (low-), 51% and 10% (intermediate-), and 25% and 2% (high-risk patients).
Fig 2.
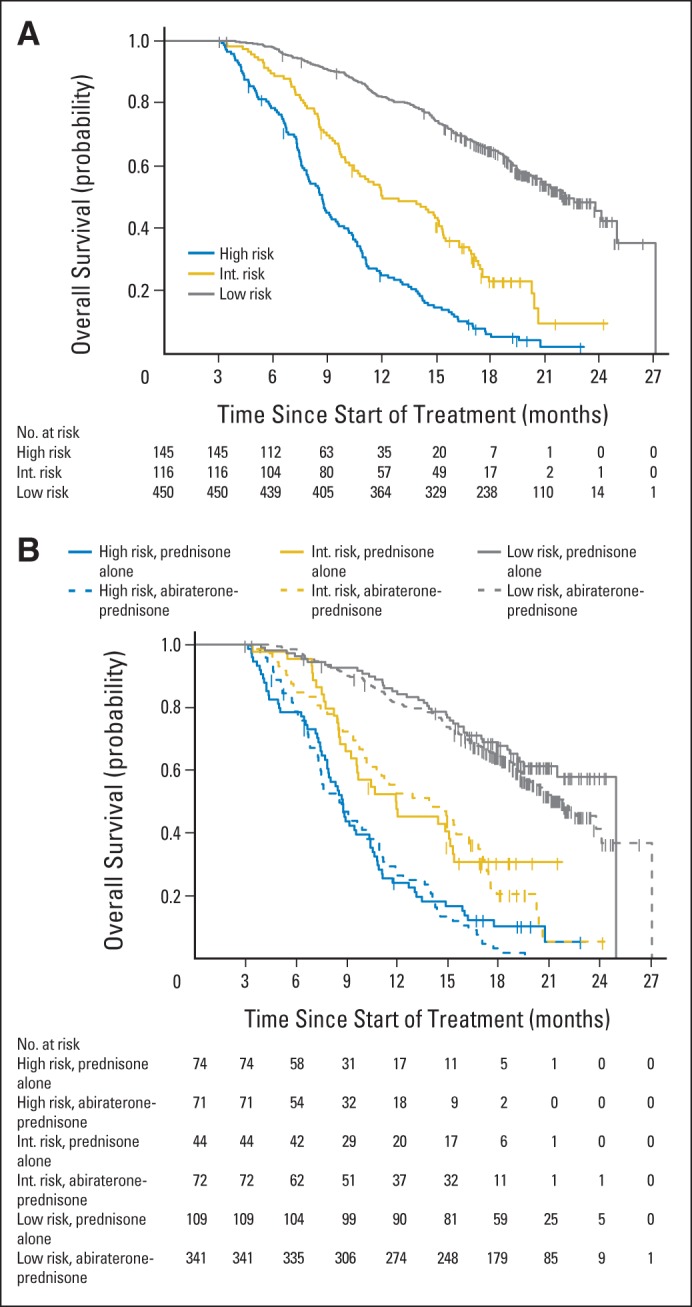
Kaplan-Meier estimates of survival for (A) surrogate risk category (n = 711) and (B) surrogate risk category and treatment group (n = 711). Int, intermediate.
Prentice25 criterion four requires that treatment assignment is independent of survival once the surrogate is accounted for. This was carried out as a test of equivalence between the Cox survival model based on treatment assignment and the surrogate, and the model based on the surrogate alone. The proportionality assumption for these models could not be rejected (global tests of proportionality, P = .13 and P = .33). Details are supplied in the Data Supplement. The P values for this test of equivalence were calculated for each month between 6 and 24 months and adjusted to account for multiple testing. The maximum adjusted P value was less than .001. This significant result showed equivalence; the Cox survival model derived with the surrogate and the treatment assignment was equivalent to the survival model based on the surrogate alone at each monthly time point between 6 and 24 months. This indicated that there was little added value to including treatment assignment in the model and that Prentice criterion four was satisfied. A depiction of the lack of treatment effect after accounting for the surrogate is provided in Figure 2B.
The sensitivity analysis, which replaced missing CTC and LDH week-12 data with earlier recorded postbaseline values from 899 patients, supported up to month 20 the attainment of the fourth Prentice criterion. The results are provided in the Data Supplement.
DISCUSSION
Prentice25 defined a surrogate as a post-treatment measure that both was prognostic for a clinical end point and captured the effect of the treatment on that end point. Establishing a surrogate for survival has the potential to shorten drug development timelines and to minimize the chance of postprotocol therapy masking the survival benefit of an experimental drug. Data from multiple trials across a range of cancers have shown that patients with detectable CTCs in blood at the start of a treatment or after treatment have inferior survival times relative to those who do not. Here we show for the first time to our knowledge that a biomarker panel containing CTC number and LDH level satisfied the Prentice criteria for individual-patient surrogacy within a randomized clinical trial where abiraterone acetate plus prednisone improved survival relative to prednisone alone (hazard ratio, 0.74; 95% CI, 0.64 to 0.86; P < .001). The surrogate categorized patients based on the 12-week levels of CTCs and LDH as low (CTCs < 5; any LDH), intermediate (CTCs ≥ 5; LDH ≤ 250), and high risk (CTCs ≥ 5 cells/7.5 mL of blood; LDH > 250 U/L). Applying the Prentice criteria, we showed: a survival advantage for patients receiving the experimental treatment (P < .001; criterion one); a more favorable change in risk for patients receiving the experimental treatment (P < .001; criterion two); that the surrogate had a high discriminatory prognostic power based on a low- to high-risk categorization (weighted c-index, 0.81), with a 1- and 2-year survival of 82% and 46% for those with CTCs ≤ 4 at 12 weeks versus 25% and 2% for patients with CTCs ≥ 5 cells/7.5 mL of blood and an abnormal LDH at 12 weeks (criterion three); and that the treatment effect on survival was eliminated when the surrogate was added to the model (criterion four). The last criterion—the most difficult to satisfy—was demonstrated using a test of conditional independence, where the survival model based on the treatment and the surrogate was equivalent to the model using the surrogate alone (Fig 2B). The results were supported by a sensitivity analysis that replaced missing 12-week biomarker data with their nearest postbaseline values.
Consistent with reported results in other series,20,22,30,31 CTC count alone and LDH level alone both showed high discriminatory power with respect to prognosis. The observation that both elevated CTC count (≥ 5 cells/7.5 mL of blood) alone and elevated LDH value alone at week 12 were associated with inferior survival times supports their use as outcome measures in phase II trials in CRPC.
Neither one alone, however, satisfied the rigorous criteria for surrogacy. That LDH level would add to CTC count is plausible, both scientifically and biologically. Tumors that continue to shed cells into circulation are likely to be more aggressive than those that do not, and although LDH level, an indicator of tumor burden, is only elevated in a small proportion of men with progressive CRPC, the impact on survival is highly negative when it is. Other biomarkers for survival reported in various CRPC nomograms,32–34 such as PSA, hemoglobin, albumin, and alkaline phosphatase, assessed either alone or in combination, did not add to the discriminatory power of the surrogate.
A common methodologic error in testing Prentice25 criterion four is to perform a test comparing the survival rates between the two treatments, adjust for the surrogate, and conclude that the criterion is satisfied if the adjusted test is not significant. However, this does not imply that the treatment had no effect on survival after this adjustment. To address this, for validation, we used a test for equivalence between the survival function that included the patient surrogate classification and treatment assignment, and the survival function based solely on the surrogate.
A limitation of our study was that only 59% (711 of 1,195) of the patients enrolled had CTC enumeration performed at week 12. However, this was addressed in part by the sensitivity analyses, which included 75% of enrolled patients and demonstrated that the Prentice25 criteria were still satisfied in this larger subset. In addition, Prentice criterion four has a causal interpretation only if there are no unmeasured confounders that affect the surrogate and the true end point. This was addressed to the extent possible by adjusting the analysis for the protocol-specified stratification factors (Eastern Cooperative Oncology Group status, bone pain index, prior chemotherapy, and type of prior progression).
Establishing surrogacy requires an analytically valid biomarker and multiple appropriately powered and controlled phase III trials. This trial is the first of a series of phase III studies designed to generate evidence to qualify a survival surrogate that can be used for regulatory submissions. Such a surrogate would shorten drug development times and eliminate the potential confounding effects of postprotocol therapy on survival. Ultimately, the validity of an outcome measure as a surrogate for survival requires assessment at the individual-patient level and trial level. Trial-level surrogacy goes beyond the Prentice25 criteria, because it requires that a treatment-induced change in the surrogate translate to a predictable treatment-induced change in survival over a whole cohort. This is typically tested using a meta-analysis of several randomized trials, with large numbers of patients, addressing the same question.35 After the initial trial, a series of trials of similar design would continue with a drug of the same class or a drug that targets the same pathway and then proceed to agents with different mechanisms in the same disease state. As examples, the demonstration of surrogacy in HIV was achieved with five trials enrolling more than 5,000 patients36 and in colorectal cancer with 18 trials enrolling more than 20,000 patients.37 We await data from additional trials to test if the CTC plus LDH biomarker panel is valid for trial-level surrogacy and subsequent testing in prospective clinical trials.
In conclusion, a biomarker panel containing CTC count and LDH level demonstrated individual patient-level surrogacy in this single phase III trial, further supporting use as a clinical trial end point. Independent phase III trials are ongoing to validate the individual patient–level surrogacy shown here and to begin the process of testing trial-level surrogacy to enable the end point to become part of regulatory submissions.
Supplementary Material
Acknowledgment
We thank Amy Plofker, Memorial Sloan Kettering Cancer Center editor, for editorial assistance. Presented in part at the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011, and 2013 European Cancer Congress, Amsterdam, the Netherlands, September 27-October 1, 2013.
Support information appears at the end of this article.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00638690.
Support
Supported by Cougar Biotechnology (now Janssen Oncology), the Prostate Cancer Foundation, and Veridex (now Janssen Diagnostics); by the Sidney Kimmel Center for Prostate and Urologic Cancers; by Memorial Sloan Kettering Cancer Center (MSKCC) Specialized Programs of Research Excellence in Prostate Cancer Grant No. P50 CA92629 (H.I.S., D.C.D., G.H.); by Department of Defense Prostate Cancer Research Program No. PC051382 (H.I.S., G.H.); by Mr William H. and Mrs Alice Goodwin and the Commonwealth Foundation for Cancer Research and the MSKCC Experimental Therapeutics Center (H.I.S., D.C.D., M.F.); by the Starr Cancer Consortium (H.I.S., D.C.D., M.F.); and by a Medical Research Council Biomarkers grant, Prostate Cancer UK, an Experimental Cancer Medicine Centre grant, and the Biomedical Research Centre (J.S.de B.).
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Arturo Molina, Johnson & Johnson (C); Weimin Peng, Janssen Research & Development (C); Robert McCormack, Janssen Pharmaceuticals (C), Veridex (C), Johnson & Johnson (C); Tomasz Burzykowski, IDDI (C); Thian Kheoh, Janssen Research & Development (C); Marc Buyse, IDDI (C) Consultant or Advisory Role: Howard I. Scher, AstraZeneca (U), Astellas Pharma (C), BIND Therapeutics (C), Bristol-Myers Squibb (U), Celgene (U), Chugai Academy for Advanced Oncology (C), Endocyte (U), Exelixis (U), Ferring Pharmaceuticals (C), Foundation Medicine (U), Genentech (U), Janssen Pharmaceuticals (U), Medivation (U), Millennium Pharmaceuticals (U), OncologySTAT (C), Palmetto GBA (C), Pfizer (U), sanofi-aventis (C), Roche/Ventana Medical Systems (U), WCG Oncology (C); Glenn Heller, Millennium Pharmaceuticals (C); Gerhardt Attard, Janssen-Cilag (C), Veridex (C), Roche/Ventana Medical Systems (C), Astellas Pharma (C), Novartis (C), Millennium Pharmaceuticals (C), Abbott Laboratories (C); Daniel C. Danila, Cougar Biotechnology (U); David Olmos, Veridex (C); Johann S. de Bono, Johnson & Johnson (C), Astellas Pharma (C), Medivation (C) Stock Ownership: Arturo Molina, Johnson & Johnson; Robert McCormack, Johnson & Johnson; Tomasz Burzykowski, IDDI; Thian Kheoh, Johnson & Johnson; Marc Buyse, IDDI Honoraria: Howard I. Scher, Chugai Academy for Advanced Oncology; Gerhardt Attard, Janssen Pharmaceuticals, Ipsen, Takeda Pharmaceuticals, sanofi-aventis, Astellas Pharma, Veridex; David Olmos, Veridex, Janssen Pharmaceutical Companies of Johnson & Johnson, Janssen-Cilag Spain; Johann S. de Bono, Johnson & Johnson Research Funding: Howard I. Scher, BIND Therapeutics, Exelixis, Janssen Pharmaceuticals, Medivation, Janssen Diagnostics; Gerhardt Attard, AstraZeneca, Genentech, Janssen Pharmaceuticals; Daniel C. Danila, Cougar Biotechnology/Johnson & Johnson; David Olmos, Fundación Científica de la Asociación Española Contra el Cáncer, Veridex; Johann S. de Bono, Janssen Pharmaceuticals Expert Testimony: None Patents, Royalties, and Licenses: Gerhardt Attard, Abiraterone awards to invention (Institute of Cancer Research) Other Remuneration: Gerhardt Attard, Janssen-Cilag, Veridex, Roche/Ventana Medical Systems, Astellas Pharma, Novartis, Millennium Pharmaceuticals, Abbott Laboratories
AUTHOR CONTRIBUTIONS
Conception and design: Howard I. Scher, Glenn Heller, Arturo Molina, Gerhardt Attard, Robert McCormack, Tomasz Burzykowski, Thian Kheoh, Marc Buyse, Johann S. de Bono
Financial support: Howard I. Scher, Arturo Molina, Robert McCormack, Johann S. de Bono
Administrative support: Howard I. Scher, Arturo Molina, Johann S. de Bono
Provision of study materials or patients: Howard I. Scher, Arturo Molina, Gerhardt Attard, Shahneen K. Sandhu, David Olmos, Johann S. de Bono
Collection and assembly of data: Howard I. Scher, Arturo Molina, Gerhardt Attard, Daniel C. Danila, Shahneen K. Sandhu, David Olmos, Ruth Riisnaes, Robert McCormack, Martin Fleisher, Johann S. de Bono
Data analysis and interpretation: Howard I. Scher, Glenn Heller, Arturo Molina, Xiaoyu Jia, Weimin Peng, Tomasz Burzykowski, Thian Kheoh, Marc Buyse, Johann S. de Bono
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 2.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 6.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 8.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 9.Parkinson DR, Dracopoli N, Petty BG, et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med. 2012;10:138. doi: 10.1186/1479-5876-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration. CellSearch Circulating Tumor Cell Kit: K073338—Premarket notification: Expanded indications for use—Metastatic prostate cancer. http://www.accessdata.fda.gov/cdrh_docs/pdf7/K073338.pdf.
- 11.US Food and Drug Administration. CellSearch Epithelial Cell Kit/CellSpotter Analyzer: K031588. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm081239.htm.
- 12.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 13.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 14.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 15.Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 17.Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96:1241–1246. doi: 10.1111/j.1464-410X.2005.05821.x. [DOI] [PubMed] [Google Scholar]
- 18.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 19.Fleming MT, Morris MJ, Heller G, et al. Post-therapy changes in PSA as an outcome measure in prostate cancer clinical trials. Nat Clin Pract Oncol. 2006;3:658–667. doi: 10.1038/ncponc0664. [DOI] [PubMed] [Google Scholar]
- 20.Omlin A, Pezaro C, Mukherji D, et al. Improved survival in a cohort of trial participants with metastatic castration-resistant prostate cancer demonstrates the need for updated prognostic nomograms. Eur Urol. 2013;64:300–306. doi: 10.1016/j.eururo.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 21.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–239. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prentice RL. Surrogate endpoints in clinical trials: Definition and operational criteria. Stat Med. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong AJ, Garrett-Mayer E, de Wit R, et al. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16:203–211. doi: 10.1158/1078-0432.CCR-09-2514. [DOI] [PubMed] [Google Scholar]
- 27.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 28.Uno H, Cai T, Pencina MJ, et al. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30:1105–1117. doi: 10.1002/sim.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 30.Goldkorn A, Ely B, Quinn DI, et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: A phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:1136–1142. doi: 10.1200/JCO.2013.51.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman OB, Jr, Symanowski JT, Loudyi A, et al. Circulating tumor cells as a predictive biomarker in patients with hormone-sensitive prostate cancer. Clin Genitourin Cancer. 2011;9:31–38. doi: 10.1016/j.clgc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Halabi S, Small EJ, Hayes DF, et al. Prognostic significance of reverse transcriptase polymerase chain reaction for prostate-specific antigen in metastatic prostate cancer: A nested study within CALGB 9583. J Clin Oncol. 2003;21:490–495. doi: 10.1200/JCO.2003.04.104. [DOI] [PubMed] [Google Scholar]
- 33.Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:671–677. doi: 10.1200/JCO.2013.52.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smaletz O, Scher HI, Small EJ, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–3982. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Buyse M, Molenberghs G, Burzykowski T, et al. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;1:49–67. doi: 10.1093/biostatistics/1.1.49. [DOI] [PubMed] [Google Scholar]
- 36.Petrella M, Montaner J, Batist G, et al. The role of surrogate markers in the clinical development of antiretroviral therapy: A model for early evaluation of targeted cancer drugs. Cancer Invest. 2004;22:149–160. doi: 10.1081/cnv-120027590. [DOI] [PubMed] [Google Scholar]
- 37.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


