Abstract
Agricultural residues like sugarcane bagasse (SCB), corn husk (CH), peanut husk (PNH), coffee cherry husk (CCH), rice bran (RB) and wheat bran (WB) are low-value byproducts of agriculture. They have been shown to contain significant levels of phenolic compounds with demonstrated antioxidant properties. In this study, the effects of two types of solvent extraction methods: solid–liquid extraction (SLE) and hot water extraction on the recovery of phenolic compounds from agricultural residues were investigated to optimize the extraction conditions based on total phenolic content (TPC), total tannin content (TTC) and total flavonoids content (TFC). Methanol (50 %) was found to be the most efficient solvent for the extraction of phenolics with higher DPPH, nitric oxide radical scavenging and reducing power activity, followed by ethanol and water. The phenolic compounds of methanolic extracts (50 %) were determined by reverse phase high performance liquid chromatography; in addition gallic acid became the major phenolic acid present in all the agricultural residues whereas ferulic acid, epicatechin, catechin, quercitin and kampferol present in lesser amounts. The present investigation suggested that agricultural residues are potent antioxidants. The overall results of this research demonstrated the potential of agricultural residues to be an abundant source of natural antioxidants suitable for further development into dietary supplements and various food additives.
Keywords: Agriculture residues, Polyphenols, Tannins, Flavonoids, Antioxidant activity
Introduction
Polyphenols and flavonoids are widely distributed in plants with significant applications in the health and food industries (Sarikaya and Ladisch 1999; Ventura et al. 2008). Such compounds include a variety of phenolic acids (hydroxybenzoic acids and hydroxycinnamic acids), flavonoids (flavonols, flavones, flavonones, isoflavones and anthocyanidins), stilbenes and lignans (Hooper and Cassidy 2006). These phytochemicals were known to reduce many chronic diseases such as cardiovascular diseases, heart diseases, diabetes, obesity and certain cancer and improve endothelial functions and reduce blood pressure (Liu 2007; Yawadio et al. 2007). Among these compounds, a strong correlation between antioxidant activity and the total phenolic content in the plants has been observed, suggesting that phenolic compounds could be the major contributor of their antioxidant capacity (Li et al. 2008).
Phenolic compounds in large quantities are known to be present in edible and non edible plants (Gharras 2009), vegetables, fruits, herbs and other plant materials which are rich in phenolics are increasingly being used for the extraction of phenolics at the industrial level. Using valuable horti crops which are human food resources, for phenolic production or extraction raises major ethical and moral issues. This would be a very costly affair for the developing and under developing countries. Expanding phenolics production could divert valuable fruits and vegetables needed to feed the people. This further would increases malnutrition in the developing and under developing countries.
Alternatively, for the production or extraction of bioactive phenolic compounds, agro industrial residues could be exploited. Large quantities from these materials, including seeds, peels, husks among others are generated every year in the form of wastes and are poorly harvested or left to decay on the land. Increased attention is being given for these materials as abundantly available and cheap renewable feedstocks for the production of value added compounds.
Antioxidants are any substance that delay or inhibits oxidative damage to a target molecule. Antioxidants prevent cell and tissue damage as they act as scavenger. Antioxidants can terminate or retard the oxidation process by scavenging free radicals. Overproduction of the free radicals can be responsible for tissue injury. Anti-oxidants are substances capable to mop up free radicals and prevent them from causing cell damage. Free radicals are responsible for causing a wide number of health problems which, include cancer, aging, heart diseases, gastric problems etc.
Antioxidant capacity is widely used as a parameter for medicinal bioactive components. Various methods are currently used to assess the antioxidant activity of plant phenolic compounds. ABTS or DPPH radical scavenging methods are common spectrophotometric procedures for determining the antioxidant capacities of components (Gulçin et al. 2010).
In the present study, waste feedstocks such as sugarcane bagasse (SCB), corn husk (CH), peanut husk (PNH), coffee cherry husk (CCH), rice bran (RB) and wheat bran (WB) were screened for the extraction of different bioactive phenolics and characterized.
Materials and methods
Materials
Agricultural residues such as sugarcane bagasse (SCB), corn husk (CH), peanut husk (PNH), rice bran (RB) and wheat bran (WB) were obtained from a local agriculture farm at Gulbarga, Karnataka, India. Coffee cherry husks (CCH) were obtained by grain mill, CFTRI, Mysore.
Chemicals
Methanol and alcohol were purchased from Merck (Darmstadt, Germany). All the chemicals and reference compounds were purchased from Sigma Aldrich (Steinheim, Germany).
Sample pretreatment
Agricultural residues were ground between 10 and 30 mesh (0.5 mm and 2.0 mm) were collected and defatted using n-hexane (ratio of solid/liquid 1/10, w/w) at room temperature and then the solvent was evaporated.
Extraction and concentration
The extraction of phenolic compounds was carried out using solvents at different polarity: 50 %, 70 % and 100 % of methanol; 50 %, 70 % and 100 % of ethanol and water. The powdered samples were added to a solvent 1:10 mixed well and kept at room temperature, for 3 days under constant stirring. The mixture was centrifuged at 6,000 rpm for 15 min and the supernatant was filtered through a filter paper. Then, the solvent was evaporated in a rotavapor. The extraction yield was expressed as dry matter percentage.
Total phenols content (TPC)
Total polyphenols analysis was performed by the colorimetric method, as described by Vazquez et al. (2008), with some modifications. The sample was re-dissolved in the extraction medium. To 100 μl of sample, 500 μl of Folin-Ciocalteu reactive, and 400 μl of 7.5 % aqueous solution of Na2CO3 were added. The mixture was kept for 30 min in the dark at room temperature. The absorbance was read at 720 nm using a UV/Vis spectrophotometer (UV-6450; Jenway, UK). Gallic acid (5–50 mM) was used for constructing the standard curve, and the results were expressed as g of gallic acid equivalents (GAE)/100 g of extract.
Total tannins content (TTC)
Total tannins analysis were performed by the colorimetric method, as described by Price and Butler (1977). To 100 μl of sample, 1 ml of 1 % potassium ferricyanide and 8 % of aqueous solution of ferric chloride was added. The mixture was kept for 5 min at room temperature. The absorbance was read at 720 nm using a UV/Vis spectrophotometer (UV-6450; Jenway, UK). Tannic acid (5–50 mM) was used for constructing the standard curve, and the results were expressed as g of tannic acid equivalents (TAE)/100 g of extract.
Total flavonoids content (TFC)
Total flavonoid content was determined by a colorimetric method (Bao et al. 2005). 0.5 ml extracts were added to 15 ml volumetric flasks containing 2 ml ddH2O and mixed with 5 % of 0.15 ml NaNO2. After reacting for 5 min, 0.15 ml 10 % AlCl3.6H2O solution was added. After another 5 min, 1 ml 1 M NaOH was added. The reaction solution was well mixed, kept for 15 min and the absorbance was determined at 415 nm. Quantification was done using the quercitin as standard and the results were expressed as g of quercitin equivalents (QE)/100 g of extract.
HPLC determination of individual polyphenols
The concentration of individual polyphenols was determined by HPLC Shimadzu LC-10A with a UV detector recording at 280 nm was used to detect the phenolic compounds. A reverse phase C-18 column (15 cm) was used with a flow rate of 0.8/min of a solvent system containing water, methanol, acetic acid were in the ratio of 80:18:2. The standard mixtures of polyphenols were prepared in the concentration of 1 mg/ml. The standard and sample of 0.02 ml were injected into the column. Peaks were identified by the retention time of the commercial standard phenolic compounds.
Antioxidant assays
DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical scavenging assay
The electron donation ability of the obtained methanol extracts of agricultural residues was measured by bleaching of the purple colored solution of DDPH (1, 1-diphenyl-2-picrylhydrazyl) radical according to the method of Sun et al. (1988). Methanolic extracts (2 ml, 10–1,000 μg/ml) were added to 0.5 ml of 0.2 mM/L DDPH. After incubation period of 30 min at room temperature, the absorbance was measured against a blank at 517 nm using UV/Vis spectrophotometer (UV-6450; Jenway, UK) versus ethanol as a blank. The antioxidant activity was calculated by the following ratio: (blank-sample/blank) × 100, where blank is the absorption of the DPPH solution and sample is the absorption of the DPPH solution after the addition of the sample.
Nitric oxide (NO) radical scavenging assay
The assay was carried out according to Isfahlan et al. (2010). The reaction mixture contained 10 mM SNP (Sodium nitroprusside), phosphate-buffered saline (pH 7.4), and the various concentrations of the samples. After incubation for 150 min at 25 °C, 1 ml of sulfanilic acid (0.33 % in 20 % glacial acetic acid) was added to 0.3 ml of the incubated solution, and the mixture allowed to stand for 5 min. NED (naphthyl ethylene diamine dihydrochloride, 0.5 ml, 0.1 % w/v) was added, and the mixture was incubated for 30 min at 25 °C. The pink chromophore, generated during diazotization of nitrite ions with sulfanilic acid and subsequent coupling with NED, was measured from the absorbance at 540 nm, using an appropriate blank.
IC50 values were calculated from the plotted graph of scavenging activity against the concentrations of the samples. IC50 is defined as the total antioxidant necessary to decrease the initial DPPH and NO radical by 50 %. IC50 was calculated for all the extracts based on the percentage of DPPH radicals scavenged. Ascorbic acid was used as the reference compound (positive control) with concentrations 50 to 500 μg/ml for both the above spectroscopic methods.
FRAP (ferric ion reducing antioxidant potential) assay
The antioxidant activity of samples was determined by the FRAP assay, according to Barriera et al. (2008). Various concentrations of extracts (100–500 μg/ml) were mixed with 2.5 ml of 200 mM sodium phosphate buffer (pH, 6.6) and 2.5 ml of 1 % potassium ferricyanide. The mixture was incubated at 50 °C for 20 min then 2.5 ml of 1 % TCA (w/w) was added. This was followed by the addition of 5 ml of distilled water and 1 ml of 0.1 % of ferric chloride. The absorbance was recorded at 700 nm after 5 min. The antioxidant activity was calculated from the calibration curve of ascorbic acid (0.1–1 mM). Results were expressed as μmol of ascorbic acid equivalents (AAE)/mg of extract.
Results and discussion
Extraction yield
The results of the extraction yields are presented in Table 1. Organic solvents such as methanol and ethanol with different ratio were used for the extraction of polyphenols and tannins from the agricultural residues. The highest yields are usually achieved with methanol and ethanol and their mixtures with water, although other solvents have been widely used in the extraction of polyphenols from plants, as ethyl acetate or acetone. Water and ethanol are the most widely used because of their low toxicity and high extraction yield, with the advantage of modulating the polarity of the solvent by using ethanol/water mixtures at different ratios. The main drawback of the aqueous extraction is the low yield in antioxidants with low polarity or liposoluble antioxidants as, for example, the carotenoids. Solubility of polyphenols depends mainly on the hydroxyl groups and the molecular size and the length of hydrocarbon. For all used solvents, methanol (50 %) proved to be the most suitable solvent for the extraction of the polyphenols as compared to the different concentrations of ethanol. The optimization procedure employed in this study resulted in a similar recovery of phenolic compounds using just one extraction stage, thereby allowing for a reduction in solvent consumption and time required for extraction. Among all agricultural residues, coffee cherry husk (CCH) showed the higher extraction yields. The largest yield value for CCH was 10.3 % obtained with 70 % methanol, while the lowest result was 3.3 % obtained using water as solvent. The yields of corn husk (CH) were 2.5 % and 6.0 % corresponding to the extraction with water and 50 % methanol respectively (Table 1).
Table 1.
Extraction yield, total polyphenols, tannins and flavonoids content of the agricultural residues
| Agricultural samples | Extraction yield (%) | Total polyphenols (g GAE/100 g extract) | Total tannins (g TAE/100 g extract) | Total flavonoids (g RE/100 g extract) |
|---|---|---|---|---|
| Sugarcane bagasse (SCB) | ||||
| Water | 2.8 ± 0.20 | 16.20 ± 0.13 | 9.25 ± 0.20 | 6.58 ± 0.15 |
| 50 % CH3OH | 6.2 ± 0.18 | 52.45 ± 0.20 | 38.18 ± 0.18 | 10.20 ± 0.21 |
| 70 % CH3OH | 5.8 ± 0.16 | 46.30 ± 0.15 | 36.12 ± 0.15 | 9.64 ± 0.20 |
| 100 % CH3OH | 4.6 ± 0.14 | 42.68 ± 0.16 | 32.33 ± 0.11 | 9.23 ± 0.18 |
| 50 % C2H5OH | 4.5 ± 0.25 | 40.15 ± 0.18 | 31.65 ± 0.22 | 8.16 ± 0.11 |
| 70 % C2H5OH | 3.8 ± 0.27 | 36.33 ± 0.25 | 28.48 ± 0.14 | 8.35 ± 0.16 |
| 100 % C2H5OH | 3.6 ± 0.30 | 33.70 ± 0.23 | 24.85 ± 0.31 | 7.13 ± 0.12 |
| Corn husk (CH) | ||||
| Water | 2.5 ± 0.28 | 12.40 ± 0.20 | 7.80 ± 0.08 | 4.84 ± 0.14 |
| 50 % CH3OH | 6.0 ± 0.22 | 48.50 ± 0.24 | 38.12 ± 0.12 | 8.23 ± 0.18 |
| 70 % CH3OH | 5.5 ± 0.16 | 45.45 ± 0.12 | 37.15 ± 0.18 | 7.82 ± 0.23 |
| 100 % CH3OH | 4.4 ± 0.12 | 41.35 ± 0.15 | 35.76 ± 0.13 | 6.80 ± 0.12 |
| 50 % C2H5OH | 4.0 ± 0.18 | 42.65 ± 0.13 | 33.65 ± 0.22 | 8.72 ± 0.11 |
| 70 % C2H5OH | 3.8 ± 0.24 | 38.70 ± 0.20 | 29.78 ± 0.25 | 7.67 ± 0.15 |
| 100 % C2H5OH | 3.6 ± 0.25 | 35.80 ± 0.22 | 29.33 ± 0.15 | 7.35 ± 0.14 |
| Peanut husk (PNH) | ||||
| Water | 2.6 ± 0.15 | 14.20 ± 0.16 | 8.88 ± 0.10 | 5.65 ± 0.08 |
| 50 % CH3OH | 6.3 ± 0.18 | 62.58 ± 0.20 | 48.28 ± 0.18 | 9.26 ± 0.12 |
| 70 % CH3OH | 6.2 ± 0.28 | 56.35 ± 0.22 | 38.85 ± 0.21 | 8.35 ± 0.18 |
| 100 % CH3OH | 5.8 ± 0.30 | 50.48 ± 0.24 | 41.92 ± 0.16 | 7.68 ± 0.15 |
| 50 % C2H5OH | 5.6 ± 0.15 | 48.72 ± 0.18 | 39.56 ± 0.13 | 8.62 ± 0.11 |
| 70 % C2H5OH | 4.5 ± 0.12 | 45.68 ± 0.12 | 37.73 ± 0.18 | 6.87 ± 0.18 |
| 100 % C2H5OH | 4.3 ± 0.20 | 42.35 ± 0.15 | 33.45 ± 0.10 | 7.73 ± 0.16 |
| Coffee cherry husk (CCH) | ||||
| Water | 3.2 ± 0.23 | 22.10 ± 0.18 | 14.28 ± 0.16 | 7.03 ± 0.08 |
| 50 % CH3OH | 9.2 ± 0.25 | 85.25 ± 0.16 | 72.56 ± 0.12 | 10.15 ± 0.10 |
| 70 % CH3OH | 10.3 ± 0.12 | 81.50 ± 0.15 | 70.63 ± 0.20 | 9.23 ± 0.18 |
| 100 % CH3OH | 9.7 ± 0.13 | 80.44 ± 0.21 | 71.36 ± 0.24 | 9.45 ± 0.13 |
| 50 % C2H5OH | 9.3 ± 0.22 | 81.38 ± 0.11 | 65.44 ± 0.08 | 8.67 ± 0.17 |
| 70 % C2H5OH | 8.5 ± 0.20 | 77.72 ± 0.15 | 62.23 ± 0.13 | 8.45 ± 0.15 |
| 100 % C2H5OH | 8.3 ± 0.18 | 72.64 ± 0.20 | 58.45 ± 0.18 | 7.78 ± 0.12 |
| Rice bran (RB) | ||||
| Water | 3.6 ± 0.30 | 15.80 ± 0.22 | 8.75 ± 0.12 | 5.76 ± 0.14 |
| 50 % CH3OH | 6.2 ± 0.25 | 46.72 ± 0.21 | 36.56 ± 0.14 | 7.75 ± 0.13 |
| 70 % CH3OH | 6.0 ± 0.17 | 44.35 ± 0.20 | 32.88 ± 0.16 | 7.75 ± 0.16 |
| 100 % CH3OH | 5.4 ± 0.24 | 40.65 ± 0.15 | 30.45 ± 0.18 | 7.42 ± 0.19 |
| 50 % C2H5OH | 5.6 ± 0.10 | 42.22 ± 0.13 | 31.65 ± 0.21 | 6.54 ± 0.22 |
| 70 % C2H5OH | 4.8 ± 0.20 | 38.36 ± 0.18 | 30.47 ± 0.23 | 6.24 ± 0.10 |
| 100 % C2H5OH | 4.3 ± 0.11 | 33.53 ± 0.20 | 28.32 ± 0.18 | 6.16 ± 0.15 |
| Wheat bran (WB) | ||||
| Water | 3.3 ± 0.16 | 16.80 ± 0.15 | 9.84 ± 0.10 | 6.57 ± 0.23 |
| 50 % CH3OH | 6.4 ± 0.21 | 53.32 ± 0.12 | 45.57 ± 0. 13 | 7.42 ± 0.18 |
| 70 % CH3OH | 5.9 ± 0.14 | 48.43 ± 0.15 | 42.56 ± 0.15 | 6.75 ± 0.13 |
| 100 % CH3OH | 5.7 ± 0.20 | 45.56 ± 0.10 | 38.23 ± 0.22 | 6.24 ± 0.20 |
| 50 % C2H5OH | 5.2 ± 0.15 | 46.73 ± 0.20 | 38.12 ± 0.20 | 5.86 ± 0.08 |
| 70 % C2H5OH | 4.7 ± 0.28 | 43.81 ± 0.15 | 37.54 ± 0.16 | 6.31 ± 0.12 |
| 100 % C2H5OH | 4.0 ± 0.22 | 40.12 ± 0.20 | 33.35 ± 0.17 | 5.86 ± 0.16 |
Each value is an average of triplicate determination; ± standard deviation
Organic solvents are commonly used for the extraction of polyphenols from plant material. The most important factor that determines the recovery of polyphenols from plant materials is the solubility of the phenolic compounds in the solvent used for the extraction process. Ethanol, methanol and acetone and their aqueous mixtures are commonly used for the extraction purposes. Zhou and Yu (2006) used 50 % acetone/water as an extraction solvent for the extraction of polyphenols from Colorado grown vegetables. Absolute methanol was used by Abas et al. (2006) and Wijngaard et al. (2009) for the extraction of phenolic antioxidants from leafy vegetables and Irish fruit and vegetable wastes, respectively, whereas Sreeramulu and Raghunath (2010) used 60 % methanol with 0.1 % HCl for the extraction of polyphenols from vegetables and tubers.
Total polyphenols and tannins and flavonoids content of the extracts
The total phenols, tannins and flavonoids content were widely higher for CCH extracts, when compared with other agricultural residues (SCB, CH, PNH, RB and WB) extracts, for all type solvent extraction. The values for CCH were 22.10; 85.50 and 81.38 g GAE/100 g of extract, for extraction with water, 50 % methanol and 50 % ethanol respectively. The content of total tannins in CCH extracts found to be 14.28; 72.56 and 62.44 g TAE/100 g of extract and the total flavonoids content was found to be 7.03; 10.15 and 8.67 g TFC/100 g for extraction with water, 50 % methanol and 50 % ethanol respectively. Among the studied solvents methanolic extract (50 %) exhibited significantly higher total phenolic content for all the agriculture residues while among them, coffee cherry husk had the highest phenolic content. This implies that the phenolic compounds in the agriculture residues might be readily soluble in aqueous methanol. The amount and quantity of phenolic molecules by extraction in solvents depends on the plant materials, the solvent used (Marinova and Yanishlieva 1997; Moure et al. 2000), as well as the contact time of extraction (Delgado et al. 2010). Awika et al. (2005) employed aqueous acetone for phenol and antioxidant activity on sorghum bran.
The extraction yield and the antioxidant activity of the extracts from plants highly depend on the solvent polarity, which determines both qualitatively and quantitatively of the extracted antioxidant compounds. The highest yields are usually achieved with ethanol and methanol and their mixtures with water.
Several authors (Lou et al. 2004; Yu et al. 2005, 2006; 2007; Wang et al. 2007) have reported that peanut skins contain phenolic compounds with demonstrated antioxidant properties. Yu et al. (2006) observed three classes of compounds in peanut skin extracts including phenolic acids, flavonoids and stilbene (resveratrol). Some authors (Yu et al. 2005, 2006; Nepote et al. 2002, 2005) have employed traditional solid–liquid extraction techniques using different organic solvents to extract antioxidants from peanut skins. Nepote et al. (2005) investigated the effects of several parameters on the extraction of phenolic compounds from peanut skins using solid–liquid extraction. In that study, optimum extraction conditions were solely based on the quantity of total phenolic compounds extracted as determined by Folin-Ciocalteu reagent, and no identification of phenolics were reported. Wang et al. (2007) extracted phenolics from peanut skins by maceration of the skins with 50 % (v/v) aqueous ethanol at room temperature and reported a total phenolics content of 90 mg/g of extract. More research is needed to develop alternative extraction procedures and to obtain a more detailed profile of the phenolic composition of peanut skin extracts.
Flavonoids are major group of polyphenols, which posses a basic C15 phenyl-benzopyrone skeleton modified with differing numbers and positions of substituents, including hydroxyl, methoxyl and glycosyl groups. The concentration of flavonoid derivatives in the studied agriculture residues is presented in Table 1. Methanol was found to be optimum solvent for the extraction of flavonoids from agriculture residues.
HPLC analysis
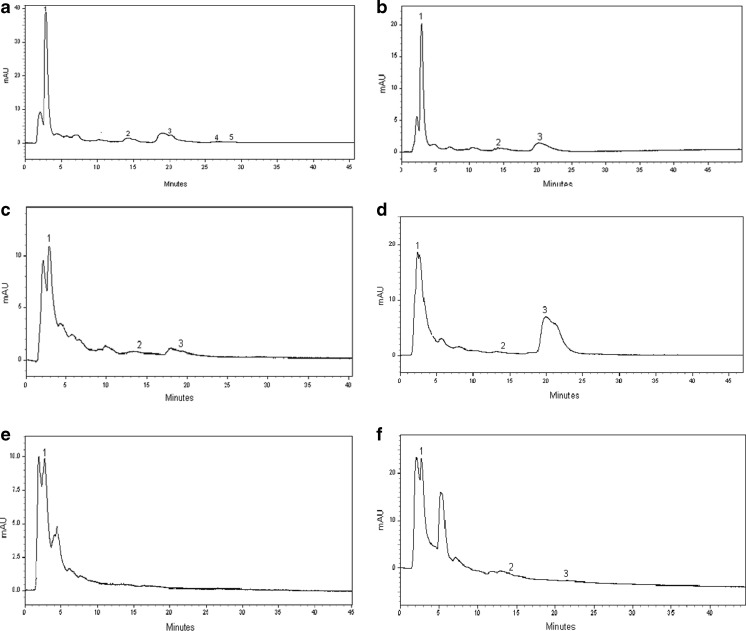
Phenolic compounds from the various agricultural residues were detected by HPLC analysis. The chromatogram of all the samples showed two major peaks (1 and 2) along with three minor peaks (3 and 5) (Fig. 1). The peaks (1 to 5) were identified as gallic acid, ferulic acid, epicatechin, quercitin and kampferol by comparing the retention time of the authentic standards.
Fig. 1.
HPLC profile of hydromethanolic extract (50 %) of agriculture residues, a sugarcane bagasse (SCB), b corn husk (CH), c peanut husk (PNH), d coffee cherry husk (CCH), e rice bran (RB) and f wheat bran (WB). Peak 1: gallic acid; peak 2: ferulic acid; peak 3: epicatechin; peak 4: quercitin and peak 5: kampferol
Antioxidant assays
DPPH radical scavenging activity
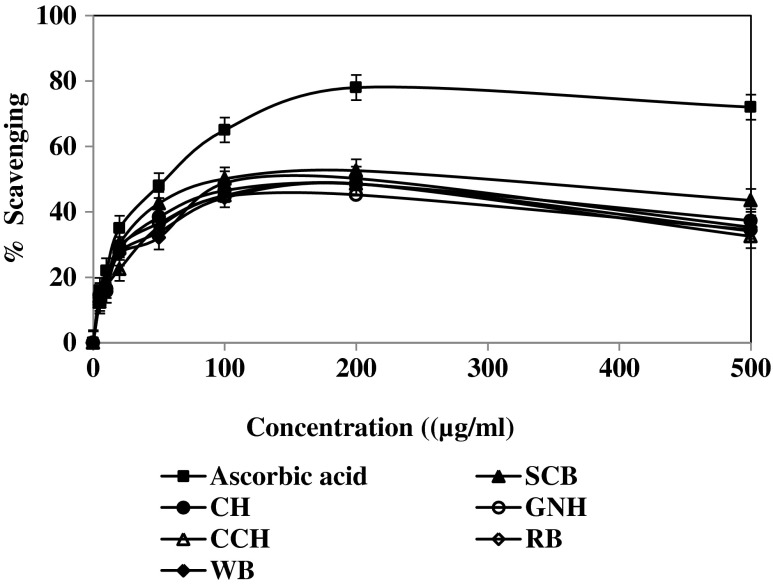
The DPPH (1, 1- diphenyl-2-picrylhydrazyl) radical scavenging activity of agricultural residues is shown in (Fig. 2). This activity was found to increase with increasing concentration of 50 % methanolic extract. It is well known that the antioxidant activity of plant extracts containing polyphenol components is due to their capacity to donate hydrogen atoms or electrons and scavenge free radicals (Sreeramulu and Raghunath 2010). Among the samples SCB showed the least scavenging activity (IC50 = 26.3 μg/ml) while PNH (IC50 = 22.6 μg/ml), CH (IC50 = 24.25 μg/ml), CCH (IC50 = 24.3 μg/ml) and WB (IC50 = 25.1 μg/ml) showed a better result than the positive control ascorbic acid (IC50 = 38.0 μg/ml). The results are summarized in Table 2. These suggested that SCB has an efficient radical scavenger, its activity being confined to the hydroxymethanolic fraction.
Fig. 2.
DPPH radical scavenging activity of agricultural residues with hydromethanol extracts (50 %). Values are the average of triplicate experiments and represented as mean ± SD
Table 2.
Antioxidant activity (IC50 values) of the agricultural residues extracted in 50 % methanol
| Agricultural residues | DPPH assay | NO assay |
|---|---|---|
| Ascorbic acida | 39.0 ± 0.02 | 38.0 ± 0.08 |
| SCB | 26.3 ± 0.02 | 27.0 ± 0.01 |
| CH | 24.25 ± 0.05 | 27.5 ± 0.04 |
| PNH | 22.6 ± 0.02 | 28.0 ± 0.07 |
| CCH | 24.3 ± 0.01 | 29.0 ± 0.01 |
| RB | 24.3 ± 0.06 | 30.0 ± 0.03 |
| WB | 25.1 ± 0.03 | 31.0 ± 0.05 |
Each value is an average of triplicate determination; ± standard deviation
aStandard drug
Agricultural residues have a great potential as source of antioxidants, many of which are polyphenols. Solvent and process variables must be carefully chosen to optimize their extraction. Antioxidant activity of every extract must be measured by several methods: radical scavenging activities (DPPH), oxidation of lipids (TBARS), micellar systems, etc., because none of them is representative of all real system; for example it is possible to obtain extracts with high radical scavenging activity but unable to protect an oil for oxidation because of its low miscibility.
The antioxidant activity of plant extracts can be in large part attributed to the presence of polyphenolic compounds located within the plant tissues. Polyphenols are attracting a great deal of attention due to evidence suggesting that an increase in their consumption in the diet may prevent cancer, strokes and neurological diseases. They are the most abundant antioxidants in our diets and it is estimated that we consume about 1 g of polyphenols per day (Scalbert and Williamson 2000). Several thousands of natural polyphenols have been identified in plants and plant foods. Polyphenolic compounds are present in high concentrations in a variety of fruits, vegetables and beverages such as tea and wine. They are also abundant in agricultural byproducts such as peanut skins, hulls and roots, grape seeds and skins and in a number of herbs and spices (rosemary, sage, thyme and oregano). Polyphenols are important to plant growth and development and provide a defense mechanism against infection and injury (Karakaya and Tas 2001). Many polyphenolic compounds have been found to have a much stronger antioxidant activity than vitamins C and E and β-carotene within the same food (Chu et al. 2002).
Nitric oxide radical scavenging activity
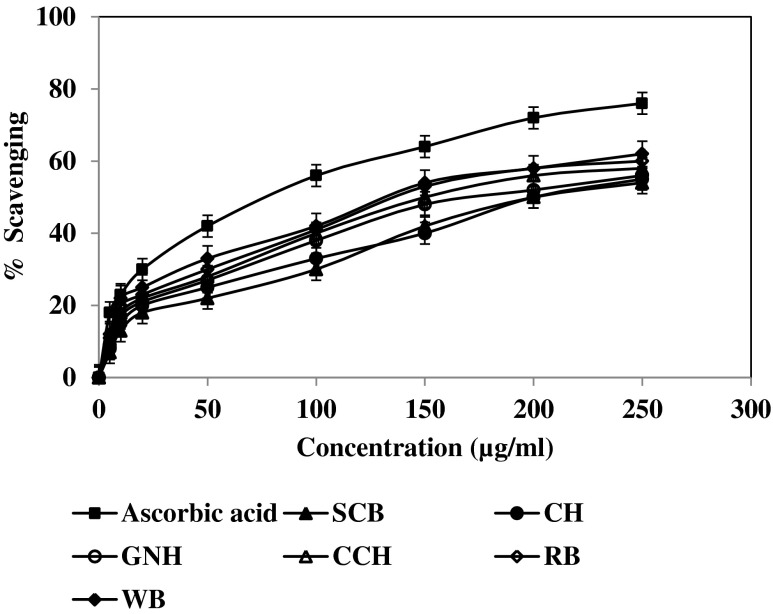
Nitric oxide (NO) has an important role in various inflammatory processes. Its sustained production is toxic to tissues and can contribute to the vascular collapse associated with septic shock. Chronic generation of nitric oxide is associated with carcinomas and various inflammatory conditions including juvenile diabetes, multiple sclerosis, arthritis, and ulcerative colitis (Tylor et al. 1997). Nitric oxide is generated from the decomposition of SNP and measured. SNP in aqueous solution at physiological pH spontaneously generates NO, which interacts with oxygen to produce nitrite ions that can be measured. All the extracts for both these methods analyzed at the same range of concentration (50–500 μg/ml). A significant decrease in the NO radical is due to the scavenging activity of the extracts. Our results showed dose-dependent NO scavenging activity by all the test samples (Fig. 3). IC50 values (Table 2) were calculated from the graphs plotted scavenging activity versus the concentrations of samples. The IC50 values of SCB (27.0 μg/ml), CH (27.5 μg/ml), PNH (28.0 μg/ml), CCH (29.0 μg/ml), RB (30.0 μg/ml) and WB (31.0 μg/ml) for scavenging NO revealed that SCB and CH were more potent than the positive control, ascorbic acid (IC50 = 38.0 μg/ml). in this case also, SCB was the most active as compare to other agricultural residues.
Fig. 3.
Nitric oxide scavenging activity of agricultural residues with hydromethanol extracts (50 %). Values are the average of triplicate experiments and represented as mean ± SD
Ferric ion reducing assay
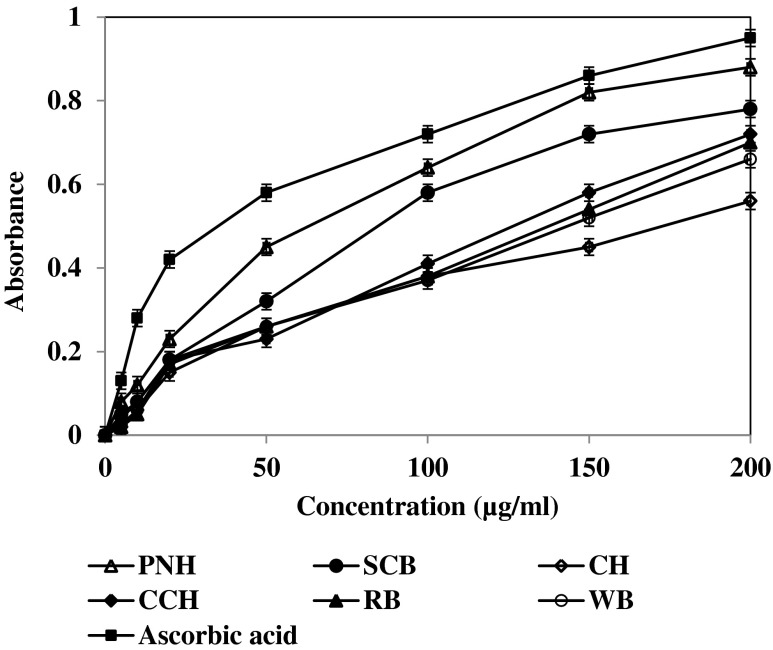
The reducing power of a compound may serve as a significant indicator of its potential antioxidant activity (Meir et al. 1995). Hence, the Fe3+ reducing powers of agricultural residues as well as its various fractions were investigated, and the results compared with that of the reference compound, Ascorbic acid. The reducing powers of the samples were found to increase concentration dependently (Fig. 4). The RP 0.5 AU was calculated and depicted in Table 3. In this assay, a higher absorbance of reaction mixture indicates higher reducing power of the sample. The absorbances of assay mixture containing a fixed concentration (200 μg/ml) of SCB, CH, PNH, CCH, RB, WB and ascorbic acid were found to be 0.39, 0.28, 0.44, 0.36,0.35, 0.33 and 0.47 respectively (Table 3). The order of the reducing powers of the samples was CH>WB>RB>CCH>SCB>PNH>ascorbic acid. Thus, all samples showed better reducing powers than ascorbic acid.
Fig. 4.
Ferrous ion reducing activity of agricultural residues with hydromethanol extracts (50 %). Values are the average of triplicate experiments and represented as mean ± SD
Table 3.
RP 0.5 AU values of agricultural residues extracted with 50 % methanol
| Agricultural residues | FRAP assay |
|---|---|
| Ascorbic acida | 0.47 ± 0.04 |
| SCB | 0.39 ± 0.03 |
| CH | 0.28 ± 0.08 |
| PNH | 0.44 ± 0.05 |
| CCH | 0.36 ± 0.03 |
| RB | 0.35 ± 0.07 |
| WB | 0.33 ± 0.02 |
Each value is an average of triplicate determination; ± standard deviation
aStandard drug
The antioxidant properties of phenolic compounds are associated with their reducing power (Jayaprakasha et al. 2001), which is associated with the presence of reductones (Duh 1998). The reducing power of agricultural residues increases significantly with phenol content (Fig. 4 & Table 3).
Conclusion
Agricultural residues have great potential and cost effective sources of antioxidants, many of which are polyphenols. To obtain the maximum yield of extraction, 50 % methanol is recommended for phenolic compounds extraction from agricultural residues. The beneficial effects of polyphenols have been ascribed to their strong antioxidant activity that is, their ability to scavenge oxygen radicals and other reactive species. These features make phenols a potentially interesting material for the development of functional foods or possible therapy for the prevention of some diseases.
Acknowledgments
This research was supported by research grants to KS from DST and UGC-SAP, the Government of India, New Delhi. VS thanks UGC-SAP for providing JRF.
Footnotes
Highlights
• Agricultural residues like sugarcane bagasse (SCB), corn husk (CH), peanut husk (PNH), coffee cherry husk (CCH), rice bran (RB) and wheat bran (WB) are used for the extraction of polyphenols.
• The effects of two types of solvent extraction methods: solid–liquid extraction (SLE) and hot water extraction on the recovery of phenolic compounds from agricultural residues were investigated to optimize the extraction conditions based on total phenolic content (TPC), total tannin content (TTC) and total flavonoids content (TFC).
• The types of polyphenols from different agricultural residues were analyzed by HPLC.
• The antioxidant activities like DPPH, NO and FRAP were also studied.
Contributor Information
S. Vijayalaxmi, Email: vijaya.gug@gmail.com
K. Sreeramulu, Phone: +91-8472-263289, FAX: +91-8472-263203, Email: ksramu@rediffmail.com
References
- Abas F, Lajis NH, Israf DA, Khozirah S, Kalsom YU. Antioxidant and nitric oxide inhibition activities of selected Malay traditional vegetables. Food Chem. 2006;985:566–573. doi: 10.1016/j.foodchem.2005.01.034. [DOI] [Google Scholar]
- Awika JM, McDonough CM, Rooney LW. Decorticating sorghum to concentrate healthy phytochemicals. J Agric Food Chem. 2005;53:6230–6234. doi: 10.1021/jf0510384. [DOI] [PubMed] [Google Scholar]
- Bao JS, Cai Y, Sun M, Wang G, Corke H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J Agric Food Chem. 2005;53:2327–2332. doi: 10.1021/jf048312z. [DOI] [PubMed] [Google Scholar]
- Barriera JCM, Ferreira ICFR, Oliviera MBPP, Pereira JA. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008;107:1106–1113. doi: 10.1016/j.foodchem.2007.09.030. [DOI] [Google Scholar]
- Chu Y, Sun J, Wu X, Liu R. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002;50:6910–9616. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- Delgado T, Malheiro R, Pereira JA, Ramalhosa E. Hazelnut (Corylus avellana L.) kernels as a source of antioxidants and their potential in relation to other nuts. Ind Crop Prod. 2010;32:623–625. doi: 10.1016/j.indcrop.2010.07.019. [DOI] [Google Scholar]
- Duh PD. Antioxidant activity of budrock (Arctium lappa L.): its scavenging effect on free radical and active oxygen. J Am Oil Chem Soc. 1998;75:455–461. doi: 10.1007/s11746-998-0248-8. [DOI] [Google Scholar]
- Gharras HE. Polyphenols: food sources, properties and applications—a review. Int J Food Sci Technol. 2009;44:2512–2518. doi: 10.1111/j.1365-2621.2009.02077.x. [DOI] [Google Scholar]
- Gulçin I, Huyut Z, Elmastas M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem. 2010;3:43–53. doi: 10.1016/j.arabjc.2009.12.008. [DOI] [Google Scholar]
- Hooper L, Cassidy A. A review of the health care potential of bioactive compounds. J Agric Food Chem. 2006;86:1805–1813. doi: 10.1002/jsfa.2599. [DOI] [Google Scholar]
- Isfahlan AJ, Mahmoodzadeh J, Hassanzadeh A, Heidari R, Jamei R. Anti-oxidant and antiradical activities of phenolic extract from Iranian almond (Prunus amygdalus L.) hulls and shells. Turk J Biol. 2010;34:165–173. [Google Scholar]
- Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001;73:285–290. doi: 10.1016/S0308-8146(00)00298-3. [DOI] [Google Scholar]
- Karakaya SESN, Tas AA. Antioxidant activity of some foods containing phenolic compounds. Int J Food Sci Nutr. 2001;52:501–508. doi: 10.1080/713671810. [DOI] [PubMed] [Google Scholar]
- Li HB, Wong CC, Cheng KW, Chen F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. Lebensm-Wiss Technol. 2008;41:385–390. doi: 10.1016/j.lwt.2007.03.011. [DOI] [Google Scholar]
- Liu RH. Whole grain phytochemicals and health. J Cereal Sci. 2007;46:207–219. doi: 10.1016/j.jcs.2007.06.010. [DOI] [Google Scholar]
- Lou H, Yuan H, Ma B, Ren D, Ji M, Oka S. Polyphenols from peanut skins and their free radical-scavenging effects. Phytochemistry. 2004;65:2391–2399. doi: 10.1016/j.phytochem.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Marinova EM, Yanishlieva NV. Antioxidant activity of extracts from selected species of the family Laminaceae in sunflower oil. Food Chem. 1997;58:245–248. doi: 10.1016/S0308-8146(96)00223-3. [DOI] [Google Scholar]
- Meir S, Kanner J, Akiri B, Philosoph-Hadas S. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem. 1995;43:1813–1819. doi: 10.1021/jf00055a012. [DOI] [Google Scholar]
- Moure A, Franco D, Sineiro J, Dominguez H, Nunez MJ, Lema JM. Evaluation of extracts from Gevuina avellana hulls as antioxidants. J Agric Food Chem. 2000;48:3890–3897. doi: 10.1021/jf000048w. [DOI] [PubMed] [Google Scholar]
- Nepote V, Grosso NR, Guzman CA. Extraction of antioxidant components from peanut skins. Grasas Y Aceites. 2002;53:391–395. doi: 10.3989/gya.2002.v53.i4.335. [DOI] [Google Scholar]
- Nepote V, Grosso NR, Guzman CA. Optimization of extraction of phenolic antioxidants from peanut skins. J Agric Food Chem. 2005;85:33–38. doi: 10.1002/jsfa.1933. [DOI] [Google Scholar]
- Price ML, Butler LG. Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. J Agric Food Chem. 1977;25:1268–1273. doi: 10.1021/jf60214a034. [DOI] [Google Scholar]
- Sarikaya A, Ladisch MR. Solid-state fermentation of lignocellulosic plant residues from Brassica napus by Pleurotus ostreatus. Appl Biochem Biotechnol. 1999;82:1–15. doi: 10.1385/ABAB:82:1:1. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073–2085. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- Sreeramulu D, Raghunath M. Antioxidant activity and phenolic content of roots, tubers and vegetables commonly consumed in India. Food Res Int. 2010;43:1017–1020. doi: 10.1016/j.foodres.2010.01.009. [DOI] [Google Scholar]
- Sun Y, Oberly LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- Tylor BS, Kion YM, Wang QI, Sharpio RA, Billiar TR, Geller DA. Nitric oxide down regulates hepatocyte-inducible nitric oxide synthase gene expression. Arch Surg. 1997;132:1177–1183. doi: 10.1001/archsurg.1997.01430350027005. [DOI] [PubMed] [Google Scholar]
- Vazquez G, Fontenla E, Santos J, Freire MS, Gonzalez-Alvarez J, Antorrena G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind Crop Prod. 2008;28:279–285. doi: 10.1016/j.indcrop.2008.03.003. [DOI] [Google Scholar]
- Ventura J, Belmares R, Aguilera-Carbo A, Gutierrez-Sanchez G, Rodriguez-Herrera R, Aguilar CN. Fungal biodegradation of tannins from Creosote Bush (Larrea tridentata) and Tar Bush (Fluorensia cernua) for gallic and ellagic acid production. Food Technol Biotechnol. 2008;46:213–217. [Google Scholar]
- Wang J, Yuan X, Jin Z, Tian Y, Song H. Free radical and reactive oxygen species scavenging activities of peanut skins extract. Food Chem. 2007;104:242–250. doi: 10.1016/j.foodchem.2006.11.035. [DOI] [Google Scholar]
- Wijngaard HH, Roble C, Brunton N. A survey of Irish fruit and vegetable waste as a source of pophenolic antioxidants. Food Chem. 2009;116:202–207. doi: 10.1016/j.foodchem.2009.02.033. [DOI] [Google Scholar]
- Yawadio R, Tanimori S, Morita N. Identification of phenolic compounds isolated from pigmented rices and their aldose reductase inhibitory activities. Food Chem. 2007;101:1616–1625. doi: 10.1016/j.foodchem.2006.04.016. [DOI] [Google Scholar]
- Yu J, Ahmedna M, Goktepe I. Effect of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005;90:199–206. doi: 10.1016/j.foodchem.2004.03.048. [DOI] [Google Scholar]
- Yu JM, Ahmedna M, Goktepe I, Dai JA. Peanut skin procyaninidins: composition and antioxidant activities as affected by processing. J Food Compos Anal. 2006;19:364–371. doi: 10.1016/j.jfca.2005.08.003. [DOI] [Google Scholar]
- Yu J, Ahmedna M, Goktepe I. Peanut skin phenolics: extraction, identification, antioxidant activity and potential applications. ACS Symp Ser. 2007;956:226–241. doi: 10.1021/bk-2007-0956.ch016. [DOI] [Google Scholar]
- Zhou K, Yu L. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT Food Sci Technol. 2006;39:1155–1162. doi: 10.1016/j.lwt.2005.07.015. [DOI] [Google Scholar]