Abstract
To explore a new and simple rapid extraction and purification technique for wheat-esterase, an ionic liquids (ILs)-based aqueous two-phase system (ATPS) was developed for the purification of wheat-esterase from wheat extracts. Effects of various process parameters such as the concentrations of [Bmim]BF4, the types and concentrations of phase-forming salt, the system pH and the temperature on partitioning of wheat-esterase were evaluated. The obtained data indicated that wheat-esterase was preferentially partitioned into the ILs-rich phase and the ATPS composed of 20 % [Bmim]BF4 (w/w) and 25 % (w/w) NaH2PO4(pH = 4.8) showed good selectivity on wheat-esterase. Under the optimum conditions, wheat-esterase was purified with an acceptable yield (88.93 %), but produced wheat-esterase was 4.23 times as pure. It was obvious that temperature shows little influence on the purification between 10 and 50 °C. Sephadex G-150FF revealed that the band intensity of contaminating proteins in ATPS fraction almost disappeared. Therefore, ILs-based ATPS was an effective method for partitioning and recovery of wheat-esterase from wheat crude extracts.
Keywords: Ionic liquid, Aqueous two-phase system, Wheat-esterase, Purification
Introduction
Typical aqueous two-phase systems (ATPS) are usually formed by mixing aqueous solutions of two structurally different polymers or one polymer and one salt above certain concentration (Li et al. 2002; Walter and Johansson 1994). Minimizing consumption of organic solvents harmful to the environment ATPS is regarded as a simple and environmentally friendly separation system. ATPS also offers many advantages, such as a low process time, low energy consumption, and an environment biocompatible to the biomolecule because each phase contains 70–90 % water, which means that biomolecules will not be denatured. Hence, ATPS has been recognized as an efficient and economical method for the separation of biomolecules (Aguilar and Rito-Palomares 2010).
Of late, ionic liquids (ILs) have emerged as alternative media for biocatalysis which are essentially liquid salts composed of organic cations and usually inorganic anions. With a melting point below 100 °C ILs remain liquid at ambient temperature or below. In contrast to conventional organic solvents ILs have unique physical properties such as a negligible vapour pressure low viscosity good dissolution power towards many substrates, high thermal and chemical stability, and a wide electrochemical potential window. (Hallett and Welton 2011; Shiddiky and Torriero 2011).
In 2003, Rogers and co-workers (Gutowski et al. 2003) reported that ILs could be used to form ATPS by mixing with inorganic salt. Since then significant progress has been made in the field of ILs-based ATPS. Since the ILs-based ATPS are particularly suitable for the extraction of biocatalysts or their products more and more investigations have been undertaken to study their abilities to extract biomolecules such as protein, incretion, antibiotic, alkaloid and emzyme (Oppermann et al. 2011).
Currently most methods for rapid detection of Organophosphorus compounds (OPs) are based on the inhibition of acetylcholinesterase (AChE) (Waibel et al. 2006; Wong et al. 2006). Methods based on AChE, generally be extracted from animal blood or tissue, have very high sensitivity. However, its widespread use is limited due to its production cost. The enzyme cost could be reduced by choosing a cheaper source of the enzyme and/or by reducing its recovery/purification cost. Plant-esterase matches cholinesterase in its ability to hydrolyze esters in OPs (Dong and Wang 2009; Qian et al. 2006a, b). Thus, it is much easier to obtain than AChE because plant-esterase can be extracted from wheat, soybean, corn, rice and other grains. So plant-esterase can be widely applied in rapid warning alerts and field analysis for OPs (Huo et al. 2009). Several methods have been developed for the purification of plant-esterase by different researchers (Cummins and Edwards 2004; De Carvalho et al. 2003; Stuhlfelder et al. 2002). However, almost all these methods involve a large number of steps such as precipitation, centrifugation, dialysis, ion-exchange chromatography and gel filtration chromatography which take higher loss of product yield (Kula et al. 1992). Furthermore, the scale-up of these methods is difficult and expensive. Hence, efficient and economical large-scale bioseparation methods are needed to provide high purity and high yield plant-esterase with biologically activity.
The aim of this study was to develop an extraction and purification method for plant-esterase from wheat by ATPS based on ILs. In this work, a preliminary study about the partition of wheat–esterase in ILs-based ATPS was carried out and relevant parameters such as the types and concentrations of phase-forming salt the system pH, and temperature which affect the partitioning behavior of the protein were investigated. The method used to optimize ATPS process parameters for wheat-esterase can be used to optimize this technique for other biomolecules as well.
Materials and methods
Materials
Wheat was acquired from College of Agriculture NEAU (Harbin, China). Low molecular weight markers and bovine serum albumin were purchased from Sigma. Sodium dodecyl sulfate (SDS), Coomassie Blue G-250,1-Naphthyl acetate (1-NA) and Fast Blue B salt were purchased from Fluka. Sephadex G-150FF were purchased from Amersham Pharmacia. 1-Butyl-3-methylimidazolium tetrafluoroborate ([Bmim] BF4) was purchased from Henan Lihua Pharmaceutical Co. Ltd. The salts and other analytical grade chemicals were purchased from Sinopharm Chemical Reagent Factory (Shanghai, China). Water used for preparation of aqueous solutions was from a Millipore Direct-Q Water system (resistivity, 18.2MΩ · cm).
All mass measurements were realized using an analytical balance (METTLER TOLEDO AL204 Analytical Balance, China) with a precision ±0.0001 g. The absorbances were measured using a spectrophotometer (Shimadzu UV-2550, Japan).
Preparation of crude extract
A 1:5 (w/w) solution of flour in water was made. The mixture was stirred for 30 min, then centrifuged at 4,000 rpm for 10 min at 4 °C. The clear supernatant was collected and then filtrated by microporous filter membrane.
Wheat-esterase activity assay
Wheat-esterase activity was determined by the colorimetric method of Van Asperen (Vanasperen 1962). The general buffer was 0.04 M sodium phosphate, pH 6.0. 1-Naphthyl acetate (1-NA) (16 mM) was used as substrate. The mixture consisting of phosphate buffer (4.0 mL), wheat-esterase solution (0.5 mL) and 1-NA (250 μL) was incubated at 30 °C for 5 min in a water bath. The hydrolysis of 1-NA was terminated by adding 1.75 mL of Fast Blue B salt–SDS solution. The absorbance at 535 nm was measured by a spectrophotometer after 5 min.
Protein determination
Protein concentration was measured by the Bradford method (Bradford 1976) using bovine serum albumin as a standard.
Preparation of ATPS
ATPSs were prepared in 10 mL centrifuge tubes by adding the appropriate amount of [Bmim]BF4, salts and plant-esterase crude extract. Distilled water was used to bring the final weight of the system to 10 g. The compounds were mixed using a Vortex mixer and centrifuged for 3 min at 2,000 rpm to increase the rate that the phases separated. The volumes of the separated phases were measured. Aliquots from each phase were used to measure wheat-esterase activity and protein concentration.
Effect of concentration of [Bmim]BF4 on partitioning of wheat-esterase
To study the effect of concentration of [Bmim]BF4, [Bmim] BF4 of different mass were mixed with 20 % (w/w) NaH2PO4 to form ATPS Partitioning was performed as described previously.
Generally, the biomolecule partition coefficient, K, was used to quantify the biomolecule partition behavior. The partition coefficient is defined as the ratio of protein concentration or wheat-esterase activity in the top phase to that in the bottom phase, as shown in Eqs. (1) and (2) (Rabelo et al. 2004).
| 1 |
| 2 |
where CT and CB are the total protein concentrations in mg/ml of the top and bottom phase, respectively, and AT and AB are the wheat-esterase activities in U/ml of the top and bottom phase, respectively.
The volume ratio as
| 3 |
where VT and VB are the volumes of top and bottom phase, respectively.
In order to evaluate the purification process, the enzyme-specific activity (SA expressed in U/mg protein), the purification factor (PF) and the wheat-esterase yield (Y) were also calculated according to the given equations (Dembczynski et al. 2010)
| 4 |
| 5 |
| 6 |
The enzyme-specific activity (SA) can be evaluated for both phases, using Eq. (4) with the corresponding wheat-esterase activity and the protein concentration in the selected phase. In Eq. (5), SAi represents the SA for the crude extract.
Effect of salts on partitioning of wheat-esterase
To study the effect of salts on partitioning of the wheat-esterase in ATPS, different salts including NaH2PO4, K2HPO4, (NH4)2SO4 and MgSO4 at different concentrations (15, 20, 25 and 30 % w/w) were mixed with 20 % (w/w) [Bmim]-BF4 in ATPS. Based on KP, KE, PF and Y, the ATPS rendering the most effective partitioning was chosen for further study.
Effect of pH on partitioning of wheat-esterase
In order to investigate the influence of pH, an IL-based ATPS consisting of 20 % (w/w) [Bmim]BF4 and 25 % (w/w) NaH2PO4was prepared. The pH of the systems was adjusted by HCl or NaOH. Based on KP, KE, PF and Y, the ATPS rendering the most effective partitioning was chosen for further study.
Effect of temperature on partitioning of wheat-esterase
The model system to evaluate the effect of temperature on wheat-esterase partitioning was prepared as described above. Phase separation was induced by storing the samples in a water bath for 1–2 h at a temperature of 10, 20, 30, 40 and 50 °C, respectively. The performance of partitioning were measured to study the effect of temperature.
Statistical analysis
All data were subjected to Analysis of Variance (ANOVA) and differences between means were evaluated by Duncan’s Multiple Range Test (Steel and Torrie 1980). SPSS statistic program (Version 18.0) was used for data analysis.
Sephadex G-150FF chromatography
After ATPS, ammonium sulfate was added (608 g/L,000 mL). The resultant mixture was kept at 4 °C for 24 h. the precipitate formed was collected by centrifugation at 4 °C for 20 min at 10,000 rpm. The precipitate was dissolved in a small amount of 50 mM sodium phosphate buffer (pH 6.0), and dialyzed against the buffer. The resultant dialysate was loaded onto a Sephadex G-150FF (1.6 cm × 80 cm) pre-equilibrated with 50 mM sodium phosphate eluting buffer (pH 6.0). The solution after Sephadex G-150FF was collected every 5 mL and assayed for protein and wheat-esterase activity.
Sodium dodecyl sulphate-gel electrophoresis
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out by following Deutscher’s method (Deutscher 1990), using a 30 % polyacrylamide slab gel. Electrophoresis was run at 50 V, 12.5 mA, for 3–4 h. The gel was stained with a solution that was 0.05 % (w/w) Coomassie Brilliant Blue R250, 50 % (v/v) methanol and 12 % (v/v) acetic acid. The gel was destained using a buffer that was identical to the staining solution except that it contained no Coomassie Brilliant Blue.
Results and discussion
Effect of concentration of [Bmim]BF4
The effect of concentration of added ILs in the systems was investigated. As indicated in Table 1, KP which was around 1 showed that the concentration of [Bmim]BF4 does not have much effect on the residual protein partitioning in the two phases. But KE was sensitive to the concentration of [Bmim] BF4. In all cases, KE was larger than KP which meant wheat-esterase were more preferably distributed to the top phase than residual proteins. The PF also showed that the purity of wheat-esterase in the top phase increased as the concentration of [Bmim]-BF4 increased till the concentration reached 20 %. At the concentration above 20 %, KE, KP and Y still increased but PF decreased. The probably reason was that more wheat-esterase was partitioned to the top phase as the concentration increased to a higher value while residual proteins partitioned to the top phase also increased. As a result PF in top phase decreased.
Table 1.
Effect of concentration of [Bmim]BF4 in ATPS on purification of wheat-esterase ( ± SD, n = 3)
| The mass fraction of ILs%(w/w) | V R | K P | K E | Y(%) | PF |
|---|---|---|---|---|---|
| 10 | 0.58 ± 0.01a* | 1.06 ± 0.02d | 2.00 ± 0.06a | 0.5355 ± 0.0105a | 1.49 ± 0.08a |
| 12 | 0.66 ± 0.01b | 1.28 ± 0.01e | 2.63 ± 0.03b | 0.6353 ± 0.0029b | 1.63 ± 0.02b |
| 15 | 0.73 ± 0.01c | 0.93 ± 0.05c | 3.78 ± 0.07c | 0.7336 ± 0.0046c | 2.40 ± 0.13d |
| 18 | 0.87 ± 0.00d | 0.63 ± 0.02a | 4.18 ± 0.03d | 0.7840 ± 0.0010d | 2.90 ± 0.06e |
| 20 | 1.11 ± 0.02e | 0.72 ± 0.02b | 7.27 ± 0.06f | 0.8893 ± 0.0012e | 4.23 ± 0.04f |
| 22 | 1.16 ± 0.01f | 0.92 ± 0.01c | 7.49 ± 0.11g | 0.8966 ± 0.0023ef | 2.13 ± 0.02c |
| 25 | 1.49 ± 0.02g | 1.05 ± 0.01d | 6.28 ± 0.10e | 0.9033 ± 0.0012f | 1.51 ± 0.04a |
V R volume ratio, K P protein distribution coefficient, K E enzyme distribution coefficient PF purified fold, Y yield
*a, b, c,……, g means within a row not sharing a common superscript differ significantly (p < 0.05)
In most cases, KE was lager than KP, which indicated that wheat-esterase was more incline to be partitioned to the top phase contrast with residual proteins. The reason of difference between the distribution of wheat-esterase and residual proteins attributed to the molecular weight, shape, volume and surface area (Dreyer et al. 2009). Another reason was the difference in distributing of charge. Partitioning in ATPS was a surface-dependent phenomenon, depending strongly on the surface characteristics of the proteins. It was mainly a process in which the exposed groups of proteins come into contact with the phase components. It has been demonstrated that electrostatic potential difference between coexisting phases is a common property at interfaces even though the phases are electrically neutral (Pfennig et al. 1998) and in this particular case the uneven distribution of Bmim+ and salts generated a difference in electrical potential between the phases. Therefore, the distribution of protein in ATPS likely depended on the differences in surface charge of wheat-esterase and residual proteins.
Effect of salts
To study the effect of salts on partitioning of the wheat-esterase in ATPS, different salts including NaH2PO4, K2HPO4, (NH4)2SO4 and MgSO4 at different concentrations (15, 20, 25 and 30 % w/w) were mixed with 20 % (w/w) [Bmim]BF4 in ATPS. The partitioning of wheat-esterase was determined and showed in Table 2.
Table 2.
Effect of phase composition in ILs–salt ATPS on purification of wheat-esterase ( ± SD, n = 3)
| Salts in ATPS(w/w) | V R | K P | K E | Y(%) | PF |
|---|---|---|---|---|---|
| NaH2PO415 % | 1.55 ± 0.04d* | 0.73 ± 0.00abc | 1.12 ± 0.03b | 63.55 ± 0.32c | 1.44 ± 0.01e |
| NaH2PO420 % | 1.44 ± 0.01d | 0.64 ± 0.02ab | 1.86 ± 0.01c | 72.80 ± 0.09e | 2.07 ± 0.01i |
| NaH2PO425 % | 1.11 ± 0.02c | 0.72 ± 0.02abc | 7.27 ± 0.06h | 88.93 ± 0.12i | 4.23 ± 0.04j |
| NaH2PO430 % | 1.07 ± 0.24bc | 1.15 ± 0.04cd | 8.62 ± 0.09i | 89.98 ± 1.86i | 1.97 ± 0.04h |
| K2HPO415 % | 1.11 ± 0.01c | 1.18 ± 0.07cd | 3.29 ± 0.08e | 78.48 ± 0.50g | 2.14 ± 0.13i |
| K2HPO420 % | 1.82 ± 0.05e | 2.05 ± 0.02e | 4.72 ± 0.29f | 89.56 ± 0.36i | 1.39 ± 0.05e |
| K2HPO425 % | 1.12 ± 0.07c | 4.99 ± 0.09fg | 5.02 ± 0.03f | 84.89 ± 0.83h | 1.28 ± 0.06d |
| K2HPO430 % | 0.88 ± 0.01b | 4.61 ± 0.04f | 2.43 ± 0.02d | 68.23 ± 0.22d | 0.85 ± 0.04a |
| (NH4)2SO415 % | 3.62 ± 0.25g | 1.02 ± 0.02abcd | 0.62 ± 0.02a | 69.16 ± 2.05d | 0.89 ± 0.03a |
| (NH4)2SO420 % | 1.57 ± 0.19d | 1.11 ± 0.03bcd | 1.89 ± 0.04c | 74.65 ± 2.02ef | 1.13 ± 0.04c |
| (NH4)2SO425 % | 0.86 ± 0.08b | 2.20 ± 0.52e | 1.81 ± 0.04c | 60.81 ± 2.68b | 1.01 ± 0.01b |
| (NH4)2SO430 % | 0.61 ± 0.07a | 5.14 ± 0.87g | 6.15 ± 0.90g | 78.64 ± 2.49g | 1.12 ± 0.03c |
| MgSO415 % | --- | --- | --- | --- | --- |
| MgSO420 % | 2.98 ± 0.20f | 1.10 ± 0.05bcd | 1.09 ± 0.02b | 76.45 ± 1.48fg | 0.94 ± 0.05ab |
| MgSO425 % | 1.17 ± 0.03c | 0.58 ± 0.03a | 1.76 ± 0.06c | 67.20 ± 0.69d | 1.72 ± 0.07g |
| MgSO430 % | 0.88 ± 0.01b | 1.33 ± 0.03d | 0.77 ± 0.02ab | 40.60 ± 0.68a | 1.62 ± 0.07f |
V R volume ratio, K P protein distribution coefficient, K E enzyme distribution coefficient, PF purified fold, Y yield
*a, b, c,……, j means within a row not sharing a common superscript differ significantly (p < 0.05)
--- no ATPS formed
After phase separation, two phases were obtained, ILs-rich top phase and salt-rich bottom phase. However, no phase separation was observed in the system containing 15 % (w/w) MgSO4. In this study, ATPSs were able to partition wheat-esterase in most of the phase compositions. Most of the KE and KP in the systems were larger than 1, which indicated that both wheat-esterase and residual proteins were preferably distributed to the top phase. Therefore, the purity of wheat-esterase in the top phase was considered as the response variable to evaluate the effect of different phase-forming salts.
In most ATPSs studied, KP increased with the increasing of the concentrations of salts and decreased after certain concentrations of salts. The presence of high concentrations of salts increased the hydrophobicity of the bottom phase (Zaslavsky et al. 1982), in which the solubility of proteins decreased significantly because the large number of salt ions competed for water molecules with proteins while the salvation spheres surrounding the proteins ionized groups were removed (McKee and Mckee 1999) and salting out of proteins took place. Consequently the majority of the proteinic components were moved into the top phase. As can be seen in Table 2. that increasing the concentrations of salts immoderately may result in a lower activity recovery and KE. One reason is the denaturation of wheat-esterase caused by the salting-out effect. Another reason is that the excess wheat-esterase precipitated at the interface of the two phases was discarded. Therefore, the type and concentration of the salt were critical for the wheat-esterase partitioning in the ATPS. System containing 20 % [Bmim]BF4 and 25 % NaH2PO4 gave the highest PF (4.23-fold), with acceptable KE(7.27) and Y (88.93 %). The result indicated that the ILs-based ATPS is suitable for purification of wheat-esterase. Therefore, the [Bmim]BF4/NaH2PO4 system was selected for further study.
Effect of pH
As an example, KP, KE, Y and PF were studied as a function of the pH of the ATPS, and the results were shown in Table 3. The pH of selected ILATPS is 4.8. Therefore, pH range of 4.8 ~ 9.0 was chosen to study the pH dependence on wheat-esterase purification. Another reason for selection of this pH range is that protein denaturation and conformational change occurred at extreme pH values. As can be seen from Table 3. the partitioning was sensitive to the system pH. KE and PF decreased sharply while KP increased, with the increasing of pH values. This can be explained by the diffenent charged state of proteins at deffenent pH.
Table 3.
Effect of pH in [Bmim]BF4–NaH2PO4 ATPS on purification of wheat-esterase ( ± SD, n = 3)
| pH | V R | K P | K E | Y(%) | PF |
|---|---|---|---|---|---|
| 4.8 | 1.11 ± 0.02a | 0.72 ± 0.02a | 7.27 ± 0.06e | 88.93 ± 0.12d | 4.23 ± 0.04d |
| 6.0 | 1.20 ± 0.02c | 1.18 ± 0.07b | 5.56 ± 0.14d | 86.94 ± 0.06c | 3.29 ± 0.20c |
| 7.0 | 1.38 ± 0.03b | 1.57 ± 0.02c | 4.86 ± 0.07c | 87.05 ± 0.11c | 2.03 ± 0.07b |
| 8.0 | 1.12 ± 0.07a | 2.18 ± 0.03d | 4.33 ± 0.13b | 82.89 ± 0.73b | 2.18 ± 0.03b |
| 9.0 | 1.19 ± 0.02b | 4.61 ± 0.04e | 2.96 ± 0.05a | 77.89 ± 0.12a | 1.50 ± 0.04a |
V R volume ratio, K P protein distribution coefficient, K E enzyme distribution coefficient, PF purified fold, Y yield
*a, b, c, d means within a row not sharing a common superscript differ significantly (p < 0.05)
In general, proteins with negative charge prefer the top phase in ATPS, while proteins with positive charge normally partition selectively to the bottom phase (Gautam and Simon 2006; Yang et al. 2008). Above the isoelectric point (pI), enzyme/protein polyanions are accepted by ILs-rich phase in ATPS (Tanuja et al. 1997). This contrasts with the rejection of inorganic polyanions and may be attributed to an inherent greater separation of point charges on amino acid sidechains over the surface of enzyme polyanions (Tanuja et al. 1997).
Glutenins and gliadins are the major wheat storage proteins with the pI 6 ~ 8 and 6.4 ~ 7.1, respectively. The pI of wheat-esterase is 4.3 ~ 4.6. This can explain that KE is much lager than KP at pH 4.8 ~ 8.As the system pH increased the difference between system and pI of wheat proteins became smaller. Therefore electropositive of wheat proteins decreased. Electrostatic interaction between the charged groups in the protein and the cation of the ionic liquids decreased. For residual proteins chance of partitioning to the top phase increased while the system pH increased. As a result, KP increased sharply. The decrease of KE may caused by loss of biological activity of wheat-esterase at high pH value.
The charged state of proteins was affected by the pH values and the isoelectric points of proteins. Therefore, it was deduced that electrostatic interactions between the amino acids on the protein surface and the IL-cation played an important role in the extraction efficiency of wheat-esterase. This purpose was proved by Dreyer in 2009 (Dreyer et al. 2009).
Effect of temperature
The purification of wheat-esterase was carried out over a temperature range of 10 ~ 50 °C, and the temperature dependence of the purified process was illustrated in Table 4. It was obvious that at temperatures range from 10 to 50 °C, VR increased in some sort as temperature increased but the overall parameter of purification remained virtually unchanged. From the results obtained it could be established that the partitioning was unsensitive to temperature. The result meant that the studied ATPS was suitable for purification of wheat-esterase at a relative wide range of temperature.
Table 4.
Effect of temperature in [Bmim]BF4–NaH2PO4 ATPS on purification of wheat-esterase ( ± SD, n = 3)
| Temperature °C | V R | K P | K E | Y(%) | PF |
|---|---|---|---|---|---|
| 10 | 1.03 ± 0.01a | 0.76 ± 0.01b | 7.33 ± 0.05b | 88.35 ± 0.07a | 4.18 ± 0.02ab |
| 20 | 1.10 ± 0.01b | 0.71 ± 0.01a | 7.23 ± 0.04a | 88.81 ± 0.12b | 4.25 ± 0.03b |
| 30 | 1.11 ± 0.02b | 0.72 ± 0.02a | 7.27 ± 0.06ab | 88.93 ± 0.12b | 4.23 ± 0.04b |
| 40 | 1.13 ± 0.01c | 0.75 ± 0.00ab | 7.22 ± 0.05a | 89.12 ± 0.06c | 4.18 ± 0.04ab |
| 50 | 1.18 ± 0.01d | 0.75 ± 0.00ab | 7.24 ± 0.01a | 89.51 ± 0.11d | 4.23 ± 0.04b |
V R volume ratio, K P protein distribution coefficient, K E enzyme distribution coefficient, PF purified fold, Y yield
*a, b, c, d means within a row not sharing a common superscript differ significantly (p < 0.05)
Performance for purification of wheat-esterase
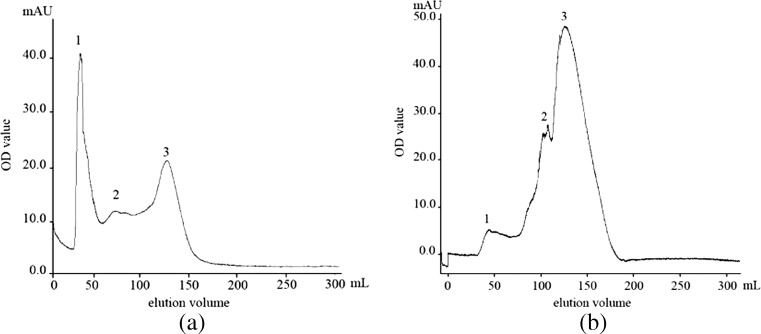
The purity of the wheat-esterase obtained from ATPS was confirmed using Sephadex G-150FF chromatography as shown in Fig. 1. Three peaks were shown in Fig. 1a and only peak 3 was proved to be caused by wheat-esterase after activity assay. In contrast to Fig. 1a, only one visible peak was found in Fig. 1b which correspond to peak 3 with elution volume. From Fig. 1, it can be observed that the majority of the contaminant proteins present in the crude extract partitioned to the bottom phase during the ATPS extraction while the wheat-esterase partitioned to the top phase. Hence an increase in the purity of wheat-esterase is observed.
Fig. 1.
Elution profile of wheat-esterase from wheat extracts (a) and ATPS (b) after Sephadex G-150FF
SDS-PAGE
The purity of the plant-esterase obtained from ATPS was confirmed using SDS-PAGE as shown in Fig. 2. Lane 3 indicated the molecular marker, while lane 1 was the crude extract of plant esterase and lane 2 was the plant-esterase after ILATPS extraction. From the SDS-PAGE, an increase in the purity of plant-esterase is observed after the ILATPS extraction.
Fig. 2.
SDS-PAGE of plant-esterase. Lane 1: crude extract of plant-esterase, lane 2: plant-esterase after the ILATPS extraction and lane 3: molecular marker
Conclusions
Purification of wheat-esterase from the crude extract using [Bmim]BF4/NaH2PO4 ATPS is reported for the first time. During this study, a systematic approach was used to find the optimized conditions to purify wheat-esterase. The process parameters involved in the purification of wheat-esterase were discussed and their optimization was described in detail. A method for purification of wheat-esterase in an ATPS was proposed. With a 20 % [Bmim]BF4 and 25 % NaH2PO4 ATPS, wheat-esterase with a purification factor of 4.23 fold and a yield of 88.93 % was obtained. This method enhances both the purity and the yield of the wheat-esterase beyond that obtained by the conventional salting-out step. Experimental results obtained here demonstrated the feasibility of an ATPS for the purification of wheat-esterase. This optimized process is expected to promote the applications of wheat-esterase in Ops detection.
Acknowledgments
The authors would like to acknowledge the financial support from the National Natural Science Foundation of China (No.31201366), Science and technology research projects of Education Department of Heilongjiang Province (No.11551063).
References
- Aguilar O, Rito-Palomares M. Aqueous two-phase systems strategies for the recovery and characterization of biological products from plants. J Sci Food Agric. 2010;90(9):1385–1392. doi: 10.1002/jsfa.3956. [DOI] [PubMed] [Google Scholar]
- Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cummins I, Edwards R. Purification and cloning of an esterase from the weed black-grass (Alopecurus myosuroides), which bioactivates aryloxyphenoxypropionate herbicides. Plant J. 2004;39(6):894–904. doi: 10.1111/j.1365-313X.2004.02174.x. [DOI] [PubMed] [Google Scholar]
- De Carvalho VM, Marques RM, Lapenta AS, Machado M. Functional classification of esterases from leaves of Aspidosperma polyneuron M. Arg. (Apocynaceae) Genet Mol Biol. 2003;26(2):195–198. doi: 10.1590/S1415-47572003000200013. [DOI] [Google Scholar]
- Dembczynski R, Bialas W, Regulski K, Jankowski T. Lysozyme extraction from hen egg white in an aqueous two-phase system composed of ethylene oxide-propylene oxide thermoseparating copolymer and potassium phosphate. Process Biochem. 2010;45(3):369–374. doi: 10.1016/j.procbio.2009.10.011. [DOI] [Google Scholar]
- Deutscher M. Guide to protein purification, vol 182. Methods in enzymology. New York: Academic Press; 1990. [Google Scholar]
- Dong HL, Wang QA (2009) Detection of oraganophosphorus by immobilized wheat esterase in chitosan bead. Paper presented at the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering
- Dreyer S, Salim P, Kragl U. Driving forces of protein partitioning in an ionic liquid-based aqueous two-phase system. Biochem Eng J. 2009;46(2):176–185. doi: 10.1016/j.bej.2009.05.005. [DOI] [Google Scholar]
- Gautam S, Simon L. Partitioning of beta-glucosidase from Trichoderma reesei in poly (ethylene glycol) and potassium phosphate aqueous two-phase systems: influence of pH and temperature. Biochem Eng J. 2006;30(1):104–108. doi: 10.1016/j.bej.2006.02.010. [DOI] [Google Scholar]
- Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD. Controlling the aqueous miscibility of ionic liquids: aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J Am Chem Soc. 2003;125(22):6632–6633. doi: 10.1021/ja0351802. [DOI] [PubMed] [Google Scholar]
- Hallett JP, Welton T. Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem Rev. 2011;111(5):3508–3576. doi: 10.1021/cr1003248. [DOI] [PubMed] [Google Scholar]
- Huo DQ, Yang LM, Hou CJ. Optical detection of dimethyl methyl-phosphonate with monosulfonate tetraphenyl porphyrin-plant-esterase complex. Sens Lett. 2009;7(1):72–78. doi: 10.1166/sl.2009.1012. [DOI] [Google Scholar]
- Kula K, Kroner H, Hustedt H. Purification of enzymes by liquid–liquid extraction. Adv Biochem Eng Biotechnol. 1992;24:73–118. [Google Scholar]
- Li L, Liu F, Kong XX, Su S, Li KA. Investigation of a liquid-liquid extraction system based on non-ionic surfactant-salt-H2O and mechanism of drug extraction. Anal Chim Acta. 2002;452(2):321–328. doi: 10.1016/S0003-2670(01)01471-4. [DOI] [Google Scholar]
- McKee T, Mckee JR. Biochemistry: an introduction. 2. New York: McGraw-Hil; 1999. [Google Scholar]
- Oppermann S, Stein F, Kragl U. Ionic liquids for two-phase systems and their application for purification, extraction and biocatalysis. Appl Microbiol Biotechnol. 2011;89(3):493–499. doi: 10.1007/s00253-010-2933-4. [DOI] [PubMed] [Google Scholar]
- Pfennig A, Schwerin A, Gaube J. Consistent view of electrolytes in aqueous two-phase systems. J Chromatogr B. 1998;711(1–2):45–52. doi: 10.1016/S0378-4347(97)00593-8. [DOI] [PubMed] [Google Scholar]
- Qian LL, He YZ, Hu YY. Determination of organophosphorus pesticide residues in vegetables by electrokinetic sequential injection analysis. Spectrosc Lett. 2006;39(6):581–592. doi: 10.1080/00387010600824652. [DOI] [Google Scholar]
- Qian LL, He YZ, Hu YY. Determination of pesticide residues in pond water by electrokinetic flow analysis and enzyme inhibition. Chin J Anal Chem. 2006;34(11):1591–1594. [Google Scholar]
- Rabelo APB, Tambourgi EB, Pessoa A. Bromelain partitioning in two-phase aqueous systems containing PEO-PPO-PEO block copolymers. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2004;807(1):61–68. doi: 10.1016/j.jchromb.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Shiddiky MJA, Torriero AAJ. Application of ionic liquids in electrochemical sensing systems. Biosensors & Bioelectronics. 2011;26(5):1775–1787. doi: 10.1016/j.bios.2010.08.064. [DOI] [PubMed] [Google Scholar]
- Steel RGD, Torrie JH. Principle and procedures of statistic: a biomaterial approach. New York: McGraw-Hill; 1980. [Google Scholar]
- Stuhlfelder C, Lottspeich F, Mueller MJ. Purification and partial amino acid sequences of an esterase from tomato. Phytochemistry. 2002;60(3):233–240. doi: 10.1016/S0031-9422(02)00126-7. [DOI] [PubMed] [Google Scholar]
- Tanuja S, Srinivas ND, Rao K, Gowthaman MK. Aqueous two-phase extraction for downstream processing of amyloglucosidase. Process Biochem. 1997;32(8):635–641. doi: 10.1016/S0032-9592(97)00009-5. [DOI] [Google Scholar]
- Vanasperen K. A study of housefly esterases by means of a sensitive colorimetric method. J Insect Physiol. 1962;8(4):401–414. doi: 10.1016/0022-1910(62)90074-4. [DOI] [Google Scholar]
- Waibel M, Schulze H, Huber N, Bachmann TT. Screen-printed bienzymatic sensor based on sol-gel immobilized nippostrongylus brasiliensis acetylcholinesterase and a cytochrome P450BM-3 (CYP102-A1) mutant. Biosensors & Bioelectronics. 2006;21(7):1132–1140. doi: 10.1016/j.bios.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Walter H, Johansson G. Aqueous two-phase systems vol 228. Methods in enzymology. New York: Academic Press; 1994. [Google Scholar]
- Wong FCM, Ahmad M, Heng LY, Peng LB. An optical biosensor for dichlovos using stacked sol-gel films containing acetylcholinesterase and a lipophilic chromoionophore. Talanta. 2006;69(4):888–893. doi: 10.1016/j.talanta.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Yang SQ, Huang ZA, Jiang ZQ, Li L. Partition and purification of a thermostable xylanase produced by paecilomyces thermophila in solid-state fermentation using aqueous two-phase systems. Process Biochem. 2008;43(1):56–61. doi: 10.1016/j.procbio.2007.10.013. [DOI] [Google Scholar]
- Zaslavsky BY, Miheeva LM, Mestechkina NM, Rogozhin SV. Physicochemical factors governing partition behavior of solutes and particles in aqueous polymeric biphasic systems 2. Effect of ionic composition on the hydration properties of the phases. J Chromatogr. 1982;253(2):149–158. doi: 10.1016/S0021-9673(01)88374-6. [DOI] [Google Scholar]