Abstract
Crude proteins of cultured mycelia and fruiting bodies of Ganoderma lucidum were investigated for antioxidant, antibacterial and DNA protective activities. It was found that the half maximal inhibitory concentration (IC50) of the mycelia protein and fruiting bodies protein extracts against 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) radical (ABTS•+) were 2.47 ± 0.01 and 2.77 ± 0.11 μg protein/ml and against 2,2-diphenylpicrylhydrazyl radical (DPPH•) were 2.5 ± 0.01 and 3.42 ± 0.01 μg protein/ml, respectively. The ferric reducing-antioxidant power (FRAP) values of those samples were 1.73 ± 0.01 and 2.62 ± 0.01 μmole trolox/μg protein respectively. Protein hydrolysates prepared by pronase exhibited a weaker antioxidant activity. Both crude proteins showed antibacterial activity, whereas only the mycelia protein extract could protect DNA damage by hydroxyl (•OH) radicals. This protein extract was partial purified by Diethyl amino ethyl (DEAE)-Sepharose column and Sulfopropyl (SP)-Sepharose column, obtained major protein with molecular weight about 45 kilo Dalton (kDa). In conclusion, G. lucidum protein extracts have promise potential for applications as antioxidant and antibacterial agents.
Keywords: Antioxidant, Mushroom, Reducing power, Protein purification, DNA protection
Introduction
Free radicals are atoms, molecules or ions with unpaired electrons (Lu et al. 2010). They are highly active to react with biological molecules including DNA, proteins, lipid membranes (Temple 2000), resulting in diseases and disorders, especially degenerative diseases such as cardiovascular diseases and cancers (Wagner et al. 1992). Free radicals can be eliminated by antioxidant molecules. Thus, these molecules have been interested. A wide range of molecules have been reported to possess antioxidant activities such as phenolic compounds, vitamins, polysaccharides, peptides, proteins, organic acids, carotenoids, alkaloids, and nucleotides (Stajić et al. 2013) Recently, proteins and peptides with antioxidant activities have been highly interested. These molecules normally contain amino acids with side chains that can donate electrons such as cysteine and tyrosine (Power et al. 2013; Sarmadi and Ismail 2010).
Mushrooms have been consumed by many cultures for centuries and have long been used in traditional Chinese medicines. Bioactive compounds of mushrooms such as polysaccharides, proteins, polysaccharide-protein complexes have been reported to possess several biological activities (Xu et al. 2011). The mushroom Ganoderma lucidum is popular medicinal mushroom and widely used for promote health and longevity. This mushroom also has been reported to have antioxidant activities (Yen and Wu 1999; Mau et al. 2002), cardioprotective effects (Sudheesh et al. 2013) and antidiabetic potency (Teng et al. 2012). However, molecules responses for those activities mostly are not proteins. There are a few investigations reported about proteins or peptides with bioactivities from this mushroom. Immunomodulatory protein derived from this mushroom has been proposed to have therapeutic effects on cancer and autoimmune diseases (Girjal et al. 2012). In the present study, the crude protein extracts from mycelia and fruiting bodies of G. lucidum which production in the Rujira Farm (Kalasin, Thailand) and distribution nationwide, were evaluated for antioxidant and antibacterial activities.
Materials and methods
Fungal cultivation
G. lucidum G2 originated from Department of Agriculture, Thailand were obtained from Rujira Mushroom Farm in Ka La Sin province (Northeast, Thailand). The fungus was cultured and maintained in potato dextrose agar (PDA). After that the fungal active growing mycelia plugs were transferred to aseptic flask containing liquid medium, in which the medium included glucose (10 g/l), yeast extract (5 g/l), peptone Type-I (5 g/l) and MgSO4 (1 g/l). The fungal mycelia were cultured for 14 days at ambient temperature and shaking at 120 revolutions per minute (rpm). Fresh fruiting bodies of the mushroom were obtained from the mushroom farm.
Bacterial strains
The tested bacteria were mostly purchased from Thailand Institute of Scientific and Technological Research (TISTR). The gram positive bacteria included Bacillus subtilis TISTR 008, Bacillus cereus TISTR 687, Staphylococus epidermidis TISTR 518, Staphyloccus aureus TISTR 1466. The gram negative bacteria were Escherichia coli TISTR 780 and Pseudomonas aeruginosa TISTR 781. All bacteria were maintained in LB (Luria-Bertani) medium.
Crude protein extraction
The mycelia of G. lucidum were washed with distilled water and homogenized in extraction solution as previously used (0.01 M HCl containing 0.15 M NaCl) (Phansri et al. 2011) in a proportion of 1 g of mycelia to 2 ml of extraction solution. The crude extract solution after filtering through cheesecloth was centrifuged (Hettich Mikro 22R, Germany) at 3,904 × g, 4 °C for 30 min. The fruiting bodies of the mushroom were washed with distilled water and cut into small pieces before homogenization with the extraction solution. Extraction of fruiting bodies was performed similar to the mycelia extraction as described above.
Protein determination
Soluble protein content was determined by the Bradford method (Bradford 1976) using a Bio-Rad protein assay reagent (Bio-Rad, USA). Bovine serum albumin (BSA) was used as a standard protein.
Antioxidant activity assays
ABTS•+ radical scavenging activity
The ABTS•+ radicals were prepared as described by Khammuang and Sarnthima (2008) with a modification, 0.02 U/ml of crude laccase from Lentinus polychrous Lév instead of Trametes versicolor laccase, was mixed with 10 mM ABTS. The reaction mixture was incubated at 32 °C for 10 min. The laccase in the reaction was deactivated by heating in boiling water for 10 min and then filtered out using a 10 kDa molecular weight cut off membrane. The filtrate containing ABTS•+ radicals was used for the antioxidant activity experiment. The ABTS•+ radicals solution was diluted with distilled water to give initial optical density at 734 nm (OD734nm) at around 0.7. The samples (various concentrations) 20 μl was added to 980 μl of ABTS•+ radical solution. The antioxidant activity was assayed by measuring the decreasing absorbance at 734 nm after incubation in the dark at room temperature for 5 min. The antioxidant activity was reported as trolox equivalent antioxidant capacity (TEAC) which calculated as shown in Eq. 1.
| 1 |
Where,
- A0
is initial absorbance of reaction,
- At
is final absorbance of reaction
- m
is slope of a plot between %Inhibition and μmole Trolox
- X
is μg protein of sample
Half maximum inhibitory concentration (IC50) values were calculated from a linear equation (y = mx) of a plot between %Inhibition (y axis) and concentrations of G. lucidum protein extracts (x axis) (Thetsrimuang et al. 2011).
DPPH• radical scavenging activity
Similar to ABTS•+ radical scavenging assay, DPPH• radical scavenging activity was measured using a modified Yamaguchi et al. (1998) as previous described (Thetsrimuang et al. 2011). The stock, 10 mM of DPPH• was diluted in ethanol until it gave an initial optical absorbance (OD515nm) of about 0.7 before using. The samples in various concentrations of 20 μl were added to 980 μl of DPPH• solution. The antioxidant capacity was calculated relatively to Trolox under the same conditions and the results were reported as μmol Trolox/μg protein of sample and also as the IC50 values as mentioned in “ABTS•+ radical scavenging activity” section.
Ferric reducing antioxidant power (FRAP)
The FRAP assay was modified method according to Benzie and Strain (1999) as previously described by Thetsrimuang and co-workers (2011). FRAP reagent was prepared by mixing 1 volume of FeCl3•6H2O solution (20 mM), 1 volume of TPTZ (2,4,6-tripyridyl-s-triazine) solution and 10 volumes of acetate buffer (0.5 M, pH 3.6). The reagent was warmed to 37 °C. The sample of 20 μl was added to 980 μl of FRAP reagent. The ability of reducing power was measured at absorbance 593 nm after initial mixing and after 90 min of reaction. Antioxidant power was expressed as μmole Trolox/μg protein of sample and FeSO4•7H2O solution was used to prepare a calibration curve. Calculations were done according to Eq. 2.
| 2 |
Where,
- A593
is absorbance of reaction at 593 nm,
- m1
is slope of a plot between A593 and μM of Fe2+
- m2
is slope of a plot between μM of Fe2+ and μmole Trolox
- X
is μg protein of sample
Proteolytic digestion by Pronase
Crude protein of mycelia extract and fruiting bodies extract were treated with pronase (Roche, USA) in a ratio of 1 μg pronase: 5 μg of protein in 50 mM Tris–HCl buffer (pH 7.5) containing 10 mM CaCl2 (conditions optimized from the company recommendation protocol). The treatment reactions were incubated at 40 °C for 30 min, 1 h, 2 h and then heated in boiling water for 10 min to deactivate the enzyme. After that, the supernatant was obtained by centrifugation (Hettich Mikro 22R, Germany) before using for antioxidant activity assay.
Antibacterial activity and minimum inhibitory concentration (MIC) assay
Overnight cultures of the reference bacterial strains were grown in LB broth. Then, 50 μl of the overnight culture were added to 50 ml of a new LB broth tube and incubated at 37 °C for 3–4 h with shaking at 180 rpm. Appropriated diluted of one milliliter of this culture containing around 1 × 108 colony was added to 5 ml warm melted LB agar. After mixing briefly, the overlay gel was poured on petri dish plate containing underlay LB agar gel. The sterile discs were then placed on plates. The crude extracts and positive control, 500 μg of kanamycin were applied onto each different disc and extraction solution was used as negative control. The plates were incubated for 5–7 h at 37 °C. After incubation, the diameters of the inhibition zone surrounding the disc were measured. The experiments were carried out in a triplicate (Phansri et al. 2011).
The minimum inhibitory concentration (MIC) determination was also performed. The tested bacteria were first grown in LB broth until an OD600 nm diluted with 0.85 % (w/v) NaCl compared to McFaland No. 0.5, in which bacteria concentration 1.5 × 108 cfu/ml were achieved. The 0.45 μm filtrated of mycelia protein extract and fruiting bodies protein extract of G. lucidum were prepared in a 2-fold serial dilution by double strength LB broth in a 96-well plate. After that, 50 μl of bacterial suspensions were added and mixed with 50 μl crude protein in 96-well a microtiter plate with a final volume of 200 μl and incubated for 18 h at 37 °C. Kanamycin at final concentration of 125 μg/ml (25 μg/200 μl) was used as positive control (Phansri et al. 2011).
Protective effects against hydroxyl radical induced DNA damage
DNA protection assay was performed as described by Siswoyo et al. (2011). The reaction was carried out in a 1.5 ml microcentrifuge tube at the total volume of 20 μl containing 0.5 μg of pGEX DNA, mixing solution (15 mM H2O2, 25 μM ascorbic acid and 40 μM FeCl3) and tested samples at various concentrations. The mixture was then incubated for 15 min at 37 °C. The reactions were then analyzed by 1 % agarose gel electrophoresis and nucleic acid was stained with GelStar™ (Lonza, USA). Trolox was used as a positive control of antioxidant compound.
Total phenol content
Total phenol content (TPC) of the crude protein extracts was assayed according to Singleton and Rossi (1965) with some modification as described previously by Thetsrimuang et al., (2011). Briefly, crude protein 500 μl was mixed with 500 μl of Folin-Ciocalteu reagent (diluted 10×) and incubated in the dark for 3 min. After 3 min, the reaction mixture was added 500 μl of 35 % (w/v) Na2CO3, and then diluted with 5 ml of distilled water. After incubation in the dark for 90 min, the reaction mixture was measured at 725 nm by spectrophotometer (Thermo spectronic Genesys 20, Model 4001/4 visible range spectrophotometer, USA). The quantification was determined based on a standard curve of gallic acid and the total phenol content was reported as GAE (mg/g protein).
Partial purification of antioxidant protein from G. lucidum
The crude mycelia protein solution was subjected to ammonium sulfate precipitation (85 % saturation). The precipitant was collected by centrifugation at 10,000 × g for 30 min at 4 °C, and then re-dissolved in a small volume of distilled water, followed by dialysis (MW cut off 2 kDa) overnight at 4 °C. The retentate was applied onto a DEAE-Sepharose column (ϕ 1.6 cm. × 10 cm. height) previously equilibrated with the 10 mM Tris–HCl buffer (pH 7.3) at flow rate 2 ml/min. Un-adsorbed proteins were washed out from the column by the same buffer at flow rate 2 ml/min and collected sample fraction at 2 ml/tube. Adsorbed proteins were eluted with a linear gradient of 0–1 M NaCl in the same buffer. The un-adsorbed proteins and adsorbed proteins were measured at 280 nm by a UV–vis spectrophotometer. The obtained protein peaks of fractions were pooled, concentrated and desalted. After that, each of pooled fractions was used to assay for antioxidant activity. The active pooled fraction was then applied to SP-Sepharose XL column (GE Healthcare, UK) equilibrated with 10 mM ammonium acetate buffer (pH 4.6) at flow rate 2 ml/min. The un-adsorbed proteins were washed out with the same buffer and the adsorbed proteins were eluted with linear gradient of 0–1 M NaCl in 10 mM ammonium acetate buffer (pH 4.6). The pooled fractions after concentrated and desalted were assayed for antioxidant activity and analyzed by a SDS-PAGE staining with silver.
Statistical analysis
Antioxidant activity and antibacterial activity experiments were carried out in triplicate. The data were reported as mean ± standard deviation (S.D.) and statistical analysis was performed with the SPSS version 16 software (SPSS Inc., Chicago, USA). T-test was applied for the comparisons and a value of p < 0.05 was considered as statistically significant.
Results and discussion
Antioxidant activities
The scavenging effect including reducing ability of crude protein from G. lucidum were evaluated by ABTS•+, DPPH• radical scavenging and FRAP assays and expressed as Trolox equivalent antioxidant capacity (TEAC) (Fig. 1). It was found that mycelia protein extract showed better scavenging activities than fruiting bodies protein extract both in ABTS•+ and DPPH• radical scavenging abilities as observed from the higher TEAC values (Fig. 1). However, fruiting bodies protein extract showed a slightly better reducing ability.
Fig. 1.
The antioxidant capacity of crude protein measured as Trolox Equivalents (μmole trolox/μg protein) assay. Each value is expressed as mean ± standard deviation (n = 3). Mean in each sample is significantly different (p < 0.05)
It was possible that in the fruiting bodies extract might contain molecules with a better reducing ability but poorer scavenging radical abilities. The crude protein extracts both from mycelia and fruiting bodies after digestion with pronase, it was observed that higher IC50 values of both crude protein extracts were obtained (Table 1 and Fig. 2). These results suggested that proteins might be key compounds responsible for radicals scavenging activities. Several amino acids compositions in protein have been reported for playing role in those properties. Phe, His and Pro containing peptides have been reported to exhibit antioxidant activity (Girjal et al. 2012).
Table 1.
Half maximal inhibitory concentrations (IC50) for radical-scavenging activity of crude protein undigested and digested from Ganoderma lucidum
| ICa50(μg protein/ml) | ||||
|---|---|---|---|---|
| Undigested | Digested | |||
| Mycelia protein | Fruiting bodies protein | Mycelia protein | Fruiting bodies protein | |
| ABTS∙+ | 2.47 ± 0.01 | 2.77 ± 0.11 | 4.41 ± 0.54 | 8.49 ± 2.37 |
| DPPH∙ | 2.50 ± 0.07 | 3.42 ± 0.35 | 10.36 ± 1.54 | 12.6 ± 0.49 |
Each value is expressed as mean ± standard deviation (n = 3). Mean within a column and row for crude protein significantly different (P < 0.05)
aIC50 value: the inhibitory concentration at which the antioxidant activity was 50 %
Fig. 2.
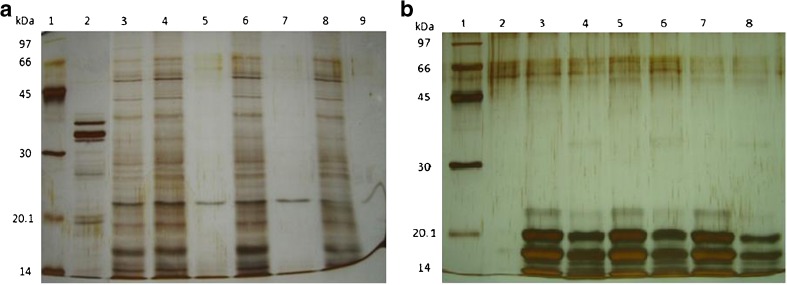
Twelve percent SDS-PAGE of Ganoderma lucidum protein extracts digested with pronase. a crude mycelia protein extract, lane 1: molecular weight markers; lane 2: pronase 4 μg; lane 3: crude mycelia protein extract; lane 4; 6; 8: control sample at 30 min, 1 h, 2 h; lane 5; 7; 9: digested sample at 30 min, 1 h and 2 h, respectively, b crude fruiting bodies protein extract digested with pronase. Lane 1: molecular weight markers, lane 2: pronase in the reaction, lane 3; 5; 7: control 30 min, 1 h, 2 h, lane 4, 6, 8: treated with pronase at 40 °C for 30 min, 1 and 2 h respectively
The very low IC50 values of crude protein extract from mycelia and fruiting bodies on DPPH• radicals were obtained in this work, 2.50 ± 0.07 μg protein/ml (or 0.48 mg extract/ml) for mycelia protein extract and 3.42 ± 0.35 μg protein/ml (or 0.60 mg extract/ml) for fruiting bodies extract. Wu and Wang (2009) reported that water soluble glycopeptides isolated from fruiting bodies of G. lucidum had the IC50 towards DPPH• radicals of about 150 μg/ml. However, it has to keep in mind that such low IC50 values from our works are probably due to contributed antioxidant activity of other components apart from proteins presented in the crude protein extracts (i.e. polyphenols etc.). Experimented data from the purified peptide should be obtained. Girjal et al. (2012) reported that purified peptides from G. lucidum fruiting bodies had the ability to inhibit DPPH• radicals 74.21 % at concentration of 40 μg/ml.
For the reducing power of both crude protein extracts as determined by FRAP assay revealed that the fruiting bodies protein extract showed more reducing ability than that of the mycelia protein extract (1.73 ± 0.01 and 2.62 ± 0.01 μmole trolox/μg protein respectively, Fig. 1). This assay is one of the total antioxidant capacity methods which do not detect glutathione and protein thiols. The differences between ferric reducing ability and the anti-radical activities of both crude protein extracts, might probably due to differences in active compositions in the crude extracts. Apart from differences in proteins (as shown in Fig. 2) and phenolic compounds, reducing sugars might be presented differently. Determination of reducing sugar in the crude protein extracts and successfully purified proteins before evaluation its antioxidant activity will be significant evidence. However, this work indicates that crude proteins from G. lucidum clearly exhibited antioxidant activity.
Antibacterial activity
Protein extracts of mycelia and fruiting bodies from G. lucidum showed interesting antibacterial activities. Since protein concentration from fruiting bodies extract was very low when compared to mycelia protein extract. Thus, protein amounts used for antibacterial tests were different. At the protein amount tested, 25 μg protein of fruiting bodies extract and 115 μg protein of mycelia extract, it was found that the fruiting bodies protein extract could inhibit all tested bacteria except for S. aureus. Whilst the mycelia protein extract could inhibit all tested bacteria except for P. aeruginosa (Table 2).
Table 2.
Antibacterial activity of the mycelia protein and fruiting bodies protein extracts of Ganoderma lucidum
| Protein extract | S. epidermidis | S. aureus | B.subtilis | B.cereus | E. coli | P. aeruginosa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibition zonea | MICb | Inhibition zonea | MICb | Inhibition zonea | MICb | Inhibition zonea | MICb | Inhibition zonea | MICb | Inhibition zonea | MICb | |
| Fruiting bodies | + | 20.0 | − | ND | + | ND | ++ | 20.0 | + | 81.5 | + | ND |
| Mycelia | +++ | 512.5 | + | ND | + | ND | +++ | 81.5 | ++ | 81.5 | − | ND |
| Kanamycin* | ++++++ | 7.8 | +++++ | ND | +++++ | ND | ++++++ | 15.6 | +++++ | 7.8 | ++++ | ND |
Bacterial growth inhibition; − No inhibition, + = inhibition zone ≥ 1 nm <3 mm, ++ = inhibition zone ≥ 3 mm <6, +++ = inhibition zone ≥ 6 mm < 8 mm, ++++ = inhibition zone ≥8 mm < 11 mm, +++++ = inhibition ≥11 mm <14 mm, ++++++ = inhibition zone ≥14 mm <17 mm
ainhibition zone (mm); bMIC (mg/ml)
*Kanamycin is positive control antibiotics. ND not determined
The MIC values of crude proteins towards tested bacteria are as shown in Table 2. The ability of fruiting bodies protein extract towards tested bacteria were S. epidermidis (20 μg protein/ml) and B. cereus (20 μg protein/ml) > E. coli (81.5 μg protein/ml). The ability of mycelia protein extract to inhibit tested bacteria were B. cereus (81.5 μg protein/ml) and E. coli (81.5 μg protein/ml) > S. epidermidis (512.5 μg protein/ml).
Protective effects of DNA damage from Fenton’s reaction
Single-strand break in supercoiled plasmid DNA, exposed to hydroxyl radical derived from Fenton’s reaction can be occurred, leads to the formation of open circular DNA. The DNA protection assay was used to evaluate protective activity of the crude protein against hydroxyl radical induced DNA damage on pGEX-1 plasmid DNA by an in vitro method. The results revealed that when amounts of the mycelia protein extract were increase, DNA damage by hydroxyl radicals tended to decrease, observed from intensity of supercoiled DNA band in agarose gel seemed to increase in proportional with amount of the mycelia protein extract (Fig. 3a). In contrast, the protective effect of DNA has been observed with the fruiting bodies protein extract better in lower protein concentrations (Fig. 3b). Standard antioxidant, Trolox (Fig. 3a, lane 3), revealed a completely disappeared of supercoiled DNA to be open chain. Even though it exhibited antioxidant activity, this compound has been reported to be a pro-oxidant (Ko et al. 1994). Our results also suggested that it might have certain compounds with pro-oxidant activity presenting in the crude protein extract of G. lucidum fruiting bodies. This is probably due to its higher reducing power than the crude protein extract of the fungal mycelia. Predomination of reducing power over scavenging activity resulted in the pro-oxidant effect of aloe-emodin at high concentrations (Tian and Hua 2005).
Fig. 3.
Inhibitory effect of Ganoderma lucidum protein extracts on DNA damage from hydroxyl radicals. a mycelia protein extract, lane 1 plasmid DNA (0.05 μg), lane 2: plasmid DNA exposed Fenton reaction, lane 3: plasmid DNA with Fenton reaction plus 200 μM trolox, lane 4; 5; 6: plasmid DNA with Fenton reaction plus mycelia protein extract 25, 100, 200 μg/ml respectively; b fruiting bodies protein, lane 1: plasmid DNA (0.05 μg), lane 2: plasmid DNA with Fenton reaction, lane 3; 4; 5: plasmid DNA plus fruiting bodies protein 2.5, 25 and 100 μg/ml respectively. OC: open chain; L: linear; SC: supercoil DNA
The ability of protein extract containing DNA protection property has been reported from Melinjo (Gnetum gnemon) seed protein which showed to protect the super coiled DNA from hydroxyl radical induced strand breaks, as reported by Siswoyo et al. (2011). Polysaccharide extracted from G. lucidum fruiting bodies has been reported in protecting against hydroxyl radical-induced DNA strand breaks (Kim and Kim 1999). Our investigation found that only protein extract from the fungal mycelia showed such property but not protein extract from its fruiting bodies. The difference of ability in protecting against DNA strand break induced by hydroxyl radicals between previous reported (Kim and Kim 1999) and our investigation may due to differences in other composition of the fruiting bodies protein extract, i.e. polysaccharides, polyphenols and etc..
Total phenolic content
Both types of G. lucidum crude protein extracts contained certain amounts of phenol compounds as shown in Table 3. When compared in term of mg GAE/g dry weight extract, it was found that TPC in both protein extracts are not significantly different (p > 0.05). However, the TPC in both extracts were different when compared in term of mg GAE/g of protein due to difference in protein content in each extract. The TPC was found higher in the fruiting bodies extract than that of the mycelia extract. However, the mycelia protein extract exhibited stronger anti-radical abilities (Fig. 1). This supported that anti-radicals of G. lucidum crude extracts might be mainly due to proteins presented in the crude extracts. In this work, phenolic compounds presented in the crude extracts might somehow play roles in its antiradical activities, unless the protein with antioxidative property could be successfully purified and characterized. There are reports of G. lucidum phenolic extracts contained phenol compounds with antioxidant activity (Mau et al. 2002; Heleno et al. 2012). Some medicinal herbs or plants have been reported potent anti-radical, reducing power and metal chelating activity suggested being due to several polyphenol compounds presented in the extracts (Prathapan et al. 2011a, b).
Table 3.
Total phenolic content of crude proteins from Ganoderma lucidum
| Sample | Total phenolic content (mg GAE/g dw. extract) | Total phenolic content (mg GAE/g of protein) |
|---|---|---|
| Fruiting bodies | 0.48 ± 0.01a | 96.0 ± 0.01 |
| Mycelia | 0.66 ± 0.01a | 70.6 ± 0.01 |
Each value is expressed as mean ± standard deviation (n = 3). Mean with the same letters are not significantly different (p > 0.05)
Partial purification of the antioxidant protein
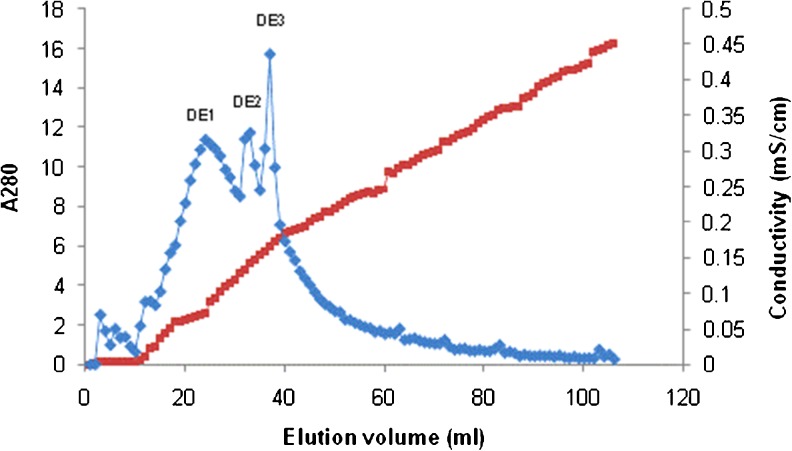
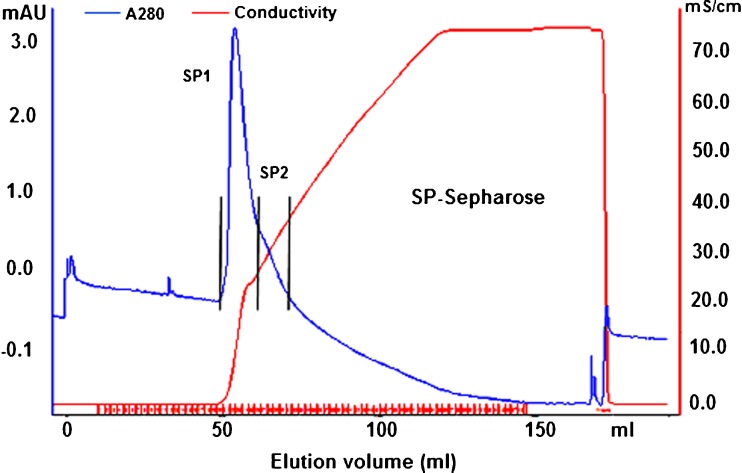
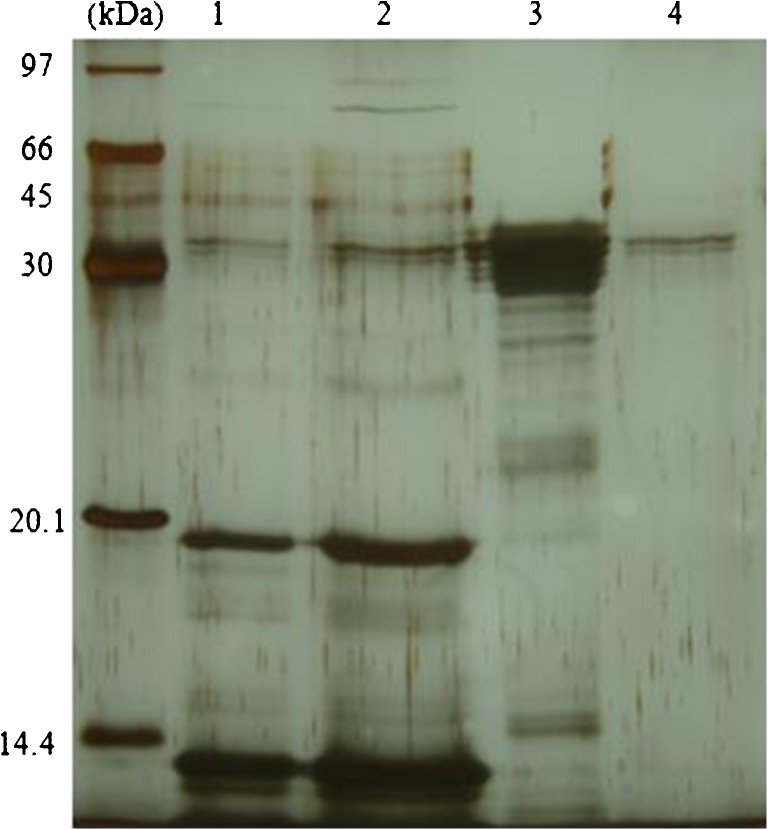
The mycelia protein of G. lucidum was selected for purification due to its easier preparation and bioactivity. It was first precipitated using 85 % saturation ammonium sulfate. Protein pellet was re-suspended in distilled water, dialyzed to remove salt and then applied onto a DEAE-Sepharose column. The elution profile showed three protein peaks according to absorbance at 280 nm (Fig. 4) and pooled fraction DE1 showed the strongest antioxidant activity against ABTS•+ radical. This fraction was dialyzed to remove salt and changing buffer and further purified by SP-Sepharose XL column. Adsorbed proteins were assigned as SP1 and SP2 (Fig. 5). Antioxidant activity observed in the adsorbed peak SP1 (ABTS•+ radical scavenging at 20.7 %) and SP2 (ABTS•+ radical scavenging at 32.2 %). After SDS-PAGE analysis, both pooled fractions showed partially purified with molecular mass about 45 kDa as shown in Fig. 6. Some reports of antioxidant peptides from G. lucidum have been reported. Peptide is major component responsible for the antioxidant activity of G. lucidum (Sun et al. 2004). Moreover, purified antioxidant peptide from fruiting bodies of G. lucidum with molecular mass about 2.8–3.35 kDa had been reported by Girjal et al. (2012).
Fig. 4.
DEAE-Sepharose profile of the mycelia protein of Ganoderma lucidum. The proteins were eluted with a linear gradient of 0–1 M NaCl in 10 mM Tris–HCl buffer (pH 7.3). DE1 showed the highest antioxidant activity
Fig. 5.
SP-Sepharose profile of DE1 fraction. SP1 and SP2 were eluted with a linear gradient of 0–1 M NaCl in 10 mM ammonium acetate buffer (pH 4.6). SP2 showed the higher antioxidant activity
Fig. 6.
Fifteen percent SDS-PAGE of the purified Ganoderma lucidum mycelia protein after SP-Sepharose XL column. Lane M: molecular weight markers; lane 1: crude mycelia protein; lane 2: un-adsorbed protein; lane 3: SP1; lane 4: SP2. Partial purified protein (SP1 and SP2) had major protein with molecular mass about 45 kDa
However, proteins with antioxidant activity have also been reported in plants with higher MW i.e. about 18 kDa purified protein from Curcuma comosa Roxb. rhizomes (Boonmee et al. 2011) and about 28 kDa purified protein from Solanum torvum seeds (Sivapriya and Leela 2007).
Conclusion
The mycelia protein extract from G. lucidum G2 had higher potential antioxidant and DNA protection than the fruiting bodies protein extract. However, the fruiting bodies protein extract had stronger reducing power and antibacterial activity. Both crude protein extracts had differences in protein content and pattern. Its protein hydrolysates prepared by pronase digestion, showed a decrease in anti-radicals activity, suggesting that proteins are responsible for anti-radical activity. Successful purification of antioxidant protein from G. lucidum is still needed to understand its molecular structure and other biological functions. To obtain fungal mycelia is easier and shorter than to obtain its fruiting bodies. Fermentation technology can be applied in the mass production for application as food supplement.
Acknowledgments
The authors would like to thank the Thailand Research Fund-Master Research Grants (TRF-MAG window I, Grant Number MRG545S046), Mahasarakham University (Grant Number 5604012/2556) and Protein and Enzyme Technology Research Unit, Center of Excellence for Innovation in Chemistry (PERCH-CIC), Department of Chemistry, Faculty of Science, Mahasarakham University.
References
- Benzie FF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–23. doi: 10.1016/S0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- Boonmee A, Srisomsap C, Karnchanatat A, Sangvanich P. An antioxidant protein in Curcuma comosa Roxb. Rhizomes. Food Chem. 2011;124:476–480. doi: 10.1016/j.foodchem.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Girjal VU, Neelagund S, Krishnappa M. Antioxidant properties of the peptides isolated from ganoderma lucidum fruiting bodies. Int J Pept Res Ther. 2012;18:319–325. doi: 10.1007/s10989-012-9303-2. [DOI] [Google Scholar]
- Heleno SA, Barros L, Martins A, Queiroz MJRP, Santos-Buelga C, Ferreira ICFR. Fruiting bodies, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: a comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Res Int. 2012;46:135–140. doi: 10.1016/j.foodres.2011.12.009. [DOI] [Google Scholar]
- Khammuang S, Sarnthima R. Laccase-aided antioxidant activity assay and antioxidant activity of selected Thai Vegetables. J Appl Sci. 2008;8:2718–2724. doi: 10.3923/jas.2008.2718.2724. [DOI] [Google Scholar]
- Kim KC, Kim IG. Ganoderma lucidum extract protects DNA from strand breakage caused by hydroxyl radical and UV irradiation. Int J Mol Med. 1999;4:273–277. [PubMed] [Google Scholar]
- Ko KM, Yick PK, Poon MK, Ip SP. Prooxidant and antioxidant effects of Trolox on ferric ion-induced oxidation of erythrocyte membrane lipids. Mol Cell Biochem. 1994;141(1):65–70. doi: 10.1007/BF00935592. [DOI] [PubMed] [Google Scholar]
- Lu JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau JL, Lin HC, Chen CC. Antioxidant properties of several medicinal mushroom. J Agric Food Chem. 2002;50:6072–6077. doi: 10.1021/jf0201273. [DOI] [PubMed] [Google Scholar]
- Phansri K, Sarnthima R, Thammassirirak S, Boonchalee P, Khammuang S. Antibacterial activity of Bauhinia acuminate L. Seed protein extract with low hemolytic activity against human erythrocytes. Chiang Mai J Sci. 2011;38:242–251. [Google Scholar]
- Power O, Jakeman P, Fitzgerald RJ. Antioxidative peptides: enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived anti-oxidative peptides. Amino Acids. 2013;44(3):797–820. doi: 10.1007/s00726-012-1393-9. [DOI] [PubMed] [Google Scholar]
- Prathapan A, Singh MK, Anusree SS, Kumar DRS, Sundaresan A, Raghu KG. Antiperoxidative, free radical scavenging and metal chelating activities of Boerhaavia diffusa L. J Food Biochem. 2011;35:1548–1554. doi: 10.1111/j.1745-4514.2010.00477.x. [DOI] [Google Scholar]
- Prathapan A, Lijo Cherian O, Nampoothiri SV, Mini S, Raghu KG. In vitro antiperoxidative, free radical scavenging and xanthine oxidase inhibitory potentials of ethyl acetate fraction of Saraca ashoka flowers. Nat Prod Res. 2011;25(3):298–309. doi: 10.1080/14786419.2010.510472. [DOI] [PubMed] [Google Scholar]
- Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31(10):1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Siswoyo TA, Mardiana E, Lee KO, Hoshokawa K. Isolation and characterization of antioxidant protein fractions from Melinjo (Gnetum gnemon) seeds. J Agric Food Chem. 2011;59:5648–5656. doi: 10.1021/jf2000647. [DOI] [PubMed] [Google Scholar]
- Sivapriya M, Leela S. Isolation and purification of a novel antioxidant protein from the water extract of Sundakai (Solanum torvum) seeds. Food Chem. 2007;104:510–517. doi: 10.1016/j.foodchem.2006.11.060. [DOI] [Google Scholar]
- Stajić M, Vukojević J, Knežević A, Laušević SD, Milovanović I. Antioxidant protective effects of mushroom metabolites. Curr Top Med Chem. 2013;13:2660–2676. doi: 10.2174/15680266113136660192. [DOI] [PubMed] [Google Scholar]
- Sudheesh NP, Ajith TA, Janardhanan KK. Ganoderma lucidum ameliorate mitochondrial damage in isoproterenol-induced myocardial infarction in rats by enhancing the activities of TCA cycle enzymes and respiratory chain complexes. Int J Cardiol. 2013;165:117–125. doi: 10.1016/j.ijcard.2011.07.103. [DOI] [PubMed] [Google Scholar]
- Sun J, He H, Xie BJ. Novel Antioxidant peptides from fermented mushroom Ganoderma lucidum. J Agric Food Chem. 2004;52(21):6646–6652. doi: 10.1021/jf0495136. [DOI] [PubMed] [Google Scholar]
- Temple NJ. Antoxidant and disease: more questions than answers. Nutr Res. 2000;20:449–459. doi: 10.1016/S0271-5317(00)00138-X. [DOI] [Google Scholar]
- Teng B-S, Wang C-D, Zhang D, Wu J-S, Pan D, Pan L-F, Yang H-J, Zhou P. Hypoglycemic effect and mechanism of a proteoglycan from Ganoderma Lucidum on streptozotocin-induced type 2 diabetic rats. Eur Rev Med Pharmacol Sci. 2012;16:166–175. [PubMed] [Google Scholar]
- Thetsrimuang C, Khammuang S, Chiablaem K, Srisomsap C, Sarnthima R. Antioxidant properties and cytotoxicity of crude poly saccharides from Lentinus polychrous Lév. Food Chem. 2011;128:634–639. doi: 10.1016/j.foodchem.2011.03.077. [DOI] [Google Scholar]
- Tian B, Hua Y. Concentration-dependence of prooxidant and antioxidant effects of aloin and aloe-emodin on DNA. Food Chem. 2005;91:413–418. doi: 10.1016/j.foodchem.2004.06.018. [DOI] [Google Scholar]
- Wagner JR, Hu CC, Ames BN. Endogenous oxidative damage of deoxycytidine in DNA. Proc Natl Acad Sci U S A. 1992;89:3380–3384. doi: 10.1073/pnas.89.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang D. A new class of natural glycopeptides with sugar moiety-dependent antioxidant activities derived from Ganoderma lucidum fruiting Bodies. J Proteome Res. 2009;8:436–442. doi: 10.1021/pr800554w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yan H, Chen J, Zhang X. Bioactive proteins from mushroom. Biotechnol Adv. 2011;29:667–674. doi: 10.1016/j.biotechadv.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Takamura H, Matoba T, Terao J. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 1998;62:1201–1204. doi: 10.1271/bbb.62.1201. [DOI] [PubMed] [Google Scholar]
- Yen GC, Wu JY. Antioxidant and radical scavenging properties of extracts from Ganoderma tsugae. Food Chem. 1999;65:375–379. doi: 10.1016/S0308-8146(98)00239-8. [DOI] [Google Scholar]