Abstract
Seasonal variation in the proximate and mineral composition of Kappaphycus alvarezii were investigated in the present study, moreover, the relationship between the nutritive components of this seaweed and the environment were also established. Carbohydrates represented the major portion of the algae (i.e. average total carbohydrate content was 23.01 ± 1.64 g/100 g DW), while the lipid content was the lowest among the constituents investigated (0.39 ± 0.04 to 0.91 ± 0.51 g/100 g DW). The protein content of K. alvarezii varied from 12.69 ± 0.6 to 23.61 ± 0.02 g/100 g DW, and the fiber content varied between 9.68 ± 0.08 to 18.57 ± 0.15 g/100 g DW. Highest total mineral content (29939.61 ± 9340.38 mg/100 g DW) was observed in April 2005, while least values were recorded in January 2006 i.e. (10997.62 ± 1120.26 mg/100 g DW). The Na/K ratio during the study ranged from 0.34 to 0.87. All the samples showed remarkable semi-refined carrageenan (SRC) yield ranging from 42.70 ± 1.07 to 63.73 ± 1.73 % (average 53.90 ± 1.37 %), and, the samples collected during December 2004 and January 2006 demonstrated maximum gel strengths i.e. 743 ± 15.28 and 783.33 ± 15.28 g·cm−2 respectively. Various environmental parameters influenced the chemical composition of K. alvarezii, and these parameters demonstrated seasonal fluctuations. Moreover, based on the nutritional composition obtained, it could be stated that this seaweed has great scope to be incorporated into several food products as an excellent nutritional supplement, or as a value additive in animal or pet food.
Keywords: Seaweed, Kappaphycus alvarezii, Proximate composition, Mineral composition, Seasonal variation
Introduction
Benthic marine macroalgae, commonly known as seaweeds, are one of the living renewable resources of the marine environment with potential food and therapeutic applications; they have been used directly or indirectly as human food in Asian countries and are considered under-exploited resources (Tseng 2004). Currently there is increasing consumer interest in products that can support or even promote health. Nutritionists acclaim seaweed as being low in calories, and rich in vitamins, minerals, and dietary fibers (Jimenez-Escrig and Sanchez-Muniz 2000).
Seaweeds have been conventionally used for a wide variety of applications such as food, fodder, fertilizer, for medicinal purpose, and phycocolloids. Approximately, 250 species of seaweeds have been commercially utilized worldwide, amongst which 150 species are favorably consumed as human food; however, in western countries they form a source of polysaccharides (agar, alginates, carrageenans) for food and pharmaceutical industry (Zemke-White and Ohno 1999; Kumari et al. 2010). In order to cope with the growing market demand of seaweeds, several of them are cultivated commercially as a source of livelihood worldwide.
Seaweeds generally show great variation in the nutrient contents, which could be related to several environmental factors such as water temperature, salinity, light and nutrients. Further, most of the environmental parameters influencing seaweed composition generally vary with season; moreover, the changes in ecological conditions can also stimulate or inhibit the biosynthesis of several nutrients (Soriano et al. 2006). Seasonal variations in the chemical composition and nutritive value have been reported in common marine seaweed from Hong Kong (Kaehler and Kennish 1996) and Ireland (Mercer et al. 1993). Though there have been several reports on biochemical composition of various seaweeds across India, but there are very few studies focusing on the temporal variations in chemical composition of seaweeds in Indian context. This study aims at presenting the seasonal variation in the proximate and mineral composition of tropical Indian red seaweed K. alvarezii, a carrageenophyte with enormous commercial value.
Materials and methods
Seaweed: sampling and processing
Kappaphycus alvarezii was collected once in a month (from September 2004 to April 2006) from the cultivation site at Okha (22°28.656ˊ N and 69°04.015 ˊE) Gujarat, Northwest coast of India. The samples were thoroughly washed with seawater followed by fresh water, and subsequently dried at 60 °C in an oven, the dried samples were ground to particle size < 1 mm and stored at room temperature in airtight plastic containers for further use.
Analysis of proximate composition
The nitrogen content of the dried seaweed was quantified using Kjeldahl procedure (Wathelet 1999) using KEL PLUS-KES 20L Digestion unit attached to a KEL PLUS-CLASSIC DX Distillation unit (M/s PELICAN Equipments, Chennai, India). The digestion was performed using sulfuric acid (96 % H2SO4; initially for 75 min at 420 °C, and again for 75 min at 370 °C); this was thereafter distilled with boric acid solution (2 %) and titrated with 0.1 M HCl. The crude protein content was estimated by multiplying the nitrogen with a factor of 6.25; while the total carbohydrate content was assayed by the phenol–sulphuric acid method using glucose as a standard (Dubois et al. 1956). Crude lipids were extracted using soxhlet extractor using 2:1 ratio of chloroform-methanol (Bligh and Dyer 1959). The fiber content of the dried seaweed was determined using a standard method outlined by AOAC (1990), here, the acid hydrolysis was carried out with sulfuric acid (0.3 N H2SO4) and the base hydrolysis was undertaken using sodium hydroxide (0.5 N NaOH). The cold extraction was performed with acetone. The sample was then dried (1 h at 110 °C) until it reached a constant weight, cooled in a desiccator, and weighed (W1); thereafter, it was placed in a muffle furnace at 550 °C for 3 h, cooled (in a desiccator) and reweighed (W2). The crude fiber percentage was calculated following the equation: % crude fiber = (W1 − W2 /W0) × 100 (wherein, W0 was the initial weight of the dried seaweed which was 2 g). The ash content was analyzed by shade drying the samples at room temperature and later in an oven at 80 °C for 1 h, thereafter, one gram of the powdered sample was accurately taken in a crucible, ashed at 550 °C in muffle furnace for 6 h to a constant weight, and the ash obtained was then quantified gravimetrically (AOAC 1995).
Analysis of minerals by ICP-OES
One gram seaweed was ashed, moistened with ten drops of distilled water (Milli-Q) and carefully dissolved in 3 ml HNO3 (1:1 v/v), followed by heating at 100–120 °C till the solution totally evaporated. The crucible was returned to muffle furnace and ashed again for 1 h at 550 °C and cooled. Subsequently the ash was dissolved in 3 ml of 10 M HCl (1:1 v/v), and the solution was filtered through Millipore syringe filter (0.25 μm) into 50 ml volumetric flask and 2 ml 0.1 N HCl was added to the filtrate and the final volume was made up to 50 ml using distilled water (Milli-Q) (Suresh Kumar et al. 2007). Determination of mineral contents (Na, K, Ca, Mg, P, S, B, Cd, Co, Cr, Cu, Fe, Mn, Zn, Hg, Mo and V) of each seaweed sample was carried out using Inductively Coupled Plasma Optical Emission Spectroscopy, ICP-OES (Perkin-Elmer, Optima 2000). The analysis of all the above minerals was carried out in triplicate. Mean and standard deviation were calculated. All the chemicals and solvents used for experiments were of analytical grade.
Environmental parameters
Selected environmental parameters were determined at the farming site during the period of experimental cultivation (September 2004 to April 2006). All determinations, including water sample collections, were conducted at a depth of 30 cm i.e. the same depth as the cultured seaweeds. Seawater temperature was recorded daily throughout the study period and the mean monthly temperature was obtained. Salinity was determined fortnightly and the average value was recorded. The seawater samples were subjected to fortnightly nutrient analysis; here, dissolved inorganic phosphate (P–PO4) and dissolved inorganic nitrate (N–NO3) were determined according to the method described by Strickland and Parsons (1972) followed by which average monthly values were calculated.
Extraction of semi-refined carrageenan
The pre-cleaned dried seaweed (10 g) was rinsed with fresh water at ambient temperature and treated with 200 ml of 8 % KOH (cooking solution), in a container (glass) maintained at approximately 70 °C for 3 h. After 3 h of processing had elapsed, the seaweed was removed from the hot aqueous KOH solution and drained (this KOH mixture containing the seaweed was handled with extreme caution as it was corrosive having a pH ranging from 12 to 14). The seaweed was then subjected to a series of fresh water washes to reduce the pH, and, to wash residual KOH from the seaweed. This process helps eliminate certain salts and residual saponification products created during the KOH cook/reaction. The processed seaweed (containing carrageenan) obtained after the final rinse, was then chopped, dried and ground (Rideout and Bernabe 1997); this processed seaweed powder referred to as semi-refined carrageenan was then used for further determinations.
The carrageenan yield (%) was determined according to the formula:
Where, Wc is the extracted carrageenan weight (g) and Wm is the dry powder or algal weight (g) used for extraction. The data were then presented as the mean yield obtained from three replicates.
Gel strength measurement
In order to evaluate the gel strength of the product, 1 g of carrageenan was soaked in a solution of 1 % KCl for 2 h. This mixture was boiled (100 °C) for 20 min, cooled and left undisturbed at room temperature to facilitate the formation of gel; thereafter, the gels were refrigerated at 10 °C overnight (approx. 10 h) in a refrigerator. Gel strength was measured at 25 °C using a Nikkansui type gel tester (Kiya Seisakusho Ltd. Tokyo, Japan) as described by Hurtado-Ponce and Umezaki (1988) and it was expressed as g·cm−2.
Statistical analysis
Analysis of variance (ANOVA) was conducted to determine the differences in seasonal variations of biochemical constituents; moreover multiple comparison tests with the least significance difference (LSD) were performed to determine significant differences.
Results and discussion
Seasonal fluctuations in various environmental parameters were observed during the study period. Apart from showing seasonal fluctuations in environmental parameters along the various coasts of India, several chemical oceanographic studies also reveal seasonal variations in nutrients and their interrelationships in the Northeastern Arabian Sea, and particularly the West coast of India (Sen Gupta et al. 1976). However, there are few studies highlighting the seasonal fluctuation in the various parameters influencing seaweed composition particularly the shoreline water of Okha coast. During the course of study a mean salinity and temperature were 34.20 ‰ and 26.55 °C respectively, which corresponded with reports of Gunalan et al. (2010) and Subba Rao et al. (2008). The seawater temperature varied from 22.5 to 30.1 °C, while the salinity fluctuated from 31.20 to 36.64 ‰. The tropical climate, warm, nutrient-enriched seawater, high light levels and high degree of water motion waters are suitable for farming Kappaphycus species, therefore, it could be projected that the prevalent environmental conditions encountered during this study were suitable for the growth of Kappaphycus alvarezii.
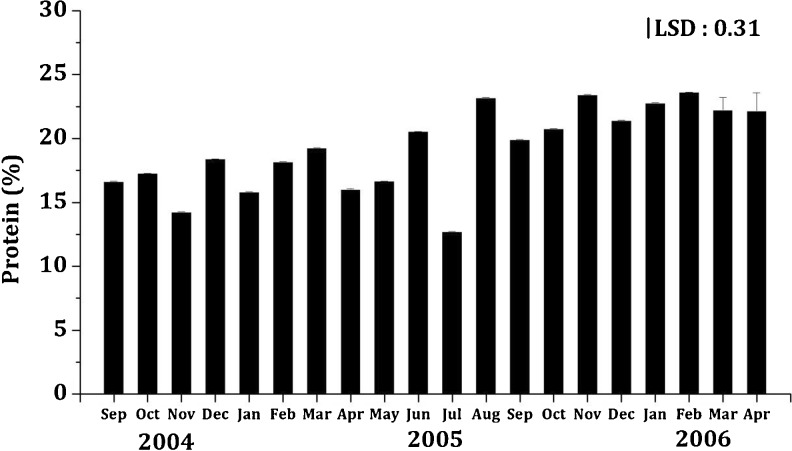
As evidenced in most studies, environmental parameters and season had a definite impact on the growth and biochemical constituent of K. alvarezii; Fig. 1 and 2 elucidate the seasonal variation in protein and carbohydrate content of K. alvarezii (P < 0.001) respectively. Subba Rao et al. (2008) reported that the growth rate of Kappaphycus alvarezii significantly correlates with salinity, nitrate and seawater temperature at various sites; however, this study reveals that various environmental parameters had a vital impact on the chemical composition of this seaweed. The average protein content of this seaweed (19.25 ± 0.15 g/100 g DW) was much higher than that reported for Sargassum vulgare (4.59–9.97 g/100 g DW; Soriano et al. 2006), Ulva lactuca (7.06 ± 0.06 g/100 g DW; Wong and Cheung 2000), Undaria pinnatifida (7.5 ± 1.9 g/100 g DW; Dawczynski et al. 2007), Caulerpa veravelensis (7.77 ± 0.59 g/100 g DW; Kumar et al. 2011), C. lentillifera (9.26 ± 0.03 g/100 g DW; Nguyen et al. 2011), Caulerpa scalpelliformis (10.50 ± 0.91 g/100 g DW; Kumar et al. 2011), Laminaria sp. (11.6 ± 0.8 g/100 g DW; Dawczynski et al. 2007), C. racemosa (12.88 ± 1.17 g/100 g DW; Kumar et al. 2011), Hizikia fusiforme (13.8 ± 6.2 g/100 g DW; Dawczynski et al. 2007) and Ulva rigida (17.8 g/100 g DW; Taboada et al. 2010). However, these values were comparable with that of Hypnea charoides (18.4 ± 0.30 g/100 g DW; Wong and Cheung 2000), Hypnea japonica (19.0 ± 0.3 g/100 g DW; Wong and Cheung 2000), and Gracilaria cervicornis (14.3–22.71 g/100 g DW; Soriano et al. 2006). El Din and El-Sherif (2012) reported the protein content of C. prolifera, C. racemosa, C. bursa, H. tuna, U. petiolata, Udotea sp., G. verrucosa, R. ardissonei, C. spinosa, D. dichotoma, S. acinarium, S. hornschuchii and S. vulgare to be 3.86, 8.23, 5.35, 11.8, 11.5, 14.5, 7.08, 4.5, 7.01, 8.15, 3.9, 6.05 and 6.15 g/100 g DW respectively, however, these values are lower than that obtained for K. alvarezii in the present study. Moreover, K. alvarezii contained relatively higher protein as compared to the seaweeds species habitually consumed as food (Fleurence 1999). Dawes (1998) vividly state that the members of Rhodophyta are characterized by greater protein content when compared to those of Phaeophyta, this could probably explain the high protein content in the species studied herein. Additionally, the mean protein content of K. alvarezii (19.25 ± 0.15 g/100 g DW) was higher than that reported for higher plants (Norziah and Ching 2000). However, reports suggest that although chemical composition of seaweeds could be influenced by factors such as species, habitat, maturity, geographical locations, environmental parameters, and season (Ito and Hori 1989; Fleurence 1999), it is essential to note the very presence of proteins and other vital nutrients such as high concentrations of essential amino acids (EAA) (e.g. argenine, aspartine and glutamine found in many seaweed species; Fleurence 1999) make them a promising candidate to be used in various food formulations or as a supplement. More essentially, even though there may be seasonal variations in the protein content and in turn the essential amino acids, however, these EAA are available throughout the year despite seasonal variations in their concentrations (Galland-Irmouli et al. 1999).
Fig. 1.
Seasonal variation in protein content (%) of K.alvarezii
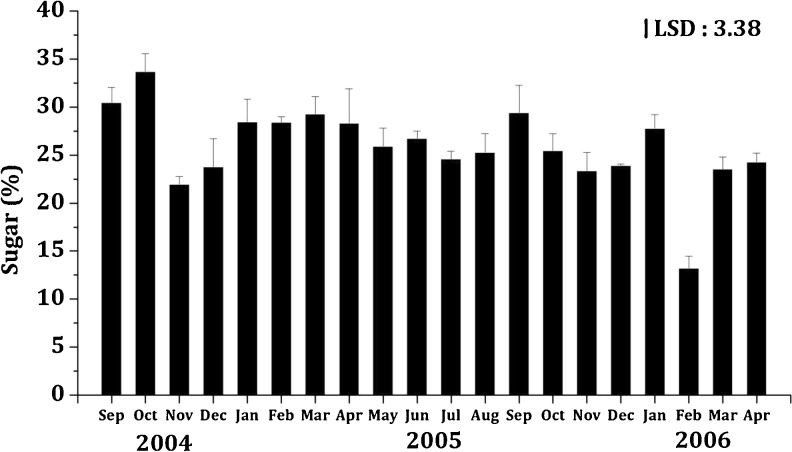
Fig. 2.
Seasonal variation in sugar content (%) of K.alvarezii
The average protein content of K. alvarezii (19.25 ± 0.15 g/100 g DW), was much less than the mean carbohydrate content (25.87 ± 1.64 g/100 g DW). The carbohydrate content of K. alvarezii (average 25.87 ± 1.64 g/100 g DW) was higher than several seaweeds, for e.g. Ulva rigida, Ulva lactuca, Caulerpa racemosa, Sargassum filipendula and Hypnea japonica (Rosenberg and Ramus 1982), C. prolifera, C. racemosa, C. bursa, H. tuna, U. petiolata, Udotea sp., G. corneum, G. verrucosa, R. ardissonei, C. spinosa, D. dichotoma, S. acinarium, S. hornschuchii and S. vulgare (El Din and El-Sherif 2012) which are reported to contain 6.40, 7.06, 3.98, 3.73, 4.28, 9.00, 6.93, 7.43, 7.40, 8.60, 8.00, 12.03, 15.77, 5.00, 20.91, 8.60, 13.67, 16.50 and 14.30 g/100 g DW of carbohydrate respectively. It is essential to note that, in the present study the occurrence of maximum carbohydrate content of K. alvarezii coincided with occurrence of maximum biomass, thereby suggesting a link between seaweed growth and carbohydrate content; similarly, Rosenberg and Ramus (1982) also related the carbohydrate synthesis to periods of maximum growth, increased photosynthetic activity and a reduction in protein content. Moreover, during the period of occurrence of maximum carbohydrate in K. alvarezii higher values of water temperature, salinity and sunlight intensity were recorded, which confirms the influence of these parameters on carbohydrate synthesis (Munda and Kremer 1977). However, a negative correlation was found in this study between total carbohydrate and phosphate (r = −0.471). On the other hand, an inverse relationship was observed between carbohydrate and protein content; Givernaud et al. (1993) observed a similar a pattern for several species of seaweeds.
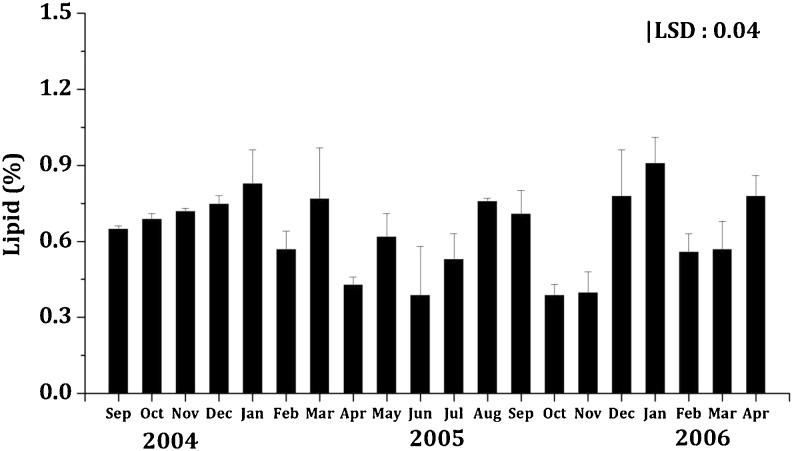
The lipid content varied from 0.39 ± 0.04 to 0.91 ± 0.51 g/100 g DW (Fig. 3) with an average of 0.64 ± 0.08 g/100 g DW. The lipid content of marine macroalgae (seaweed) has been reported to generally range between 1 and 6 g/100 g DW (Dawczynski et al. 2007). However, in the present investigation on the carragenophytic seaweed K. alvarezii, the lipid content ranged from 0.39 ± 0.04 to 0.91 ± 0.51 g/100 g DW (Fig. 3) which was comparable with Sargassum polyschides (0.7 ± 0.09 g/100 g DW), Corallina officinalis (0.3 ± 0.2 g/100 g DW; Sara Marsham et al. 2005) as well as Hizikia sp. and Arame (0.7–0.9 g/100 g DW; Kolb et al. 1999); however, it was lower than Palmaria sp. (1.8 ± 0.14 g/100 g DW), Hypnea elongata (0.97 ± 0.07 g/100 g DW) and Undaria pinnatifida (1.05 ± 0.01 g/100 g DW) (Sanchez-Machado et al. 2004). Moreover, there was a positive correlation between the water temperature and the lipid content (r = 0.554).
Fig. 3.
Seasonal variation in lipid content (%) of K.alvarezii
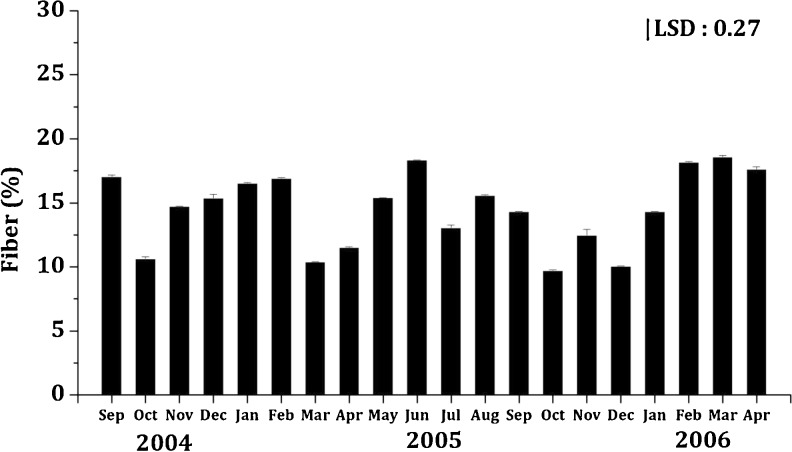
Seaweeds are reported to be rich in fiber as compared to most fruits and vegetables; moreover, consumption of seaweeds helps increase the intake of dietary fiber and lower the occurrence of some chronic diseases (diabetes, obesity, heart diseases, cancers, etc.). In addition, consumption of seaweed is known to promote the growth of beneficial intestinal flora and also protect them; it also reduces the overall glycemic response, greatly increases stool volume and reduces the risk of colon cancer (Southgate 1990; Gupta and Abu-Ghannam 2011). K. alvarezii was particularly rich in fiber. Its fiber content ranged from 9.68 ± 0.08 to 18.57 ± 0.15 g/100 g DW (Fig. 4). This was higher than that reported by Robledo and Freile-Pelegrin (1994); they reported Sargassum vulgare (mean 7.40 ± 1.61 g/100 g DW) and Gracilaria cervicornis (mean 5.65 ± 0.74 g/100 g DW) to have fiber content comparable to few other brown and red macroalgal species. On the other hand, the average fiber content of K. alvarezii (14.52 ± 0.11 g/100 g DW) was comparable with Gelidiella acerosa (13.45 ± 1.07 g/100 g DW) and lower than Sargassum wightii (17 ± 1.19 g/100 g DW) (Syad et al. 2013).
Fig. 4.
Seasonal variation in fiber content (%) of K.alvarezii
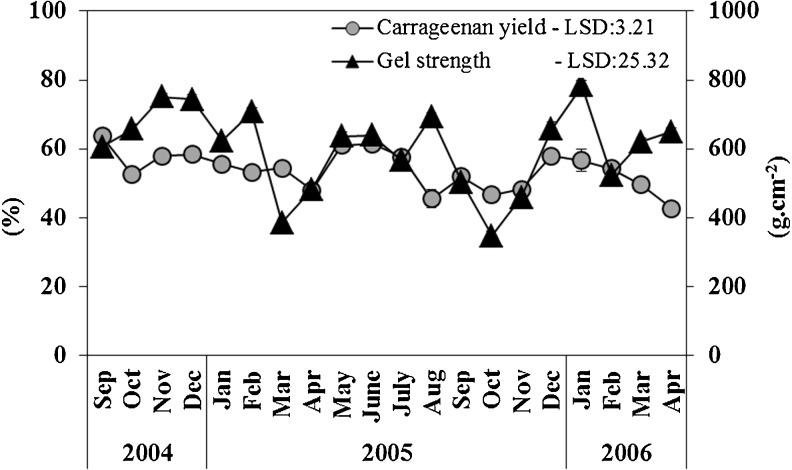
The average SRC yield obtained in the present study (53.90 ± 1.37 %) was comparable with western Pacific commercial sample (57 %; Hayashi et al. 2007). Subba Rao et al. (2008), cite several reports mentioning lower semi-refined carrageenan content (31–43 %) for the same seaweed farmed in the subtropical waters of Sao Paulo State, Brazil, and moderate yields for materials from Vietnam (34.5 to 45.3 %; Ohno et al. 1996) and Indonesia (45 %; Ohno et al. 1996), however, higher yields are reported from Philippines (54.6 %; Trono and Ohno 1986). On the other hand, the SRC obtained in this study also demonstrated remarkable gel strength ranging from 348.33 ± 12.58 to 783.33 ± 15.28 g·cm−2 with an average of 602.00 ± 12.13 g·cm−2 which justifies its potential use in several pet food products. All the K. alvarezii samples yielded good amount of SRC i.e. the SRC yields ranged between 42.70 ± 1.07 to 63.73 ± 1.73 % (Fig. 5). A positive correlation could be derived between the carrageenan yield and gel strength (r = 0.578; Table 1); nonetheless, the nitrate present in seawater had a positive correlation with carrageenan yield (r = 0.465).
Fig. 5.
Seasonal variation in semi-refined carrageenan yield (%) and gel strength (g·cm−2)
Table 1.
Correlation coefficients (n = 20) of the environmental parameters and the chemical composition of K. alvarezii
| Salinity | Phosphate | Nitrate | Biomass | Gel strength | Carrageenan Yield | Protein | Carbohydrate | Lipid | Fiber | Ash | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water °C | 0.314 | −0.169 | 0.139 | 0.005** | 0.338 | 0.175 | 0.152 | −0.042 | 0.554* | 0.170 | −0.259 |
| Salinity | 0.197 | −0.216 | 0.193 | −0.136 | −0.426 | 0.373 | −0.352 | 0.041 | −0.127 | 0.077 | |
| Phosphate | 0.380 | −0.346 | −0.278 | −0.008 | −0.174 | −0.471* | −0.267 | −0.076 | 0.071 | ||
| Nitrate | −0.307 | 0.399 | 0.465* | −0.698** | 0.004 | 0.055 | 0.017 | −0.049 | |||
| Biomass | −0.059 | −0.196 | 0.140 | −0.171 | 0.113 | 0.367 | 0.234 | ||||
| Gel strength | 0.578* | −0.246 | 0.095 | 0.423 | 0.191 | −0.155 | |||||
| Carrageenan yield | −0.476* | 0.097 | 0.167 | 0.093 | 0.116 | ||||||
| Protein | −0.355 | 0.023 | 0.144 | 0.046 | |||||||
| Sugar | 0.173 | −0.313 | −0.153 | ||||||||
| Lipid | 0.052 | 0.196 | |||||||||
| Fiber | −0.205 | ||||||||||
| Ash | 1.000 |
*Significance (p < 0.05)
**Significance (p < 0.01)
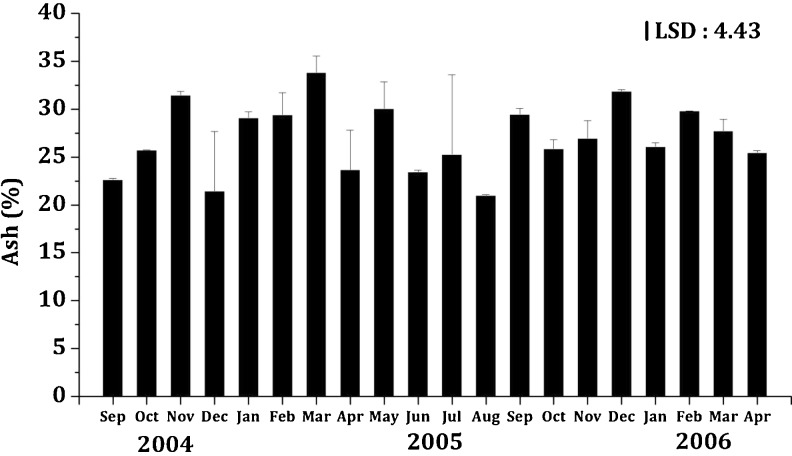
The ash content of K. alvarezii ranged from 20.99 ± 0.08 to 33.81 ± 1.71 g/100 g DW (Fig. 6). The ash contents of the seaweeds are usually much higher than land vegetables other than spinach (Rupeŕez 2002; Omotoso 2006). However, the ash content is known to vary with species, geographical locations and season (Kaehler and Kennish 1996). The average ash content of K. alvarezii recorded in this study (27.00 ± 1.62 g/100 g DW; Fig. 6) was considerably higher than Hypnea japonica (22.1 ± 0.72 g/100 g DW), Hypnea charoides (22.8 ± 2.23 g/100 g DW), Ulva lactuca (21.3 ± 2.78 g/100 g DW), Hypnea pannosa (15.3 g/100 g DW), Ulva lactuca (24.6 g/100 g DW), Ulva pertusa (24.7 g/100 g DW), Ulva lactuca (11.0 ± 0.1 g/100 g DW), Durvillaea Antarctica (17.9 ± 1.2 g/100 g DW), Caulerpa lentillifera (22.20 ± 0.27 g/100 g DW), Gelidiella acerosa (10.3 ± 4.9 g/100 g DW) and Sargassum wightii (25 ± 2 g/100 g DW) (Dawczynski et al. 2007; Mushollaeni 2011; Taboada et al. 2010; Syad et al. 2013). Generally, the ash content of a sample is known to reflect its mineral content. Mushollaeni (2011) state that the presence of ash content in seaweed showed that there were mineral salt in the sample; similarly, this study also confirms that K. alvarezii (which demonstrated high ash content) was also rich in minerals.
Fig. 6.
Seasonal variation in ash content (%) of K.alvarezii
Seaweed are rich (8–40 %) in several essential minerals and trace elements required for human nutrition (Indegaard and Ostgaard 1991); in fact, several reports suggest that the mineral content in seaweeds is higher than edible land plants (Ortega-Calvo et al. 1993; USDA 2001). However, it is obvious that occurrence and amounts of these may vary with species, moreover, within the same species there may also be seasonal fluctuations in the composition of these elements. Seasonal variation in mineral composition of K. alvarezii have been elucidated in Table 2; the highest mineral content (29939.61 ± 9340.38 mg/100 g DW) was recorded in the month of April 2005, while the lowest value (10997.62 ± 1120.26 mg/100 g DW) was recorded in January 2006. Among the 17 minerals analyzed, K. alvarezii demonstrated highest S content (11,240 ± 730 mg/100 g DW), and lowest quantities of Mo (0.04 ± 0.02 mg/100 g DW). Apart from remarkable macro-mineral content (Na + K + Ca + Mg; 7,910 ± 2,950 mg/100 g DW), K. alvarezii was also rich in selected micronutrients (Fe + Zn + Mn + Cu; 69.61 ± 0.16 mg/100 g DW). The accumulation of Na and K salts generally depends upon the physiology as well as season; in this study the Na/K ratio of K. alvarezii ranged from 0.34 to 0.87. The macro-mineral content (Na + K + Ca + Mg) of K. alvarezii (7,910 ± 2,950 mg/100 g DW) was lower than the values reported for the brown seaweeds such as Fucus (11,723 ± 126 mg/100 g DW), Laminaria (17,061 ± 182 mg/100 g DW), Wakame (17,875 ± 382 mg/100 g DW), and for the red seaweeds such as Chondrus (8,606 ± 90 mg/100 g DW) and Nori (8,082 ± 214 mg/100 g DW) (Indegaard and Ostgaard 1991); however, it was higher than many land vegetables (carrot 3,276 mg/100 g DW, sweet corn 1,347 mg/100 g DW, potato 6,015 mg/100 g DW, tomato 3,429 mg/100 g DW), but spinach (9679 mg/100 g DW) is exceptional. Looking into the Mg content of all the samples, it could be stated that K. alvarezii could prove to be a good source for Mg, which is essential for regulating central nervous system excitability and normal functions. In this context, Syad et al. (2013) reported that low levels of Mg contribute to the heavy metal deposition in the brain that leads Parkinson’s, multiple sclerosis and Alzheimer’s disease; hence inclusion of this seaweed in the diet could evade these mentioned problems. Marine algae are also interesting candidates as Fe sources, especially in countries where the algal production is feasible; K. alvarezii also comprised significant quantities of Fe. In this study, the selected micronutrients (Fe + Zn + Mn + Cu) in the seaweed was higher (69.61 ± 0.16 mg/100 g DW) than the land plant sweet corn (Fe + Zn + Mn + Cu, 4.9 mg/100 g DW) and the seaweed Laminaria (5.1 mg/100 g DW) (Food and Nutrition Board 1981).
Table 2.
Macro (g/100 g DW), micro and trace elements (mg/100 g DW) of K.alvarezii determined by ICP-OES
| Mineral | Sep-04 | Oct-04 | Nov-04 | Dec-04 | Jan-05 | Feb-05 | Mar-05 | Apr-05 | May-05 | June-05 | July-05 | Aug-05 | Sep-05 | Oct-05 | Nov-05 | Dec-05 | Jan-06 | Feb-06 | Mar-06 | Apr-06 | Average | LSD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | 2.49 ± 0.20 | 1.11 ± 0.29 | 2.56 ± 0.17 | 3.79 ± 0.17 | 3.27 ± 0.25 | 2.38 ± 0.36 | 2.61 ± 0.27 | 2.71 ± 0.31 | 2.05 ± 0.1 | 2.07 ± 0.17 | 2.06 ± 0.25 | 2.56 ± 1.53 | 2.13 ± 0.15 | 2.71 ± 0.15 | 2.34 ± 0.93 | 1.92 ± 0.61 | 1.39 ± 0.15 | 1.62 ± 0.15 | 1.13 ± 0.58 | 1.65 ± 0.38 | 2.23 ± 0.36 | 1.10 |
| K | 3.40 ± 0.17 | 2.93 ± 0.21 | 4.08 ± 0.23 | 6.41 ± 0.17 | 5.27 ± 0.30 | 4.96 ± 0.46 | 5.85 ± 0.31 | 6.69 ± 0.26 | 3.46 ± 2.08 | 5.21 ± 0.2 | 3.5 ± 0.12 | 7.59 ± 1.53 | 3.26 ± 0.38 | 3.28 ± 0.31 | 2.68 ± 0.25 | 2.78 ± 0.64 | 2.06 ± 0.1 | 3.01 ± 0.06 | 3.01 ± 0.15 | 2.42 ± 0.82 | 4.10 ± 0.44 | 2.60 |
| Ca | 0.82 ± 0.33 | 0.82 ± 0.17 | 0.80 ± 0.06 | 0.96 ± 0.12 | 0.80 ± 0.23 | 0.81 ± 0.15 | 0.83 ± 0.23 | 0.76 ± 0.42 | 0.98 ± 0.25 | 0.89 ± 0.06 | 0.89 ± 0.16 | 0.75 ± 2.52 | 0.97 ± 0.36 | 1.12 ± 0.35 | 0.92 ± 0.2 | 0.99 ± 0.15 | 0.76 ± 0.21 | 0.55 ± 0.17 | 0.53 ± 0.21 | 0.88 ± 0.18 | 0.84 ± 0.33 | 0.25 |
| Mg | 0.74 ± 0.18 | 0.66 ± 0.24 | 0.69 ± 0.17 | 0.84 ± 0.12 | 0.91 ± 1.16 | 0.77 ± 0.71 | 0.71 ± 0.12 | 0.78 ± 0.15 | 0.92 ± 0.21 | 0.76 ± 0.12 | 0.89 ± 0.46 | 0.72 ± 0.3 | 0.76 ± 0.16 | 0.77 ± 0.2 | 0.75 ± 0.21 | 0.76 ± 0.3 | 0.56 ± 0.2 | 0.65 ± 0.58 | 0.54 ± 0.15 | 0.66 ± 0.62 | 0.74 ± 1.82 | 0.25 |
| P | 0.10 ± 0.00 | 0.09 ± 0.00 | 0.11 ± 0.00 | 0.13 ± 0.00 | 0.17 ± 0.00 | 0.17 ± 0.00 | 0.12 ± 0.00 | 0.10 ± 0.00 | 0.13 ± 0.00 | 0.12 ± 0.00 | 0.17 ± 0.00 | 0.15 ± 0.00 | 0.12 ± 0.00 | 0.12 ± 0.00 | 0.25 ± 0.00 | 0.05 ± 0.00 | 0.09 ± 0.00 | 0.06 ± 0.00 | 0.10 ± 0.00 | 0.11 ± 0.00 | 0.12 ± 0.00 | 0.07 |
| S | 5.43 ± 0.46 | 11.38 ± 0.20 | 11.37 ± 0.35 | 13.76 ± 0.52 | 13.06 ± 0.50 | 13.47 ± 0.79 | 15.39 ± 0.64 | 9.59 ± 0.46 | 12.13 ± 0.46 | 11.84 ± 0.46 | 12.18 ± 0.17 | 18.03 ± 3.46 | 13.67 ± 2.04 | 11.3 ± 0.79 | 9.24 ± 0.35 | 10.36 ± 0.76 | 6.12 ± 0.46 | 9.23 ± 0.3 | 8.96 ± 0.79 | 8.24 ± 0.61 | 11.24 ± 0.73 | 5.23 |
| Total | 12.98 ± 1.13 | 16.96 ± 1.11 | 19.61 ± 0.98 | 25.89 ± 1.1 | 23.48 ± 2.44 | 22.56 ± 2.47 | 25.51 ± 1.57 | 20.63 ± 1.6 | 19.67 ± 3.1 | 20.89 ± 1.01 | 19.65 ± 1.16 | 29.8 ± 9.34 | 20.91 ± 3.09 | 19.3 ± 1.8 | 16.18 ± 1.94 | 16.86 ± 2.46 | 10.98 ± 1.12 | 15.12 ± 1.26 | 14.27 ± 1.88 | 13.96 ± 2.61 | 19.27 ± 3.68 | |
| B | 5.75 ± 0.02 | 1.74 ± 0.03 | 0.19 ± 0.01 | 0.60 ± 0.02 | 2.06 ± 0.02 | 5.68 ± 0.04 | 5.81 ± 0.02 | 6.14 ± 0.02 | 2.03 ± 0.02 | 4.56 ± 0.02 | 5.65 ± 0.02 | 4.67 ± 0.02 | 4.87 ± 0.02 | 2.16 ± 0.04 | 0.16 ± 0.03 | 0.93 ± 0.04 | 0.33 ± 0.06 | 1.07 ± 0.12 | 1.23 ± 0.06 | 0.63 ± 0.05 | 2.81 ± 0.03 | 3.70 |
| Cd | 1.35 ± 0.01 | 1.54 ± 0.01 | 0.74 ± 0.02 | 0.80 ± 0.01 | 3.96 ± 0.03 | 2.07 ± 0.03 | 0.63 ± 0.02 | 0.83 ± 0.02 | 1.53 ± 0.01 | 1.85 ± 0.02 | 0.92 ± 0.02 | 0.58 ± 0.02 | 1.03 ± 0.12 | 0.67 ± 0.03 | 0.4 ± 0.01 | 0.22 ± 0.02 | 0.23 ± 0.06 | 0.24 ± 0.01 | 0.23 ± 0.02 | 0.23 ± 0.02 | 1.00 ± 0.03 | 1.47 |
| Co | 0.48 ± 0.02 | 0.48 ± 0.01 | 0.71 ± 0.01 | 0.64 ± 0.02 | 0.20 ± 0.01 | 0.76 ± 0.03 | 0.19 ± 0.02 | 0.18 ± 0.02 | 2.65 ± 0.03 | 0.16 ± 0.03 | 2.14 ± 0.02 | 0.28 ± 0.02 | 0.6 ± 0.02 | 0.47 ± 0.06 | 0.76 ± 0.02 | 0.13 ± 0.06 | 0.1 ± 0.03 | 0.14 ± 0.01 | 0.06 ± 0.01 | 0.12 ± 0.03 | 0.56 ± 0.02 | 1.11 |
| Cr | 2.66 ± 0.02 | 4.31 ± 0.02 | 5.36 ± 0.01 | 7.28 ± 0.01 | 0.74 ± 0.03 | 3.59 ± 0.02 | 1.73 ± 0.15 | 1.35 ± 0.04 | 13.47 ± 0.31 | 1.53 ± 0.03 | 13.54 ± 0.01 | 5.34 ± 0.04 | 5.26 ± 0.02 | 1.57 ± 0.02 | 5.16 ± 0.03 | 0.87 ± 0.03 | 1.14 ± 0.0 | 1.33 ± 0.23 | 0.37 ± 0.01 | 1 ± 0.02 | 3.88 ± 0.05 | 6.3 |
| Cu | 0.62 ± 0.03 | 1.03 ± 0.02 | 1.07 ± 0.02 | 0.76 ± 0.01 | 0.76 ± 0.07 | 0.65 ± 0.03 | 0.58 ± 0.02 | 0.55 ± 0.06 | 3.77 ± 0.01 | 0.38 ± 0.01 | 0.65 ± 0.02 | 0.58 ± 0.02 | 0.55 ± 0.03 | 0.55 ± 0.03 | 0.77 ± 0.02 | 0.6 ± 0.1 | 0.35 ± 0.02 | 0.22 ± 0.01 | 0.19 ± 0.02 | 0.47 ± 0.02 | 0.76 ± 0.03 | 1.22 |
| Fe | 49.16 ± 0.09 | 68.80 ± 0.06 | 94.50 ± 0.17 | 98.85 ± 0.01 | 132.73 ± 0.04 | 101.64 ± 0.08 | 34.57 ± 0.03 | 24.67 ± 0.02 | 111.83 ± 0.12 | 27.47 ± 0.25 | 64.78 ± 0.01 | 123.6 ± 0.17 | 90.2 ± 0.02 | 45.5 ± 0.03 | 126.77 ± 0.23 | 48.56 ± 0.02 | 13.83 ± 0.03 | 21.15 ± 0.02 | 9.09 ± 0.02 | 31.19 ± 0.02 | 65.94 ± 0.07 | 81.78 |
| Mn | 1.35 ± 0.02 | 0.16 ± 0.02 | 2.26 ± 0.04 | 1.67 ± 0.01 | 0.66 ± 0.04 | 1.67 ± 0.02 | 1.03 ± 0.02 | 0.75 ± 0.03 | 1.24 ± 0.01 | 0.79 ± 0.01 | 0.69 ± 0.01 | 1.6 ± 0.02 | 1.28 ± 0.04 | 1.17 ± 0.04 | 1.48 ± 0.02 | 1.25 ± 0.02 | 0.5 ± 0.01 | 0.57 ± 0.01 | 0.36 ± 0.02 | 0.88 ± 0.02 | 1.10 ± 0.02 | 0.88 |
| Zn | 2.21 ± 0.02 | 1.07 ± 0.02 | 2.26 ± 0.01 | 1.34 ± 0.03 | 1.65 ± 0.04 | 2.25 ± 0.02 | 2.17 ± 0.21 | 1.45 ± 0.02 | 2.26 ± 0.05 | 1.4 ± 0.02 | 1.43 ± 0.02 | 2.76 ± 0.03 | 1.8 ± 0.1 | 1.77 ± 0.02 | 3.37 ± 0.02 | 2.96 ± 0.04 | 1.04 ± 0.01 | 1.09 ± 0.01 | 0.81 ± 0.02 | 1.99 ± 0.03 | 1.85 ± 0.04 | 1.16 |
| Hg | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.22 ± 0.01 | 0.09 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.01 ± 0.01 | 0.08 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.0 | 0.05 ± 0.0 | 0.01 ± 0.0 | 0.01 ± 0.01 | 0.1 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.03 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.08 |
| Mo | 0.05 ± 0.01 | 0.16 ± 0.01 | 0.01 ± 0.01 | 0.10 ± 0.01 | 0.05 ± 0.03 | 0.06 ± 0.05 | 0.03 ± 0.01 | 0.02 ± 0.12 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.04 ± 0.02 | 0.07 ± 0.01 | 0.01 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.0 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.06 |
| V | 0.11 ± 0.01 | 1.78 ± 0.02 | 0.33 ± 0.02 | 0.37 ± 0.12 | 0.04 ± 0.04 | 0.11 ± 0.09 | 0.11 ± 0.01 | 0.08 ± 0.01 | 0.1 ± 0.1 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.14 ± 0.03 | 0.16 ± 0.02 | 0.13 ± 0.01 | 0.15 ± 0.02 | 0.13 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.02 | 0.09 ± 0.01 | 0.21 ± 0.03 | 0.62 |
| Total | 63.75 ± 0.24 | 81.11 ± 0.23 | 107.65 ± 0.32 | 112.49 ± 0.26 | 142.9 ± 0.35 | 118.51 ± 0.42 | 46.87 ± 0.50 | 36.03 ± 0.34 | 138.99 ± 0.68 | 38.3 ± 0.42 | 89.97 ± 0.15 | 139.61 ± 0.38 | 105.8 ± 0.41 | 54.07 ± 0.3 | 139.13 ± 0.42 | 55.72 ± 0.36 | 17.62 ± 0.26 | 25.92 ± 0.45 | 12.43 ± 0.22 | 36.66 ± 0.24 | 78.20 ± 0.35 | |
| Grand Total (mg/100 g) | 13043.75 ± 1130.24 | 17041.11 ± 1110.23 | 19717.65 ± 980.32 | 26002.49 ± 1100.26 | 23622.90 ± 2440.35 | 22678.51 ± 2470.42 | 25556.87 ± 1570.50 | 20666.03 ± 1600.34 | 19808.99 ± 3100.68 | 20928.30 ± 1010.42 | 19739.97 ± 1160.15 | 29939.61 ± 9340.38 | 21015.80 ± 3090.04 | 19354.07 ± 1800.30 | 16319.13 ± 1940.42 | 16915.72 ± 2460.36 | 10997.62 ± 1120.26 | 15145.92 ± 1260.45 | 14282.43 ± 1880.22 | 13996.66 ± 2610.24 |
K. alvarezii showed a relatively higher sodium content than as compared to that reported for terrestrial vegetables (carrots, sweet corn, green peas, potato and tomato) (Food and Nutrition Board 1981). Generally, the intake of sodium chloride and diets with high Na/K ratio are related to the incidence of hypertension; here, the Na/K ratio of K. alvarezii ranged from 0.34 to 0.87. Ortega-Calvo et al. (1993) studied Spirulina and five other eukaryotic seaweeds used in food in Spain, and reported Na/K ratios were below 1.5 in all seaweeds studied (0.33–1.34), which is interesting from the point of nutrition; nevertheless, the Na/K ratio in olives and sausages are 43.63 and 4.89, respectively (USDA 2001).
Seaweeds have been investigated for various elements and heavy metals in the past; in fact, reports suggest that most of the trace elements present in the algal biomass are heavy metals (As, Cd, Cu, Hg, Pb and Zn), but their content is generally below the toxic limit in most seaweeds (Ortega-Calvo et al. 1993). However, it is essential to quantify the mineral, ash and heavy metal content in any seaweed before recommending its use in food formulation. In the USA, algal products have to comply with the following maximum limits: 45 % ash, 40 ppm heavy metals (Dietary Reference Intakes 2004). In the present study the Cu + Zn content ranged from 1.47 to 4.31 mg/100 g DW, which is below the toxic limits permitted for macroalgae for human consumption (Ortega-Calvo et al. 1993). Indegaard and Ostgaard (1991) suggested incorporation of algal products in the daily diet of adults, and recommend intake values of some trace elements (Fe: 10–18 mg, Zn: 15 mg, Mn: 2.5–5 mg and Cu: 2–3 mg. 100 g dry weight). Table 3 provides a comparison of the various recommended dietary allowances of macronutrients, minerals and trace elements and their presence in K. alvarezii (1 g); as elucidated this seaweed indeed qualifies to be incorporated as a food ingredient.
Table 3.
Recommended dietary allowances of macronutrients, minerals and trace elements of K. alvarezii
| Macronutrients, minerals and trace elements | Recommended Dietary Allowances per daya | Average content per gram in K. alvarezii |
|---|---|---|
| Macronutrients (g) | ||
| Protein | 9–71 | 0.17 |
| Carbohydrate | 60–210 | 0.23 |
| Fiber | 19–29 | 0.12 |
| Fats | ND | 0.007 |
| Macro elements (mg) | ||
| Boron | <20 | 0.03 |
| Calcium | 210–1,000 | 8.42 |
| Iron | 6–27 | 0.63 |
| Magnesium | 30–320 | 7.42 |
| Manganese | 0.003–2.6 | 0.011 |
| Phosphorus | 100–1,250 | 1.23 |
| Zinc | 2–12 | 0.019 |
| Microelements (μg) | ||
| Cadmium | 50–150 | 10.02 |
| Chromium | 5.5–45 | 38.87 |
| Copper | 200–1,300 | 7.55 |
| Molybdenum | 2–50 | 43 |
| Mercury | 40 | 0.46 |
Based on the study conducted herein, it could be stated that K. alvarezii could be an excellent source of essential macronutrients and minerals, therefore, it could probably compensate for the frequently low minerals content of food; moreover, it also comprises significant quantities of protein and fiber. Hence, it could be preferentially used as an essential value-added food supplement or spice to the vegetarian or omnivorous diet.
Conclusion
Seaweeds have been traditionally used for variegated purposes including several food applications. Studies on temporal variations in the proximate and mineral composition of K. alvarezii conducted herein reveal the consistent presence of fiber and minerals (micro and macro nutrient) in this widely cultivated seaweed, thereby suggesting its use as a potential food supplement. K. alvarezii having high nutritional value, could either be used in the food industry as a source of these essential ingredients, or could be used as such for oral consumption after proper processing. However, its commercial value could also be enhanced by improving its quality and further increasing the probability of its use in a wider range of novel seaweed-based products. Nevertheless, it could definitely be incorporated into various animal and pet food products as a vital value additive supplement.
Acknowledgments
The authors are grateful to Dr. Pushpito Ghosh, Director, Central Salt & Marine Chemicals Research Institute, Bhavnagar, Gujarat, India for his constant support and encouragement. They also appreciate Discipline Coordinator, Marine Biotechnology and Ecology Discipline, for providing research facilities and profusely thank the Department of Biotechnology (Sanction No: BT/PR 3309/PID/03/139/2002), New Delhi, India for providing financial assistance.
References
- AOAC . Official methods of analysis, Association of Official Analytical Chemists. 15. Gaithersburg: AOAC Press; 1990. [Google Scholar]
- AOAC (1995) Official methods of analysis. In: Horwitz W (ed) Association of Official Analytical Chemists, Washington, DC
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Concon JM. Food toxicology, part A (vol. 582) New York: Marcel Dekker Inc; 1988. pp. 1049–1073. [Google Scholar]
- Dawczynski C, Schubert R, Jahreis G. Amino acids, fatty acids, and dietary fiber in edible seaweed products. Food Chem. 2007;103:891–899. doi: 10.1016/j.foodchem.2006.09.041. [DOI] [Google Scholar]
- Dawes CJ. Marine botany. New York: Wiley; 1998. [Google Scholar]
- Dietary Reference Intakes (2004) Food and Nutrition Board, Institute of Medicine, National Academies, Washington DC. Available from: http://www.iom.edu
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric methods for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- El Din NGS, El-Sherif ZM. Nutritional value of some algae from the north-western Mediterranean coast of Egypt. J Appl Phycol. 2012;24:613–626. doi: 10.1007/s10811-012-9831-3. [DOI] [Google Scholar]
- Fleurence J. Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol. 1999;10:25–28. doi: 10.1016/S0924-2244(99)00015-1. [DOI] [Google Scholar]
- Food and Nutrition Board . Food chemical codex. 3. Washington, DC: National Academy Press; 1981. [Google Scholar]
- Food and Nutrition Board (1989) National Academy of Sciences-Recommended Dietary Allowances, Revised. Available from: http://www.diet-and-health.net/Nutrients/rdas.html
- Galland-Irmouli AV, Fleurence J, Lamghari R, Lucon M, Rouxel C, Barbaroux O, Bronowicki JP, Villaume C, Guéant JL. Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse) J Nutr Biochem. 1999;10:353–359. doi: 10.1016/S0955-2863(99)00014-5. [DOI] [PubMed] [Google Scholar]
- Givernaud MA, Givernaud T, Morvan H, Cosson J. Annual variations of the biochemical composition of Gelidium latifolium (greville) Thuret et Bornet. Hydrobiologia. 1993;260/261:607–612. doi: 10.1007/BF00049078. [DOI] [Google Scholar]
- Gunalan B, Kotiya AS, Jetani KL. Comparison of Kappaphycus alvarezii growth at two different places of Saurashtra Region. Eur J Appl Sci. 2010;2(1):10–12. [Google Scholar]
- Gupta S, Abu-Ghannam N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci Technol. 2011;22:315–326. doi: 10.1016/j.tifs.2011.03.011. [DOI] [Google Scholar]
- Hayashi L, Paula EJD, Chow F. Growth rate and carrageenan analyses in four strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales) farmed in the subtropical waters of São Paulo State Brazil. J Appl Phycol. 2007;19:393–399. doi: 10.1007/s10811-006-9135-6. [DOI] [Google Scholar]
- Hurtado-Ponce AQ, Umezaki I. Physical properties of agar gel from Gracilaria (Rhodophyta) of the Philippines. Bot Mar. 1988;31:171–174. doi: 10.1515/botm.1988.31.2.171. [DOI] [Google Scholar]
- Indegaard M, Ostgaard K. Polysaccharides for food and pharmaceutical uses. In: Guiry MD, Blunden G, editors. Seaweed resources in Europe: uses and potential. Chichester: John Wiley & Sons Ltd; 1991. pp. 169–183. [Google Scholar]
- Ito K, Hori K. Seaweed: chemical composition and potential uses. Food Rev Int. 1989;5:101–144. doi: 10.1080/87559128909540845. [DOI] [Google Scholar]
- Jimenez-Escrig A, Sanchez-Muniz FJ. Dietary fiber from edible seaweeds: chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr Res. 2000;20(4):585–598. doi: 10.1016/S0271-5317(00)00149-4. [DOI] [Google Scholar]
- Kaehler S, Kennish R. Summer and winter comparisons in the nutritional value of 447 marine macroalgae from Hong Kong. Bot Mar. 1996;39:11–17. doi: 10.1515/botm.1996.39.1-6.11. [DOI] [Google Scholar]
- Kolb N, Vallorani L, Stocchi V. Chemical composition and evaluation of protein quality by amino acid score method of edible brown marine algae Arame (Eisenia bicyclis) and Hijiki (Hijikia fusiforme) Acta Aliment. 1999;28:213–222. doi: 10.1556/AAlim.28.1999.3.1. [DOI] [Google Scholar]
- Suresh Kumar K, Ganesan K, Subba Rao PV (2007) Phycoremediation of heavy metals by the three-color forms of Kappaphycus alvarezii. J Hazard Mater 143:590–592 [DOI] [PubMed]
- Kumar M, Gupta V, Kumari P, Reddy CRK, Jha B. Assessment of nutrient composition and antioxidant potential of Caulerpaceae seaweeds. J Food Compos Anal. 2011;24:270–278. doi: 10.1016/j.jfca.2010.07.007. [DOI] [Google Scholar]
- Kumari P, Kumar M, Gupta V, Reddy CRK, Jha B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010;120:749–757. doi: 10.1016/j.foodchem.2009.11.006. [DOI] [Google Scholar]
- Mercer JP, Mai KS, Donlon J. Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata Linnaeus and Haliotis discus hannaiIno. I. Effects of algal diets on growth and biochemical composition. Invertebr Reprod Dev. 1993;23:2–3. doi: 10.1080/07924259.1993.9672298. [DOI] [Google Scholar]
- Munda IM, Kremer BP. Chemical composition and physiological properties of fucoids under conditions of reduced salinity. Mar Biol. 1977;42:9–15. doi: 10.1007/BF00392009. [DOI] [Google Scholar]
- Mushollaeni W. The physicochemical characteristics of sodium alginate from Indonesian brown seaweeds. Afr J Food Sci. 2011;5(6):349–352. [Google Scholar]
- Nguyen VT, Ueng JP, Tsai GJ. Proximate composition, total phenolic content, and antioxidant activity of seagrape (Caulerpa lentillifera) J Food Sci. 2011;76:C950–C958. doi: 10.1111/j.1750-3841.2011.02289.x. [DOI] [PubMed] [Google Scholar]
- Norziah MH, Ching CY. Nutritional composition of edible seaweed Gracilaria changgi. Food Chem. 2000;68:69–76. doi: 10.1016/S0308-8146(99)00161-2. [DOI] [Google Scholar]
- Ohno M, Nang HO, Hirase S. Cultivation and carrageenan yield and quality of Kappaphycus alvarezii in the waters of Vietnam. J Appl Phycol. 1996;8:431–437. doi: 10.1007/BF02178588. [DOI] [Google Scholar]
- Omotoso OT. Nutritional quality, functional properties and anti-nutrient compositions of the larva of Cirina forda (Westwood) (Lepidoptera: Saturniidae) J Zhejiang Univ Sci B. 2006;7(1):51–55. doi: 10.1631/jzus.2006.B0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Calvo JJ, Mazuelos C, Hermosin B, Saiz-Jimenez C. Chemical composition of Spirulina and Eukaryotic algae food products marketed in Spain. J Appl Phycol. 1993;5:425–435. doi: 10.1007/BF02182735. [DOI] [Google Scholar]
- Phaneuf D, Cote I, Dumas P, Ferron LA, LeBlanc A. Evaluation of the contamination of marine algae (Seaweed) from the St. Lawrence River and likely to be consumed by humans. Environ Res A. 1999;80:S175–S182. doi: 10.1006/enrs.1998.3915. [DOI] [PubMed] [Google Scholar]
- Recommended Daily Intakes of Various Food Supplements (2007) Lenntech Water & Luchtbehandeling Holding B.V., Netherlands. (Available from: http://www.lenntech.com/recommended-daily-intake.htm on 15th August 2007)
- Rideout CS, Bernabe MG. US patent 5,801,240, field March 7, 1997, and issued September 1, 1998
- Robledo D, Freile-Pelegrin Y. Chemical and mineral composition of six potentially edible seaweed species of Yucatán. Bot Mar. 1994;40:301–306. [Google Scholar]
- Rosenberg G, Ramus J. Ecological growth strategies in the seaweeds Gracilaria follifera (Rhodophyceae) and Ulva sp. (Chlorophyceae): soluble nitrogen and reserve carbohydrates. Mar Biol. 1982;66:251–259. doi: 10.1007/BF00397030. [DOI] [Google Scholar]
- Rupeŕez P. Mineral content of edible marine seaweeds. Food Chem. 2002;79:23–26. doi: 10.1016/S0308-8146(02)00171-1. [DOI] [Google Scholar]
- Sanchez-Machado DI, Lopez-Cervantes J, Lopez-Hernandez J, Paseiro-Losada P. Fatty acids, total lipids, protein and ash contents of processed edible seaweeds. Food Chem. 2004;85:439–444. doi: 10.1016/j.foodchem.2003.08.001. [DOI] [Google Scholar]
- Sara Marsham S, Scott GW, Tobin ML. Comparison of nutritive chemistry of a range of temperate seaweeds. Food Chem. 2005;100:1331–1336. doi: 10.1016/j.foodchem.2005.11.029. [DOI] [Google Scholar]
- Sen Gupta R, Sankaranarayanan VN, De Sousa SN, Fondekar SP. Chemical oceanography of the Arabian Sea. Part III: studies on nutrient fraction and stoichiometric relationships in the northern and eastern basins. Indian J Mar Sci. 1976;5:58–71. [Google Scholar]
- Soriano EM, Fonseca PC, Carneiro MAA, Moreira WSC. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour Technol. 2006;97:2402–2406. doi: 10.1016/j.biortech.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Southgate DAT. Dietary fiber and health. In: Southgate DAT, Waldron K, Johnson IT, Fen-wick GR, editors. Dietary fiber: chemical and biological aspects. Cambridge: The Royal Society of Chemistry; 1990. pp. 10–19. [Google Scholar]
- Strickland JDH, Parsons TR. A practical handbook of seawater analysis. Bull Fish Res Board Can. 1972;67:1–311. [Google Scholar]
- Subba Rao PV, Suresh Kumar K, Ganesan K, Thakur MC (2008) Feasibility of cultivation of Kappaphycus alvarezii (Doty) Doty at different localities on the Northwest coast of India. Aquac Res 39:1107–1114
- Syad AN, Shunmugiah KP, Kasi PD. Seaweeds as nutritional supplements: analysis of nutritional profile, physicochemical properties and proximate composition of G. acerosa and S. wightii. Biomed Prev Nutr. 2013;3:139–144. doi: 10.1016/j.bionut.2012.12.002. [DOI] [Google Scholar]
- Taboada C, Millán R, Míguez I. Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. J Sci Food Agric. 2010;90:445–449. doi: 10.1002/jsfa.3933. [DOI] [PubMed] [Google Scholar]
- Trono GC, Ohno M. Seasonality in the biomass production of the Eucheuma strains in Northern Bohol, Philippines. In: Umezaki I, editor. Scientific survey of marine algae and their resources in the Philippine islands. Japan: Monbushio International Scientific Research Program; 1986. pp. 71–80. [Google Scholar]
- Tseng CK. The past, present and future ofphycology in China. Hydrobiologia. 2004;512:11–20. doi: 10.1023/B:HYDR.0000020363.37807.ec. [DOI] [Google Scholar]
- USDA (2001) Agricultural research service. Nutrient Database for Standard Reference, Release 14
- Wathelet B. Nutritional analysis for proteins and amino acids in beans (Phaseolus sp.) Biotechnol Agron Soc Environ. 1999;3:197–200. [Google Scholar]
- Wong KH, Cheung PCK. Nutritional evaluation of some subtropical red and green seaweeds. Part I. Proximate composition, amino acid profiles and some physico-chemical properties. Food Chem. 2000;71:475–482. doi: 10.1016/S0308-8146(00)00175-8. [DOI] [Google Scholar]
- Zemke-White LW, Ohno M. World seaweed utilisation: an end-of-century summary. J Appl Phycol. 1999;11:369–376. doi: 10.1023/A:1008197610793. [DOI] [Google Scholar]