Abstract
The effect of grinding cum blanching (GCB) of sprouted soybean at different temperatures on milk and tofu quality was studied. Three temperatures (121 °C-T1,100 °C-T2 and 80 °C-T3) for GCB were used to produce soymilk and tofu from sprouted soybean which were analysed for the yield, nutritional, anti-nutritional profile, colour attributes, particle size, organoleptic quality and texture profile. Unsprouted Soybeans with GCB at 121 °C served as control (C). There was significant difference (P < 0.5) in trypsin inhibitor content in milk and ranged from 4.1 mg/g in T3 to 1.4 mg/g in T1. Optimal reduction in TI of 75–80 % was achieved in T2. There was significant difference (P < 0.5) in protein extractability and ranged from 84.4 % in C to 93.9 % in T2. Hardness (N) of tofu was around 11.22 in C and reduced to 8.9, 8.6 and 4.4 in T1, T2 and T3 respectively. L values of soymilk ranged from 83.4 in C to 85.8 in T3; in tofu from 83.1(T3) to 87.2 (C) and decreased with the increase in heating temperature and time. Particle size d [3, 2] and volume d [4, 3] between treatments varied significantly (P < 0.0001 and P < 0.0038). Overall acceptability scores on 9 point hedonic scale for all treatments for milk and tofu were above 5. The texture scores of tofu for T3 were very low due to its soft structure. From the above investigations 100 °C was the optimal temperature for GCB of sprouted soybean for the production of good quality soymilk and tofu.
Keywords: Sprouting, Soymilk, Tofu, Nutrients, Trypsin inhibitor, Phytic acid, Texture, Organoleptic quality, Particle size
Introduction
Soymilk was first invented in China during 179 B.C. to 122 B.C. (Shurtleff and Aoyagi 1984; Wang and Hasseltine 1982). Soymilk is composed of 94 % moisture, 3.0 % protein, 1.5 % fat, 1.5 % carbohydrates, and ash (de Man et al. 1987). The conventional process of making soymilk involves use of intense heat for the extraction of soybeans and to destroy trypsin inhibitor. Although thermal treatment inactivates effectively trypsin inhibitor, it denatures soybean proteins, results in amino acid degradation and other deteriorative reactions. Moreover, certain flavours, colours, vitamins and nutrients can also be affected by heat treatment (Adams 1991; Borhan and Snyder 1979). Therefore, inactivation of trypsin inhibitor by non-thermal pre-treatments becomes interesting to avoid quality loss of soymilk by decreasing thermal processing.
Sprouting of soybean reduces trypsin inhibitor (TI) and phytic acid, whose reduction otherwise necessitates intense heat (for TI) or methods like ultrafiltration (for phytic acid). Since sprouting is a natural non-thermal non-chemical process, its use as a pre-treatment results in better quality-processed products like milk and tofu. Similarly tofu from sprouted beans has a higher tofu index, good colour and textural properties along with high acceptance on the 9-point hedonic scale (Agrahar-Murugkar 2011). In our previous studies (Agrahar-Murugkar 2011) where the effect of using sprouting as a pre-treatment for production of soymilk and tofu was studied, the time and temperature used for production of soymilk was conventional (121 °C for 30 min). It was recommended in that study that evaluation of time and/ temperature reductions could be tried out along with sprouting to reduce the heat requirement and intensity, which could result in better nutritional and functional quality products. Processing can cause changes in the sensory appeal and the nutritive value of soybeans and soy products. To minimize adverse changes, thermal processing at or below 100 °C for short periods are recommended (Lokuruka 2011).
Therefore the present study aims at investigating temperatures lower than 121 °C (conventional temperature) for grinding cum blanching of soybean to assess the optimal heat requirement along with sprouting to get good quality soymilk and tofu in terms of the yield, nutritional quality, anti-nutritional profile, colour attributes, particle size, organoleptic quality and texture profile.
Materials and methods
Sprouting of soybean
Soybean variety JS9650 grown in Central Institute of Agricultural Engineering farm was selected as material for analysing effect of different process parameters on yield and quality of soymilk and tofu. This cultivar was harvested in November 2011 and stored at 20 °C for further studies. Soybean seeds were cleaned thoroughly and made free from dust, dirt, stubbles and foreign matter. Damaged seeds with cracked hull etc were discarded and the seeds were surface sterilized with 0.1 % (w/v) potassium permanganate solution for 5 min. They were then rinsed with distilled water to remove any traces of potassium permanganate. Seeds were soaked in distilled water for 4 h at room temperature (RT). Then, the excess water was drained, sample further rinsed with distilled water, seeds placed in a single layer on filter paper in sterile petridishes and placed in the Seed Germinator (Indosaw India) at 25 °C, 90 % RH for 72 h as per the method of Agrahar-Murugkar and Jha (2009).
Soymilk preparation
Soymilk was prepared in the grinding cum blanching (GCB) unit using the Soycow model SC 100 (Gupta and Gupta 1990) method. Around 1 kg of soybean per batch was taken both for control and treatments which is the minimum amount required for the Soy Cow model. For control soymilk, dehulled soy splits were soaked for 4 h in water at RT and then fed into the GCB unit. For the preparation of test soymilk, sprouted soybeans containing 60 % moisture were dehulled manually, the hulls discarded and the seeds and cotyledons fed into the unit. Water was added to the soybean in the ratio 5:1 in the unit and a pressure of 1 kg/cm2 (15 psi) was generated by infusing culinary steam at a pressure of 1–3 kg /cm2 (15 to 54 psi). The infused steam replaced the air in the unit, minimizing contact of soybean with oxygen. Grinding cum blanching was started and continued till the required temperature (given below) was achieved. After the pressure from unit was released, the slurry was filtered and milk extracted. The temperature in the GCB unit using the conventional method was 121 °C at which temperature trypsin inhibitor is completely destroyed. Dehulled soybean treated in the GCB unit upto 121 °C served as control (C). Treatments included
T1 = sprouted seeds in the GCB unit upto 121 °C to examine the effect of only sprouting at conventional temperature.
T2 = sprouted seeds in the GCB unit upto 100 °C to examine the combined effect of lower temperature of GCB and sprouting and
T3 = sprouted seeds in the GCB unit upto 80 °C to examine the combined effect of lower temperature of GCB and sprouting.
In our pilot plant the GCB process for control and T1 took 25 min to reach 121 °C. It took 15 min for T2 and 10 min for T3.
Tofu preparation
Milk obtained from the above processes was cooled to around 80 °C subsequently 2.5 % citric acid was added (of the total milk obtained). The mixture was coagulated and the tofu was separated from the whey using a muslin cloth after which it was pressed in a tofu press.
Analyses
The moisture content and dry matter content of soymilk and tofu was determined by the method given by AOAC (1984). The relative viscosity of soymilk was determined by the method given by Ranganna (1977). Soymilk yield was determined by the method of Gesinde et al. (2008). Soymilk index is the summation of soymilk yield (ml g−1 of seed), total solids (g kg−1), soymilk protein (g kg−1) and whiteness index (Wi) (Bharadwaj et al. 1999). The yield of tofu was calculated as the percentage of weight (g) of fresh tofu obtained from a specified amount of the soymilk used for its preparation (Prabhakaran et al. 2006. Tofu index is the summation of tofu yield (g mL−1), tofu strength (N), tofu protein (g kg−1) and whiteness index (Wi) (Bharadwaj et al. 1999). Percent protein extractability was calculated as the protein percentage, which was extracted from soybean seeds into soymilk (Bharadwaj et al. 1999. The solid content and crude protein and fat contents of the soymilk and tofu were estimated by the standard methods (AOAC 1995). Trypsin inhibitor (TI) activity was determined by standard procedure of Kakade et al. (1974) as modified by Hammerstrand et al. (1981). The phytate content of tofu was assayed using Latta and Eskin (1980).
Determination of colour
Soymilk and tofu samples were taken for the measurement of colour using lab scan XE spectrocolorimeter (model no LX16244, Hunter Associate Laboratory Virginia, USA) in terms of CIE ‘L’ (lightness) ‘a’ (redness and greenness) and ‘b’ (yellowness and blueness), Yi (Yellowness index) and Wi (Whiteness index) following the method of Chantrapornchai et al. (1998). ΔE (colour difference) values were calculated using the following formula:
where ΔL, Δa and Δb are the differences in the specified tristimulus coordinate between the test soymilk and tofu with commercial soymilk and tofu (ΔE).
Particle size analysis of soymilk
The soymilk samples from tests and control were analyzed for particle size distribution and average particle sizes (d [3,2] – surface-weighted mean diameter – Sauter Mean Diameter and d [4,3] – volume-weighted mean diameter – De Brouckere Mean Diameter) using a particle size analyzer (Mastersizer, Malvern Inc., Worcestershire, United Kingdom). Mean diameters d [3,2] and d [4,3] were defined by:
where ni is the number of droplets of diameter di.
Particle size distribution was calculated by laser diffraction method using a refractive index ratio of 1.47. Soy milk samples were diluted with water to obtain 10–14 % obscuration. The absorption value was set up at 0.001. All measurements were carried out at 25 °C. All analyses were performed in triplicate.
Texture analysis of tofu
The texture profile of tofu was determined using the Texture Analyzer (TA- XT2i) of Stable Microsystem equipped with a 5 kg load cell. The analyser is linked to a computer that recorded data via a software program Texture Expert (Texture Technologies Corp., New York, USA). Samples of length, width and height of 25.4 mm were cut from the central portion of tofu cake with a stainless steel cutter. A flat plate probe of 75 mm diameter was used. The pre, test and post- test speed was 1 mm/min. Each sample was compressed twice to 50 % of its height with trigger force of 0.05 N and 5 s time gap between the two compressions. Data was acquired at 200 points per sec. Force versus displacement was recorded. Hardness, cohesiveness, springiness and chewiness of tofu were determined using the method described by Kotwaliwale et al. (2007).
Sensory evaluation of tofu
Sensory evaluation was done on freshly made soymilk and tofu. Ten semi- trained panellists evaluated the sensory attribute of fresh milk and tofu. Panellists were familiar with product sensory evaluation, most having participated in previous related projects. Milk was served in glasses and tofu was cut into cubic samples and placed on a plastic plate with a random number (Cai and Chang 1998). The attributes evaluated were colour, flavour, taste, texture (for tofu), appearance and overall acceptability. The colour attribute included only colour of the product while appearance included colour, size and shape (tofu), clarity (soymilk) and interior appearance (tofu). For each sample, panelists scored their liking of theses characteristics using the nine point Hedonic scale (1 = dislike extremely, 2 = dislike very much, 3 = dislike moderately, 4 = dislike slightly, 5 = neither like nor dislike, 6 = like slightly, 7 = like moderately, 8 = like very much and 9 = like extremely).
The experiment was conducted using Completely Randomized Design (CRD) with replications in triplicate. The statistical analyses were performed using SAS 9.3. The General Linear Model (GLM) procedure was used to study the effect of treatments on the parameters studied.
Results and discussion
The nutrient and anti-nutrient profile of soymilk and tofu from different GCB temperatures is given in Table 1. The moisture, protein and fat contents of both soymilk and tofu were not significantly affected by processing temperature. The slight variation in the amounts of protein and fat between control and test samples were because of phenomena of sprouting. These observations could be explained considering the fact that during sprouting, there is a breakdown of reserve protein to give NH3, which accumulates, in the form of amide such as glutamic acid and aspartic acid (Hsu et al. 1980), while depletion of the fat stored is due to the catabolic activities of the seeds during sprouting (Mostafa et al. 1987). There was significant reduction (P < 0.5) in the levels of trypsin inhibitor, which was a cumulative effect of sprouting as well as heat treatment. During seed germination, the concentration of protease inhibitors, in general and Bowman Birk Inhibitor (BBI) in particular, decreases as a result of BBI digestion by protease K1 and B2 (Papastoitsis and Wilson 1991) and a reduction of around 60 % after 72 h of sprouting has been reported (Agrahar-Murugkar and Jha 2009). Although BBI languished for many years as an anti-nutrient, clinical trials have shown that BBI has now become an important protein for disease prevention (Kennedy 1998; Armstrong et al. 2000a, b; Meyskens 2001; Tolcher et al. 2001). Extensive in-vitro toxicity and clinical studies of BBI at levels normally found in food and at higher levels have confirmed that BBI is safe (Kennedy 1998; Malkowicz et al. 2001) and is now considered a functional food (Losso et al. 2004). Therefore instead of using intensive heat treatment to completely destroy trypsin inhibitor and in turn BBI, optimal heat during processing retaining 20–25 % of the complex is desirable. The levels of trypsin inhibitor in milk ranged from 4.1 mg/g in T3, which was sprouted and had minimal heat intervention at 80 °C to 1.4 mg/g in T1, which was sprouted and was processed at 121 °C. The trypsin inhibitor in the C milk was 1.7 mg/g where reduction in trypsin inhibitor was only due to heat treatment. Optimal reductions of around 75 % in trypsin inhibitor were achieved in T2. Similarly in tofu a combination of sprouting and heat reduced the Trypsin inhibitor in T1 to around 87 %. The levels of phytic acid in soymilk were significantly different between all the groups. The difference between control (C) and test samples was nearly 42 % due to sprouting which reduces the phytic acid content which is confirmed by other investigators due to the possibility of increased phytase activity by 3 to 5-fold in some cereal grains and legume seeds (Egli et al. 2002; Dave et al. 2008; Agrahar-Murugkar and Jha 2009).
Table 1.
Nutrient and anti-nutrient profile of soymilk and tofu from different process parameters
| Moisture% | Protein (fb) % | Fat (fb)% | TI mg/g (db)* | % reduction of TI from seed* | Phytic acid mg% (db)* | |
|---|---|---|---|---|---|---|
| Soymilk | ||||||
| Control | 94.3 ± 0.03 | 2.6 ± 0.01 | 1.4 ± 0.28 | 1.7a ± 0.03 | 84.54c ± 2.34 | 6.7c ± 0.07 |
| T1 | 94.1 ± 0.04 | 2.7 ± 0.02 | 1.1 ± 0.01 | 1.4a ± 0.27 | 87.27c ± 3.12 | 4.6a ± 0.06 |
| T2 | 94.1 ± 0.71 | 2.7 ± 0.06 | 1.1 ± 0.01 | 2.0b ± 0.121 | 82.97b ± 2.16 | 4.6a ± 0.083 |
| T3 | 94.3 ± 0.07 | 2.5 ± 0.01 | 1.2 ± 0.03 | 4.1c ± 0.08 | 62.72a ± 3.13 | 5.1b ±0.032 |
| Tofu | ||||||
| Control | 71.1 ± 0.92 | 15.2 ± 0.07 | 8.7 ± 0.28 | 1.0a ± 0.03 | 88.18c ± 2.88 | 6.8c ± 0.06 |
| T1 | 72.0 ± 0.42 | 15.5 ± 0.40 | 7.4 ± 0.24 | 1.3a ± 0.02 | 90.90d ± 2.56 | 3.9a ± 0.07 |
| T2 | 72.3 ± 1.39 | 15.9 ± 0.02 | 7.4 ± 0.24 | 1.7b ± 0.56 | 85.53b ± 3.65 | 4.7b ± 0.11 |
| T3 | 73.9 ± 0.76 | 14.6 ± 0.05 | 7.1 ± 0.11 | 2.9c ± 0.11 | 73.63a ± 2.89 | 5.0b ± 0.01 |
Values given are mean ± SD
*Significantly different at P < 0.5, *Superscripts indicates significant differences (P < 0.5) between columns (n = 3)
fb fresh basis, db dry basis, TI trypsin inhibitor
Control: Dehulled soybean treated in the GCB unit upto 121 °C
T1 = sprouted seeds in the (grinding cum blanching unit) GCB unit upto 121 °C
T2 = sprouted seeds in the GCB unit upto 100 °C
T3 = sprouted seeds in the GCB unit upto 80 °C
The characteristics of soymilk and tofu are given in Table 2. The differences in the yields (ml/g) between control and test samples were not significant (P < 0.5) and ranged from 7.1 to 7.3. The viscosity of soymilk ranged from 1.91 in T3 to 2.51 in C and varied significantly (P < 0.5). The difference in viscosity of soymilk from sprouted and unsprouted seeds may be due to several endogenous endopeptidases that are found to be active in soybean seedlings during sprouting, some of which are responsible for the hydrolyzation of soybean storage protein subunits (Qi et al. 1994; Kawai et al. 1997) causing a decrease in viscosity. The lowest viscosity of 1.91 was seen in T3 where the lowest temperature of 80 °C was used which is in line with studies where comparison between lower and higher temperatures for extractions were studied and revealed that hot extraction results in a significantly higher viscosity (Vishwanathan et al. 2011; Toda et al. 2007). Extractability of soymilk depends on solubility, temperature and time of grinding (Vishwanathan et al. 2011). There was significant difference (P < 0.5) in the extractability and ranged from 84.4 % in control to 93.9 % in T2. Solubility of seeds increase on sprouting due to breakdown of proteins and decrease in particle size leading to increased extractability of test samples. Among the test samples, higher duration of grinding and temperature, higher was the surface area and greater was the recovery in extraction process (Vishwanathan et al. 2011), which could be the reason for the extractability of soymilk in test samples in T1, and 2 being higher than the milk from T3. The soymilk index (SMI) was obtained by summing yield, total solids, protein content and whiteness index of soymilk (Bharadwaj et al. 1999) and it ranged from 938.5 in control to T3 to 1066.47 in T2. There was a significant difference (P < 0.5) in the SMI of all the samples, which can be attributed to slight increase in yields and protein contents of the soymilk from sprouted samples and is in line with previous studies (Agrahar-Murugkar 2011). The reduction in SMI in T3 is due to lower yields and protein content of soymilk. The reason for this may be the lower temperature of 80 °C with a shorter time period of 10 min in the GCB unit did not facilitate optimum extraction reducing yields, protein and extractability despite sprouting. There was a significant (P < 0.5) decrease in the yields of tofu between control and test samples and ranged from 0.13 in T3 to 0.24 in control. The decrease in T1 and T2 was around 16 % whereas the decrease in T3 was 45 % in comparison to control. Hardness of tofu was around 11.22 N in control and reduced to 8.89, 8.60 and 4.38 in T1, T2 and T3 respectively. Recently, researchers have reported that gels made from soybean glycinin are harder than the b-conglycinin one. As sprouting takes place the molecular structure of soybean storage protein is looser since the acidic chains of glycinin (11S) are degraded gradually. After that the cross-linking of soybean protein three-dimensional network decreases, and softer, less hard gels are formed (Yang and Li 2010). The decrease in hardness of tofu in sprouted samples could also be related to decreasing phytic acid levels after sprouting. Increased phytic acid levels increase hardness of tofu (Saio et al. 1969).
Table 2.
Characteristics of soymilk and tofu from different process parameters
| Soymilk | Tofu | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yield (ml/g) | Viscosity* | Extractability%* | SMI* | Yield *(g/ml) | Tofu Index* | Hardness* | Cohesiveness* | Springiness* | Chewiness* | |
| Control | 7.1 ± 0.03 | 2.51 c ± 0.31 | 84.4a ± 2.31 | 938.48a ± 5.02 | 0.24c ± 0.02 | 542.46b ± 2.36 | 11.22c ± 4.21 | 0.36a ± 0.05 | 0.38a ± 0.14 | 3.24b ± 1.20 |
| T1 | 7.2 ± 0.04 | 2.15b ± 0.67 | 93.5b ±1.69 | 1048.16b ± 4.66 | 0.20b ± 0.03 | 523.59a ± 1.99 | 8.89b ± 0.31 | 0.86b ± 0.05 | 0.47b ± 0.33 | 4.05c ± 2.45 |
| T2 | 7.3 ± 0.08 | 2.15b ± 0.40 | 93.9b ±2.06 | 1066.47c ± 5.13 | 0.20b ± 0.02 | 559.40c ± 2.03 | 8.60b ± 1.42 | 0.83b ± 0.08 | 0.52bc ± 0.28 | 2.67a ± 1.94 |
| T3 | 7.1 ± 0.06 | 1.91a ±0.53 | 86.0a ±2.33 | 938.50a ± 3.22 | 0.13a ± 0.01 | 522.31a ± 2.19 | 4.38a ± 0.66 | 1.41c ± 0.33 | 0.62c ± 0.28 | 3.86bc ± 1.98 |
Values given are mean ± SD
*Significantly different at P < 0.5
Superscripts indicates significant differences (P < 0.5) between columns (n = 3)
SMI soy milk index
Control: Dehulled soybean treated in the GCB unit upto 121 °C
T1 = sprouted seeds in the (grinding cum blanching unit) GCB unit upto 121 °C
T2 = sprouted seeds in the GCB unit upto 100 °C
T3 = sprouted seeds in the GCB unit upto 80 °C
The colour attributes of milk and tofu is given in Table 3. The colours of soymilk of the test samples were compared with the commercial soymilk. L values ranged from 83.39 in C to 85.76 in T3 and decreased with the increase in heating temperature and time, following first-order kinetics. Similarly in tofu the L values followed the same trend as soymilk ranging from 83.07(T3) to 87.22 (C). This could be due to non-enzymatic browning, mainly due to the Maillard reaction, which occurs in soymilk during heat treatment (Kwok et al. 1999). The measurement of browning is useful in evaluating the quality of soymilk due to the fact that proteins participate in the reaction (Johnson et al. 1981). The ΔE ranged from 4.61 (T2) showing least difference to 7.61 (T1) showing greatest difference with the commercial soymilk. In tofu the ΔE on comparison with commercial tofu was 6.41 in T3 to 10.34 in control. With regard to the colour, either white or creamy white colour is the desirable soymilk and tofu characteristic (Hou and Chang 2004), which is what was observed in our experiment as well.
Table 3.
Colour attributes of milk and tofu
| L | a | b | Yi | Wi | ΔE | |
|---|---|---|---|---|---|---|
| Milk | ||||||
| Commercial soymilk | 84.5 | −2.31 | 8.07 | – | – | |
| Control | 83.39 ± 1.20 | −0.06 ± 0.01 | 13.41 ± 0.12 | 22.22 ± 1.03 | 7.27 ± 1.20 | 5.90 |
| T1 | 84.50 ± 1.22 | −0.46 ± 0.02 | 15.45 ± 0.32 | 18.26 ± 0.42 | 7.59 ± 0.24 | 7.61 |
| T2 | 85.64 ± 1.33 | −0.49 ± 0.03 | 12.11 ± 0.0.21 | 22.49 ± 0.33 | 7.30 ± 0.98 | 4.61 |
| T3 | 85.76 ± 1.05 | −0.41 ± 0.01 | 13.07 ± 0.22 | 27.3 ± 1.21 | 7.37 ± 0.36 | 5.47 |
| Tofu | ||||||
| Commercial tofu | 80.1 | −2.31 | 18.8 | NA | NA | – |
| Control | 87.22 ± 1.25 | 2.94 ± 0.01 | 13.44 ± 3.02 | 26.20 ± 6.66 | −39.20 ± 0.32 | 10.34 |
| T1 | 85.20 ± 2.50 | 0.97 ± 0.05 | 16.40 ± 1.02 | 24.20 ± 5.76 | 5.10 ± 0.42 | 8.18 |
| T2 | 85.54 ± 1.10 | 2.04 ± 0.23 | 18.28 ± 2.11 | 26.67 ± 8.02 | −23.40 ± 0.33 | 6.98 |
| T3 | 83.07 ± 1.02 | 2.62 ± 0.02 | 15.97 ± 2.46 | 32.28 ± 4.44 | −41.50 ± 1.02 | 6.41 |
Values given are mean ± SD NA –not available
Yi yellowness index, Wi whiteness index
Control: Dehulled soybean treated in the GCB unit upto 121 °C
T1 = sprouted seeds in the (grinding cum blanching unit) GCB unit upto 121 °C
T2 = sprouted seeds in the GCB unit upto 100 °C
T3 = sprouted seeds in the GCB unit upto 80 °C
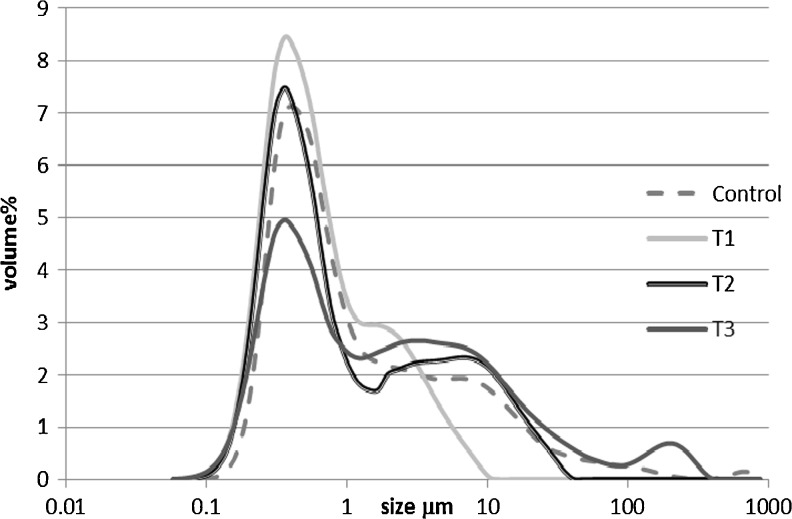
Soymilk contains colloidal fat and protein and in some cases cellular particles. The size and distribution of these particles, which affects mouth feel is an important quality parameter. The particle size d [3, 2] and particle volume d [4, 3] of soymilk are given in Table 4 and the particle size distribution in shown in Fig. 1. There was significant difference in the particle size (P < 0.0001) and volume (P < 0.0038) between the treatments. d [3,2] ranged from 0.45 in T1 to 0.68 in T3. The particle size distribution (PSD) shows that all the graphs were bimodal with one large peak at around 0.5 ηm and the sec. at around 10 ηm. The difference in d [3, 2] between C and T1 both processed at 120 °C was due to the process of sprouting which breaks down particles and decreases particle size (Qi et al. 1994). However differences in particle size and volume between test samples was due to process parameters especially time taken for grinding. Increased time of grinding resulted in smaller and more uniform particles and so the time of grinding was inversely proportional to the particle size. This is evident in the PSD where in T1 the particle sizes are uniform and less than 10 ηm. In T2 the maximum particle size is around 50 ηm and in T3 it is more than 100 ηm.
Table 4.
Particle size d [3, 2] and particle volume d [4, 3] of soymilk from different process parameters
| d [3,2]* | d [4,3]* | |
|---|---|---|
| Control | 0.61a | 5.07ab |
| T1 | 0.45b | 1.02b |
| T2 | 0.53b | 2.93b |
| T3 | 0.68a | 12.45a |
*Significantly different at P < 0.0001 and P < 0.0038
Superscripts indicates differences (P < 0.5) between columns (n = 10)
Control: Dehulled soybean treated in the GCB unit upto 121 °C
T1 = sprouted seeds in the (grinding cum blanching unit) GCB unit upto 121 °C
T2 = sprouted seeds in the GCB unit upto 100 °C
T3 = sprouted seeds in the GCB unit upto 80 °C
Fig. 1.
Particle size distribution (Mastersizer, Malvern Inc., Worcestershire, United Kingdom) of soymilk from different process parameters. Control: Dehulled soybean treated in the GCB unit upto 121 °C T1 = sprouted seeds in the (grinding cum blanching unit) GCB unit upto 121 °C T2 = sprouted seeds in the GCB unit upto 100 °C T3 = sprouted seeds in the GCB unit upto 80 °C
The organoleptic and sensory scores of the milk and tofu are given in Table 5. The overall acceptability of milk ranged from 6.6 in control and T3 to 6.8 in T2 which is not statistically significant showing that all milk samples were at par with the controls. However the taste scores in milk are the lowest in C and T3 (6.5) probably due to the chalkiness attribute which is related to the sensation of chalky powder in the mouth after the ingestion of the product, which has been attributed to the larger particles present in soymilk that are retained in the pores and in the mucous membranes of the mouth during ingestion (Wei 1984) as has been seen in the particle size analysis. In the case of tofu however, all the parameters of evaluation were very low in the case of T3. This could be because of the consistency of tofu not being firm which has been demonstrated in the case of texture. Apart from the taste and texture of tofu in T3 the tofu from all other tests showed an acceptable score of above 5.
Table 5.
Organoleptic and sensory scores of milk and tofu
| Taste | Texture | Flavour | Colour | Appearance | Overall acceptability | |
|---|---|---|---|---|---|---|
| Milk | ||||||
| Control | 6.5 ± 0.33 | – | 6.2 ± 0.65 | 6.7 ± 0.81 | 7.0 ± 0.33 | 6.6 ± 0.13 |
| T1 | 6.6 ± 1.02 | 6.5 ± 1.32 | 6.8 ± 1.03 | 6.7 ± 0.12 | 6.7 ± 0.52 | |
| T2 | 6.8 ± 0.98 | 6.7 ± 1.04 | 6.8 ± 0.05 | 6.8 ± 0.45 | 6.8 ± 0.21 | |
| T3 | 6.5 ± 0.99 | – | 6.5 ± 0.88 | 6.7 ± 0.23 | 6.7 ± 0.35 | 6.6 ± 0.34 |
| Tofu | ||||||
| Control | 6.9 ± 1.25 | 6.9 ± 0.33 | 6.7 ± 0.46 | 7.1 ± 0.33 | 7.2 ± 0.26 | 7.0 ± 0.56 |
| T1 | 6.7 ± 0.68 | 7.2 ± 0.79 | 6.7 ± 1.01 | 6.9 ± 1.03 | 7.1 ± 0.41 | 6.9 ± 0.26 |
| T2 | 7.2 ± 1.00 | 7.3 ± 0.55 | 6.8 ± 0.66 | 7.1 ± 0.44 | 7.1 ± 0.69 | 7.1 ± 0.55 |
| T3 | 4.5 ± 0.56 | 4.5 ± 0.71 | 6.5 ± 0.49 | 5.6 ± 0.22 | 6.2 ± 0.11 | 5.5 ± 0.12 |
Values given are mean ± SD
Control: Dehulled soybean treated in the GCB unit upto 121 °C
T1 = sprouted seeds in the (grinding cum blanching unit) GCB unit upto 121 °C
T2 = sprouted seeds in the GCB unit upto 100 °C
T3 = sprouted seeds in the GCB unit upto 80 °C
Conclusion
Sprouting of soybean prior to feeding in the grinding cum blanching unit produced soymilk and tofu with better quality products in terms improved nutritional profiles, higher soymilk index and higher tofu index, good colour characteristics and high acceptability by panellists in sensory evaluation due to absence of beany flavour and odour. Reducing the temperature of grinding cum blanching to 100 °C reduced the time taken and energy required for the process, and further improved quality of soymilk and tofu. The soymilk had reductions of trypsin inhibitor by 75 % retaining 25 % of trypsin inhibitor containing BBI, which is now recognised as a functional food. The soymilk also had increased extractability of proteins, smaller particle size, better colour profile and good sensory scores. The tofu from this soymilk was of a higher quality. Therefore sprouting as a pre-treatment followed by a grinding cum blanching temperature of 100 °C resulted in saving of energy, time and resulted in high quality soymilk and tofu.
References
- Adams JB. Enzyme inactivation during heat processing of food-stuffs. Int J Food Sci Technol. 1991;26:1–20. doi: 10.1111/j.1365-2621.1991.tb01136.x. [DOI] [Google Scholar]
- Agrahar-Murugkar D. Effect of sprouting of soybean on the chemical composition and quality of soymilk and tofu. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrahar-Murugkar D, Jha K. Effect of sprouting on nutritional and functional characteristics of soybean (Glycine max L) J Food Sci Technol. 2009;46(3):240–243. doi: 10.1007/s13197-010-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 12. Washington DC: Association of Official Analytical Chemists; 1984. [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 16. Washington DC: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Armstrong WB, Kennedy AR, Wan XS, Atiba J, McClaren CE, Meyskens FL., Jr Single-dose administration of Bowman-Birk inhibitor concentrate in patients with oral leukoplakia. Cancer Epidemiol Biomark Prev. 2000;9(1):43–47. [PubMed] [Google Scholar]
- Armstrong WB, Kennedy AR, Wan SX, Taylor TH, Nguyen QA, Jensen J, Thompson W, Lagerberg W, Meyskens FL., Jr Clinical modulation of oral leukoplakia and protease activity by Bowman-Birk inhibitor concentrate in a phase IIa chemoprevention trial. Clin Cancer Res. 2000;6:4684–4691. [PubMed] [Google Scholar]
- Bharadwaj HL, Bhagsari AS, Joshi JM, Rangappa M, Sapre VT, Rao MSS. Yield and quality of soymilk and tofu made from soybean genotypes grown at four locations. Crop Sci. 1999;39:401–405. doi: 10.2135/cropsci1999.0011183X0039000200017x. [DOI] [Google Scholar]
- Borhan M, Snyder HE. Lipoxygenase destruction in whole soybeans by combinations of heating and soaking in ethanol. J Food Sci. 1979;44(2):586–590. doi: 10.1111/j.1365-2621.1979.tb03842.x. [DOI] [Google Scholar]
- Cai TD, Chang KC. Characteristic of production-scale tofu as affected by soymilk coagulation method: propeller blade size, mixing time and coagulant concentrations. Food Res Int. 1998;31:289–295. doi: 10.1016/S0963-9969(98)00091-X. [DOI] [Google Scholar]
- Chantrapornchai W, Clydesdale F, Mc Clements DJ. Influence of droplet characteristics on the colour of food emulsions. J Agric Food Chem. 1998;96(8):2914–2920. doi: 10.1021/jf980278z. [DOI] [Google Scholar]
- Dave S, Yadav BK, Tarafdar JC. Phytate phosphorus and mineral changes during soaking, boiling and sprouting of legumes and pearl millet. J Food Sci Technol. 2008;45:344–348. [Google Scholar]
- deMan L, deMan JM, Buzzell RI. Composition and properties of soymilk made from Ontario light hilum soybeans. Can Inst Food Sci Tech J. 1987;20:363–367. doi: 10.1016/S0315-5463(87)71332-7. [DOI] [Google Scholar]
- Egli I, Davidsson MA, Juillerat D, Barclay RF, Hurrel A. The influence of soaking and sprouting on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feeding. J Food Sci. 2002;67(9):3484–3488. doi: 10.1111/j.1365-2621.2002.tb09609.x. [DOI] [Google Scholar]
- Gesinde AF, Oyawoye OM, Adebisi A. Comparative studies on the quality and quantity of soymilk from different varieties of soybean. Pak J Nutr. 2008;7(1):157–160. doi: 10.3923/pjn.2008.157.160. [DOI] [Google Scholar]
- Gupta, RP, Gupta, RR (1990) Food Processing in Oxygen Free Environment, US Patent number 4915972
- Hammerstrand GE, Black LT, Glover JD. Trypsin inhibitor in soy products: modification of standard analytical procedures. Cereal Chem. 1981;15:215–218. [Google Scholar]
- Hou HJ, Chang KC. Storage conditions affect soybean colour, chemical composition and tofu qualities. J Food Process Preserv. 2004;28(6):473–488. doi: 10.1111/j.1745-4549.2004.24015.x. [DOI] [Google Scholar]
- Hsu D, Leung HK, Finney PL, Morad MM. Effects of sprouting of nutritive value and baking properties of dry peas, lentils and faba beans. J Food Sci. 1980;45(1):87–92. doi: 10.1111/j.1365-2621.1980.tb03877.x. [DOI] [Google Scholar]
- Johnson LA, Deyoe CW, Hoover WJ. Yield and quality of soymilk processed by steam-infusion cooking. J Food Sci. 1981;46:239–243. doi: 10.1111/j.1365-2621.1981.tb14572.x. [DOI] [Google Scholar]
- Kakade ML, Simons N, Liner IE. An evaluation of natural versus synthetic substrates for measuring the antitryptic activity of soybean samples. Cereal Chem. 1974;46:518–526. [Google Scholar]
- Kawai M, Suzuki S, Asano M, Miwa T, Shibai H. Characterization of 30-kDa fragments derived from beta-conglycinin degradation process during germination and seedling growth of soybean. Biosci Biotechnol Biochem. 1997;61:794–799. doi: 10.1271/bbb.61.794. [DOI] [PubMed] [Google Scholar]
- Kennedy AR. The Bowman-Birk inhibitor from soybeans as an anticarcinogenic agent. Am J Clin Nutr. 1998;68:1406S–1412S. doi: 10.1093/ajcn/68.6.1406S. [DOI] [PubMed] [Google Scholar]
- Kotwaliwale N, Bakane P, Verma A. Changes in textural and optical properties of oyster mushroom during hot air drying. J Food Eng. 2007;78(4):1207–1211. doi: 10.1016/j.jfoodeng.2005.12.033. [DOI] [Google Scholar]
- Kwok KC, MacDougall DB, Niranjan K. Reaction kinetics of heat-induced colour changes in soymilk. J Food Eng. 1999;40:15–20. doi: 10.1016/S0260-8774(99)00031-X. [DOI] [Google Scholar]
- Latta M, Eskin MA. A simple and rapid colourimetric method for phytate determination. J Agric Food Chem. 1980;28:1313–1315. doi: 10.1021/jf60232a049. [DOI] [Google Scholar]
- Lokuruka M. Effects of processing on soybean nutrients and potential impact on consumer health: an overview. Afr J Food Agric Nutr Dev. 2011;11(4):5000–5017. [Google Scholar]
- Losso JN, Munene CN, Bansode RR. Inhibition of metalloproteinase-1 activity by the soybean Bowman-Birk inhibitor. Biotechnol Lett. 2004;26:901–905. doi: 10.1023/B:bile.0000025900.33812.7c. [DOI] [PubMed] [Google Scholar]
- Malkowicz SB, McKenna WG, Vaughn DJ, Wan XS, Propert KJ, Rockwell K, Marks SH, Wein AJ, Kennedy AR. Effects of Bowman-Birk inhibitor concentrate (BBIC) in patients with benign prostatic hyperplasia. Prostate. 2001;48:16–28. doi: 10.1002/pros.1077. [DOI] [PubMed] [Google Scholar]
- Meyskens FL. Development of Bowman-Birk inhibitor for chemoprevention of oral head and neck cancer. Ann N Y Acad Sci. 2001;952:116–123. doi: 10.1111/j.1749-6632.2001.tb02732.x. [DOI] [PubMed] [Google Scholar]
- Mostafa MM, Rahma EH, Rady AH. Biochemical and nutritional changes in soybean during sprouting. J Food Biochem. 1987;23:257–275. doi: 10.1016/0308-8146(87)90113-0. [DOI] [Google Scholar]
- Papastoitsis G, Wilson KA. Initiation of the degradation of the soybean Kunitz and Bowman-Birk trypsin inhibitors by a cysteine protease. Plant Physiol. 1991;96:1086–1092. doi: 10.1104/pp.96.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran MP, Perera CO, Valiyaveettil S. Effect of different coagulants on the isoflavone levels and physical properties of prepared firm tofu. J Food Chem. 2006;99(3):492–499. doi: 10.1016/j.foodchem.2005.08.011. [DOI] [Google Scholar]
- Qi X, Chen R, Wilson KA, Tan-Wilson AL. Characterization of a soybean [beta]-conglycinin-degrading protease cleavage site. Plant Physiol. 1994;104:127–133. doi: 10.1104/pp.104.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganna S (1977) Handbook of analysis of quality control for fruits and vegetable products, 2nd edn. New Delhi, Tata McGraw-Hill Publishing Co. Wiley, New York, p 149–178
- Saio K, Kamiya M, Watanabe T. Food processing characteristics of soybean 11S and 7S. Part I. Effect of differences of protein components among soybean varieties on formation of tofu-gel. Agric Biol Chem. 1969;33:1301. doi: 10.1271/bbb1961.33.1301. [DOI] [Google Scholar]
- Shurtleff W, Aoyagi A. Soymilk industry and market. California: Soyfoods Center; 1984. [Google Scholar]
- Toda K, Chiba K, Ono T. Effect of components extracted from okara on the physicochemical properties of soymilk and tofu texture. J Food Sci. 2007;72:108–C113. doi: 10.1111/j.1750-3841.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Tolcher AW, Kennedy AR, Padley RJ, Majeed N, Pollak M, Kantoff PW. Other novel agents: rationale and current status as chemopreventive agents. Urology. 2001;57:86–89. doi: 10.1016/S0090-4295(00)00948-1. [DOI] [PubMed] [Google Scholar]
- Vishwanathan KH, Singh V, Subramanian R. Wet grinding characteristics of soybean for soymilk extraction. J Food Eng. 2011;106:28–34. doi: 10.1016/j.jfoodeng.2011.04.002. [DOI] [Google Scholar]
- Wang HL, Hasseltine CW. Coagulation conditions in tofu processing. Process Biochem. 1982;17:7–12. [Google Scholar]
- Wei LS. Soymilk—new processing, packaging expand markets. J Am Oil Chem Soc. 1984;61(12):1784–1809. doi: 10.1007/BF02540803. [DOI] [Google Scholar]
- Yang M, Li L. Characteristics of probiotic Soy yogurt. Food Technol Biotechnol. 2010;48(4):490–496. [Google Scholar]