Abstract
The inhibitory effect of grape seed powder (GSP) on lipid oxidation in chicken nuggets during frozen storage for 5 months was investigated. Chicken nuggets were prepared by dipping into batter containing GSP and pre-fried at 180 °C and then stored at −18 °C. Prefried chicken nugget crusts showed antioxidant properties. Primary oxidation products, determined by the peroxide value (POV) and conjugated diene (CD) concentration, gradually increased until month 2 and then declined, which is an indication of secondary lipid oxidation. Thiobarbituric acid reactive substance (TBARS) values slowly increased during the first 2 months of storage and then slightly decreased. However, at the end of the storage period, the levels were increased to 0.4 mg MDA/kg meat and were lowest in 10 % GSP (0.104 mg MDA/kg meat). The para-anisidine values (pAV) increased in all samples during storage. Generally, samples treated with GSP had lower POV, pAV, TBARS, and CD values compared to the control. These findings indicated that GSP significantly (p < 0.05) retarded lipid oxidation in precooked chicken nuggets.
Keywords: Grape seed, Lipid oxidation, Batter coating, Chicken nugget, Deep-fat frying
Introduction
Chicken meat and products have many desirable nutritional characteristics, such as a low lipid content and high amounts of polyunsaturated fatty acids compared to most types of red meat that can be further enhanced by specific dietary strategies. The susceptibility of lipids to oxidation is one of the major causes of quality deterioration in many types of natural and processed foods (Jittrepotch et al. 2006). Lipid oxidation in raw and cooked meat products is often synonymous with warmed over flavor (WOF), which is the disappearance of fresh meat flavor; the development of stale, rancid flavors; changes in the rheological properties and solubility; and potential formation of toxic compounds such as 4-hydroxy-nonenal in precooked or cooked stored meats. Cooked meat develops a rancid flavor more rapidly than raw meat during storage (Han et al. 1995) because thermal processes promote lipid oxidation by disrupting cell membranes and releasing pro-oxidants, thereby inducing WOF, which rapidly develops in cooked meat products during cold storage and subsequent reheating. The formation of volatile carbonyl compounds like hexanal, pentanal, 2, 4-decadienal, 2, 3-octanedione, and 2-octenal as secondary oxidation products in precooked/cooked meat samples is attributed to the oxidation of unsaturated lipids (Juntachote et al. 2006). Due to increasing demand for consumption of battered and breaded chicken products, it is important to control lipid oxidation during processing and subsequent storage of table ready, reheatable food products to prevent the development of WOF.
The possible toxicity and ongoing consumer concern over synthetic antioxidants have increased interest in the use of natural antioxidants to extend the shelf life of foods (Prasetyo et al. 2008). Various plant materials containing polyphenolic compounds are effective antioxidants and can decelerate the development of WOF in meat products (Kulkarni et al. 2011; Chen and Waimaleongora 1999; Ahn et al. 2002; Nissen et al. 2004; Han and Rhee 2005; Fernandez-Lopez et al. 2003).
Grape seed has been identified as antioxidant owing to its complex matrix containing approximately 40 % fiber, 16 % oil, 11 % proteins, and 7 % complex phenols including tannins, in addition to sugars, mineral salts, etc. (Kim et al. 2006). Grape seed extract (GSE) is sold commercially as a dietary supplement and listed on the “Everything Added to Food in the United States (EAFUS)” list (Perumalla and Hettiarachchyc 2011). Chedea et al. (2011) showed that aqueous GSE is rich in the known chemopreventive neutraceuticals catechin, epicatechin, and procyanidins. Antioxidative effects of grape seed extracts and other plant extracts have been widely searched and showed positive effects on reducing the amount of primary lipid oxidation products (e.g., lipid hydroperoxides and hexanal) and secondary lipid oxidation products (e.g., TBARS) effectively in raw and cooked meat products, such as beef (Banon et al. 2007), ground beef (Rojas and Brewer 2007), chicken (Lau and King 2003; Conchillo et al. 2005; Rababah et al. 2006; Brannan 2009), fish (Pazos et al. 2005), turkey breast (Mielnik et al. 2006), and pork (Carpenter et al. 2007). Little work has been focus on evaluating effect of these plants when used as powder in batter formulation. It would be beneficial to study the potential nutritional advantage of adding grape seed powder (GSP) directly into batter with other ingredients. For this consideration, in this study; an alternative way to suppress lipid oxidation during deep frying and frozen storage by adding grape seed powder directly into the batter was investigated. Therefore, we investigated whether inclusion of grape seed powder in batter which are exposed to high heat during processing could improve the oxidative stability of nuggets as well as phenolic extracts by measuring the changes in conjugated diene (CD), thiobarbituric acid reactive substances (TBARS), para-anisidine (PA) and peroxide (PO) values during frozen storage.
Materials and methods
Ingredients
Wheat flour, corn flour, de-boned chicken breast, and sunflower oil as the frying medium were purchased from commercial markets. The hydrocolloid used in this study was hydroxypropyl methylcellulose (HPMC) and was obtained from Sigma-Aldrich Co. (Sigma-Aldrich, St. Louis, MO, USA). GSP (Naturin Natural Products Ltd., Izmir, Turkey) used in the experiments comprised of 11.33 ± 0.86 % moisture, 10.77 ± 0.25 % protein, and 13.66 ± 2.01 % oil, and had 1.51 ± 0.05 (mmol trolox/mg sample) antioxidant capacity. Chicken breast meat was stored at +4 °C and flour samples were stored at room temperature until their use in experiments.
Sample preparation and frying
Chicken breasts were supplied fresh and quickly prepared for the battering and pre-frying steps. Samples (5-cm long, 2-cm wide, and 1-cm thick) were cut manually and controlled to have a uniform weight. A tempura/puff batter style was used in the experiments. Chicken nuggets (about 15 g) were individually immersed into different batter formulas for 30 s and allowed to drip for 10 s. The basic formula contained equal amounts of corn and wheat flours. The solid: water ratio of the batter was 1:1.2 (w/v), and each type of batter contained 1 g salt, 1 g HPMC, and 0.5 g leavening agent per 100 g corn and wheat flour mixture. GSE has GRAS (Generally Recognized As Safe) status and a no-observed-adverse effect level in rats of 1.78 g/kg of body weight/day, which is a much higher concentration than normally used in food applications (Bentivegna and Whitney 2002). Basic flour mix was replaced with increasing GSP amounts of 1–10 % of the flour mix containing wheat flour, corn flour, leavening agent, salt, and HPMC to determine the effects of natural antioxidants. The control batter formulation did not include any grape seed or its products. All dry ingredients were thoroughly pre-blended and mixed with water using a hand mixer (Model K1433, Arcelik Inc., Turkey) at the lowest speed for 2 min. The chicken nuggets were pre-fried at 180 °C for 30 s in a conventional deep-fat fryer (Oleoclean; Tefal, Rumilly, France) using sunflower oil with a 2.1 L oil capacity. Pre-fried chicken nuggets were placed in plastic bags and stored at −18 °C in a freezer. Samples were obtained before freezing (at day zero) and at 1 month intervals during frozen storage for a period of 5 months to determine the changes in the primary and secondary oxidation product concentrations. For each sampling time, samples were thawed at 4 °C for 2.5 h before analysis.
Antioxidant activity measurements
The crusts of pre-fried chicken nuggets were de-fatted by hexane and freeze-dried to obtain ground powder after stored at −24 °C for 1 week. Total antioxidant capacity was determined by using the trolox equivalent antioxidant capacity (TEAC) assay according to Re et al. (1999). The percentage inhibition of absorbance at 734 nm was calculated and plotted as a function of concentration of antioxidants and of trolox for the standard reference data.
Lipid extraction
To extract lipids from samples, the Bligh and Dyer (1959) method was used with slight modifications. An aliquot of sample (30 g) was homogenized with 100 ml methanol and chloroform solvent mixture at a ratio of 1:2 (v/v) for 1 min. The mixture was filtered into a separating funnel and the procedure was repeated with the resulting solid using 100 ml of the same solvent solution. After filtering and retaining the liquid, 25 ml of 0.4 % CaCl2 solution was added to the separating funnel. The mixture was thoroughly mixed, deaerated, and allowed to stand for 24 h. The lower organic phase was collected and evaporated in a rotary vacuum evaporator (IKA RV 05-ST; IKA-Werke GmbH and Co., Staufen, KG, Germany) at 40 °C. The extracted lipid was used for the peroxide values (POVs), para-anisidine values (pAVs), and conjugated diene (CD) analyses.
Lipid oxidation measurement
Lipid oxidation during frozen storage was evaluated by measuring CDs, POVs for primary oxidation, and TBARS, pAVs for secondary oxidation. All analyses were performed in triplicate.
Conjugated diene concentrations (CD) were determined based on an increase in absorbance, measured at 232 nm (IUPAC 1987). The CD concentration of extracted lipids was calculated using a molar extinction coefficient of 25,200 M−1 cm−1 and expressed as μmol mg−1 meat lipid sample.
The peroxide values (POVs) of samples were determined using the IUPAC (1987) method and expressed as mill equivalents of active oxygen per kilogram (meqO2 kg−1), as given in the formula below:
| 1 |
where V is milliliters of thiosulfate solution (corrected to take into account the blank test); T is the normality of the sodium thiosulfate solution, and m is the mass, in grams, of the test portion.
The extent of lipid oxidation was determined by a TBARS assay using the distillation method of Tarladgis et al. (1960). TBARS value was calculated from a standard curve of malondialdehyde (MDA) prepared by acidification of 1, 1, 3, 3-tetraethoxypropane and the results were expressed as milligrams MDA per kilogram meat.
pAVs were determined by the IUPAC (1987) method. Extracted chicken nugget lipid was dissolved in isooctane and absorbance of the mixture was measured at 350 nm using a UV/Vis spectrophotometer (As). Para-anisidine reagent (1 ml) was added to 5 ml of the mixture and stored in the dark for 10 min before reading (Ab) at 350 nm. The results were calculated using the following formula:
| 2 |
where m represents the amount of chicken nuggets in grams. As is the absorbance of the fat solution after reaction with the para-anisidine reagent, and Ab is the absorbance of the fat solution.
Statistical analysis
Means were compared using a two-way analysis of variance using SPSS 16.0 for Windows (SPSS Inc. Chicago, IL, USA). Significant differences among treatment means were determined by Duncan’s multiple range test (p < 0.05).
Results and discussion
Antioxidant activities of pre-fried chicken nugget crust samples
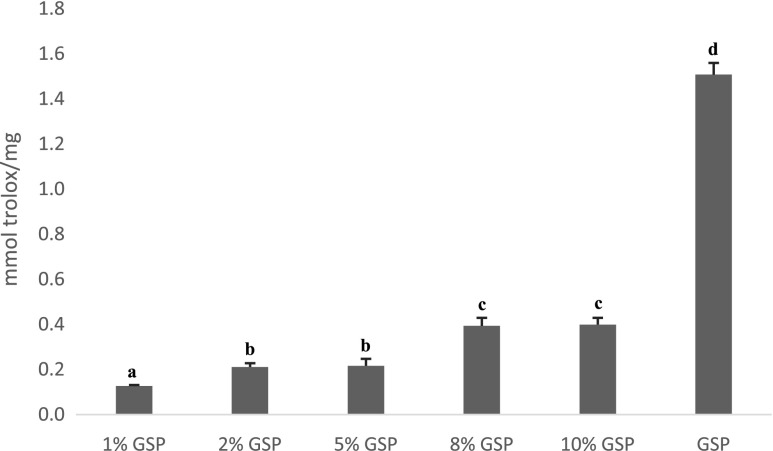
Antioxidant activities of battered samples after pre-frying step were shown in Fig. 1 Samples with higher GSP amounts had higher antioxidant activity values as expected. Grape seeds are a rich source of monomeric phenolic compounds (Saito et al. 1998), and radical scavenging activity of proanthocyanidins had been reported (Ariga et al. 1988; Ricardo da Silva et al. 1991). Kim et al. (2006) found that heat treatment liberated phenolic compounds in grape seed extracts except treatments at 200 °C. Contrary to Kim et al. (2006) it was found that antioxidant activities of samples were decreased but not eliminated after pre-frying. But in this study GSP were used directly in battering treatment instead using GSE to differentiate any effect. The difference may be due to the phenolic compounds in the grape seeds being bound to other components of the seeds while in the GSE, the phenolic compounds are free and susceptible to the thermal treatments. Study conducted by Kim et al. (2007) on GSE reported subjecting grape seed to 150 °C for 40 min increased its phenolic content by 50 %, but raising the temperature to 200 °C reduced its phenolic content and antioxidant activity. Simple heat treatment can convert insoluble phenolic compounds to soluble phenolics. This indicates that phenolic compounds of same sample may be resulted in different activities depend on the condition of heating and physical form of grape seed and poly phenols may behave differently in mixture due to interactions than when they occur individually.
Fig. 1.
Trolox equivalent antioxidant capacity (TEAC) values. a-d Means with different letters are significantly different (p<0.05)
Changes in the CD concentration during frozen storage of precooked chicken nuggets
The autoxidation products of oils and fats display characteristic spectra in the ultraviolet region: linolenic hydroperoxide and CD, which may result from lipid decomposition, show an absorption band at about 232 nm. The altered CD values in control and treated samples during frozen storage are presented in Table 1. CD analysis revealed that the treatments and storage period significantly affected the lipid oxidation of precooked samples (p < 0.05) with a significant interaction (p < 0.05). The highest CD values were observed in control samples (p < 0.05) during the entire storage period. For the same storage period, CD values decreased with an increased percentage of GSP. The highest values were recorded in all samples at 2 months of storage and no significant differences (p > 0.05) in CD concentrations were detected between any of the treatment groups and the control sample at month 2. The formation of CDs increases in parallel with the production of hydroperoxides, which are observed in the early stages of lipid oxidation and then transformed into secondary substances that reduce absorptivity (Frankel 2005). Generally, CD concentrations increased in all samples until month 2 and decreased thereafter (Table 1). These findings indicated that the decomposition rate of hydroperoxides was higher than CD formation rate. Conjugated hydroperoxides are expected to decompose to the secondary products. Therefore, the decrease in the CD concentration may be accompanied by an increase in TBARS in precooked chicken nuggets in the present study. Similar trends were observed in previous studies of cooked ground pork containing lotus and barley leaves (Choe et al. 2011), cooked chicken meat containing combinations of natural antioxidants (Sampaio et al. 2012), cooked pork patties containing holy basil and galangal (Juntachote et al. 2006), and cooked pork patties treated with whey and soy protein hydro lysates (Pena-Ramos and Xiong 2003).
Table 1.
Conjugated diene concentrations in precooked chicken nuggets untreated and treated with grape seed powder during frozen storage (μmol mg−1)
| Treatment | Storage period (month) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Control | 0.39 ± 0.01aA | 0.18 ± 0.01bA | 0.51 ± 0.04cA | 0.19 ± 0.01bA | 0.19 ± 0.01bA | 0.18 ± 0.01bA |
| 1 % GSP | 0.19 ± 0.02aB | 0.17 ± 0.01aB | 0.41 ± 0.03bB | 0.15 ± 0.01aB | 0.18 ± 0.05aA | 0.17 ± 0.01aA |
| 2 % GSP | 0.20 ± 0.04aB | 0.16 ± 0.01aBC | 0.40 ± 0.01bB | 0.17 ± 0.01aA | 0.17 ± 0.07aA | 0.08 ± 0.01cB |
| 5 % GSP | 0.13 ± 0.02aC | 0.15 ± 0.01aC | 0.39 ± 0.04bB | 0.14 ± 0.01aBC | 0.14 ± 0.04aA | 0.08 ± 0.01cB |
| 8 % GSP | 0.12 ± 0.01aCD | 0.13 ± 0.01aD | 0.38 ± 0.04bB | 0.13 ± 0.01aBC | 0.13 ± 0.01aA | 0.07 ± 0.01cB |
| 10 % GSP | 0.09 ± 0.01aD | 0.09 ± 0.01aE | 0.37 ± 0.01bB | 0.12 ± 0.02cC | 0.12 ± 0.01cA | 0.07 ± 0.02aB |
Data are means of three samples ± standard deviation
A-E Means within columns with different superscript letters are significantly different (p < 0.05)
a-c Means within rows with different superscript letters are significantly different (p < 0.05)
Changes in POVs during frozen storage of precooked chicken nuggets
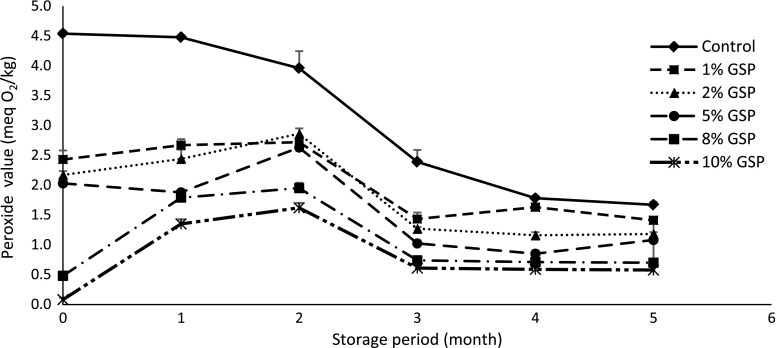
Hydroperoxides are the primary products of lipid oxidation (Sun et al. 2011). The POVs of the samples are shown in Fig. 2 and ranged from 0.5 to 4.5 meq of peroxides/kg sample during storage. There was a significant main effect for treatment and storage period on lipid oxidation with a significant interaction (p < 0.05). All samples treated with GSP had significantly (p < 0.05) lower POVs than the control during storage as an indication of retarding peroxidation, probably because antioxidant constituents of GSP terminated free radical chain reaction. The POVs generally decreased with an increase in the amount of GSP.
Fig. 2.
Peroxide values in precooked chicken nuggets containing grape seed powder and control
The POVs of the control sample and those treated with GSP increased in the early stages of storage and then decreased during later stages of storage (Fig. 2). The rate of hydroperoxide formation may have been lower than that of hydroperoxide decomposition, resulting in a decline in POVs after about a month.
The increase in POV during storage may be due to the catalysis of intracellular compounds after the precooking step. This might be because heat reduces the volatile oil content, which has antioxidant activity, or because phenolic compounds react with different substances. Furthermore, the presence of salt in the batter preparation might also affect the progression of peroxidation by destroying the cell structure (Shahidi 2005). Heat damage to the cell membrane is partially responsible for the release or activation of transition metal ions. These changes markedly decrease the oxidative stability of muscle foods (Ma et al. 2007). The increased POVs of the samples indicated that the samples were in the propagation stage of lipid oxidation and that the formed hydroperoxide decomposed at a lower rate.
The values obtained in this study are consistent with those obtained by Conchillo et al. (2005), who observed that POVs reached 38 to 40 meqO2 kg−1 in frozen samples stored for 3 months. On the other hand, Abd El-Alim et al. (1999) reported that the POVs reached 0.55 meqO2 kg−1 at 1 month, increased to 11.56 meqO2 kg−1 at 3 months, and decreased after 6 months in pork patties treated with NaCl during frozen storage. The acceptable limit for human consumption is 10 meq/kg with reference to the European Pharmacopoeia and Norwegian Medicinal Standard. Thus, POVs for all treatments were in the acceptable range at the end of storage.
Changes in TBARS values during storage of precooked chicken nuggets
TBARS values represent the content of secondary lipid oxidation products, such as aldehydes, carbonyls, and hydrocarbons, which are considered to be responsible for the off-flavors in meat (St. Angelo 1996). Analysis of variance indicated a significant effect of GSP inclusion and storage period on TBARS values (p < 0.05). Also, there was a non-significant interaction between GSP inclusion level and storage period on TBARS values. The effect of GSP on the TBARS values of the precooked chicken nuggets during frozen storage is shown in Table 2. TBARS values of all treated samples were significantly lower (p < 0.05) than those of the control on day zero. This is an indication that GSP inclusion retarded oxidation during the precooking stage. The samples including 10 % GSP had TBARS levels lower than 40 % when compared to the control during 5 months frozen storage. These results were in agreement with Rababah et al. (2006) and (Shirahigue et al. 2010), who found a reduction in TBARS in chicken meat with GSE during refrigerated storage. Recently many storage experiments were carried out to prove the antioxidative effects of plant materials and some of them investigated a considerable reduction in TBARS during the first part of the storage period. But generally at the end of the storage TBARS values had increased to values found in control samples (Wong et al. 1995; Resurrecceccion and Reynolds 1990). Kulkarni et al. (2011) reported similar results with precooked, frozen, reheated beef sausage treated with GSE. Similarly, Selani et al. (2011) demonstrated that GSE and peel extracts were effective in retarding lipid oxidation of raw and cooked chicken meat during frozen storage. According to Al-Kahtani et al. (1996), meat products containing less than 3 mg MDA/kg sample, are well preserved concerning to oxidative changes. Therefore, the GSP concentrations used in this experiment were sufficient for maintaining oxidative stability.
Table 2.
Thiobarbituric acid reactive substances values in precooked chicken nuggets untreated and treated with grape seed powder during frozen storage (mg MDA kg−1)
| Treatment | Storage period (month) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Control | 0.27 ± 0.07aA | 0.27 ± 0.04aA | 0.36 ± 0.01bA | 0.25 ± 0.05aA | 0.17 ± 0.01cA | 0.28 ± 0.02aA |
| 1 % GSP | 0.25 ± 0.11aAB | 0.25 ± 0.09aA | 0.37 ± 0.03bA | 0.23 ± 0.12aA | 0.18 ± 0.03cA | 0.27 ± 0.01aA |
| 2 % GSP | 0.24 ± 0.01abAB | 0.25 ± 0.01abA | 0.31 ± 0.02cB | 0.23 ± 0.01bA | 0.17 ± 0.02dA | 0.27 ± 0.01eA |
| 5 % GSP | 0.22 ± 0.01abAB | 0.24 ± 0.05acAB | 0.30 ± 0.06cB | 0.21 ± 0.03abA | 0.16 ± 0.01bA | 0.25 ± 0.03acAB |
| 8 % GSP | 0.20 ± 0.01aAB | 0.22 ± 0.03aAB | 0.30 ± 0.04bB | 0.19 ± 0.04aA | 0.10 ± 0.01cB | 0.22 ± 0.03aB |
| 10 % GSP | 0.15 ± 0.01abB | 0.16 ± 0.01abB | 0.18 ± 0.02bC | 0.15 ± 0.06abA | 0.10 ± 0.05aB | 0.16 ± 0.02abC |
Data are means of three samples ± standard deviation
A-C Means within columns with different superscript letters are significantly different (p < 0.05)
a-e Means within rows with different superscript letters are significantly different (p < 0.05)
The TBARS levels continued to increase in all samples up to month 2 however, after reaching maximum values, a decrease was observed subsequently (Table 2). The lowest TBARS values were in the sample containing 10 % GSP, indicating that GSP effectively retards lipid oxidation during frozen storage and can be used as an alternative to commercial antioxidants. Similarly, Brannan (2008) reported an increase in the TBARS values of chicken meat during refrigerated storage. The ability of grape seed to inhibit lipid oxidation is likely related to its rich polyphenol content, especially proanthocyanidin. It might be due to the fact that proanthocyanidin has both antioxidant activity and abilities to sequester radicals, chelate metals, and synergize with other antioxidats (Lu and Foo 1999). The formation of MDA as a secondary product of lipid oxidation resulted in an increase in TBARS values of stored chicken nuggets from the early through the mid stages of storage, but the TBARS values declined toward the late stage, due mainly to the reaction of MDA with protein in the precooked chicken nuggets (Tarladgis et al. 1960; Giron-Calle et al. 2003). Under some conditions, myosin reacts with malonaldehyde which is an oxidation product of polyunsaturated. This protein malonaldehyde reaction was thought as a potential mechanism of protein denaturation in muscle, especially during frozen storage. The reaction of amino groups of myosin is much faster at frozen storage than refrigerated storage because of concentration and catalytic effect involving the ice structure (Buttkus 1967). Therefore decreased levels of MDA concentration toward the late stage of storage may be seen.
Changes in pAV during storage of precooked chicken nuggets
The pAVs which indicate aldehyde content as a secondary oxidation product are given in Table 3. There was a significant main effect (p < 0.05) of GSP inclusion and the storage period on pAVs in precooked chicken nuggets, but there was no significant interaction between the storage period and GSP %. pAVs in the control increased from 5.99 ± 0.13 to 21.21 ± 0.05 μmol/μg during the 5-month frozen storage period, whereas pAVs increased from 4.69 ± 0.21 to 17.10 ± 0.13 0.03 μmol/μg with the addition of GSP at 10 %. The pAVs of the treated samples were lower than that of the control and they were increased steadily in all samples during storage, indicating the accumulation of aldehydes in chicken nuggets during storage. These findings indicated that the primary lipid oxidation products decomposed into secondary oxidation products. A sharp increase in pAV was observed in all samples after a month. The highest pAV levels were detected in control samples at the end of the storage period (p < 0.05). Similar results were reported by Yerlikaya and Gokoglu (2010) that GSE added samples showed a smaller increase in pAVs during 5 months of frozen storage compared to high increase in control bonito fillets.
Table 3.
Para-anisidine value of precooked chicken nuggets untreated and treated with grape seed powder during frozen storage
| Treatment | Storage period (month) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Control | 5.99 ± 0.13aA | 7.48 ± 1.82aA | 17.63 ± 0.12bA | 17.07 ± 0.11bA | 19.16 ± 0.01bcA | 21.20 ± 0.05cA |
| 1 % GSP | 5.79 ± 1.32aA | 6.72 ± 0.07aAB | 14.15 ± 0.10bB | 13.30 ± 0.06bB | 19.96 ± 0.02cB | 20.62 ± 2.89cA |
| 2 % GSP | 5.34 ± 0.25aAB | 7.00 ± 0.35aAB | 14.09 ± 2.75bB | 15.13 ± 0.04bACB | 17.98 ± 0.03cC | 19.54 ± 0.11cAB |
| 5 % GSP | 5.27 ± 0.06aAB | 6.96 ± 0.06aAB | 14.07 ± 0.26bcB | 14.32 ± 0.14cCB | 17.05 ± 0.02dD | 19.34 ± 0.07eAB |
| 8 % GSP | 5.15 ± 0.07aAB | 5.79 ± 0.22aB | 13.92 ± 0.05bB | 15.85 ± 0.04cAC | 16.84 ± 0.06cE | 17.50 ± 0.02cBC |
| 10 % GSP | 4.69 ± 0.21aB | 5.77 ± 0.01aB | 12.45 ± 2.73bB | 15.30 ± 3.36cACB | 16.32 ± 0.03cF | 17.10 ± 0.03cC |
Data are means of three samples ± standard deviation
A-F Means within columns with different superscript letters are significantly different (p < 0.05)
a–e Means within rows with different letters are significantly different (p < 0.05)
Aldehydes, ketones, and other oxidation products are produced at the end of the chain reaction occurred in the progression of lipid peroxidation. However, one of the most important preservation techniques for processed or unprocessed meat products is frozen storage, this method did not suppressed completely but did minimize many detrimental changes. Results indicated that the oxidation products were produced in all samples but it was more rapid in control compare to samples including GSP. So, the combination of frozen storage with using a natural antioxidant did prevent lipid oxidation in some degree.
Conclusions
Antioxidant capacity in chicken nugget crusts prepared with GSP was persistent even after pre-frying step. GSP significantly reduced lipid oxidation of chicken nuggets during frozen storage in a concentration-dependent manner, similar to GSE. Based on the TBARS values, POVs, pAVs, and CD concentration, GSP provided oxidative stability to the product throughout the 5-months storage period. Increasing GSP levels resulted in decreased TBARS values, POVs, pAVs, and CD concentration as compared to control. Although all batters including GSP did retard lipid oxidation, samples with 10 % GSP showed the lowest oxidation values. Generally, oxidation stability values of batters including 10 % and 8 % GSP were not significantly different during frozen storage. Thus, considering oxidation stability and measured antioxidant capacity, batter formulation can be replaced with 8 % GSP. However, further studies are needed to determine the quality characteristics such as color, texture, pick up, oil and moisture content and the acceptability of product for consumer.
Acknowledgments
Financial support provided by The Scientific and Technological Research Council of Turkey, TUBITAK (Project No. 110 O 512) and the Ege University Scientific Research Fund, EBILTEM (Project No. 2011 BIL 017) was greatly appreciated.
Contributor Information
Ece Cagdas, Email: ececagdas@sdu.edu.tr.
Seher Kumcuoglu, Phone: +90-232-3113023, FAX: +90-232-3427592, Email: seher.kumcuoglu@ege.edu.tr.
References
- Abd El-Alim SSL, Lugasi A, Hovari J, Dworschak E. Culinary herbs inhibit lipid oxidation in raw and cooked minced meat patties during storage. J Sci Food Agric. 1999;79:277–285. doi: 10.1002/(SICI)1097-0010(199902)79:2<277::AID-JSFA181>3.0.CO;2-S. [DOI] [Google Scholar]
- Ahn J, Grun IU, Fernando LN. Antioxidant properties of natural plant extracts containing polyphenolic compounds in cooked ground beef. J Food Sci. 2002;6:1364–1369. doi: 10.1111/j.1365-2621.2002.tb10290.x. [DOI] [Google Scholar]
- Al-Kahtani HA, Abu-Tarboush HM, Bajaber AS, Atia M, Abou-Arab AA, El-Mojaddidi MA. Chemical changes after irradiation and post-irradiation in tilapia and Spanish mackerel. J Food Sci. 1996;61:729–733. doi: 10.1111/j.1365-2621.1996.tb12191.x. [DOI] [PubMed] [Google Scholar]
- Ariga T, Koshiyama I, Fukushima D. Antioxidative properties of procyanidins B-1 and B-3 from azuki beans in aqueous systems. Agric Biol Chem. 1988;52:2717–2722. doi: 10.1271/bbb1961.52.2717. [DOI] [Google Scholar]
- Banon S, Diaz P, Rodriguez M, Garrido MD, Price A. Ascorbate, green tea and grape seed extracts increase the shelf life of low sulphite beef patties. Meat Sci. 2007;77:626–633. doi: 10.1016/j.meatsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Bentivegna SS, Whitney KM. Subchronic 3-month oral toxicity study of grape seed and grape skin extracts. Food Chem Toxicol. 2002;40:1731–1743. doi: 10.1016/S0278-6915(02)00155-2. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. J Biochem Phys. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brannan RG. Effect of grape seed extract on physicochemical properties of ground, salted, chicken thigh meat during refrigerated storage at different relative humidity levels. J Food Sci. 2008;73:36–40. doi: 10.1111/j.1750-3841.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- Brannan RG. Effect of grape seed extract on descriptive sensory analysis of ground chicken during refrigerated storage. Meat Sci. 2009;81:589–595. doi: 10.1016/j.meatsci.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Buttkus H. The reaction of myosin with malonaldehyde. J Food Sci. 1967;32(4):432–434. doi: 10.1111/j.1365-2621.1967.tb09703.x. [DOI] [Google Scholar]
- Carpenter R, O’Grady MN, O’Callaghan YC, O’Brien NM, Kerry JP. Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 2007;76:604–610. doi: 10.1016/j.meatsci.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Chedea VS, Echim C, Braicu C, Andjelkovic M, Verhe R, Socaciu C. Composition in polyphenols and stability of the aqueous grape seed extract from the Romanian variety “merlot recas”. J Food Biochem. 2011;35:92–108. doi: 10.1111/j.1745-4514.2010.00368.x. [DOI] [Google Scholar]
- Chen TC, Waimaleongora EK. Effect of pH on TBA values of ground raw poultry meat. J Food Sci. 1999;46:1946–1947. doi: 10.1111/j.1365-2621.1981.tb04525.x. [DOI] [Google Scholar]
- Choe JH, Jang A, Lee ES, Choi JH, Choi YS, Han DJ, Kim HY, Lee MA, Shim SY, Kim CJ. Oxidative and color stability of cooked ground pork containing lotus leaf (Nelumbo nucifera) and barley leaf (Hordeum vulgare) powder during refrigerated storage. Meat Sci. 2011;87:12–18. doi: 10.1016/j.meatsci.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Conchillo A, Ansorena D, Astiasaran I. Intensity of lipid oxidation and formation of cholesterol oxidation products during frozen storage of raw and cooked chicken. J Sci Food Agric. 2005;85:141–146. doi: 10.1002/jsfa.1969. [DOI] [Google Scholar]
- Fernandez-Lopez J, Sevilla L, Sayas-Barbera ME, Navarro Marin F, Perez-Alvarez JA. Evaluation of the antioxidant potential of hyssop (Hyssopus officinalis L.) and rosemary (Rosmarinus officinalis L.) extract in cooked pork meat. J Food Sci. 2003;68:660–664. doi: 10.1111/j.1365-2621.2003.tb05727.x. [DOI] [Google Scholar]
- Frankel EN. Lipid oxidation. Scotland: The Oily Press Dundee; 2005. [Google Scholar]
- Giron-Calle J, Alaiz M, Millan F, Ruiz-Gutierrez V, Vioque E. Bound malondialdehyde in foods: Bioavailability of N, N”-di-(4-methyl-1,4-dihydropyridine-3,5-dicarbaldehyde) lysine. J Agric Food Chem. 2003;51:4799–4803. doi: 10.1021/jf0343027. [DOI] [PubMed] [Google Scholar]
- Han J, Rhee KS. Antioxidant properties of selected oriental non culinary/nutraceutical herb extracts as evaluated in raw and cooked meat. Meat Sci. 2005;36:169–189. doi: 10.1016/j.meatsci.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Han D, Mcmillin KW, Godber JS, Bidner TD, Younathn MT, Hart LT. Lipid stability of beef model systems with heating and iron fraction. J Food Sci. 1995;60:599–603. doi: 10.1111/j.1365-2621.1995.tb09836.x. [DOI] [Google Scholar]
- IUPAC . Standard methods for the analysis of oils, fats and derivates. Oxford: Blackwell Scientific Publications; 1987. International Union of Pure and Applied Chemistry Method Number 2.501, 2.504, 2.505; pp. 138–139. [Google Scholar]
- Jittrepotch N, Ushio H, Ohshima T. Effects of EDTA and a combined use of nitrite and ascorbate on lipid oxidation in cooked Japanese sardine (Sardinops melanostictus) during refrigerated storage. Food Chem. 2006;99:70–82. doi: 10.1016/j.foodchem.2005.07.021. [DOI] [Google Scholar]
- Juntachote T, Berghofer E, Siebenhandl S, Bauer F. The anti oxidative properties of Holy basil and Galangal in cooked ground pork. Meat Sci. 2006;72:446–456. doi: 10.1016/j.meatsci.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Kim SY, Jeong SM, Park WP, Nam KC, Ahn DU, Lee SC. Effect of heating conditions of grape seeds on the antioxidant activity of grape seed extracts. Food Chem. 2006;97:472–479. doi: 10.1016/j.foodchem.2005.05.027. [DOI] [Google Scholar]
- Kim ES, Liang YR, Jin J, Sun QF, Lu JL, Du YY, Lin C. Impact of heating on chemical composition of green tea liquor. Food Chem. 2007;103:1263–1267. doi: 10.1016/j.foodchem.2006.10.031. [DOI] [Google Scholar]
- Kulkarni S, De Santos FA, Kattamuri S, Rossi SJ, Brewer MS. Effect of grape seed extract on oxidative, color and sensory stability of a pre-cooked, frozen, re-heated beef sausage model system. Meat Sci. 2011;88:139–144. doi: 10.1016/j.meatsci.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Lau DW, King AJ. Pre- and post-mortem use of grape seed extract in dark poultry meat to inhibit development of thiobarbituric acid reactive substances. J Agric Food Chem. 2003;51:1602–1607. doi: 10.1021/jf020740m. [DOI] [PubMed] [Google Scholar]
- Lu Y, Foo LY. The polyphenol constituents of grape pomace. Food Chem. 1999;65:1–8. doi: 10.1016/S0308-8146(98)00245-3. [DOI] [Google Scholar]
- Ma HJ, Ledward DA, Zamri AI, Frazier RA, Zhou GH. Effects of high pressure/thermal treatment on lipid oxidation in beef and chicken muscle. Food Chem. 2007;104:1575–1579. doi: 10.1016/j.foodchem.2007.03.006. [DOI] [Google Scholar]
- Mielnik MB, Olsen E, Vogt G, Adeline D, Skrede G. Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT Food Sci Technol. 2006;39:191–198. doi: 10.1016/j.lwt.2005.02.003. [DOI] [Google Scholar]
- Nissen L, Byrne D, Bertelsen G, Skibsted L. The anti oxidative activity of plant extracts in cooked pork patties as evaluated by descriptive sensory profiling and chemical analysis. Meat Sci. 2004;68:485–95. doi: 10.1016/j.meatsci.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Pazos M, Gallardo JM, Torres JL, Medina I. Activity of grape polyphenols as inhibitors of the oxidation of fish lipids and frozen fish muscle. Food Chem. 2005;92:547–557. doi: 10.1016/j.foodchem.2004.07.036. [DOI] [Google Scholar]
- Pena-Ramos EA, Xiong YLL. Whey and soy protein hydro lysates inhibit lipid oxidation in cooked pork patties. Meat Sci. 2003;64:259–263. doi: 10.1016/S0309-1740(02)00187-0. [DOI] [PubMed] [Google Scholar]
- Perumalla AVS, Hettiarachchyc NS. Green tea and grape seed extracts-potential applications in food safety and quality. Food Res Int. 2011;44:827–839. doi: 10.1016/j.foodres.2011.01.022. [DOI] [Google Scholar]
- Prasetyo M, Chia M, Hughey C, Were LM. Utilization of electron beam irradiated almond skin powder as a natural antioxidant in ground top round beef. J Food Sci. 2008;73:T1–T6. doi: 10.1111/j.1750-3841.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- Rababah T, Hettiarachchy NS, Horax R, Cho MJ, Davis B, Dickson J. Thiobarbituric acid reactive substances and volatile compounds in chicken breast meat infused with plant extracts and subjected to electron beam irradiation. Poult Sci. 2006;8:1107–1113. doi: 10.1093/ps/85.6.1107. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9/10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Resurrecceccion AVA, Reynolds AE. Evaluation of natural antioxidants in frankurters containing chicken and pork. J Food Sci. 1990;55:629–631. doi: 10.1111/j.1365-2621.1990.tb05193.x. [DOI] [Google Scholar]
- Ricardo da Silva JM, Darmon N, Fenandez Y, Mitjavila S. Oxygen free radical scavenger capacity in aqueous models of deferent procyanidins from grape seeds. J Agric Food Chem. 1991;39:1549–1552. doi: 10.1021/jf00009a002. [DOI] [Google Scholar]
- Rojas MC, Brewer MS. Effect of natural antioxidants on oxidative stability of cooked, refrigerated beef and pork. J Food Sci. 2007;72:S282–S288. doi: 10.1111/j.1750-3841.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Saito M, Hosoyama H, Ariga T, Kataoka S, Yamaji N. Antiulcer activity of grape seed extract and procyanidins. J Agric Food Chem. 1998;46:1460–1464. doi: 10.1021/jf9709156. [DOI] [Google Scholar]
- Sampaio GR, Saldanha T, Soares RAM, Torres EAFS. Effect of natural antioxidant combinations on lipid oxidation in cooked chicken meat during refrigerated storage. Food Chem. 2012;135:1383–1390. doi: 10.1016/j.foodchem.2012.05.103. [DOI] [PubMed] [Google Scholar]
- Selani MM, Contreras-Castillo CJ, Shirahigue LD, Gallo CR, Plata-Oviedo M, Montes-Villanueva ND. Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Sci. 2011;88:397–403. doi: 10.1016/j.meatsci.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Shahidi F. Lipid derived flavors in meat products. In: Kerry J, Kerry J, Ledward D, editors. Meat processing, improving quality. North America: CRC Press; 2005. pp. 105–117. [Google Scholar]
- Shirahigue LD, Plata-Oviedo M, Alencar SM, D'arce MABR, Vieira TMFD, Oldoni TLC, Contreras-Castillo CJ. Wine industry residue as antioxidant in cooked chicken meat. Int J Food Sci Technol. 2010;45:863–870. [Google Scholar]
- St. Angelo AJ. Lipid oxidation in foods. Crit Rev Food Sci Nutr. 1996;36:175–224. doi: 10.1080/10408399609527723. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang WD, Chen HW, Li C. Autoxidation of unsaturated lipids in food emulsion. Crit Rev Food Sci Nutr. 2011;51:453–466. doi: 10.1080/10408391003672086. [DOI] [PubMed] [Google Scholar]
- Tarladgis BG, Watts BM, Younathan MT, Dugan L. A distillation method for the quantitative determination of malondialdehyde in rancid foods. J Am Oil Chem Soc. 1960;37:44–48. doi: 10.1007/BF02630824. [DOI] [Google Scholar]
- Wong JW, Hashimoto K, nad Shibamoto T. Antioxidant activities of rosemary and sage extracts and vitamin E in a model meat system. J Agric Food Chem. 1995;43:2707–2712. doi: 10.1021/jf00058a029. [DOI] [Google Scholar]
- Yerlikaya P, Gokoglu N. Inhibition effects of green tea and grape seed extracts on lipid oxidation in bonito fillets during frozen storage. Int J Food Sci Technol. 2010;45:252–257. doi: 10.1111/j.1365-2621.2009.02128.x. [DOI] [Google Scholar]