Abstract
Kiwi fruits (Actinidia deliciosa C.F. Liang et A.R. Ferguson) were treated by prestorage conditioning (20 °C for 2 days), 1-methylcyclopropene (1-MCP, 1 ppm for 16 h) and conditioning plus 1-MCP. After the treatment the fruits were immediately stored at 0 °C during 24 weeks. Flesh firmness gradually decreased with storage time and the rate of its loss was lower in 1-MCP and conditioning plus 1-MCP treatments than those of control or conditioning. However, SSC, acidity and pH did not change among treatments. Starch content decreased during the storage time regardless of treatments. Oppositely the amount of reducing sugars increased at the same duration of the treatments. Rate and incidence of fruit decay was the lowest in fruit treated with conditioning plus 1-MCP treatment. Fruit decay mainly caused pathogen Botrytis cinerea and its rate significantly decreased with conditioning plus 1-MCP treatment. Ethylene and respiration abruptly increased after 8 weeks of storage, but their contents were lower in 1-MCP and conditioning plus 1-MCP. Total soluble phenolics, flavonoids, and total antioxidant capacities were much higher than in other treatments. Kiwi fruits treated with conditioning plus 1-MCP extended the shelf life by reducing the rate of fruit decay and softening during the storage. The bioactive compounds and total antioxidant status of fruits increased during the treatment.
Keywords: Firmness, Starch, Fruit decay, Ethylene, Antioxidants
Introduction
Kiwi fruit is one of the main crops in south-east region of Korea. In the recent years kiwi fruit became an important commercial crop and its growing area has spread in United States, Italy, Spain and Japan (Park et al. 2006). Kiwi fruit consumption in Korea is about 35,000 t per year, where 70 % of the consumed fruits are imported from New Zealand, Chile and USA. In order to reduce the amount of kiwi fruit import, it is necessary to extend consumption duration due to long term storage. Shelf life of kiwi fruit is mainly influenced by the rate of softening and fruit decay during cold and controlled atmosphere storage (Park and Kim 1995; Park 1996; Park (2002); Park 2003). Kiwi fruits are highly sensitive to ethylene. At ethylene concentration of 0.03 ppm was induced the premature ripening and flesh softening.
Before the advent of the 1-methylcyclopropene (1-MCP) the long-term cold storage was limited (Agar et al. 2000). 1-MCP is a synthetic cyclic olefin that blocks access of ethylene to its receptor and therefore, prevents major undesirable effects on ethylene sensitive plant tissues (Sisler and Serek 1997). The rate of ripening and premature softening of a wide range of fruits, vegetables and floriculture crops can be controlled with 1-MCP postharvest treatment Athanasios and Evangelos (2007). The use of such treatment depends on the physiological characteristics of each commodity (Blankenship and Dole 2003; Boquete et al. 2004). The effect of ethylene and 1-methylcyclopropene on the quality of different fruits and vegetables was studied recently itself and also in combination (Kramer et al. 2012; Sivakumar et al. 2012; Zhang et al. 2013). Boquete et al. (2004) and Kim et al. (2001) determined that application of 1-MCP in kiwi fruits reduces ethylene production and softening during cold storage and subsequently exposed to 20 °C. Low concentrations of 1-MCP from 2.5 ppm to 1 ppm in most commodity are the most effective, but this depends also on temperature treatment. The most commonly applied is 20–25°C, but lower temperatures can be used in some commodities (Mir et al. 2001).
Generally, optimal treatment durations of 12–24 h in fruits were sufficient to achieve a full response (Ku and Wills 1999b). It was shown that 1-MCP effectiveness in fruits and vegetables were influenced by the cultivar, developmental stage, time from harvest to treatment and its concentration (Wills and Ku 2002).
Botrytis and Botryosphaeria rot occur in most areas of the world where kiwi fruits are grown and the most important decay causing fungi found on stored kiwi fruits in Korea (Park 2003; Park and Jung 2005). Also, fruit softening was induced by high evolution of ethylene resulting from either Botrytis cinerea or Botryosphaeria dothidea infection (Bautista-Banos et al. 1997). Although some chemicals are effective in retarding and preventing fruits rots caused by B. cinerea and B. dothidea, but the use of pre- and post-treatment chemicals is becoming limited due to the consumer concerns on the safety of foods.
Prestorage conditioning has been used for many years to control fungal disease. Fawcett and Barger (1927) showed that a prestorage conditioning of Citrus fruits to 30°C for 1 day suppressed both softening and decay; mostly due to penicillum spp. Similar beneficial effects on decay control were obtained in apple (Sam et al. 1993), orange (Schirra et al. 1997), and ‘yuzu’ (Citrus ichangensis x C. reticulata). It is believed to be a hybrid of sour mandarin and Ichang papeda (Park and Jung 1996). Pennycook and Manning (1992) and Bautista-Banos et al. (1997) reported that curing kiwi fruits for 2 or 3 days at 10 oC and 20 °C reduced decay caused by B. cinerea and maintained fruit firmness during 6 months of storage at 0 °C. A hot water dip at 46 °C for 15 min was also effective on reducing the decay of kiwi fruit (Cheah et al. 1992). However, in order to suppress Botrytis rot elevated CO2 and fumigation with SO2 were used to control the disease in kiwi fruits (Park and Jung 2005; Cheah et al. 1992). The changes in the bioactive compounds and antioxidant capacities are important for different fruit nutrition, especially in our case for kiwi fruit (Gorinstein et al. 2002; Kundan et al. 2011). In our previous reports the changes in the bioactivity were shown after growing and treatment of kiwi fruits (Park et al. 2006; Park et al. 2012a; Park et al. 2012b).
The objective of this study was to evaluate the effects of prestorage conditioning, 1-MCP and conditioning plus 1-MCP treatment on the firmness, sugar content, fruit decay, respiration and ethylene production, and activity of kiwi fruits during storage.
Materials and methods
Plant material
‘Hayward’ kiwi fruits were harvested at a commercial orchard near Haenam county, Jeonnam province, and transported to postharvest laboratory at the Mokpo National University. The harvest date was October 24, 2010. Three lots of 140 healthy fruits of 100–120 g were constituted, discarding all fruits with defects of damaged. The initial SSC and firmness were 7.8 % and 31.5 N, respectively.
In pretreatment, the sorted fruits were conditioned at 20 °C for 2 days with RH 85 % in a growth chamber (Percival Scientific, Inc., Perry, IA, USA).
1-MCP treatment
Fruits were put into the 20 L glass jars and were treated with 1.0 ppm 1-MCP (active Ingredient 3.3 % SmartFresh™) for 16 h at 20 °C. 1-MCP was released by adding distilled water to SmartFresh™ powder according to the manufacturer instructions. A part of the plant material was not subjected to pretreatment and hence used as control. After MCP treatment, fruits were wrapped in 60 μm PE film bags and then stored in cold room in darkness (0 °C, 90 % RH) for 24 weeks.
Physico-chemical properties
Every 4 weeks of storage, kiwi fruits were sampled in order to evaluate firmness, soluble solids content (SSC), titratable acidity, pH, starch content, reducing sugar, L-ascorbic acid, fruit decay, CO2 and ethylene production, and soluble polyphenols, flavonoids and antioxidant activities.
Ten fruits from each replicate were analyzed for firmness by measuring penetration force in kilogram using a fruit firmness tester (Model KM, Fruit Test Tech, Japan). Fruit firmness was measured in a skinned area of the equatorial region of the fruit by means of a 5 mm diameter spherical probe. The mean values of the firmness were expressed as newtons (N). After that, ten peeled fruits per replication were homogenized and filtered through a cheesecloth to obtain a clear juice for determination of SSC, pH and acidity. The SSC was measured using a Refractometer (Atago Com. Ltd., Tokyo, Japan), pH with a pH meter (Model PB-11, Sartorius, Japan). The acidity was measured by a 4 mL juice diluted with 20 mL of distilled water and titrated with 0.1 N NaOH solution using phenolphthalein as an indicator.
Starch was determined in 10 g of flesh kiwi fruit extracted with 20 mL of 90 % ethanol and filtered through Whatman No. 4 filter paper (110 mm pore size). The extract solution was evaporated by means of a rotary evaporator and then 5 mL of 90 % ethanol were added. Starch concentration was obtained from values of absorbance (calibration curve), measured at 525 nm on a spectrophotometer (Hewlett-Packard, model 8452A, USA). Reducing sugar was extracted from juice and analyzed with the modified method of 3.5- dinitrosalicylic acid (Park and Park 1997).
Fruits were counted as decayed when decay spot exceeded 5 mm on the fruit skin or flesh. Also, decay fruits were sorted with the rot symptom by visual appearance.
The fruit decay (FD) was defined by equation:
in which N is fruit number. The degree of FD was calculated as:
where N is a fruit number, A = 1–5 %, B = 6–25, C = 26–50, and D = 51–100 % of decayed area, respectively. In degree of fruit decay there are four classes: slight (1–5), small (6–25 %), moderate (26–50 %), and severe (51–100 %).
Carbon dioxide and ethylene production
For measurement of carbon dioxide and ethylene production, five fruits were sealed in a 1.8 L glass jar for 24 h and head space gas was sampled with a 1 mL syringe. Ethylene and respiration contents were determined using gas chromatography (Hewlett Packard, USA) according to modified method of Park and Kim (2002).
Total soluble polyphenols and flavonoids
Total soluble polyphenols were extracted with ethanol at room temperature during 1 h at concentration 25 mg/ml. The polyphenols were determined by Folin-Ciocalteu method with absorbance measurement at 750 nm with spectrophotometer (Hewlett-Packard, model 8452A, Rockvile, USA). The results were expressed (Singleton et al. 1999) as mg of gallic acid equivalents (GAE) per g dry weight (DW). Total flavonoid content was determined by an aluminum chloride colorimetric method with some modifications. The absorbance was measured immediately against the blank at 510 nm. The results are expressed as milligrams of catechin equivalents.
Determination of antioxidant activity (AA)
The AA was determined by three complementary assays:
2, 2-Azino-bis (3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt (ABTS•+) method for the screening of antioxidant activity is reported as a decolorization assay applicable to both lipophilic and hydrophilic antioxidants, including flavonoids, hydroxycinnamates, carotenoids, and plasma antioxidants. The pre-formed radical monocation ABTS is generated by oxidation of ABTS with potassium persulfate and is reduced in the presence of such hydrogen-donating antioxidants. The influences of both the concentration of antioxidant and duration of reaction on the inhibition of the radical cation absorption are taken into account when determining the antioxidant activity. ABTS•+ radical cation was generated by the interaction of ABTS (7 mmol/L) and K2S2O8 (2.45 mmol/L). This solution was diluted with water until the absorbance in the samples reached 0.7 at 734 nm.
Ferric-reducing/antioxidant power (FRAP) assay measures the ability of the antioxidants in the investigated samples to reduce ferric-tripiridyltriazine (Fe3+-TPTZ) to a ferrous form (Fe2+). FRAP reagent (2.5 mL of a 10 mmol ferric-tripiridyltriazine solution in 40 mmol HCl plus 2.5 mL of 20 mmol FeCl3xH2O and 25 mL of 0.3 mol/L acetate buffer, pH 3.6) of 900 μL was mixed with 90 μL of distilled water and 30 μL of kiwi fruit extract samples as the appropriate reagent blank. The absorbance was measured at 595 nm.
Cupric reducing antioxidant capacity (CUPRAC): This assay is based on utilizing the copper (II)-neocuproine [Cu (II)-Nc] reagent as the chromogenic oxidizing agent. To the mixture of 1 mL of copper (II)-neocuproine and NH4Ac buffer solution, acidified and non acidified water extracts (or standard) solution (x, in mL) and distilled water [(1.1-x) mL] were added to make the final volume of 4.1 mL. The absorbance at 450 nm was recorded against a reagent blank. (Apak et al. 2004; Preshita et al. 2013; Ozgen et al. 2006).
Statistical analyses
To verify the statistical significance, mean ± SD of ten independent measurements were calculated. Differences between groups were tested by one way ANOVA. In the assessment of the antioxidant activity and polyphenols correlation coefficients (R2) were calculated. Linear regressions were also calculated. P-values of <0.05 were considered significant.
Results and discussion
Firmness and ethylene production
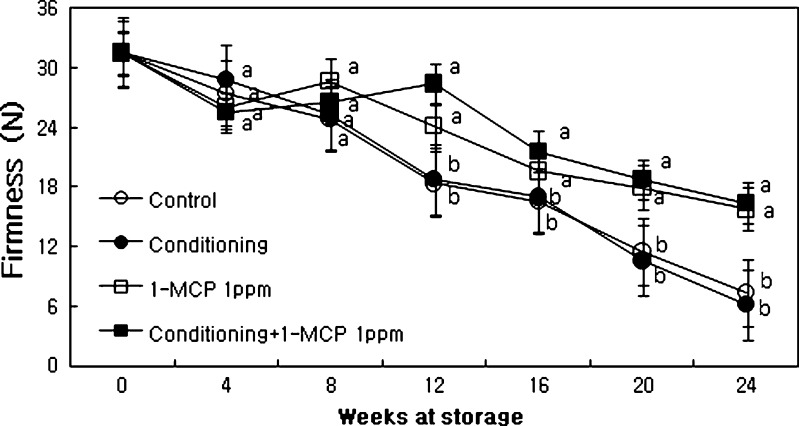
Flesh firmness decreased gradually with storage time and reached lower levels at the end stage of storage in both conditioning and control compared to either 1-MCP or conditioning plus 1-MCP treatments (Fig. 1). Fruits treated with 1-MCP enhanced firmness, about 20 % higher than control fruits. Application of 1 ppm of 1-MCP was sufficient to delay kiwi fruit softening during cold storage. Kim et al. (2001) found that 1-MCP reduced softening of intact kiwi fruit and the effect tended to be nearly the same with different concentrations. Similar results for kiwi fruits were reported by Boquete et al. (2004). In this experiment, fruits treated with either 1 ppm or conditioning with 1 ppm of 1-MCP enhanced flesh firmness in comparison with control or conditioning. The rate of firmness loss is related to the decrease of ethylene production and the reaction of the fruits when they were treated conditioning with 1-MCP. Wound occurred in stem of fruit during harvesting. Conditioning cured the wound. Therefore the decay rate decreases by curing the wound in order to prevent fungi to penetrate into the flesh. In order to decrease firmness loss during storage, careful handlings and pretreatments of fruits are required. Our results are consistent with Sharma et al. (2012), where the experiments were conducted to observe the effect of different concentrations of 1-MCP on the post-harvest life and quality of ‘Allison’ kiwifruit (Actinidia deliciosa). The results indicated that 2.0 μl/l it was the highest mean fruit firmness value (32.7 ± 0.2 N), and began to ripen only after 12 days in storage, whereas untreated fruit started ripening on the 6th day of storage.
Fig. 1.
Physico-chemical and chemical changes in firmness of ‘Hayward’ kiwi fruit as influenced by conditioning, 1-MCP and conditioning plus 1-MCP treatments during storage (0°C; %RH, 90). Each observation is a mean ± SD; n = 10; values with different letters are significantly different at p < 0.05
Physico-chemical properties
SSC gradually increased with storage time even when fruits were placed at cold storage. In control fruits, SSC increased from 9.8 % (4 weeks after storage) to 15.2 % (24 weeks after storage), while SSC in fruits treated with 1-MCP reached similar values to those of control fruits after 8 weeks. Therefore, the increase of SSC in fruit treated with 1-MCP underwent further retardation. In this experiment, 1-MCP had no effects on SSC, acidity and pH during storage.
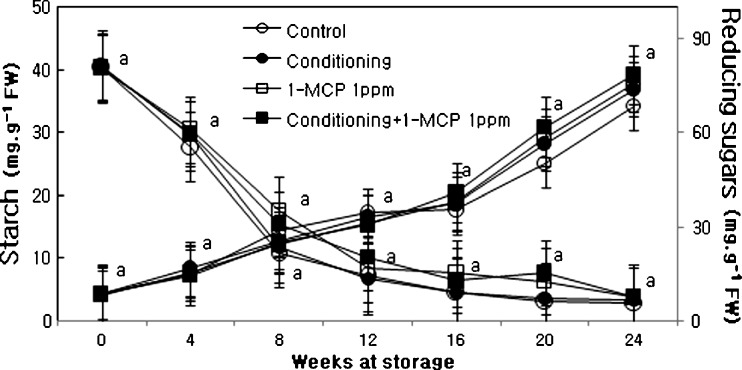
Starch content abruptly decreased during 4 weeks of storage in all treatments and its content was slightly higher in 1-MCP and conditioning plus 1-MCP than those of control or conditioning (Fig. 2). 1-MCP treatments showed slight effects on delaying of starch hydrolysis during storage compared to control. The amount of reducing sugars increased significantly at initial time (Fig. 2) and then gradually increased with the progress of storage period in both 1-MCP treatment and control fruits. The decreasing starch content is closely related with firmness loss during storage in kiwifruits (Park and Kim 1995; Park 1996). This experiment showed that flesh firmness enhanced by 1-MCP treatment changes with increasing of starch content during storage. Several explanations are available for the tendency of firmness in correlation with starch content when the treatment was carried out. 1-MCP treated kiwi fruits showed the lesser decrease of the starch content. The relatively high content of starch is related to the enhanced kiwi fruit firmness.
Fig. 2.
Changes in starch and reducing sugars of ‘Hayward’ kiwi fruit as influenced by conditioning, 1-MCP and conditioning plus 1-MCP treatments during storage (0oC; %RH, 90). Each observation is a mean ± SD; n = 10; values with different letters are significantly different at p < 0.05
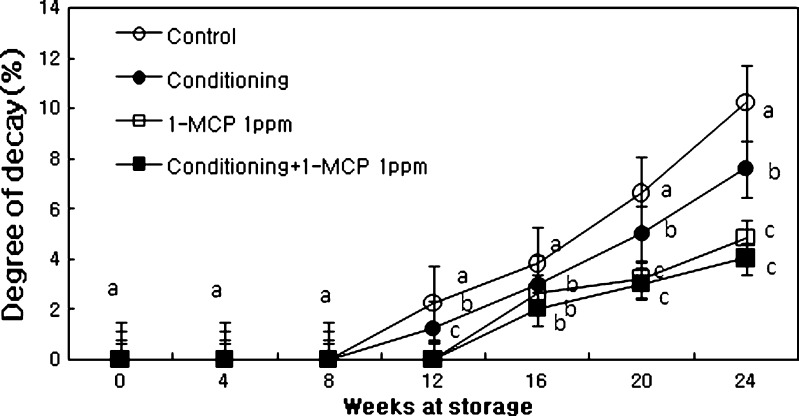
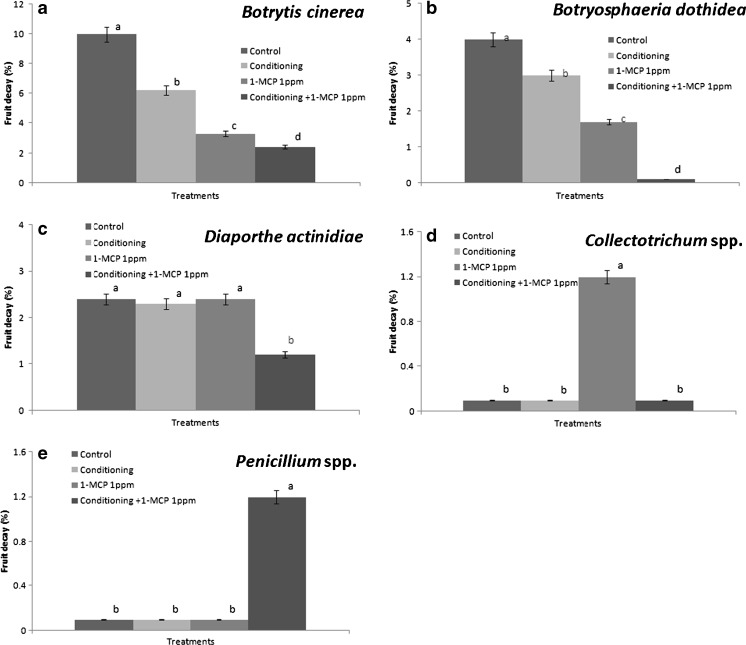
The degree of fruit decay (FD) significantly increased the end stage of storage and was influenced by different treatments. The degree of FD (Fig. 3) was much lower in fruits treated with 1-MCP and conditioning plus 1-MCP treatment than those of control or conditioning. FD pathogen during the storage is the highest by Botrytis cinerea, followed by Botryosphaeria dothidea, Diaporthe actinidiae and Collectotrichum spp., regardless of treatments (Fig. 4a–e). FD increased with the fruit ripening during storage. Treatments of conditioning plus 1-MCP reduced the fruit decay caused by B. cinerea, B. dothidea and D. actinidae.
Fig. 3.
Changes in degree of decay of ‘Hayward’ kiwifruit as influenced by conditioning, 1-MCP and conditioning plus 1-MCP treatments during storage (0oC; %RH, 90). Each observation is a mean ± SD; n = 10; values with different letters are significantly different at p < 0.05
Fig. 4.
Changes of fruit decay pathogen by Botrytis cinerea (A), Botryosphaeria dothidea (B), Diaporthe actinidiae (C), Collectotrichum spp. (D), Penicillium spp.(E) of ‘Hayward’ kiwi fruit as influenced by conditioning, 1-MCP and conditioning plus 1-MCP treatments as the final results at the 24 th week of storage (0°C; %RH, 90). Each observation is a mean ± SD; n = 10; values with different letters are significantly different at p < 0.05
Prestorage conditioning has been reported to reduce fruit decay in grapefruits (Brown et al. 1991), ‘yuzu’ (Park and Jung 1996) and non-astringent persimmon (Park and Kim 2002).
Pennycook and Manning (1992) reported that incidence of stem end rot in kiwi fruits was reduced from 46 % to 6 % by an increased holding period at ambient temperature. Sommer et al. (1983) stated that Botrytis rot significantly decreased when fruit curing picking scar at 10 °C compared to 0 °C, 5 °C, and 20 °C. Bautista-Banos et al. (1997) showed that temperature of 0 °C or 5 °C had no curing effect over a 3 days period, while 10 °C appeared to be most effective curing temperature to reduce subsequent disease development. It has appeared that kiwi fruit storage rot results from infection which occurs before storage but, remains quiescent until fruit ripen in storage. Stem scar may be contaminated with fungal spores at harvest. Temperature of 0°C and 5°C probably suppressed the development of resistance mechanism in kiwi fruits which have a range of metabolic defense mechanism as reported by Poole and McLeod (1991). Although, B. cinerea grows more slowly at 0°C or 5°C than at 20°C, such low temperatures could have inactivated or dramatically slowed the enzyme based defense responses of the kiwi fruit. Previous work (Park 2003) on picking scar curing has shown that a curing effect can be obtained by storing at 10°C or 20°C for 2 or 3 days, respectively. In this experiment, 1-MCP treatment following conditioning of picking scar decreased fruit decay with decreasing fruit softening resulting from low content of ethylene production. Combined conditioning with 1-MCP treatment before storage is necessary in order to get the synergistic effect for disease control.
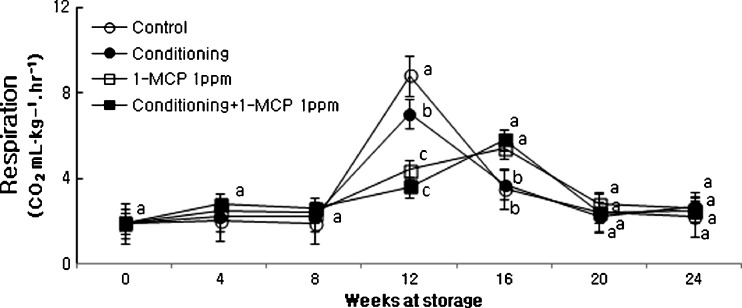
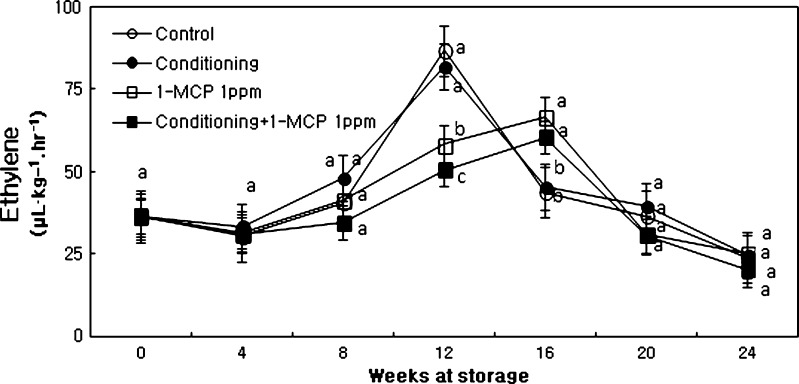
In CO2 (Fig. 5) and ethylene (Fig. 6) evolution these contents abruptly increased at initial time and then peaked at the middle stage of storage regardless of treatments. The delaying of increasing pattern of ethylene production was corresponded with the pattern of respiration in fruits treated with 1-MCP or conditioning plus 1-MCP treatment. Fruits treated 1-MCP displayed much lower ethylene production and peak time compared to control throughout the storage duration (Fig. 6).
Fig. 5.
Changes in CO2 production of ‘Hayward’ kiwi fruit as influenced by conditioning, 1-MCP and conditioning plus 1-MCP treatments during storage (0oC; %RH, 90). Each observation is a mean ± SD; n = 10; values with different letters are significantly different at p < 0.05
Fig. 6.
Changes in ethylene production of ‘Hayward’ kiwi fruit as influenced by conditioning, 1-MCP and conditioning plus 1-MCP treatments during storage (0°C; %RH, 90). Each observation is a mean ± SD; n = 10; values with different letters are significantly different at p < 0.05
Kiwi fruits are extremely sensitive to ethylene and soften quickly when exposed to as low as 0.1 ppm ethylene concentration (Crisosto et al. 2000). Delay of firmness loss at early stage of storage by 1-MCP treatment and close relationship between 1-MCP reaction and ethylene production have been well documented for kiwi fruit (Kim et al. 2001), strawberry (Ku and Wills 1999a; Tian et al. 2000), tomato (Wills and Ku 2002), banana (Pelayo et al. 2003) and mango (Jiang and Joyce 2000). In this experiment, softening of kiwi fruit was delayed with decreasing ethylene production when fruits were treated with 1-MCP or conditioning plus 1-MCP treatment (Boquete et al. 2004; Kim et al. 2001). Boquete et al. (2004) showed that peak ethylene production was measured at 17th day. Ethylene production by treated fruit remained low and they did not show an ethylene climacteric during 32 days of storage at 20◦ C. Control fruit softened rapidly to 11.8 N in 4 days but fruit treated with 1-MCP did not soften to a similar extent until after 18 days. Treated fruit did not soften as much as control fruit but firmness of treated fruit after 32 days was considered appropriate for their consumption. SSC remained low in 1-MCP treated fruit for about 14 days. Our assignments were consistent with their results. Kim and Lee (2005) supported our results and Boquete et al. (2004) that when applied at harvest 1-MCP had little effect on C2H4 induced softening after fruit removal from 0°C. However when 1-MCP was applied after fruit removal from 0°C and then challenged with C2H4, softening rate was reduced to a level similar to controls and fruit treated with MCP alone.
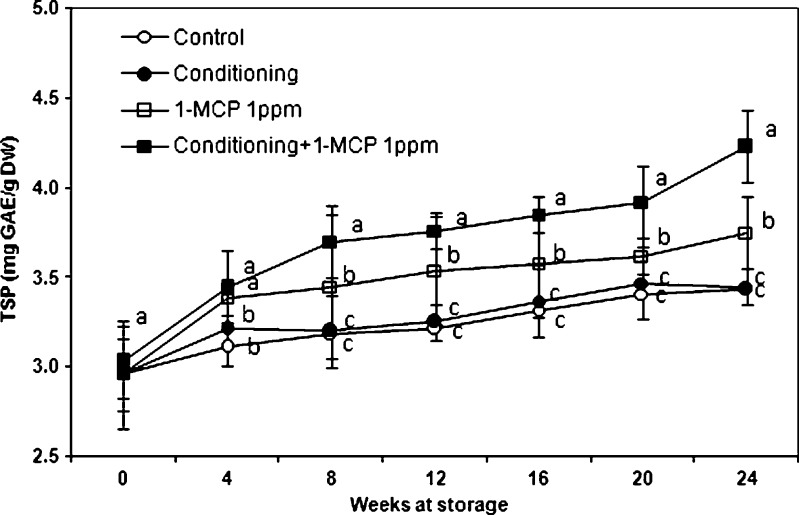
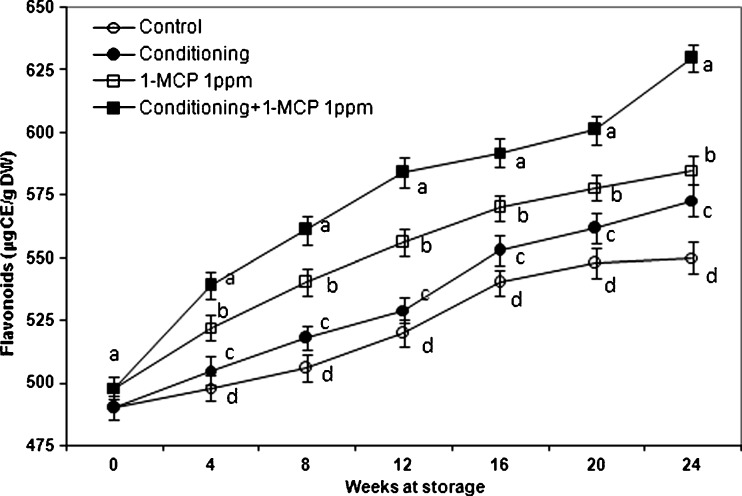
Total soluble phenolics, flavonoids and antioxidant activities
Our results of phenolics (Fig. 7), flavonoids (Fig. 8) and antioxidants (Table 1) during different treatments are comparable with those shown in Sharma et al. (2012), where the total phenolics content (24.3 ± 0.3 mg GAE/100 g FW) and the antioxidant activities (12.5 ± 0.03 μmol Trolox/g FW) were higher in 1-MCP-treated fruit than in untreated fruit. These results are in accordance with our data (Park et al. 2006; Park et al. 2012a; Park et al. 2012b), but were slightly higher than in previous report, because the kiwi fruits were grown under different conditions and the used extraction procedures differ as well. Antioxidant capacity and total phenol contents differ in kiwi fruit gathered at two harvest times, showing different maturity (Tavarini et al. 2008). Scalzo et al. (2005) underlined the importance of appropriate and well-balanced growing conditions, maturity, rootstock which influences seasonal antioxidant activity and polyphenol content. The use of various solvents for extraction of bioactive compounds influences the results (Preshita et al. 2013). The correlation coefficient (R2) between polyphenols and each of antioxidant values was relatively high as the final results at the 24th week of storage and varied from 0.8116 to 1 (Table 2). The obtained data are consistent with Zhang et al. (2013), where exposure of 1-MCP significantly delayed accumulation of total soluble phenolics, flavonoids in avocado, and total antioxidant activities levels increased. The results indicate that changes in antioxidant parameters of avocado fruit are not markedly influenced by 1-MCP, but are delayed or altered in proportion to the general suppression of ripening as indicated by ethylene production and fruit softening trends. In our case the antioxidant activity increased during treatment with 1-MCP. This supports also the previous report by Zhang et al. (2013), where the effects of ethylene-action suppression on antioxidant parameters during ripening vary considerably among different fruits. Our results are in agreement with Sun et al. (2012), where the 1-MCP treatment significantly extended the shelf life, delayed weight loss and decreased the rate of softening in Chinese kale (Brassica alboglabra Bailey). Ethylene production was suppressed and the respiration rate declined. Moreover, 1-MCP treatment postponed the decrease of ascorbic acid. 1-MCP treatment of bolting stems also delayed the accumulation of total phenolics, and maintained a high level of antioxidant capacity. These results demonstrate that 1-MCP treatment is a good practice for extending shelf life, maintaining the appearance and nutrient value, and reducing the loss of health-promoting compounds, particularly antioxidants in the Chinese kale. Qiu et al. (2009) confirmed similar results in ‘Sunrise’ apples, where the 1-MCP treatment resulted in improved fruit quality. 1-MCP used for the postharvest treatment of kiwi fruit enhanced its shelf-life and marketability by approximately of 6 days (Jhalegar et al. 2012; Sharma et al. 2012), which is in agreement with our data.
Fig. 7.
Changes in total soluble phenolics (TSP) of ‘Hayward’ kiwi fruit as influenced by conditioning, 1-MCP and conditioning plus 1-MCP treatments during storage (0°C; %RH, 90). Each observation is a mean ± SD; n = 10; values with different letters are significantly different at p < 0.05
Fig. 8.
Changes in total flavonoids of ‘Hayward’ kiwi fruit as influenced by conditioning, 1-MCP and conditioning plus 1-MCP treatments during storage (0oC; %RH, 90). Each observation is a mean ± SD; n = 10; values with different letters are significantly different at p < 0.05
Table 1.
Changes in antioxidant activity (AA) with different radical scavenging assays of ‘Hayward’ kiwi fruit as influenced by conditioning, 1-MCP and conditioning plus 1-MCP treatments during storage
| Samples | Weeks of storage | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | 20 | 24 | |
| Control ABTS | 13.27 ± 1.3a | 13.34 ± 1.3a | 13.49 ± 1.2a | 13.51 ± 1.4a | 13.54 ± 1.2a | 13.59 ± 1.3a | 13.61 ± 1.4a |
| Co ABTS | 13.29 ± 1.1a | 13.41 ± 1.2a | 13.68 ± 0.9a | 13.75 ± 1.1a | 13.98 ± 1.5a | 14.18 ± 1.2b | 14.56 ± 1.5b |
| MCP ABTS | 13.29 ± 1.4a | 13.52 ± 1.1a | 13.87 ± 1.3a | 14.22 ± 1.4b | 14.39 ± 1.2b | 14.67 ± 1.1b | 14.87 ± 1.4b |
| Co + MCP ABTS | 13.32 ± 1.2a | 13.67 ± 1.5a | 13.98 ± 1.1a | 14.54 ± 1.6ab | 14.69 ± 0.9ab | 14.78 ± 1.2ab | 15.01 ± 1.6ab |
| Control FRAP | 8.16 ± 0.9a | 8.20 ± 0.5a | 8.27 ± 0.8a | 8.31 ± 0.9a | 8.38 ± 0.7a | 8.41 ± 0.8a | 8.45 ± 0.9a |
| Co FRAP | 8.18 ± 1.1a | 8.22 ± 0.8a | 8.32 ± 0.7a | 8.41 ± 0.7a | 8.54 ± 0.8a | 8.62 ± 0.8a | 8.72 ± 0.8a |
| MCP FRAP | 8.18 ± 0.7a | 8.32 ± 0.9a | 8.48 ± 0.8a | 8.56 ± 0.8a | 8.65 ± 0.7a | 8.75 ± 0.7a | 8.87 ± 0.7a |
| Co + MCP FRAP | 8.21 ± 0.8a | 8.45 ± 0.7a | 8.68 ± 0.8a | 8.79 ± 0.9ab | 8.87 ± 0.6ab | 8.91 ± 0.7ab | 9.23 ± 0.7b |
| Control CUPRAC | 12.89 ± 0.9a | 12.91 ± 1.1a | 12.95 ± 1.2a | 13.01 ± 1.6a | 13.21 ± 1.1ab | 13.25 ± 1.3b | 13.28 ± 1.3b |
| Co CUPRAC | 12.91 ± 0.8a | 13.11 ± 0.9ab | 13.78 ± 1.4ab | 13.86 ± 1.3ab | 13.91 ± 0.9b | 13.99 ± 1.2b | 14.21 ± 1.5b |
| MCP CUPRAC | 12.92 ± 0.7a | 13.41 ± 1.2ab | 13.87 ± 1.4ab | 13.97 ± 1.2ab | 14.23 ± 1.2b | 14.45 ± 1.4b | 14.53 ± 1.2b |
| Co + MCP CUPRAC | 12.94 ± 1.1a | 13.65 ± 1.4a | 14.56 ± 1.2b | 14.65 ± 1.3b | 14.75 ± 1.2b | 14.81 ± 1.1b | 14.89 ± 1.1b |
ABTS •+ 2, 2-Azino-bis (3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt, FRAP Ferric-reducing/antioxidant power, CUPRAC Cupric reducing antioxidant capacity; Control, without any treatment; Co Prestorage conditioning at 20°C for 2 days, MCP 1-methylcyclopropene, 1 ppm for 16 h; Co + MCP Conditioning +1 ppm for 16 h, DW Values of ABTS, FRAP, CUPRAC are in μM TE/g, TE 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, Trolox, equivalent, DW dry weight
Each observation is a mean ± SD; n = 10; values with different letters are significantly different at p < 0.05
Table 2.
Correlation coefficients (R2) between polyphenols and the overall antioxidant activities of ‘Hayward’ kiwi fruit as influenced by conditioning, 1-MCP and conditioning plus 1-MCP treatments as the final results at the 24 th week of storage
| Samples | ABTS | FRAP | CUPRAC |
|---|---|---|---|
| Control | 0.9181 | 0.9684 | 1.0000 |
| Co | 0.9219 | 0.9436 | 0.8540 |
| MCP | 0.9526 | 0.9748 | 0.9701 |
| Co + MCP | 0.9226 | 0.9216 | 0.8116 |
ABTS •+ 2, 2-Azino-bis (3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt, FRAP Ferric-reducing/antioxidant power, CUPRAC Cupric reducing antioxidant capacity; Control, without any treatment; Co Prestorage conditioning at 20°C for 2 days, MCP 1-methylcyclopropene, 1 ppm for 16 h, Co + MCP Conditioning +1 ppm for 16 h
Conclusions
Utilization of 1-MCP extended shelf life in kiwi fruit with maintaining firmness by decreasing ethylene production and its reaction during storage. Prestorage conditioning with 1-MCP application in kiwi fruit effectively reduced the fruit softening and fruit decay resulting from low ethylene production and wound healing.
Acknowledgments
This paper was partly supported by the Regional Research Development Program from Rural Development Administration (RDA), in Korea, 2014. The authors are thankful to Dr. Elena Katrich (School of Pharmacy, Hebrew University of Jerusalem) for her technical assistance in determination of antioxidant activity.
Abbreviations
- DW
Dry weight
- SSC
Soluble solids content
- AA
Antioxidant activity
- ABTS+
2, 2-Azino-bis (3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt
- 1-MCP
1-methylcyclopropene
- FRAP
Ferric-reducing/antioxidant power
- CUPRAC
Cupric reducing antioxidant capacity
- GAE
Gallic acid equivalents
- CE
Catechin equivalent
- TE
Trolox equivalent
Contributor Information
Yong Seo Park, Phone: +82-61-4502376, FAX: +82-61-4532309, Email: ypark@mokpo.ac.kr.
Shela Gorinstein, Phone: +972-2-6758690, FAX: +972-2-6757076, Email: gorin@cc.huji.ac.il.
References
- Agar IT, Biasi WV, Mitcham, EJ (2000) Temperature and exposure time during ethylene conditioning affect ripening of Bartlett pears. J Agric Food Chem 48:165–170 [DOI] [PubMed]
- Apak R, Guclu K, Ozyurek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Athanasios K, Evangelos S. Effect of 1-MCP pre-storage treatment on ethylene and CO2 production and quality of ‘Hayward’ kiwi fruit during shelf-life after short, medium and long term cold storage. Postharv Biol Technol. 2007;46:174–180. doi: 10.1016/j.postharvbio.2007.05.002. [DOI] [Google Scholar]
- Bautista-Banos S, Long PG, Ganesh S. Curing of kiwi fruit for control of postharvest infection by Botrytis cinerea. Postharv Biol Technol. 1997;12:137–145. doi: 10.1016/S0925-5214(97)00043-4. [DOI] [Google Scholar]
- Blankenship SM, Dole JM. 1-Methylcyclopropene: a review. Postharv Biol Technol. 2003;8:1–25. doi: 10.1016/S0925-5214(02)00246-6. [DOI] [Google Scholar]
- Boquete EJ, Trinchero GD, Fraschina AA, Vidella F, Sozzi SGO. Ripening of ‘Hayward’ kiwi fruit treated with 1-methylcyclopropene after cold storage. Postharv Biol Technol. 2004;32:57–65. doi: 10.1016/j.postharvbio.2003.09.013. [DOI] [Google Scholar]
- Brown GE, Miller WR, McDonald RE. Control of green mold in marsh grapefruit with vapor heat quarantine treatment. Proc Fla State Hortic Soc. 1991;104:115–117. [Google Scholar]
- Cheah LH, Irving DE, Hunt AW, Corrigan VR. Effects of hot water dips on Botrytis storage rot and quality of kiwi fruit. Postharv Biol Technol. 1992;2:1–6. doi: 10.1016/0925-5214(92)90021-G. [DOI] [Google Scholar]
- Crisosto CH, Mitcham EJ, Kader AA. Produce facts kiwi fruit. Management of fruit ripening. Postharv Horticult Res. 2000;9:40–41. [Google Scholar]
- Fawcett HS, Barger WR. Relationship of temperature to growth of Penicillium italicum and P. digitatum to Citrus fruit decay produced by these fungi. J Agric Res. 1927;35:925–931. [Google Scholar]
- Gorinstein S, Martin-Belloso O, Lojek A, Ciz M, Soliva-Fortuny R, Park YS, Caspi A, Libman I, Trakhtenberg S. Comparative content of some phytochemicals in Spanish apples, peaches and pears. J Scien Food Agric. 2002;82:1166–1170. doi: 10.1002/jsfa.1178. [DOI] [Google Scholar]
- Jhalegar MJ, Sharma RR, Pal RK, Sharma S. Effect of 1-MCP on shelf-life and quality of kiwi fruit stored under ambient conditions. Indian J Hort. 2012;69:258–262. [Google Scholar]
- Jiang Y, Joyce D. Effects of 1-methylcyclopropene alone and in combination with polyethylene bags on the postharvest life of mango fruit. Annals Appl Biol. 2000;137:321–327. doi: 10.1111/j.1744-7348.2000.tb00073.x. [DOI] [Google Scholar]
- Kim HO, Hewett EW, Lallu N. Softening and ethylene production of kiwi fruit reduced with 1-methylcyclopropene. Acta Horticult. 2001;553:167–170. [Google Scholar]
- Kim YK, Lee JM (2005) Extension of storage and shelf-life of sweet persimmon with 1-MCP. In Park YM, Kang SM (eds) Proc. IIIrd IS on Persimmon. Acta Hort Ishs 685:165–174
- Kramer M, Bufler G, Ulrich D, Leitenberger M, Conrad J, Carle R, Dietmar R, Kammerer DR. Effect of ethylene and 1-methylcyclopropene on bitter compounds in carrots (Daucus carota L.) Postharv Biol Technol. 2012;73:28–36. doi: 10.1016/j.postharvbio.2012.05.009. [DOI] [Google Scholar]
- Ku VVV, Wills RBH (1999a) 1-methylcyclopropene can differentially affect the postharvest life of strawberries exposed to ethylene. Hortscience 34:119–120
- Ku VVV, Wills RBH. Effect of 1-methylcyclopropene on the storage life of broccoli. Postharv Biol Technol. 1999;17:127–132. doi: 10.1016/S0925-5214(99)00042-3. [DOI] [Google Scholar]
- Kundan K, Pathak KA, Shukla R, Bharali R. Effect of storage temperature on physico-chemical and sensory attributes of purple passion fruit (Passiflora edulis Sims) J Food Sci Technol. 2011;48:484–488. doi: 10.1007/s13197-010-0189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir NA, Curell E, Khan N, Whitaker M, Beaudry RM. Harvest maturity, storage temperature, and 1-MCP application frequency alter firmness retention and chlorophyll fluorescence of ‘Redchief Delicious’ apples. J Amer Soc Horticult Sci. 2001;126:618–624. [Google Scholar]
- Ozgen M, Reese RN, Tulio AZ, Jr, Scheerens JC, Miller AR. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agric Food Chem. 2006;54:1151–1157. doi: 10.1021/jf051960d. [DOI] [PubMed] [Google Scholar]
- Park YS. The shelf life of kiwi fruit in room temperature and cold storage following controlled atmosphere storage. J Kor Soc Horticult Sci. 1996;37:58–63. [Google Scholar]
- Park YS. Storability of fresh-cut kiwi fruit slices influenced by storage temperature. J Kor Soc Horticult Sci. 2002;43:728–732. [Google Scholar]
- Park YS. Changes in the rates of fruit softening and decay of kiwi fruit influenced by prestorage conditioning and hot air treatment during storage. J Kor Soc Horticult Sci. 2003;44:670–674. [Google Scholar]
- Park YS, Jung ST. Effects of storage temperature and preheating on the shelf life of yuzu during storage. J Kor Soc Horticult Sci. 1996;37:285–291. [Google Scholar]
- Park YS, Jung SY. Prestorage conditioning and CO2 pretreatment for control of postharvest rot in kiwi fruit inoculated with Botrytis cineria and Botryosphaeria dothidea. J Kor Soc Horticult Sci. 2005;46:49–54. [Google Scholar]
- Park YS, Kim BW. Changes in fruit firmness, fruit composition, respiration and ethylene production of kiwi fruit during storage. J Kor Soc Horticult Sci. 1995;36:67–73. [Google Scholar]
- Park YS, Kim SY. Effects of prestorage conditioning and hot water dip on fruit quality of non-astringent ‘Fuyu’ persimmon fruit during storage. J Kor Soc Horticult Sci. 2002;43:58–63. [Google Scholar]
- Park YS, Park MY. Effects of time and degree of fruit thinning on the fruit quality, yield and return bloom in kiwi fruit. J Kor Soc Horticult Sci. 1997;38:60–65. [Google Scholar]
- Park YS, Jung ST, Gorinstein S. Ethylene treatment of ‘Hayward’ kiwi fruits during ripening and its influence on ethylene biosynthesis and antioxidant activity. Sci Horticult. 2006;108:22–28. doi: 10.1016/j.scienta.2006.01.001. [DOI] [Google Scholar]
- Park YS, Ham KS, Kang SG, Park YK, Namiesnik J, Leontowicz H, Leontowicz M, Ezra A, Trakhtenberg S, Gorinstein S. Organic and conventional kiwi fruit, myths versus reality: antioxidant, antiproliferative, and health effects. J Agric Food Chem. 2012;60:6984–6993. doi: 10.1021/jf3010614. [DOI] [PubMed] [Google Scholar]
- Park YS, Heo BG, Ham KS, Kang SG, Park YK, Nemirovski A, Tashma Z, Gorinstein S. Analytical determination of bioactive compounds as an indication of fruit quality. J AOAC Intern. 2012;95:1725–1732. doi: 10.5740/jaoacint.12-130. [DOI] [PubMed] [Google Scholar]
- Pelayo CEV, de Vilas-Boas B, Benichou M, Kader AA. Variability in responses of partially ripe bananas to 1-metylcyclopropene. Postharv Biol Technol. 2003;28:75–85. doi: 10.1016/S0925-5214(02)00124-2. [DOI] [Google Scholar]
- Pennycook SR, Manning MA. Picking wound curing to reduce Botrytis storage rot of kiwi fruit. New Zealand J Crop Horticult Sci. 1992;20:357–360. doi: 10.1080/01140671.1992.10421779. [DOI] [Google Scholar]
- Poole PR, McLeod LC. Inhibition of infection Botrytis cinerea in kiwi fruit tissue. Acta Horticult. 1991;297:599–603. [Google Scholar]
- Preshita P, Sharma N, Joseph GS, Singh RP. Antioxidant activity and detection of (−) epicatechin in the methanolic extract of stem of Tinospora cordifolia. J Food Sci Technol. 2013;50:567–572. doi: 10.1007/s13197-011-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Lu C, Li X, Toivonen PMA. Effect of 1-MCP on quality and antioxidant capacity of in vitro digests from ‘Sunrise’ apples stored at different temperatures. Food Res Intern. 2009;42:337–342. doi: 10.1016/j.foodres.2008.12.007. [DOI] [Google Scholar]
- Sams CE, Lewis RJ, Benshalom N. Firmness and decay of apples following postharvest pressure infiltration of calcium and heat treatment. J Amer Soc Horticult Sci. 1993;90:152–154. [Google Scholar]
- Scalzo J, Politi A, Pellegrini N, Mezzetti B, Battino M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition. 2005;21:207–213. doi: 10.1016/j.nut.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Schirra M, Agabbio M, D'hallewin G, Pala M, Ruggiu R (1997) Response of Tarocco oranges to picking date, postharvest hot water dips, and chilling storage temperature. J Agric Food Chem 45:3216–3220
- Sharma RR, Jhalegar MJ, Pal RK. Response of kiwi fruit (Actinidia deliciosa cv. Allison) to post-harvest treatment with 1-methylcyclopropene. J Hort Sci Biotech. 2012;87:278–284. [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzym. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at the receptor level: recent development. Plant Physiol. 1997;100:577–582. doi: 10.1111/j.1399-3054.1997.tb03063.x. [DOI] [Google Scholar]
- Sivakumar D, Van Deventer F, Terry LA, Polenta GA, Korsten L. Combination of 1-methylcyclopropene treatment and controlled atmosphere storage retains overall fruit quality and bioactive compounds in mango. J Sci Food Agric. 2012;92:821–830. doi: 10.1002/jsfa.4653. [DOI] [PubMed] [Google Scholar]
- Sommer NF, Fortlage RJ, Edwards DC. Minimizing postharvest disease of kiwi fruit. California Agric. 1983;37:16–18. [Google Scholar]
- Sun B, Yan H, Liu N, Wei J, Wang Q. Effect of 1-MCP treatment on postharvest quality characters, antioxidants and glucosinolates of Chinese kale. Food Chem. 2012;131:519–526. doi: 10.1016/j.foodchem.2011.09.016. [DOI] [Google Scholar]
- Tavarini S, Degl’Innocenti E, Remorini D, Massai R, Guidi L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwi fruit. Food Chem. 2008;107:282–288. doi: 10.1016/j.foodchem.2007.08.015. [DOI] [Google Scholar]
- Tian MS, Prakash S, Elgar HJ, Young H, Burmeister DM, Ross GS. Responses of strawberry fruit to 1-methylcyclopropene (1-MCP) and ethylene. Plant Growth Regul. 2000;32:83–90. doi: 10.1023/A:1006409719333. [DOI] [Google Scholar]
- Wills RBH, Ku VVV. Use of 1-MCP to extend the time to ripen of green tomatoes and postharvest life of ripe tomatoes. Postharv Biol Technol. 2002;26:85–90. doi: 10.1016/S0925-5214(01)00201-0. [DOI] [Google Scholar]
- Zhang Z, Huber DJ, Rao J. Antioxidant systems of ripening avocado (Persea Americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharv Biol Technol. 2013;76:58–64. doi: 10.1016/j.postharvbio.2012.09.003. [DOI] [Google Scholar]