Abstract
Purple carrot juice was clarified by microfiltration. Two modes of filtration, batch concentration and total recycle were tested and the effect of microfiltration process on permeate flux and membrane fouling was studied. Intrinsic membrane resistance was negligible compared with the fouling resistances, which was less than 5 % of total resistance. Determination of membrane hydraulic permeability showed that water cleaning could permit a recovery of about 7 % of initial hydraulic flux. The analysis of color parameters of feed, permeate and concentrate juice during filtration shows that the a* and b* values decrease for the permeate corresponding respectively to changes from green to red and from blue to yellow. The total sugar and reducing sugars increase in permeate and decrease in concentrate. This work showed that it was possible to clarify the purple carrot juice by microfiltration with a real amelioration of the juice appearance.
Keywords: Carrot juice, Microfiltration, Fouling, Resistances, Clarification
Introduction
The demand for vegetable foods is increasing all over the word. They are important components of a balanced human diet and provide us with significant levels of some micronutrients. Epidemiological studies showed that the consumption of vegetables can supply protection against certain diseases including cancer, cardiovascular diseases, cataracts, macular degeneration and diabetes (Cortés et al. 2005). Therefore, there has been an important expansion in the industrial processing of vegetables, mainly in the manufacturing of juices (Vandresen et al. 2009). The carrot is a nutritious root vegetable widely known for various medicinal properties (Sharma et al. 2006). Its characteristics and color are related to the content of β-carotene properly protected by inactivation of oxidative enzymes Quitão-Teixeira et al. (2008). However, its utilization is limited in time. Indeed, in Tunisia, it is consumed mostly in its fresh form or cooked. The development and study of new technologies to obtain high quality products from this vegetable is essential to encourage its production and consumption. Carrot juice has a high nutritional value, and it is used as a natural source of provitamin A in the carotenoids drinks (Demir et al. 2004; Yoon et al. 2005). Carrot juice is a good raw material for the production of non milk based probiotic drink (Kun et al. 2008). As reported by Schieber et al. (2001), there is a steady increase of carrot juice consumption in many countries.
Purple carrots are grossly underutilized and do not find much consumer acceptance as a vegetable. The juice of carrot purple is very thick with a cloudy appearance. The aim of our study was to develop a process that would be suitable for manufacturing a juice for natural colorant.
In the process of fruit juices, the major application of membranes concerns the clarification. Membrane based processes help with the creation of novel products and keep sensory properties of intermediate products reasonably good. Microfiltration and ultrafiltration are reported for the clarification and/or concentration of fruit like pineapple, passion fruit (De Oliveira et al. 2012; Pereira et al. 2002; Vaillant et al. 1999, kiwi, umbu, watermelon (Gomes et al. 2013; Ushikubo et al. 2006, 2007), pulpal (apricot, peach, pear) and red fruits (strawberry, blackcurrant, raspberry, cherry, pomegranate, tomato) (Baklouti et al. 2012; Daufin et al. 2001; Mirsaeedghazi et al. 2012; Razi et al. 2012). The aim of this paper is to evaluate the effect of different process conditions, such as operating mode and transmembrane pressure (TMP) on permeate flux during carrot juice filtration. Moreover, the different type of fouling and their contribution to the total resistance were realized and the evaluation of the hydraulic permeabilities, after filtration and during cleaning procedure was also performed. Finally juice quality in term of color, proteins, total and reducing sugars were determined during the microfiltration process.
Materials and methods
Juice extraction
Purple variety carrots were acquired at a local market (Sousse, Tunisia). The washed carrots were immersed in a 100 ppm sodium hypochlorite solution for 30 min and washed again. They were, topped, cut and squeezed in a home juicer (Black & Decker JE 55/B1). After extraction, the pomace was filtered in a 100 mesh cloth filter. This method gave an average juice yield of 75–80 % (w/w). The juice was stored at −18 °C and was defrosted to room temperature before use.
Crossflow filtration pilot and membrane
Microfiltration experiments were performed using a lab-scale pilot plant equipped with a 0.4 m length Kerasep multitubular membrane containing 19 channels with an internal diameter of 2.5 10−3 m. The filtration area was 0.056 m2, the mean pore diameter was 0.2 μm. The feed raw juice of 5 L was recycled by a centrifugal pump. TMP was controlled by the pressure gauges and calculated as:
| 1 |
Where Pf, Pr and Pp are respectively the pressure of the feed, the retentate and the filtrate Microfiltration experiments were performed using a lab-scale pilot plant whose scheme is shown in Fig. 1. In the batch concentration mode, the permeate is collected separately and the concentrate is recycled to the feed tank. However, in total recycle experiments, both permeate and concentrate were returned to the feed tank to keep concentrations constant. All experiments were run at 25 °C to preserve the juice characteristics.
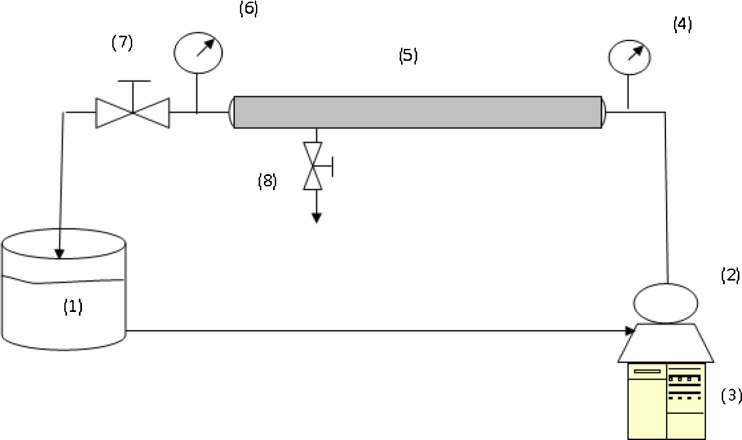
Fig. 1.
Scheme of pilot plant used in experiments. 1 Feed tank, 2,3 centrifugal pump, 4,6 Pressure Gauges, 5 membrane module, 7 valve, 8 permeate
Before each experiment, the integrity of the membrane was checked by the measurement of its permeability with distilled water at 20 °C. Previous experiments (results not shown) have been performed to determine the optimal operating conditions for the clarification process (TMP 180 kPa, axial feed flow rate 171 l.h−1 and at a temperature of 25 °C).
Filtration laws
The Darcy’s low (Eq. 2) models the juice flow through a porous environment during a filtration operation. The permeate flux through the membrane was calculated by the following equations:
| 2 |
where J is the permeate flux (l.m−2 h−1.), TMP the transmembrane pressure (Pa), μ the dynamic viscosity of the feed (Pa.s) and Rt the total resistance to flow (m−1). The membrane total resistance (Rt) is the sum of several resistances given by:
| 3 |
where Rm is the intrinsic resistance of the clean membrane, Rf is the fouling layer resistance. Rf can be considered as the sum of the reversible (Rrf) and irreversible (Rif) fouling resistances. The Rrf resistance is due to concentration polarization and deposition of solids (cake layer) on the membrane surface, and therefore, it can be removed by cleaning with water after the filtration run. On the contrary, the Rif resistance is due to pore blocking and adsorption of materials on the membrane surface and pores, which cannot be removed by water cleaning. The intrinsic and the fouling layer resistance for membrane were calculated using the Eqs. (4) and (5):
| 4 |
| 5 |
where Jo represents the flux of distilled water, calculated at the beginning of the filtration experiment (Jiraratananon and Chanachai 1996; De Bruijn et al. 2002; Cassano et al. 2007). The flux loss was calculated using the equation:
| 6 |
where J0 : the initial filtration flux and Js : the stabilized filtration flux.
Measurement of hydraulic permeability and membrane regeneration
Water flux was measured at different values of TMP and in fixed conditions of temperature (25 °C) and 171 l.h−1 for flow rate. As reported by Cassano et al. (2003), the hydraulic permeability of the membrane was determined by the slope of the straight lines obtained plotting the water flux values versus the applied TMP (Lp = J/TMP). The value obtained for clean membrane is referred to as L0p. The hydraulic permeability measured after the carrot juice filtration was indicated as L1p.
The membranes were carefully cleaned immediately after each experiment in order to recover the initial permeability. The pilot unit was rinsed with hot water (50 °C). The hydraulic permeability measured afterwards was L2p.
The fouling index was calculated by comparing the hydraulic permeability before and after the treatment of carrot juice according to the following equation:
| 7 |
Where L1p and L0p are the hydraulic permeabilities measured after and before treatment of the carrot juice respectively (Mänttäri and Nyström 2007). Then, a 10 g.l−1 sodium hydroxide solution (80 °C during 30 min) was circulated through it. After rinsing with distilled water until neutral pH, 5 ml.l−1 nitric acid solution at 60 °C was circulated during 30 min. At the end of each cleaning procedure the membrane was rinsed with distilled water. The water flux rates under the processing conditions were measured to estimate the irreversible fouling (Rif) using the Eq. (5). The total resistance (Rt) was calculated with the stabilized permeate flow rate according to the Eq. (2). The resistance caused by concentration polarization and cake layer was estimated by the Eq. (3).
Physicochemical analysis of juice
Total soluble solids were determined at 20 °C with a hand refractometer Abbe-Zeiss and the values were expressed as °Brix. The reducing and total sugars were determined by the DNS method (Miller 1959). The total solids and ashes were determined by drying the samples, respectively, at 105 ± 2 °C and 600 ± 15 °C until constant weight.
The pH was measured at 20 °C. Protein content was determined by the method of Bradford (1976), using bovine serum albumin as standard. The color analysis of the juices according to the surface color parameters L*(lightness), a*(green/red), and b*(blue/yellow) were determined using Lovibond PFX 195-Tintometer. Parameters a* and b* were used to calculate the H (Hue) value (H = arctang (b*/a*)) and C (chroma) (C = (a*2 + b*2)1/2).
Color difference values were calculated to study color changes. ΔE* (total color difference) was defined as: ΔE = [(L*i − L*0)2 + (a*i − a*0)2+ (b*i − b*0)2]0.5 where L*0, a*0 and b*0 are the values of the juices at zero time.
Results and discussion
Effect of operating mode
Operating mode influence on permeate flux
To evaluate the effect of juice concentration on filtration performances, the microfiltration runs were performed according to two operating mode: the total recycle and the batch concentration mode. As shown in Fig. 2, for the two microfiltration modes, the flux value decrease was very fast during the first 20 min of the runs. Then, a smaller decrease of permeate was noticed until approximately 50 min of filtration. Finally, a pseudo-steady state was reached. It can be noticed that, in spite of the difference in concentration during filtration time, the two curves are very similar. No significant changes were observed in terms of flow rates.
Fig. 2.
Microfiltration of carrot juice in total recycle mode (white up-pointing triangle) and in concentration mode (black circle) at T°:25 °C; U: 171 l.h−1 and TMP: 180 kPa
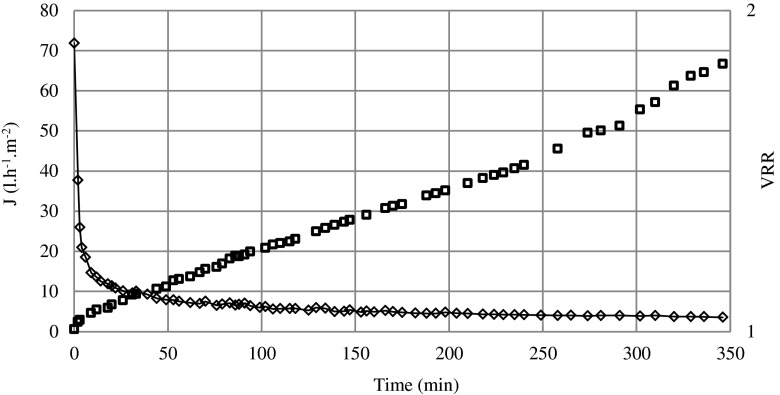
Figure 3 shows the time course of the permeate flux and the volume reduction ratio in the batch concentration mode at TMP of 180 kPa, at a temperature of 25 °C and at flow rate of 171 l.h-1. The initial permeate flux was about 72 l.h−1.m−2; it decreased gradually with the operating time by increasing the volume reduction ratio (VRR) due to concentration polarization and gel formation. A 95 % reduction of the initial flux was obtained after a 350 min run corresponding to a final VRR of 1.8.
Fig. 3.
MF of carrot juice, time course of the permeate flux and of the VRR (operating conditions T°:25 °C; U: 171 l.h−1; TMP: 180 kPa (white square) VRR versus time and (white diamond) for flux versus time during batch concentration mode
Analysis of resistances
Table 1 show the membrane and the different fouling resistances for the two MF modes calculated according to the resistance in-series model. It can be noted that, obviously, the main part of total resistance was caused by fouling. Intrinsic membrane resistance, Rm = 4.43.1012 m−1, was negligible compared to the fouling resistances (Rf = 128 and 180 1012 m−1 respectively for total recycle and batch concentration modes). For batch concentration mode, the reversible resistance contributed to 83 % of the total resistance while its contribution was only about of 52.6 % in the case of total recycle mode. Moreover, the irreversible resistance was two fold more important in total recycle mode than in batch concentration mode respectively 61 and 30.1012 m−1.
Table 1.
Determination of resistances and permeabilities of membrane
| MF Total recycle mode | MF Batch concentration mode | |
|---|---|---|
| Resistances | ||
| Rt (·1012).(m−1) | 132 | 184 |
| Rm (1012).(m−1) | 4.43 | 4.43 |
| Rf (1012).(m−1) | 128 | 180 |
| Rrev (1012).(m−1) | 67 | 150 |
| Rirrrev (1012).(m−1) | 61 | 30 |
| Rrev/Rf (%) | 52.66 | 83.33 |
| Rirrev/Rf (%) | 47.34 | 16.67 |
| Permeabilities before and after cleaning processes | ||
| Clean membrane (L0) | 91.11 | 91.11 |
| After treatment with juice (L1) | 3.06 | 2.22 |
| After cleaning with water (L2) | 6.67 | 13.21 |
| After cleaning with alkaline solution | 24.01 | 23.10 |
| Fouling index (%) | 96.64 | 97.56 |
(n = 2)
Analysis of permeabilities
Table 1 shows hydraulic permeabilities of the microfiltration membrane measured before and after cleaning procedures for the two filtration modes. The values of permeate flux obtained for distilled water before and after the microfiltration runs and the cleaning treatments are reported in Fig. 4. The hydraulic permeability can be taken as the indicator of cleaning efficiency. Indeed, if the fouling compounds have been efficiently removed from the membrane, the same filtration performance can be expected for the next experiment. It can be noticed the fouling index calculated for the two operating mode is about 97 % after the microfiltration of the juice. After cleaning with distilled water, the recovery of the initial permeability was about 14 and 7 % for the batch concentration mode and the total recycle mode, respectively. Indeed, it can be assumed that, due to juice concentration, the thickness of the reversible fouling layers was more important during the batch concentration mode. Moreover, it was observed that, after an alkaline cleaning treatment was performed, the hydraulic permeability of the membrane was about 25 % of the initial one (Fig. 4a and b).
Fig. 4.
Distilled water permeate flux of membrane before and after carrot juice MF and cleaning treatments (Operating conditions: conditions: T°:25; U = 171 l.h − 1). a according to Total recycle mode and b according to batch concentration mode. (white square) After cleaning with NaOH (black diamond) After cleaning with water (white circle) Before treatment with juice
Effect of transmembrane pressure
In total recycle mode, the permeate flux values, are plotted versus TMP (Fig. 5). As shown, the pressure increases from 100 to 200 KPa, led to a variation in permeate flux. According to Cassano et al. (2007) at low pressure, shear forces tend to minimize particle deposition and the juice is proportional to the applied PTM. When the foulants particles start to deposit on the membrane surface, flux decreases. Further increase in pressure induces an increase of the thickness of the particle layer without a corresponding increase in flux. In these conditions, a limiting flux is reached at a TMP value of about 180 KPa. At a sec time, the PTM was decreased. However, the fouling layer on the membrane surface is already so consolidated that the shear forces could no more remove surface and, consequently, causes permeate decrease (Cassano et al. 2007; Ushikubo et al. 2007).
Fig. 5.
Microfiltration of carrot juice: Permeate flux (white circle) at different applied pressure P (black diamond) versus time (operating conditions: U = 171 l.h−1; T = 25 °C)
Carrot juice quality in batch concentration mode
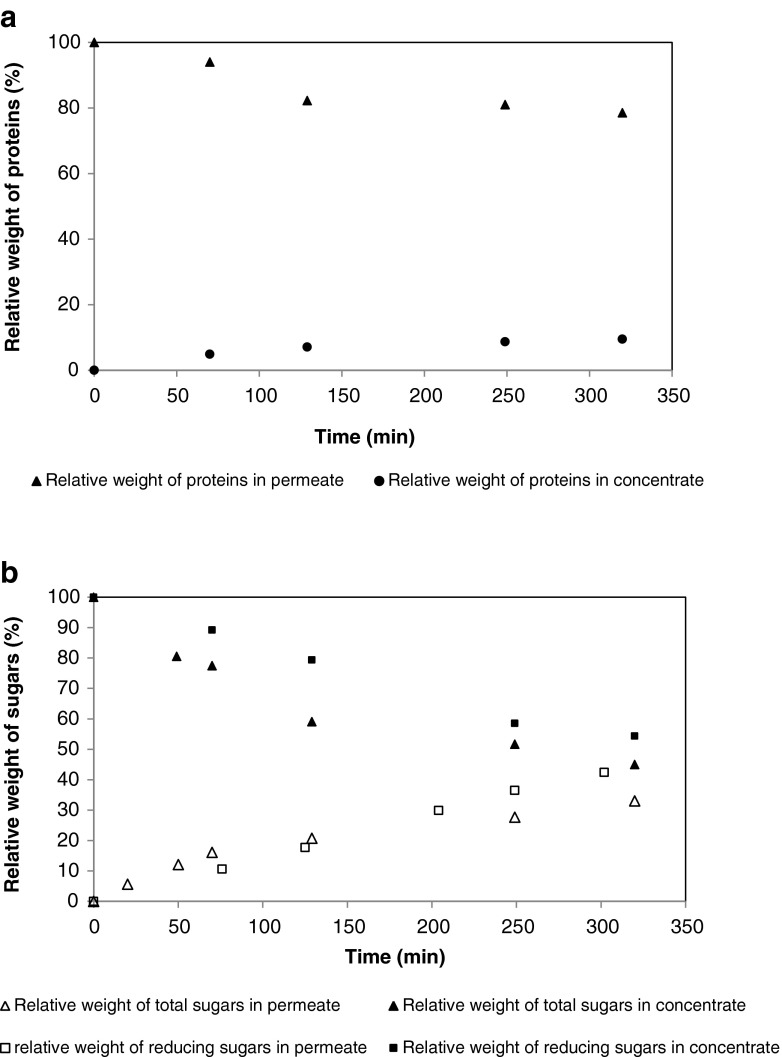
In Fig. 6a the relative weight of proteins in permeate and concentrate during carrot juice clarification are shown. During all the runs the percentage of proteins increase in permeate and decrease in concentrate and tend to stabilize (after 300 min of filtration) at a rate of 10 % in permeate and 75 % in concentrate. It could be assumed that there was an important retention of these compounds. The same phenomenon was reported by Severo et al. (2007) for wine clarification. In the beginning of microfiltration process proteins due to their high molecular weight are adsorbed on the membrane pores and on surface so the proteins don’t pass in permeate. By action of cake formation, a constant rate of proteins passed into the pores so the protein concentration in permeate was constant after 300 min of filtration.
Fig. 6.
Protein curves (a) and sugar curves (b) for permeate and concentrate
Total and reducing sugars relative weight in permeate and concentrate during the carrot juice microfiltration are shown in Fig. 6b. The sugars increase in permeate and decrease in concentrate; however the retention of total sugars is more important than for reducing sugars. At the end of filtration time, 45 % of total sugars are collected in permeate and in the concentrate, we note only 30 % of the initially sugars present in the feed (Fig. 6b), so 22 % of total sugars were retained on and in the membrane. Reducing sugars pass in permeate at 42 %, in the concentrate it remains 55 % of reducing sugars, this can be due to their low molecular weight, and they are not retained.
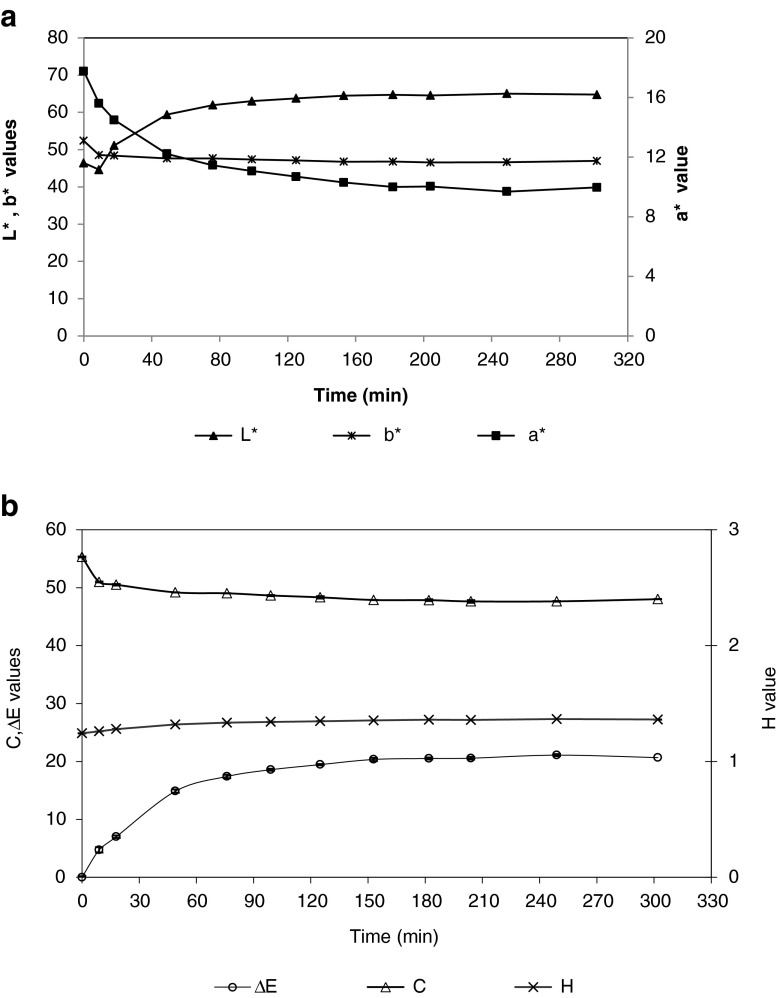
For the evaluation of color, the CIELAB parameters L*, a* and b* were measured during the carrot juice clarification and the parameters hue (H), chroma (C) and the difference in color (ΔE) were calculated. In Fig. 7a and b the parameters L*, a*, b*, C, H and ΔE are presented. The L* value tending from 0 for dark to 100 for white vary with filtration process. The L value initially at 46.4, highly increase in permeate and reach 65. The a* and b* values decrease in permeate tending respectively from green to red and from blue to yellow. A correlation exists between the carotenoids contents and the color of juice. The value of a* is directly correlated to carotenoids content in carrot purée according to Patras et al. (2009).
Fig. 7.
Evolution of juice colour parameters in the permeate during microfiltration (batch concentration mode)
In Fig. 7a the a* value decreases during filtration passing from 17 to 10 in the last 150 min of filtration which can traduce a partial retention of carotenoids in concentrate. For L* and b* we observe also a stabilization of color values at 150 min of filtration.
It was observed a decrease in chroma values from 55 to 48 which means a decline in color intensity. The color difference increase during the first 120 min from 0 to 20 this means a significant change in total color during microfiltration.
The physico-chemical characteristics of feed, permeate and concentrate juice are reported in Table 2. Total soluble solids appear to be higher in the concentrate, this behavior was also reported by Cassano et al. (2010) for prickly pear juice and is probably due to the presence of suspended solids content in the pulpy products that can be interfere with the measurement of the refractive index (Vaillant et al. 1999). This corroborate the finding results where we note an increase of total solids in the concentrate comparing to feed and permeate.
Table 2.
Physico-chemical characteristics of carrot juice submitted to MF treatment in batch concentration mode
| Parameters | Feed | Permeate | Concentrate |
|---|---|---|---|
| pH | 6.60 ± 0.02 | 6.17 ± 0.01 | 6.13 ± 0.01 |
| Total soluble solids (°Brix) | 7.6 ± 0.0 | 7.0 ± 0.0 | 8.0 ± 0.0 |
| Total solids (g) | 304.83 ± 2.79 | 108.39 ± 2.04 | 179.61 ± 2.11 |
| Ash (g) | 38.47 ± 0.04 | 16.81 ± 0.04 | 19.71 ± 0.07 |
(n = 3)
Conclusion
Based on above results, it could be concluded that membrane filtration is a good alternative for purple carrot juice clarification. Purple carrot juice clarified by microfiltration show a fouling index similar in total recycle and batch concentration modes. As for proteins, the total sugars percentage was higher in concentrate than in permeate probably due to retention phenomena, however, for reducing sugars, this effect was less pronounced. Colour of juice is better after microfiltration process with a decrease in total solids, making it more illuminant. The nature of particles retained must be elucidated therefore; investigations on the chemical composition and specially the nature of fibers retained are currently under way.
Nomenclature
- MF
Microfiltration
- J
Flux (L.h−1.m−2)
- J0
Flux of distilled water for the clean membrane (L.h−1.m−2)
- Js
Stabilized filtration flux
- ΔJ
Flux loss
- Lp
Permeability
- L0p
Hydraulic permeability of clean membrane
- L1p
Hydraulic permeability after juice filtration
- L2p
Hydraulic permeability after rinsing with hot water
- TMP
Transmembrane pressure (bar)
- Rt
The total resistance to flow (m−1)
- Pf
Pressure of the feed (bar)
- Pr
Pressure of the retentate (bar)
- Pp
Pressure of the filtrate (bar)
- Rm
Membrane intrinsic resistance of the clean membrane (m−1)
- Rf
Fouling resistance (m−1)
- Rrf
Reversible fouling resistance (m−1)
- Rif
Irreversible fouling resistance (m−1)
- V0
Feed initial volume (m3)
- Vp
Filtrate volume (m3)
- VRR
Volume reduction ratio
- μ
Dynamic viscosity (Pa.s)
- L*
(Lightness)
- a*
(Green/red)
- b*
(Blue/yellow)
- C
Chroma
- H
Hue
- ∆E
Total color difference
References
- Baklouti S, Ellouze Gorbel R, Mokni A, Chaabouni S. Clarification of pomegranate juice by ultrafiltration: study of juice quality and of the fouling mechanism. Fruits. 2012;67(3):215–225. doi: 10.1051/fruits/2012010. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cassano A, Drioli E, Galaverna G, Marchelli R, Di Silvestro G, Cagnasso P. Clarification and concentration of citrus and carrot juices by integrated membrane processes. J Food Eng. 2003;57:153–163. doi: 10.1016/S0260-8774(02)00293-5. [DOI] [Google Scholar]
- Cassano A, Donato L, Drioli E. Ultrafiltration of kiwifruit juice: operating parameters, juice quality and membrane fouling. J Food Eng. 2007;79:613–621. doi: 10.1016/j.jfoodeng.2006.02.020. [DOI] [Google Scholar]
- Cassano A, Conidi C, Drioli E. Physico-chemical parameters of cactus pear (Opuntia ficus-indica) juice clarified by microfiltration and ultra filtration processes. Desalination. 2010;250:1101–1104. doi: 10.1016/j.desal.2009.09.117. [DOI] [Google Scholar]
- Cortés C, Esteve JM, Frígola A, Torregrosa F. Changes in carotenoids including geometrical isomers and ascorbic acid content in orange-carrot juice during frozen storage. Eur Food Res Technol. 2005;221:125–131. doi: 10.1007/s00217-004-1117-9. [DOI] [Google Scholar]
- Daufin G, Escudier JP, Carrere H, Berot S, Fillaudeau L, Decloux M. Recent and emerging applications of membrane processes in the food and dairy industry. Food Bioprod Process. 2001;79:98–102. [Google Scholar]
- De Bruijn J, Venegas A, Borquez R. Influence of crossflow ultrafiltration on membrane fouling and apple juice quality. Desalination. 2002;148:131–136. doi: 10.1016/S0011-9164(02)00666-5. [DOI] [Google Scholar]
- De Oliveira RC, Doce RC, De Barros STD. Clarification of passion fruit juice by microfiltration: analyses of operating parameters, study of membrane fouling and juice quality. J Food Eng. 2012;111(2):432–439. doi: 10.1016/j.jfoodeng.2012.01.021. [DOI] [Google Scholar]
- Demir N, Acar J, Bahceci KS. Effects of storage on quality of carrot juices produced with lactofermentation and acidification. Eur Food Res Technol. 2004;218:465–468. doi: 10.1007/s00217-004-0883-8. [DOI] [Google Scholar]
- Gomes FS, Costa PA, Campos MBD, Tonon RV, Couri S, Cabral LMC. Watermelon juice pretreatment with microfiltration process for obtaining lycopene. Int J Food Sci Technol. 2013;48(3):601–608. doi: 10.1111/ijfs.12005. [DOI] [Google Scholar]
- Jiraratananon R, Chanachai A. A study of fouling in the ultrafiltration of passion fruit juice. J Membr Sci. 1996;111:39–48. doi: 10.1016/0376-7388(95)00270-7. [DOI] [Google Scholar]
- Kun S, Rezessy-Szabo JM, Nguyen QD, Hoschke A. Changes of microbiological population and some components in carrot juice during fermentation with selected Bifidobacterium strains. Process Biochem. 2008;43(8):819–821. doi: 10.1016/j.procbio.2008.03.008. [DOI] [Google Scholar]
- Mänttäri M, Nyström M. Membrane filtration for tertiary treatment of biologically treated effluents from the pulp and paper industry. Water Sci Technol. 2007;55:99–107. doi: 10.2166/wst.2007.217. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalycilic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mirsaeedghazi H, Mousavi SM, Emam-Djomeh Z, Rezaei K, Aroujalian A, Navidbakhsh M. Comparison between ultrafiltration and microfiltration in the clarification of pomegranate juice. J Food Process Eng. 2012;35(3):424–436. doi: 10.1111/j.1745-4530.2010.00598.x. [DOI] [Google Scholar]
- Patras A, Brunton N, Da Pieve S, Butler F, Downey G. Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Innov Food Sci Emerg Technol. 2009;10:16–22. doi: 10.1016/j.ifset.2008.09.008. [DOI] [Google Scholar]
- Pereira CC, Rufino JM, Habert AC, Nobrega R, Cabral LMC, Borges CP. Membrane for processing tropical fruit juice. Desalination. 2002;148:57–60. doi: 10.1016/S0011-9164(02)00653-7. [DOI] [Google Scholar]
- Quitão-Teixeira LJ, Aguiló-Aguayo I, Ramos AM, Martín-Belloso O. Inactivation of oidative enzymes by high-intensity pulsed electric field for retention of color in carrot juice. Food Bioprocess Technol. 2008;1:364–373. doi: 10.1007/s11947-007-0018-x. [DOI] [Google Scholar]
- Razi B, Aroujalian A, Fathizadeh M. Modeling of fouling layer deposition in cross-flow microfiltration during tomato juice clarification. Food Bioprod Process. 2012;90:841–848. doi: 10.1016/j.fbp.2012.05.004. [DOI] [Google Scholar]
- Schieber A, Stintzing FC, Carle R. By-products of plant food processing as a source of functional compounds-recent development. Trends Food Sci Technol. 2001;12:401–413. doi: 10.1016/S0924-2244(02)00012-2. [DOI] [Google Scholar]
- Severo JB, Almeida SS, Narain N, Souza RR, Santana JCC, Tambourgi EB. Wine clarification from Spondias mombin L. pulp by hollow fiber membrane system. Process Biochem. 2007;42:1516–1520. doi: 10.1016/j.procbio.2007.08.003. [DOI] [Google Scholar]
- Sharma HK, Kaur J, Sarkar BC, Singh C, Singh B, Shitandi AA. Optimization of pretreatment conditions of carrots to maximize juice recovery by response surface methodology. J Eng Sci Technol. 2006;1:158–165. [Google Scholar]
- Ushikubo FY, Watanabe AP, Viotto LA. Microfiltration of umbu (Spondias tuberosa Arr. Cam.) juice using polypropylene membrane. Desalination. 2006;200:549–551. doi: 10.1016/j.desal.2006.03.431. [DOI] [Google Scholar]
- Ushikubo FY, Watanabe AP, Viotto LA. Microfiltration of umbu (Spondias tuberosa Arr. Cam.) juice. J Membr Sci. 2007;288:61–66. doi: 10.1016/j.memsci.2006.11.003. [DOI] [Google Scholar]
- Vaillant F, Millan P, O’Brien G, Dornier M, Decloux M, Reynes M. Crossflow microfiltration of passion fruit juice after partial enzymatic liquefaction. J Food Eng. 1999;42:215–224. doi: 10.1016/S0260-8774(99)00124-7. [DOI] [Google Scholar]
- Vandresen S, Quadri MGN, De Souza JAR, Dachamir H. Temperature effect on the rheological behavior of carrot juices. J Food Eng. 2009;92:269–274. doi: 10.1016/j.jfoodeng.2008.11.010. [DOI] [Google Scholar]
- Yoon KY, Cha M, Shin SR, Kim KS. Enzymatic production of a soluble-fiber hydrolyzate from carrot pomace and its sugar composition. Food Chem. 2005;92:151–157. doi: 10.1016/j.foodchem.2004.07.014. [DOI] [Google Scholar]