Abstract
The potential of antioxidant activity of the green algae (Chaetomorpha sp.) was studied in this work. The optimum processing conditions for the extraction of antioxidant compounds from dried green algae were determined using response surface methodology (RSM). A central composite design (CCD) was applied to determine the effects of three process variables as follows: solvent concentration (percent), extraction time (min) and microwave power (w) on total phenolic contents, ferric reducing power, 2’2-dipheny-l-picrylhydrazyl (DPPH) radical scavenging activity and total antioxidant capacity assays. The independent variables were coded at five levels and CCD included 20 experimental runs with six replications at the center point. The statistical analysis of data was performed using design expert software and second-order polynomial models generated after analysis of variance (ANOVA) applied for predicting the responses. The results revealed that the highest total phenol content and reducing power were 1.09 and 0.12 mg of tannic acid equivalent/g dry weight, respectively. The maximum antioxidant activity was 0.19 mg ascorbic acid equivalent/g dry weight and DPPH was 99.8 % under MAE. The optimum conditions using RSM for the predicted responses were: microwave power 300 W, extraction time 8 min and solvent concentration 25 %, respectively. Furthermore the actual experimental values were adjacent to the corresponding predicted values which demonstrated fitness of the employed models and suitability of RSM in extraction parameters optimization.
Keywords: Antioxidant capacity, Central composite design, Ferric reducing power, Phenolic compounds, Response surface methodology
Introduction
Reactive oxygen species (ROS) as very unstable molecules (Pan et al. 2008) are one of the main factors for beginning biomolecules oxidation (Kumar et al. 2008) including proteins, carbohydrates and polyunsaturated fatty acids (Fan et al. 2011) which lead to injury and death of the cells, called oxidative stress and cause diseases such as cancer (Ganesan et al. 2008), diabetes and nervous disorders like Alzheimer’s and Parkinson’s (Chew et al. 2008). Numerous studies focused on natural antioxidants. Phenolic compounds are one of the most important chemical groups greatly distributed in different species of plants (Li et al. 2009). They are known for their antioxidant role in plants (Ballard et al. 2010). Phenolic acids and flavonoids are the main groups of polyphenols with critical functions in the prevention of human diseases (Proestos and Komaitis 2008).
More recently, it has been revealed that seaweeds can be a rich source of natural polyphenolic compounds with antioxidant activity (Wang et al. 2009; Rodríguez-Bernaldo de Quirós et al. 2010; Ganesan et al. 2008) and among all substances that can be obtained from seaweeds, these groups of compounds are probably of the most interests. Phlorotanins from marine algae are a preventive defense against pathogenic agents, marine grazers, light and degenerative effects of the sun’s ultraviolet radiations (Blanc et al. 2011). Marine algae are the focus of this research because of the presence of exclusive bioactive compounds generated due to their unique and extreme habitat (Li et al. 2009). Seaweed extracts have been mentioned in several documents to have antioxidant activity. Airanthi et al. (2011) reported fucoxanthin and phenolics are responsible for the antioxidative activity of several brown seaweeds. Wang et al. (2009) studied ten island seaweed species and reported that polyphenols are the main responsible for free radical scavenging activity. Zubia et al. (2009) evaluated antioxidant and antimicrobial activity of ten brown seaweeds and reported there is a correlation between phenolics and antioxidant activities. Souza et al. (2011), Matanjun et al. (2008) have been also evaluated the antioxidant property of different species of seaeeds.
Microwave-assisted extraction employs microwave energy to extract target compounds from different solid matrix. The focused temperature and pressure can lead to a selective target diffusion during extraction process from material to the surrounding solvent with an elevated extraction rate (Spigno and De Faveri 2009). It has been used as an alternative and new technique for the extraction of different compounds from food and various organic materials. More recently, many papers have been published on the employment of MAE for extraction of secondary metabolites from plants (Bonny et al. 2009). Some researchers (Pan Xuejun and Huizhou 2003; Hayat et al. 2009; Liu et al. 2010; Ballard et al. 2010; Krishnaswamy et al. 2012) have employed MAE extraction from various materials for their antioxidant activities. Compared to conventional methods, microwave assisted extraction has been mentioned in literature as an technique with many advantages including less solvent consumption, shorter extraction time and more extraction recovery and finally higher quality of the final product (Camel 2000). This has recently led to an increasing demand for applying MAE instead of conventional extraction techniques (Ballard et al. 2010).
Iranian coasts and its rich marine sources with an exclusive climatic condition aren’t been yet completely exploited. There are a number of species in our coastline that are been used locally and some other has no considerable application. Finding other high value substances in these unexploited resources, leading to a commercial advantages and also bring people a higher standards of life. This kind of seaweed was collected from bushehr coastline, in tidal zone, in summer because of a large quantity of it. Chaetomorpha sp. is a native species for our country which has adapted to its special climate and hasn’t any value-added usage now.
The aim of the present study was to determine the antioxidant properties of MAE of the Persian Gulf green algae (Chaetomorpha sp.) using response surface methodology to optimize processing conditions and to evaluate the effect of different initial factors on extraction procedure. Moreover, total phenolic content, total antioxidant capacity, reduction of power activity and free radicals scavenging activity assays were also investigated in our study.
Material and methods
Chemicals
Folin-Ciocalteu’s phenol reagent and sodium carbonate was purchased from Merck. 2, 2-Diphenyl-1-picrylhydrazyl (DPPH), was purchased from Sigma Aldrich Chemical Co. All solvents and other chemicals were of analytical grade.
Collection of seaweed samples
Chaetomorpha sp. was collected freshly from the coast of Bushehr (Bushehr province, Iran). Samples were thoroughly washed with seawater and tap water to remove epiphytes, sand and other particles, and then were shade-dried for three days. The samples were transported to a laboratory, freeze-dried (OPERON, OPR-FDU-7012, Korea) and pulverized completely to powder form (60 μ in size) in an electric grinder (Mulinex, La Molinette, 1000 w, France) .
Extraction procedure
The process of microwave-assisted extraction of green algae was performed in a domestic microwave oven (ME3410W. Samsung). Dried samples (2.5 g) were irradiated with 50 ml of different acetone/water ratios (pure acetone 100, 30, 50, 70 % and pure water 100 %) for different extraction times (4, 6, 8, 10, 12 min). After the extraction procedure, the algal extracts were filtered with whatman filter paper (no.4). The mixture was centrifuged (Z206A-HERMLE, Germany) at 300 rpm for 10 min and then supernatants were separated and extracts were stored at −20 °C (maximum 2 days) until antioxidant experiments were done.
Total phenolic content
Phenolic contents of seaweed extracts were determined by the method of (Taga and silvia, MEEaPDE 1984). Briefly, 200 μl aliquot of sample were mixed with 4.0 ml of 2 % Na2CO3. After incubation for 2 min at room temperature, 200 μl of 50 % Folin-Ciocalteau’s phenol reagent were added; the reaction mixture was mixed thoroughly and allowed to stand for 30 min at room temperature in the dark. Absorbance of the samples were measured at 720 nm using spectrophotometer (Lambda PerkinElmer precisely, USA). Measurements were based on calibration curve with tannic acid. Phenolic contents were expressed as mg tannic acid equivalents per gram dry weight.
| 1 |
Reducing power
Reducing power of extract obtained from green seaweeds were determined by the method described by (Chew et al. 2008), Briefly, 1.0 ml of sample was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml potassium ferricyanide (1 %). Reaction mixture was incubated at 50 C for 20 min. After incubation, 2.5 ml of trichloroacetic acid (10 %) was added and centrifuged at 3,000 rpm, 10 min. From the supernatant, 2.5 ml solution was mixed with 2.5 ml of distilled water and 0.5 ml FeCl3 (0.1 %). Absorbance of the sample solutions were read at 700 nm. Measurement was based on calibration curve with tannic acid. The results were expressed as mg tannic acid equivalents per gram dry weight.
| 2 |
Total antioxidant activity
Total antioxidant activities of extracts were determined according to the method of (Prieto et al. 1999) with some modification, 0.6 ml of sample solution was mixed with 6.0 ml reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). Reaction mixture was incubated at 95 °C for 90 min under water bath. Absorbance of the sample mixtures were measured at 695 nm. Measurements were based on ascorbic acid. Total antioxidant activities were expressed as mg ascorbic acid equivalents per gram dry weight.
| 3 |
DPPH radical scavenging activity
The scavenging effects of extract were determined as described by (Brand-Williams et al. 1995), Briefly, 2.0 ml of 0.16 mM DPPH solution (in methanol) were added to 2.0 ml of sample. The mixture was vortexed for 1 min and left to stand in room temperature for 30 min in the dark. The absorbance of the sample solutions were measured at 517 nm. The scavenging effect (percent) was calculated by using the formulae offered by (Duan et al. 2006). Sample blank and control samples were performed according to the method.
Experimental design
The recovery of the microwave assisted-extraction can be affected by different parameters such as irradiation time, microwave power and extraction solvent (Li et al. 2012). Optimization of MAE of phenolic compounds was performed using RSM. A central composite design can reveal the relation between responses and process variables that lead to the optimization of extraction process (Li et al. 2012). The most excellent combination of variables was achieved using CCD for the antioxidant properties. TPC, FRAP, DPPH and TAC values were responses which have been displayed as Y1, Y2, Y3 and Y4 respectively. Three independent variables were microwave power (X1), extraction time (X2) and acetone concentration (X3) which were selected according to the preliminary experimental data. Each independent variable included five levels (Table 1). The general second order polynomial models (Shown in Eq-4) were used for the regression analysis of data.
| 4 |
Table 1.
The coded values and actual values (uncoded) for independent variables
| Independent variables | Parameters levels | ||||
|---|---|---|---|---|---|
| − | −1 | 0 | +1 | + | |
| Microwave power, w (x 1) | 200 | 300 | 400 | 500 | 600 |
| Irradiation time, min (x 1) | 4 | 6 | 8 | 10 | 12 |
| Acetone:H2O ratio, % (x 1) | 0 | 25 | 50 | 75 | 100 |
Where Y is the predicted response, β0 a con βi the linear coefficient, βii the quadratic coefficient, βij the interaction coefficient of variables i and j, and Xi and Xj are independent variables that affect responses. The software uses above quadratic model to create response surface. The fitness of the model was demonstrated by appraising the lack of fit, coefficient of determination (R2) and analysis of variance (ANOVA) was performed for all response variables in order to determine significance of the model and parameters contributing in model (p <0.05). The coded values used in the analysis of response surface and the parallel parameter values are shown in Table 2. The design included 20 experimental points, with six replications at the center point. Table 2 shows the coded and uncoded values for the experimental design.
Table 2.
The coded and corresponding real values of experimental parameters and their actual responses in central composite design
| Standard order | Run | Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | Response 3 | Response 4 | Yield |
|---|---|---|---|---|---|---|---|---|---|
| Microwave power (W) | Irridation Time (min) | Acetone:H2O (%) | TPCa | FRAPa | DPPHb | TACc | mg/100 g dry weight | ||
| 1 | 13 | 300 (−1) | 6 (−1) | 25 (−1) | 1.096 | 0.122 | 92.859 | 0.188 | 0.21 |
| 2 | 18 | 500 (1) | 6 (−1) | 25 (−1) | 0.951 | 0.085 | 98.697 | 0.168 | 0.22 |
| 3 | 14 | 300 (−1) | 10 (1) | 25 (−1) | 0.984 | 0.109 | 96.089 | 0.178 | 0.22 |
| 4 | 9 | 500 (1) | 10 (1) | 25 (−1) | 0.996 | 0.104 | 99.543 | 0.168 | 0.22 |
| 5 | 15 | 300 (−1) | 6 (−1) | 75 (1) | 0.328 | 0.068 | 70.652 | 0.063 | 0.13 |
| 6 | 11 | 500 (1) | 6 (−1) | 75 (1) | 0.463 | 0.089 | 81.448 | 0.086 | 0.15 |
| 7 | 19 | 300 (−1) | 10 (1) | 75 (1) | 0.385 | 0.051 | 79.047 | 0.077 | 0.13 |
| 8 | 2 | 500 (1) | 10 (1) | 75 (1) | 0.537 | 0.105 | 88.815 | 0.098 | 0.16 |
| 9 | 16 | 200 (−2) | 8 (0) | 50 (0) | 0.674 | 0.077 | 99.433 | 0.120 | 0.16 |
| 10 | 5 | 600 (2) | 8 (0) | 50 (0) | 0.809 | 0.093 | 99.870 | 0.131 | 0.19 |
| 11 | 1 | 400 (0) | 4 (−2) | 50 (0) | 0.745 | 0.077 | 64.407 | 0.114 | 0.18 |
| 12 | 4 | 400 (0) | 12 (2) | 50 (0) | 0.778 | 0.085 | 73.979 | 0.136 | 0.20 |
| 13 | 8 | 400 (0) | 8 (0) | 0 (−2) | 1.012 | 0.106 | 95.012 | 0.195 | 0.24 |
| 14 | 20 | 400 (0) | 8 (0) | 100 (2) | 0.028 | 0.054 | 61.306 | 0.021 | 0.10 |
| 15 | 3 | 400 (0) | 8 (0) | 50 (0) | 0.801 | 0.094 | 86.855 | 0.107 | 0.17 |
| 16 | 17 | 400 (0) | 8 (0) | 50 (0) | 0.804 | 0.107 | 89.916 | 0.142 | 0.17 |
| 17 | 7 | 400 (0) | 8 (0) | 50 (0) | 0.796 | 0.095 | 87.384 | 0.107 | 0.19 |
| 18 | 12 | 400 (0) | 8 (0) | 50 (0) | 0.783 | 0.103 | 89.688 | 0.134 | 0.18 |
| 19 | 10 | 400 (0) | 8 (0) | 50 (0) | 0.804 | 0.110 | 88.970 | 0.133 | 0.19 |
| 20 | 6 | 400 (0) | 8 (0) | 50 (0) | 0.854 | 0.095 | 88.298 | 0.143 | 0.18 |
a All data were expressed as mg tannic acid equivalent per gram dry weight algae
b All data were expressed as Radical scavenging activity (%)
c All data were expressed as mg ascorbic acid equivalent per gram dry weight algae
Results and discussion
In the present study, MAE was employed for antioxidant extract from green algae Chaetomorpha sp. The effectual parameters were optimized using central composite design with response surface methodology. The main independent factors with influencing effect on antioxidant content of crude extract from Chaetomorpha sp. using MAE for optimization were considered as power (ranged from 200 to 600 W), irradiation time (ranged from 2 to 12 min) and acetone concentration (ranged 0 to 100 %). All data received from experimental condition with 20 runs and predicted using response surface analysis model made based on obtained data (Table 2).
Fitting the model
It is clear we need adequate data about process and parameters influencing process to establish a practical model. The software gave us the regression equations with analysis of variances (ANOVA) as follow:
These regression equations revealed the relationship between values of total phenol contents, ferric reducing power, DPPH radical scavenging activity and total antioxidant capacity of the Green algae (Chaetomorpha sp.) as responses and were influenced by initial factors including: power, irradiation time and acetone concentration which were coded as x1, x2 and x3, respectively. The model generated coefficients of regression based on analysis of variance (ANOVA) have been given in Table 3. The statistical significance of the model equation was determined by the F-test (ANOVA). The higher F-values and the smaller P-values demonstrate that the applied model and its different contributing terms are more significant. “prob > F” with the values less than 0.05 also revealed that the level of significant regression is more than 95 % coefficient. The results of the analysis of variances (ANOVA) for the models are shown in Table 3.
Table 3.
The results of analysis on variance (ANOVA) for the effects of independents variables
| Coefficient | Response | |||||||
|---|---|---|---|---|---|---|---|---|
| TPC | FRAP | DPPH | TAC | |||||
| Prob > F | F.value | Prob > F | F.value | Prob > F | F.value | Prob > F | F.value | |
| β 0 | 0.81 | – | 0.1 | – | 88.52 | – | 0.13 | – |
| β 1 | 0.0185 | 7.9 | 0.014 | 8.52 | <0.0001 | 113.03 | 0.4529 | 0.6 |
| β 2 | 0.4047 | 0.76 | 0.4092 | 0.74 | <0.0001 | 96.33 | 0.2068 | 1.77 |
| β 3 | <0.0001 | 809.05 | <0.0001 | 82.63 | <0.0001 | 1,149 | <0.0001 | 251.31 |
| β 12 | 0.0675 | – | 0.0021 | 16 | <0.0001 | 1.47 | 0.038 | 5.34 |
| β 13 | 0.1939 | – | <0.0001 | 51.34 | <0.0001 | 16.11 | – | – |
| β 23 | <0.0001 | – | – | – | <0.0001 | 17.32 | 0.294 | 1.2 |
| β 1 2 | 0.1331 | 4.2 | 0.0116 | 9.13 | 0.2593 | 235.65 | – | – |
| β 2 2 | 0.0027 | 1.92 | 0.0026 | 15.02 | 0.0039 | 568.24 | – | – |
| β 3 2 | 0.0917 | 89.21 | 0.0015 | 17.59 | 0.0032 | 163.28 | 0.0699 | 3.9 |
| Model | <0.0001 | – | <0.0001 | – | <0.0001 | – | <0.0001 | – |
| Lack of fit | 0.0864 ns | – | 0.8332 ns | – | 0.9801 ns | – | 0.9901 ns | – |
| R 2 | 0.98 | – | 0.94 | – | 0.99 | – | 0.95 | – |
| Adj–R 2 | 0.97 | – | 0.90 | – | 0.99 | – | 0.93 | – |
| Pre–R 2 | 0.92 | – | 0.83 | – | 0.99 | – | 0.91 | – |
| CVpercent | 5.15 | – | 6.29 | – | 1.17 | – | 9.13 | – |
Tables 3 exhibits the effect of main factors and their interactions which were analyzed using response surface methodology (Patil et al. 2011). P-values with low value (<0.0001) revealed that regression model is more significant. The propriety and fitness of the models were evaluated by determination of the coefficient (R2). In our study, as shown in Table 3 determination of coefficient for total phenol content, ferric reducing power, radical scavenging activity and total antioxidant capacity were 0.98, 0.94, 0.99 and 0.95, respectively, which demonstrated the significance of these regressions. Predicted determination of the coefficient with the values of 0.92, 0.83, 0.99 and 0.91 and adjusted determination with values of 0.97, 0.90, 0.99 and 0.93 for the above responses were in rational agreement. On the other hand, coefficient of variance with low values shows high accuracy in the applied models and the experiments.
Effects of process variables
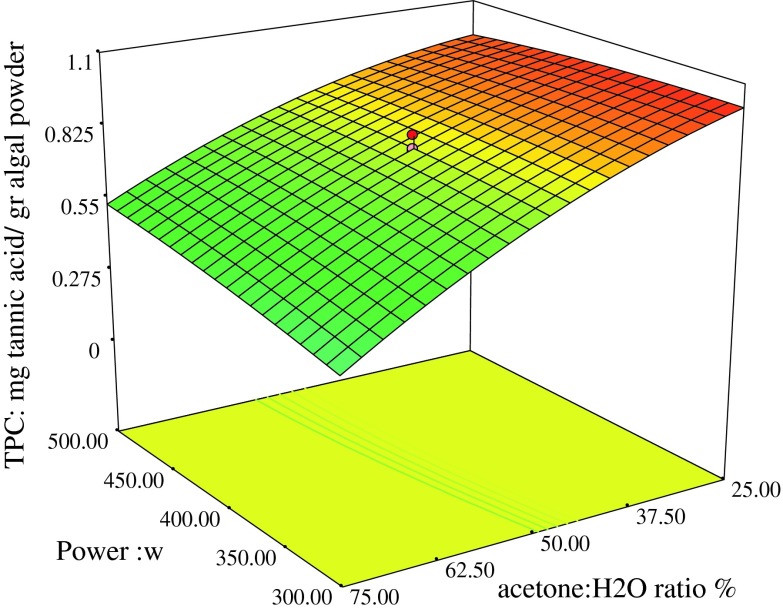
To investigate the effects of process variables and their interactive effects on antioxidant content of the algal crude extract, the three-dimensional plots were depicted in Figs. 1–4. These three dimensional response surface plots can exhibit how the initial factors affected different responses, more clearly. Figures 1–4 show the effects of different experimental parameters (microwave power, extraction time and acetone concentration) based on the obtained responses. According to analysis of the data demonstrated in Table 3, it was concluded that acetone concentration had a main effect on TPC, FRAP, DPPH scavenging activity and TAC with a positive linear effect (p <0.01). Figure 1 shows the effects of acetone concentration and power on total phenol content. Effect of the acetone concentration can be related to the solvent type, because acetone is a microwave-transparent solvent documented is the best selection for extraction of phenolics from plant tissue as we use microwave energy for extraction and this is because of the better absorption of energy causing rupture of plant cell walls and finally releasing target compounds into surrounding extraction solvent (Proestos and Komaitis 2008). As shown in Table 4, these factors have significant effects (p <0.05) on responses. Decrease in the acetone concentration caused an increase in TPC. This effect may be related to the change of solvent polarity with change in acetone concentration. The polarity of acetone–water mixture increased continuously with the addition of water to extraction solvent and caused extraction of phenolic compounds with more polarity characteristics. Moreover polarity of solvents can affect the composition of phenolic compounds (Tabaraki and Nateghi 2011). In addition, when dried extraction materials with low moisture contents, were exposed to the microwave heating, this low moisture evaporated that led to an increasing pressure within cells and resulted in wall rupture and finally diffusion of bioactive components into the extraction solvent which evaluate phenolic compound extraction (Ballard et al. 2010). The simultaneous effects of time and power on reducing power are shown in Fig. 2a. It was found that longer extraction time, led to a higher ferric reducing power. It’s been reported that in longer extraction time, phenolic compounds have time enough to dissolve in surrounding solvent. It was also mentioned that lower extraction time and low power may decrease extraction mass transfer into extraction solvent (Li et al. 2012). The FRAP mainly depended on acetone concentration as its effect was significant (p < 0.01). It was reported that microwave power, solvent concentration, extraction time and the interaction between microwave power, solvent concentration and time, had a significant effect on the antioxidant activity involving DPPH and FRAP. The diffusion of the antioxidants from the matrix to the surrounding extraction solvent is increased when the extraction time increases (Krishnaswamy et al. 2012). As evident in Fig. 2b, the FRAP value decreased when the acetone concentration increased. When solvent concentration increased, the polarity of the solvent changed resulting in an increasing rate of impurities extraction which affected antioxidant properties of extract and even decreased its ferric reducing power activities. Decrease in diffusion because of the protein’s coagulation may lead to low FRAP value of phenolic compounds (Li et al. 2012).
Fig. 1.
TPC (mg tannic acid/g algal powder), Power (W) and acetone: H2O (%)
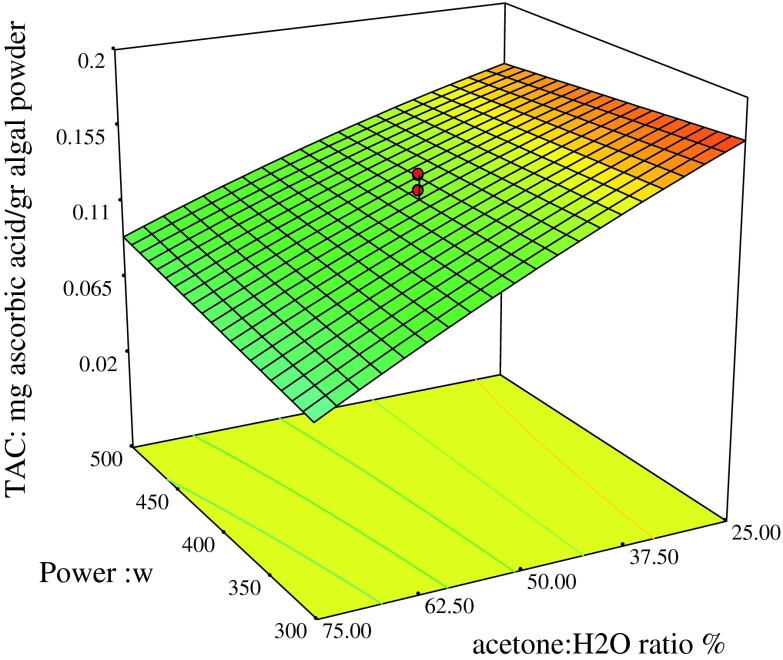
Fig. 4.
TAC (mg Ascorbic acid/g algal powder), acetone: H2O (%) and Power (W)
Table 4.
The best value of various responses under optimized condition
| TAC | DPPH | FRAP | TPC | x 3 | x 2 | x 1 |
|---|---|---|---|---|---|---|
| Acetone concentration | Time | power | ||||
| 0.38 | 99.38 | 0.086 | 0.98 | 25 % | 8 | 300 |
Fig. 2.
a FRAP (mg tannic acid/g algal powder), Time (min) and Power (W). b FRAP (mg tannic acid/g algal powder), acetone: H2O (%) and Power (W)
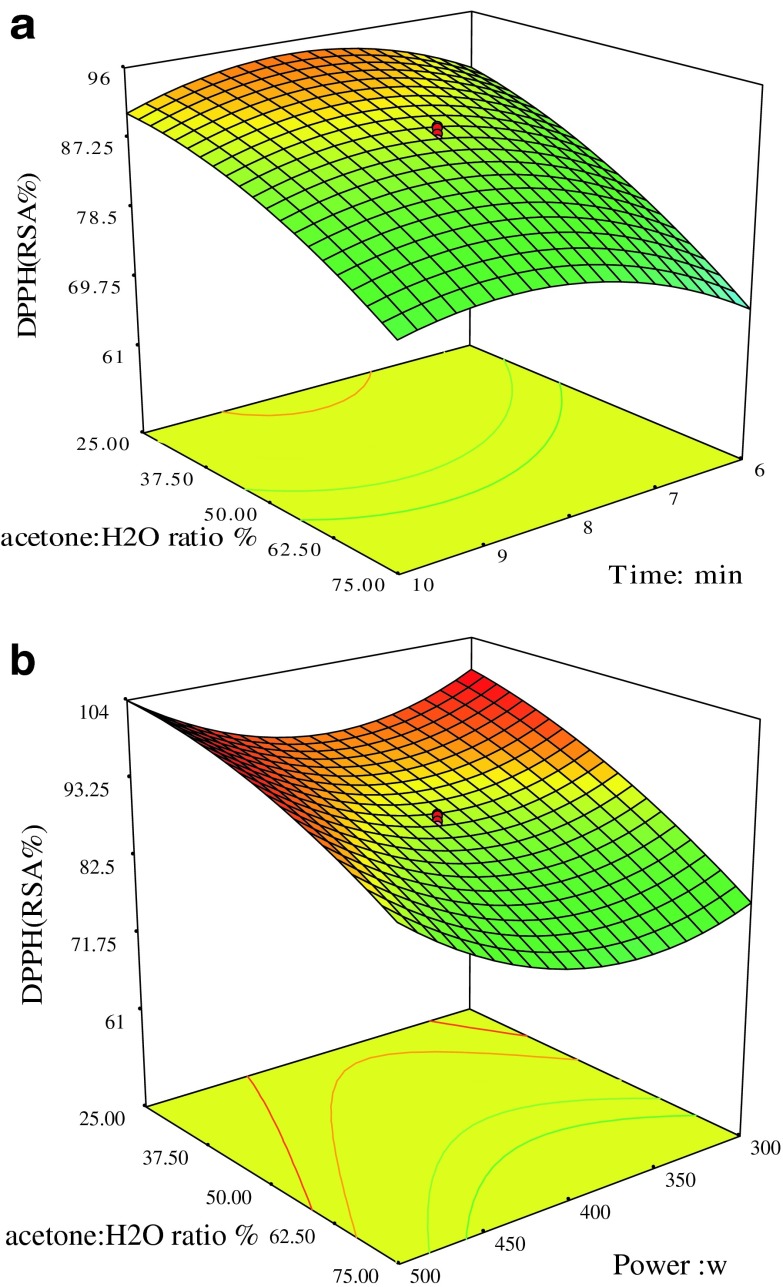
The influence of initial solvent concentration, time and power on DPPH radical scavenging activity is shown in Figs. 3a and b. It was found according to the results of ANOVA shown in Table 3 the interaction between power, acetone concentration and time were significant (p <0.01). As revealed in Fig. 3a, increasing in extraction time to a specific value had a positive effect on enhancement of the DPPH because it can increase diffusion of the target compounds from matrix samples (Krishnaswamy et al. 2012) and give them a break to dissolve into extraction solvent (Tabaraki and Nateghi 2011) but lengthy extraction duration can lead to the demolition of antioxidant compounds (Krishnaswamy et al. 2012). As shown in Fig. 3b, decrease in acetone concentration and increase in the microwave power led to an increase in DPPH radical scavenging activity. The linear terms of initial factors had positive influence on DPPH assay. The interaction between solvent concentration and microwave power is shown in Fig. 4a. It was found that decrease in the acetone concentration can improve total antioxidant activity. Solvent extraction has an important and basic effect on extraction of natural products. Different solvent compositions are capable of modifying phenolics extraction profile and finally their antioxidative activity (Li et al. 2012). When polarity of a solvent is changed, it may be led to the extraction of impurities and reduction of antioxidant activities (Huang et al. 2009). The response surface plot of antioxidant assay indicates that the extraction power had no significant effect as it can be concluded from Table 3, while the linear term of acetone concentration showed positive influence and was very significant (p <0.01).
Fig. 3.
a DPPH (RSA %), acetone: H2O (%) and Time (min). b DPPH (RSA %), acetone: H2O (%) and Power (W)
Verification experiments
Under optimized process, power (300 w), time (8 min) and acetone concentration (25 %), the best values of different responses TPC, FRAP, DPPH and TAC are shown in Table 4. In order to evaluate the sufficiency of the models, verification experiments were carried out at the predicted conditions derived from analysis of RSM with desirability within 97 % coefficient of experimental values. Under desired condition each parameter and response was chosen as its optimized value. The actual results and their contrast predicted values are presented in Table 5. The experimental values were rationally adjacent to the predicted values in different antioxidant responses and no significant differences were found between them, which confirmed the suitability and fitness of the predicted responses and made it clear that the employed models were adequate enough to predict the optimum condition for different TPC, FRAP, DPPH and TAC assays.
Table 5.
Optimum condition for antioxidant components extraction using microwave assisted extraction (MAE): TPC, FRAP, DPPH, TAC
| Optimum condition | |
| Power (w) | 300 |
| Irradiation time (min) | 8 |
| Solvent concentration percent | 25 |
| Predicted values | |
| TPC, mg tannic acid/gram dry weight algae | 1.02 |
| FRAP, mg tannic acid/gram dry weight algae | 0.12 |
| DPPH, Radical scavenging activity (percent) | 99.17 |
| TAC, mg ascorbic acid/gram dry weight algae | 0.18 |
| Actual values | |
| TPC, mg tannic acid/gram dry weight algae | 0.98 |
| FRAP, mg tannic acid/gram dry weight algae | 0.086 |
| DPPH, Radical scavenging activity (percent) | 99.38 |
| TAC, mg ascorbic acid/gram dry weight algae | 0.16 |
Conclusion
The high degree relationship of the models demonstrate that second order polynomial model could be applied to optimize the extraction of phenolic and antioxidant compounds from the green algae (Chaetomorpha sp.) to evaluate the antioxidant activity. Aqueous acetone was shown to be an effective solvent for antioxidant and phenolic component extraction from the green algae. In this study, we optimize the extraction condition to have maximum recovery with less energy, solvent consumption and extraction time in microwave assisted-extraction as a novel technique to extract high value products from marine sources. Therefore, the optimized extraction condition for TPC, FRAP, DPPH and TAC were in the following order: power (300 w), extraction time (8 min) and acetone concentration (25 %). The values of the predicted data were in agreement with the experimental value.
Abbreviation
- MAE
as microwave assisted-extraction
- RSM
as response surface methodology
- TPC
as total phenol content
- FRAP
as ferric reducing power
- TAC
as total antioxidant capacity
References
- Airanthi MKW-A, Hosokawa M, Miyashita K. Comparative antioxidant activity of edible japanese brown seaweeds. J Food Sci. 2011;76(1):C104–C111. doi: 10.1111/j.1750-3841.2010.01915.x. [DOI] [PubMed] [Google Scholar]
- Ballard TS, Mallikarjunan P, Zhou K, O’Keefe S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010;120(4):1185–1192. doi: 10.1016/j.foodchem.2009.11.063. [DOI] [Google Scholar]
- Blanc N, Hauchard D, Audibert L, Ar Gall E. Radical-scavenging capacity of phenol fractions in the brown seaweed ascophyllum nodosum: an electrochemical approach. Talanta. 2011;84(2):513–518. doi: 10.1016/j.talanta.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Bonny S, Hitti E, Boustie J, Bernard A, Tomasi S. Optimization of a microwave-assisted extraction of secondary metabolites from crustose lichens with quantitative spectrophotodensitometry analysis. J Chromatogr A. 2009;1216(45):7651–7656. doi: 10.1016/j.chroma.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Camel V. Microwave-assisted solvent extraction of environmental samples. TrAC Trends Anal Chem. 2000;19(4):229–248. doi: 10.1016/S0165-9936(99)00185-5. [DOI] [Google Scholar]
- Chew YL, Lim YY, Omar M, Khoo KS. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT Food Sci Technol. 2008;41(6):1067–1072. doi: 10.1016/j.lwt.2007.06.013. [DOI] [Google Scholar]
- Duan X-J, Zhang W-W, Li X-M, Wang B-G. Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem. 2006;95(1):37–43. doi: 10.1016/j.foodchem.2004.12.015. [DOI] [Google Scholar]
- Fan D, Hodges DM, Zhang J, Kirby CW, Ji X, Locke SJ, Critchley AT, Prithiviraj B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011;124(1):195–202. doi: 10.1016/j.foodchem.2010.06.008. [DOI] [Google Scholar]
- Ganesan P, Kumar CS, Bhaskar N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol. 2008;99(8):2717–2723. doi: 10.1016/j.biortech.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Hayat K, Hussain S, Abbas S, Farooq U, Ding B, Xia S, Jia C, Zhang X, Xia W. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep Purif Technol. 2009;70(1):63–70. doi: 10.1016/j.seppur.2009.08.012. [DOI] [Google Scholar]
- Huang W, Xue A, Niu H, Jia Z, Wang J. Optimised ultrasonic-assisted extraction of flavonoids from Folium eucommiae and evaluation of antioxidant activity in multi-test systems in vitro. Food Chem. 2009;114(3):1147–1154. doi: 10.1016/j.foodchem.2008.10.079. [DOI] [Google Scholar]
- Krishnaswamy, K., Orsat, V., Gariépy, Y., Thangavel, K. (2012) Optimization of Microwave-Assisted Extraction of Phenolic Antioxidants from Grape Seeds (Vitis vinifera). Food bioprocess tech :1–15
- Kumar KS, Ganesan K, Rao PVS. Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty - An edible seaweed. Food Chem. 2008;107(1):289–295. doi: 10.1016/j.foodchem.2007.08.016. [DOI] [Google Scholar]
- Li Y, Qian Z-J, Ryu B, Lee S-H, Kim M-M, Kim S-K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg Med Chem. 2009;17(5):1963–1973. doi: 10.1016/j.bmc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Li H, Deng Z, Wu T, Liu R, Loewen S, Tsao R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012;130(4):928–936. doi: 10.1016/j.foodchem.2011.08.019. [DOI] [Google Scholar]
- Liu J-L, Yuan J-F, Zhang Z-Q. Microwave-assisted extraction optimised with response surface methodology and antioxidant activity of polyphenols from hawthorn (Crataegus pinnatifida Bge.) fruit. Int J Food Sci Technol. 2010;45(11):2400–2406. doi: 10.1111/j.1365-2621.2010.02416.x. [DOI] [Google Scholar]
- Matanjun P, Mohamed S, Mustapha N, Muhammad K, Ming C. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J Appl Phycol. 2008;20(4):367–373. doi: 10.1007/s10811-007-9264-6. [DOI] [Google Scholar]
- Pan Xuejun NG, Huizhou L. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem Eng Process. 2003;42:129/133. [Google Scholar]
- Pan Y, Wang K, Huang S, Wang H, Mu X, He C, Ji X, Zhang J, Huang F. Antioxidant activity of microwave-assisted extract of longan (Dimocarpus Longan) peel. Food Chem. 2008;106(3):1264–1270. doi: 10.1016/j.foodchem.2007.07.033. [DOI] [Google Scholar]
- Patil PD, Gude VG, Mannarswamy A, Cooke P, Munson-McGee S, Nirmalakhandan N, Lammers P, Deng S. Optimization of microwave-assisted transesterification of dry algal biomass using response surface methodology. Bioresour Technol. 2011;102(2):1399–1405. doi: 10.1016/j.biortech.2010.09.046. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Proestos C, Komaitis M. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT Food Sci Technol. 2008;41(4):652–659. doi: 10.1016/j.lwt.2007.04.013. [DOI] [Google Scholar]
- Rodríguez-Bernaldo de Quirós A, Frecha-Ferreiro S, Vidal-Pérez A, López-Hernández J. Antioxidant compounds in edible brown seaweeds. Eur Food Res Technol. 2010;231(3):495–498. doi: 10.1007/s00217-010-1295-6. [DOI] [Google Scholar]
- Souza BWS, Cerqueira MA, Martins JT, Quintas MAC, Ferreira ACS, Teixeira JA, Vicente AA. Antioxidant potential of two red seaweeds from the Brazilian Coasts. J Agric Food Chem. 2011;59(10):5589–5594. doi: 10.1021/jf200999n. [DOI] [PubMed] [Google Scholar]
- Spigno G, De Faveri DM. Microwave-assisted extraction of tea phenols: a phenomenological study. J Food Eng. 2009;93(2):210–217. doi: 10.1016/j.jfoodeng.2009.01.006. [DOI] [Google Scholar]
- Tabaraki R, Nateghi A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason Sonochem. 2011;18(6):1279–1286. doi: 10.1016/j.ultsonch.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Taga, M., silvia, MEEaPDE (1984) Chia Seeds as a Source of Natural Lipid Antioxidants. JAOCS ~ vol. 61, no, 5
- Wang T, Jónsdóttir R, Ólafsdóttir G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009;116(1):240–248. doi: 10.1016/j.foodchem.2009.02.041. [DOI] [Google Scholar]
- Zubia M, Fabre MS, Kerjean V, Lann KL, Stiger-Pouvreau V, Fauchon M, Deslandes E. Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem. 2009;116(3):693–701. doi: 10.1016/j.foodchem.2009.03.025. [DOI] [Google Scholar]