Abstract
The stability of antioxidants in extruded and nixtamalized blue maize flours with calcium hydroxide [Ca(OH)2] and calcium lactate [C6H10O6Ca] were evaluated. Extruded blue maize flours batches were obtained by mixing blue maize flours separately with different Ca(OH)2 (0.1, 0.2 and 0.3 %) and C6H10O6Ca (0.3, 0.6 and 0.9 %) concentrations respectively and extruded to obtain the extruded flours. For nixtamalized flours, the maize grains were cooked at 1 % Ca(OH)2 and 2.95 % C6H10O6Ca concentrations respectively. Color, antioxidant activity, total phenolics, total anthocyanins and individual anthocyanins, contents were analyzed. Color, antioxidant activity, anthocyanins contents and total phenolics decreased as the calcium hydroxide concentration increased. In contrast, increasing the calcium lactate concentration on the extruded flours had the opposite effect. The extrusion process retained 57–47 %, 72–62 % and 79–65 % of the anthocyanins content, total phenolic content and antioxidant activity, respectively. These retention rates were higher than those of the nixtamalized flours using the same calcium sources. Cyanidin-3-glucoside and pelargonidin-3-glucoside were identified in the maize kernel and flours. Cyanidin-3-glucoside concentration was increased by both extrusion and nixtamalization processed with either of the two calcium sources. In contrast, pelargonidin-3-glucoside concentration decreased by both processes. Other anthocyanins were observed, but they were not identified.
Keywords: Blue maize, Extrusion, Nixtamalization, Antioxidants, Anthocyanins
Introduction
Pigmented maize nixtamalization is a common practice in small communities in Latin American countries. However, the processing of pigmented maize has grown in the last decade due to the demand of consumers for nixtamalized products high in antioxidants such as tortillas, snacks and tortilla chips, among others. The colors of the pigmented maize such as blue maize grains are attributed to anthocyanins contained in their pericarp and aleurone layer (Urías-Peraldí et al. 2013). These natural pigments are water-soluble and are important because, besides giving color, they have biological activity and have been associated with several health benefits such as prevention of cardiovascular diseases, as well as cancer, obesity and diabetes control and in the improvement of visual and brain functions, among others disorders (Tsuda 2012). In its natural form the anthocyanin structure is esterified to one or several sugars; when anthocyanins contain an acyl group these are named acyl anthocyanins (Salinas-Moreno et al. 2003). In aqueous phase anthocyanins exist in a mixture of four molecular species, the concentration of these forms varies according to the pH. At pH below 2 the anthocyanins exist predominantly as the red flavylium cation, while at pH of 3 to 6, the colorless carbinol or pseudobase is formed, and at neutral and slightly acidic pH the quinoidal base is generated, showing blue or violet color which becomes the colorless chalcone (He and Giusti 2010; Tsuda 2012). Thereby, in acidic media (at low pH values) the antocyanins are more stable than in alkaline solutions where they become more susceptible to degradation, this condition can be compared with the nixtamalization process, provoking changes in these anthocyanins and therefore in their color. Among the different anthocyanin species reported in blue and purple corn the major anthocyanin present in these grains is the cyanidin-3-glucoside (Salinas-Moreno et al. 2003; Pedreschi and Cisneros-Zeballos 2007). Likewise, pelargonidin, and peonidin glucosides have been found in maize plants whereas acyl antocyanins has been reported in processed maize products (Salinas-Moreno et al. 2003; De Pascual-Teresa et al. 2002).
Maize nixtamalization is a process that gives the physical, chemical and sensorial characteristics of the maize products. It consists in the cooking and steeping of maize grain in a calcium hydroxide [Ca(OH)2] solution. The cooking liquor, or nejayote is drained; the grain is washed, milled and finally dried to produce instant flour. Instant corn flour is used to make tortillas and derivative products (Ruiz-Gutiérrez et al. 2012). However, the nixtamalization of pigmented maize has the disadvantage that the bioactive compounds, like the anthocyanins are degraded in the alkaline pH of the cooking solution (Salinas-Moreno et al. 2003; Del Pozo-Insfran et al. 2007) and at the same time free phenolic compounds are released from the pericarp into the cooking solution (Cortés-Gómez et al. 2006; López-Martínez et al. 2011). The alkaline medium provokes some chemical changes in the molecular structure of the anthocyanins resulting in color changes (Salinas-Moreno et al. 2003; Cortés-Gómez et al. 2006), additionally, nejayote discharges generates environmental pollution problems.
The use of combined or individual compounds, with or without calcium present in the cooking medium has been reported (Maya et al. 2010; Ruiz-Gutiérrez et al. 2012). Recently, a study showed that the use of calcium lactate [C6H10O6Ca] in nixtamalization resulted in tortillas with good acceptance for the consumers (Ruiz-Gutiérrez et al. 2012). Calcium lactate produce neutral pH values representing an advantage in the pigmented maize nixtamalization because that could reduce the degradation of anthocyanins as compared to the traditional nixtamalization process with alkaline Ca(OH)2.
An alternative technology to produce instant flours is the extrusion process. This is a low cost, energy efficient, highly productive, not pollutant and versatile technology, which generate high quality products. Some reports have described the use of extrusion-cooking for the production of instant corn flours (Reyes-Moreno et al. 2003; Gutiérrez-Dorado et al. 2008). Previous studies have reported the effects of both lime cooking and extrusion on the antioxidant content in blue maize tortilla (Mora-Rochín et al. 2010; Aguayo-Rojas et al. 2012). Furthermore, in a recent study realized in our laboratory (Sánchez-Madrigal et al. 2014) the addition of C6H10O6Ca on the functional and physical properties of extruded and nixtamalized blue maize flours were evaluated. It was found that C6H10O6Ca was a convenient alternative source to lime in the extrusion and nixtamalization of blue maize, this due to the improvement in the rheological properties of instant maize flours. However, the effect of C6H10O6Ca on the retention of bioactive compounds during blue maize processing to obtain instant flours high in antioxidants have not been reported.
The aim of this study was to investigate the effect of Ca(OH)2 and C6H10O6Ca at different concentrations on the antioxidant stability of extruded and nixtamalized blue maize flours.
Materials and methods
Chemicals and reagents
Cyanidin-3-glucoside, cyanidin-3-galactoside, pelargonidin-3-glucoside, malvidin-3-glucoside, Folin-Ciocalteu’s phenol reagent, gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH•), and 6-hidroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from the Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). HPLC-grade solvents (water and methanol) were obtained from J.T. Baker (Mexico City, Mexico), formic acid was obtained from Fluka. Other analytical grade solvents used for extractions were purchased from J.T. Baker.
Materials
Blue maize (Zea mays L.) from region of Babicora, Chihuahua State, Mexico, was used. Calcium hydroxide [Ca(OH)2] and calcium lactate [C6H10O6Ca] were used.
Flour preparation for extrusion
A lot (850 g) of blue maize was placed in a hammer mill (Pulvex model 200, Mexico) equipped with a 5 mm sieve to break the kernels and obtain 2–3 mm grits and fine powder. Each lot was mixed with different concentrations of Ca(OH)2 (0.1, 0.2 and 0.3 %) and C6H10O6Ca (0.3, 0.6 and 0.9 %) and water was added to reach a moisture content of 30 g/100 g. A batch without added calcium was prepared similarly to serve as control (EF control). Each batch was packed for a tempered in a polyethylene bag and stored for 14 h at 4 °C.
Extrusion process
The extrusion experiments were performed with a single-screw extruder that was designed and constructed at CINVESTAV-IPN (Queretaro, Mexico). It was equipped with barrel with a 25 mm diameter and 428 mm length, a 1:1 extruder screw compression ratio, and a die opening of 4.0 mm. The barrel was separated into independent electrically heated feed and compression zones. The die end of the barrel was also electrically heated. The extruder speed was 30 Hz and the feed rate was 30 g/min, which remained constant. Samples tempered of blue maize for each treatment of Ca(OH)2 or C6H10O6Ca were extruded maintaining temperature at 50, 70 and 80 °C in the feed, compression and die end barrel zones respectively. The extrudates were dried at 45 °C for 48 h in an oven (Felisa, Mexico) until a moisture content of 0.09–0.11 g/kg was achieved.
Extruded blue maize flours
The extrudates were milled in a hammer mill with a 0.8 mm sieve and then the flours were passed through a 60-mesh sieve to obtain uniform particle size. Extruded blue maize flours (EFs) were stored in the dark at 4 °C in hermetically sealed plastic bags until analysis.
Nixtamalized blue maize flours
Two different nixtamalized blue maize flours (NFs) were prepared. Lots of 1 kg of blue maize were nixtamalized using two different calcium solutions. Each lot was cooked in a solution of 1 % [Ca(OH)2], and the other was cooked in a solution of 2.95 % [C6H10O6Ca] in a 1:3 maize:solution ratio at 90 °C for 40 min followed by a 15 h period of steeping. The cooking liquor (nejayote) was discarded, and the cooked grain (nixtamal) was washed twice with water to remove excess Ca(OH)2 or C6H10O6Ca. The nixtamal was milled in a stone mill (FUMASA, Mexico), dried in a flash dryer that was designed and constructed at CINVESTAV-IPN (Queretaro, Mexico), with an input temperature of 295 °C and an output temperature of 90 °C. The dehydrated flours were milled again in a hammer mill (Pulvex model 200, Mexico) with a 0.8 mm sieve to equalize the particle size of extruded flours. After that the flours were passed through a 60-mesh sieve to obtain uniform particle size. The sifted flour was stored in the dark at 4 °C in hermetically plastic bags for analysis.
pH of blue maize flours
The pH values were determined by the standard AACC (2000) method. This parameter was determinates by triplicate and report an average value.
Color of blue maize flours
The color was measured with a Konica Minolta CR-400/410 (Minolta Co., Osaka, Japan) colorimeter, which was calibrated with a plate with X = 94.9, y = 0.3185 and x = 0.3124 values. The L* (luminosity), a* (green to red) and b* (blue to yellow) parameters of the flours were determined from 10 measurements for each treatment product (Ruiz-Gutiérrez et al. 2012).
Total anthocyanins content
The total anthocyanins content (TA) was determined according to method described by Abdel-Aal and Hucl (1999). 1.5 g of sample was homogenized with 12 mL of an acidified methanol solution (methanol and 1 N HCl, 85:15, v/v). The solution was adjusted to pH 1 with 4 N HCl and stirred for 30 min and centrifuged at 3,200 × g (Thermo IEC model CL3-R, USA) for 45 min. The supernatant was poured into a 25 mL volumetric flask and brought to that volume with acidified methanol. The absorbance at 535 nm was obtained using a reagent blank. TA content was expressed as mg of cyanidin-3-glucoside equivalents/100 g and was calculated by the following formula:
where C is the concentration of the total anthocyanins (mg of cyanidin-3-glucoside equivalents per 100 g of sample), ε is the molar absorptivity (cyanidin-3-glucoside = 25,965/cm/M), and MW is the molecular weight of the cyanidin-3-glucoside, (449.2 g/mol). This determination was performed in triplicate for each extract.
Total phenolics content
The total phenolics content (TP) was determined according to the Folin-Ciocalteu colorimetric method described by Singleton et al. (1999), with some modifications. Gallic acid was used to obtain the calibration curve and deionized water was used as solvent. The extracts were prepared according to Dykes et al. (2005). A sample of 0.5 g was homogenized with 25 mL of 1 % HCl/methanol (v/v) and stirred for 2 h. The extracts were centrifuged at 3,200 × g (Thermo IEC model CL3-R, USA) for 30 min and then were decanted. A mixture of 30 μL of the extract, 3 mL of deionized water and 200 μL of Folin-Ciocalteu’s phenol reagent was prepared and allowed to stand for 10 min at room temperature. The reaction was neutralized with 600 μL of a 20 % sodium carbonate solution. This mixture was incubated for 20 min at 40 °C in a water bath and then cooled on ice. The absorbance was measured at 760 nm using a spectrophotometer (PerkinElmer model Lambda 25 UV/VIS, USA). The values were compared with those from a standard curve prepared with known concentration of gallic acid. The results were expressed as mg of gallic acid equivalents per 100 g of sample (mg GAE/100 g). This determination was performed in triplicate for each extract.
Determination of antioxidant activity
The antioxidant activity (AA) was measured using the free radical method of Brand-Williams et al. (1995). The free radical used in this study was 2,2-diphenyl-1-picrylhydrazyl (DPPH•). The extracts were prepared similarly to those for the determination of the phenolic content except that only methanol was used. An aliquot of 3.9 mL of 60 μM DPPH• in methanol was added to 0.1 mL of the extract. The mixture was shaken vigorously and allowed to stand at room temperature in the dark for 3 h, at which time the decrease in absorbance at 515 nm was measured using a spectrophotometer (PerkinElmer model Lambda 25 UV/VIS, USA.). The results were expressed as μmol of Trolox equivalents per gram of sample (μmol TE/g). This determination was performed in triplicate for each extract.
HPLC analysis of anthocyanins
The extracts were prepared according to Kühnen et al. (2011) with some modifications. 400 mg of sample was homogenized with 1 mL of 1 % HCl/methanol(v/v) and stirred in the dark for 24 h at 4 °C. The resulting extracts were centrifuged at 1,538 × g (Eppendorf AG model 5415D, Hamburg, Germany) for 10 min and decanted. The supernatant was centrifuged at 8,855 × g for 20 min. The extracts were poured into amber vials for HPLC analysis. All of the extracts were prepared in triplicate. The analysis of the anthocyanins was performed using an HPLC system equipped with a diode array detector and an autosampler (DAD Varian ProStar model 410, Palo Alto, CA). A Spherisorb ODS-2 column (5 μm, 250 × 46 mm; Waters, Ireland) was used. Aqueous 5 % formic acid (solvent A) and 100 % methanol (solvent B) were used as the elution solvents. The samples were eluted according to the following gradient: 0–30 min with 14 % solvent B and 30–45 min with 45 % solvent B. The flow rate was 1.5 mL/min, and the injection volume was 20 μL. The chromatograms were processed at 525 nm and the spectrum between 250 and 550 nm was obtained. The anthocyanins in the sample were identified by their retention time using the following standards: cyanidin-3-glucoside, cyanidin-3-galactoside, pelargonidin-3-glucoside and malvidin-3-glucoside. The proportion of each anthocyanin in the extract was calculated from the percentage area of each peak in the chromatogram.
Statistical analysis
The results were analyzed by nested ANOVA, where factor A was the calcium source [Ca(OH)2 and C6H10O6Ca] and factor B that was nested in A was the concentration of each calcium source [0.1 %, 0.2 % and 0.3 % for Ca(OH)2 and 0.3 %, 0.6 % and 0.9 % for C6H10O6Ca]. In addition, a contrast analysis of the mean differences between the results for EFs and NFs was performed. Significance was defined as P < 0.05 using SAS software version 9.2 (2007) (SAS Institute Inc., Cary, NC, USA).
Results and discussion
Color
The L* parameter of EFs was significantly affected (P < 0.05) by both calcium sources (Table 1) and only significant effects are observed for the different concentrations of Ca(OH)2 in EFs in this parameter. The L* value decreased as the Ca(OH)2 concentration increased; higher Ca(OH)2 concentrations produced darker flours. In addition, the L* value of EF control was not significantly different with most of the EFs; presented only differences with the EFs prepared with 0.2 % and 0.3 % of Ca(OH)2. The L* parameter of both NFs was significantly different from each other (P < 0.05) and from that of the EFs; being the NFs more luminous than EFs.
Table 1.
Results of color of blue maize flours with different calcium sources
| Flours | [Conc] (%) | Color | |||
|---|---|---|---|---|---|
| L* | a* | b* | ΔE | ||
| EF Ca(OH)2 | 0.10 | 66.41 ± 0.22c | 6.34 ± 0.35c | −1.36 ± 0.14f | 32.32 ± 0.13bc |
| 0.20 | 65.09 ± 0.44d | 5.04 ± 0.12d | −1.13 ± 0.19e | 33.37 ± 0.40b | |
| 0.30 | 63.78 ± 0.03e | 3.97 ± 0.28e | −0.56 ± 0.03d | 34.48 ± 0.12a | |
| EF C6H10O6Ca | 0.30 | 66.11 ± 0.21c | 6.72 ± 0.27c | −0.12 ± 0.02c | 32.60 ± 0.26bc |
| 0.60 | 66.28 ± 0.10c | 7.75 ± 0.40b | −0.54 ± 0.06d | 32.69 ± 0.01bc | |
| 0.90 | 66.75 ± 0.48c | 8.51 ± 0.09a | −0.73 ± 0.07d | 32.43 ± 0.49bc | |
| EF control | 0.00 | 66.94 ± 1.05c | 8.48 ± 0.06a | −1.27 ± 0.08ef | 32.28 ± 1.19c |
| NF Ca(OH)2 | 1.00 | 69.81 ± 0.04b | 3.00 ± 0.05f | 2.93 ± 0.01a | 28.38 ± 0.03d |
| NF C6H10O6Ca | 2.95 | 72.10 ± 0.31a | 6.49 ± 0.07c | 1.10 ± 0.01b | 26.69 ± 0.28e |
Means values ± standard error of triplicate of two repetition treatments. Means by column and treatment with different letters shows significant difference, contrasts test (P < 0.05)
EF Extruded Flours, NF Nixtamalized Flours
The a* parameter of color shown in Table 1 was affected by the increase of both calcium sources concentrations. The a* parameter values decreased as the Ca(OH)2 concentration increased, and it increased as the C6H10O6Ca concentration increased. These results indicate that the red coloration of EFs decreased as the Ca(OH)2 concentration increased, whereas increasing the C6H10O6Ca concentration in EFs provoked an intense reddish coloration. The EF control showed the same reddish coloration as that observed in EF with the highest concentration of C6H10O6Ca. The NF prepared with Ca(OH)2 exhibited an a* value significantly different (P < 0.05) from those of the rest of flours, presenting the lowest reddish coloration. The NF prepared with C6H10O6Ca presented a similar reddish coloration to the EFs processed with the lowest concentrations of both calcium sources.
The b* parameter of extruded and nixtamalized blue maize flours was significantly affected (P < 0.05) by calcium sources and their concentrations (Table 1). The values for this parameter decreased as the Ca(OH)2 concentration increased and increased as the C6H10O6Ca concentration increased. Increases in the Ca(OH)2 concentration decreased the blue coloration of EFs, showing blue-gray hues. Whereas that increases of the C6H10O6Ca concentration increased the blue coloration in the EFs. Despite this, the blue coloration achieved was lower compared with the EF prepared with Ca(OH)2. Similar blue coloration was achieved in the EF with 0.3 % Ca(OH)2 and EFs prepared with 0.6 % and 0.9 % C6H10O6Ca. The b* values for both NFs were significantly different (P < 0.05) from those of all EFs, with positive values that indicated an inclination to yellowish coloration, typical hues of traditional nixtamalized flours.
The color difference (ΔE) of EFs was significantly affected (P < 0.05) only by the Ca(OH)2 concentration (Table 1). The EF control showed ΔE similar (P < 0.05) to EF prepared with 0.1 % Ca(OH)2 and all EFs prepared with C6H10O6Ca. The ΔE values for both NFs were significantly different (P < 0.05) to all EFs, showing the lowest values in color difference. The ΔE of NFs indicate higher color degradation due to long exposure time required for nixtamalization process. In general, EFs presented higher ΔE values than did the NFs, indicating that the extrusion process retained more the blue color.
Total anthocyanins content
The total anthocyanins content in blue maize extruded flours was significantly affected (P < 0.05) by both calcium sources and their concentrations (Table 2). The TA decreased with the increase of the Ca(OH)2 concentrations and increased as the C6H10O6Ca concentrations increased achieving the maximum values at 0.1 % and 0.9 %, respectively. These results could be related to the pH changes provoked by increasing concentrations Ca(OH)2 and C6H10O6Ca. These pH changes are related to stability of anthocyanins in flours being more stable in acidic media than in alkaline media (Brouillard 1982; He and Giusti 2010). The results obtained in color of flours are closely related with the anthocyanins structural changes, due to the prevailing pH in the cooking medium, resulting in different colors and hues at different pH values (Brouillard 1982; He and Giusti 2010; Tsuda 2012). EFs and NFs produced by either process with and without calcium sources exhibited a significant lower of anthocyanins content compared with raw blue maize. EFs retained more anthocyanins content (47–59 %) than NFs (33–39 %).The higher anthocyanins retention of EFs can be attributed to the short-term heat exposure during extrusion process, although the high temperatures cause anthocyanins degradation (Hirt et al. 2014). In contrast, during nixtamalization process, the kernels are exposed to high temperatures for a long period in alkaline conditions (pH = 10). These two factors synergistically contribute to anthocyanins loss. Additionally, it should be noted that anthocyanins are located mainly in pericarp and aleurone layer of the grain (Urías-Peraldí et al. 2013) and that most of these parts of the grain are degraded during nixtamalization (Salinas-Moreno et al. 2003) and leached into the nejayote (Cortés-Gómez et al. 2006; López-Martínez et al. 2011). Some investigations reported anthocyanins losses of 55.3–56 % (Mora-Rochín et al. 2010; López-Martínez et al. 2011), 80–82 % (Cortés-Gómez et al. 2006), and even 73–100 % (Salinas-Moreno et al. 2003) occurring during traditional nixtamalization process. However, the differences among these results for anthocyanins losses could be due mainly to nixtamalization conditions used and the variety of kernel used.
Table 2.
Total anthocyanins, phenolics content and antioxidant activity of blue maize flours with different calcium sources
| Flours | [Conc] (%) | TA (mg/100 g) | RA (%) | TP (mg GAE/100 g) | RA (%) | AA (μmol TE/g) | RA (%) | pH |
|---|---|---|---|---|---|---|---|---|
| EF Ca(OH)2 | 0.10 | 34.45 ± 0.12b | 56.28 | 216.41 ± 1.75b | 72.51 | 2.03 ± 0.02b | 78.69 | 6.57 ± 0.02c |
| 0.20 | 33.28 ± 0.02c | 54.37 | 201.86 ± 2.34de | 67.64 | 1.89 ± 0.01c | 73.26 | 6.79 ± 0.02b | |
| 0.30 | 28.98 ± 0.04g | 47.34 | 184.24 ± 1.02f | 61.73 | 1.74 ± 0.01d | 67.45 | 7.01 ± 0.01a | |
| EF C6H10O6Ca | 0.30 | 30.12 ± 0.68f | 49.20 | 197.18 ± 1.54e | 66.07 | 1.68 ± 0.02e | 65.12 | 6.02 ± 0.03f |
| 0.60 | 31.31 ± 0.09e | 51.15 | 206.98 ± 2.46cd | 69.35 | 1.79 ± 0.05d | 69.38 | 5.84 ± 0.01g | |
| 0.90 | 32.24 ± 0.31d | 52.67 | 213.59 ± 1.80bc | 71.57 | 1.86 ± 0.01c | 72.10 | 5.66 ± 0.02h | |
| EF control | 0.00 | 36.25 ± 0.32a | 59.22 | 261.39 ± 6.39a | 87.58 | 2.25 ± 0.01a | 87.21 | 6.24 ± 0.04d |
| NF Ca(OH)2 | 1.00 | 20.58 ± 0.14i | 33.62 | 142.20 ± 1.48g | 47.65 | 1.57 ± 0.01f | 60.86 | 6.82 ± 0.01b |
| NF C6H10O6Ca | 2.95 | 24.09 ± 0.21h | 39.35 | 132.42 ± 1.44h | 44.37 | 1.43 ± 0.02g | 55.43 | 6.10 ± 0.01e |
| Blue maize | 0.00 | 61.22 ± 1.23 | 100.00 | 298.47 ± 6.55 | 100.00 | 2.58 ± 0.04 | 100.00 | 5.87 ± 0.04 |
Means values ± standard error of triplicate of two repetition treatments. Means by column and treatment with different letters shows significant difference, contrasts test (P < 0.05)
EF Extruded Flours, NF Nixtamalized Flours, TA Total anthocyanins, TP Total phenolics, AA Antioxidant activity, RA Retention anthocyanins percent
Total phenolics content and antioxidant activity
The extrusion process caused higher retention in total phenolics content and antioxidant activity in the instant blue maize flours whereas the nixtamalization process caused a higher degradation in the content and activity of these bioactive compounds (Table 2). EFs prepared with both calcium sources and without calcium showed higher retention of polyphenols (61.7–87.5 %) and antioxidant activity (65.1–87.2 %) than NFs (55.4–60.8 %). These losses can be attributed to combined effect of alkalinity and temperature during nixtamalization process, as well as physical losses of pericarp and bioactive compounds including the phenolic compounds leaching into the nejayote (Del Pozo-Insfran et al. 2007; López-Martínez et al. 2011). The phenolic compounds, which are bound to hemicellulose in the cell wall of pericarp, aleurone layer and kernels germ (Adom et al. 2005; De la Parra et al. 2007; Mora-Rochín et al. 2010) are hydrolyzed and released into the cooking liquid during thermal-alkaline treatment.
On the other hand EFs produced with Ca(OH)2 caused a decrease of both TP and AA values as Ca(OH)2 increased. In contrast, increasing the C6H10O6Ca concentration led to increases in both TP and AA content. The highest retention values for TP and AA in EFs with calcium added was achieved with 0.1 % Ca(OH)2 and 0.9 % C6H10O6Ca, respectively.
Analysis of anthocyanins profile by HPLC
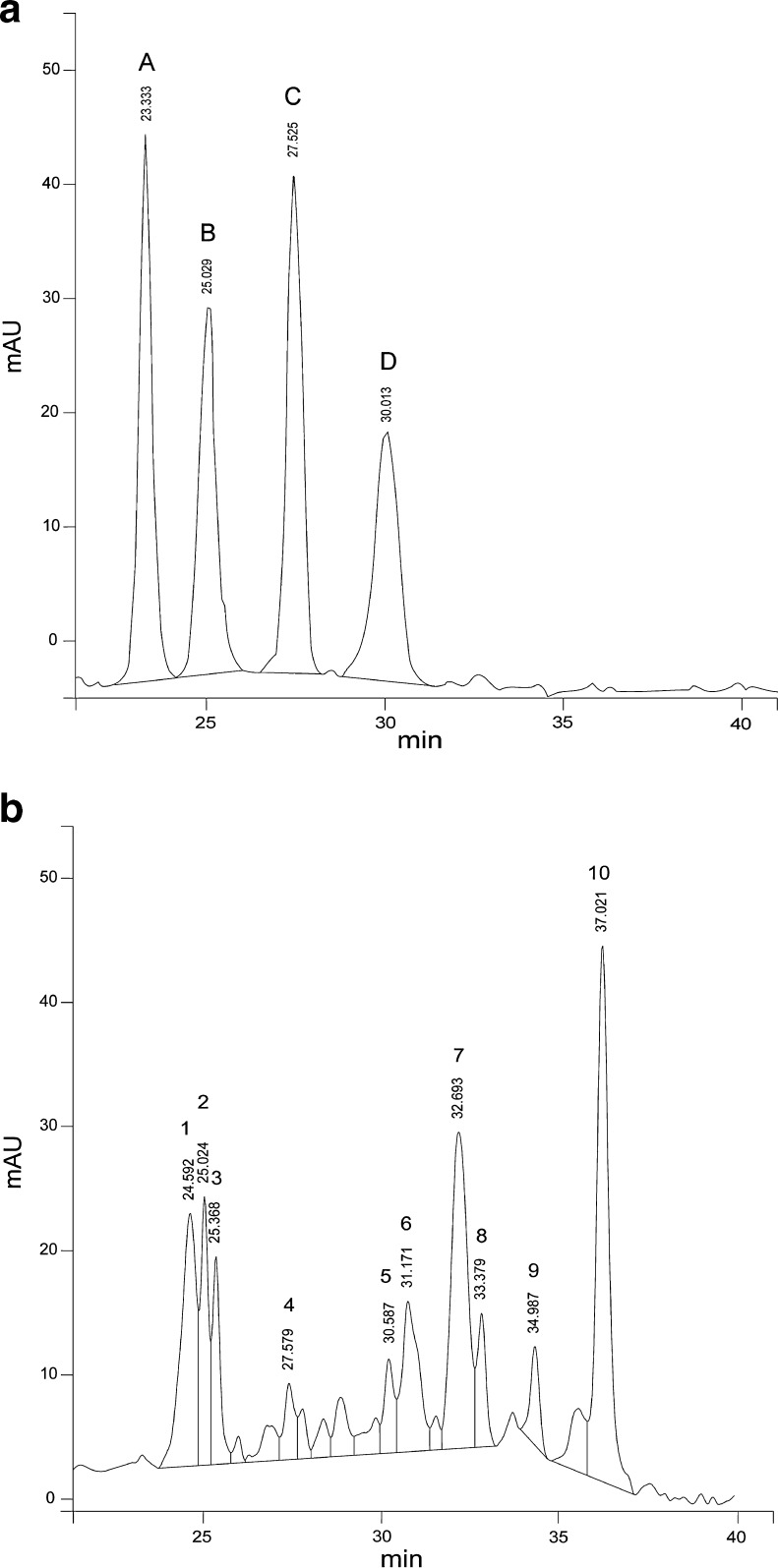
Figure 1a shows the chromatographic profile of the anthocyanins standards: cyanidin-3-galactoside (peak A, 23.33 min), cyanidin-3-glucoside (peak B, 25.02 min), pelargonidin-3-glucoside (peak C, 27.52 min) and malvidin-3-glucoside (peak D, 30.01 min). Figure 1b shows the individual chromatogram of anthocyanins present in raw maize extract. Cyanidin-3-glucoside (peak 2) and pelargonidin-3-glucoside (peak 4), identified by their retention times, were present in concentrations of 8.30 mg/100 g and 3.42 mg/100 g, respectively. However, as shown in Fig. 1b, several unidentified anthocyanins were detected in raw maize, which appear as peaks 1, 3, 5, 6, 7, 8, 9 and 10. The components with retention times of 32.69 min and 37.02 min (peaks 7 and 10) were present in relatively high proportions (21.90 % and 25.07 %, respectively) and may be acyl-type anthocyanins, although it could not be confirmed in this study. Related studies of maize had reported the presence of non-acylated and acylated anthocyanins (De Pascual-Teresa et al. 2002; Salinas-Moreno et al. 2003; Cortés-Gómez et al. 2006; Pedreschi and Cisneros-Zeballos 2007). Salinas-Moreno et al. (2005) identified non-acylated and acylated anthocyanins, and demonstrated that acylated anthocyanins exhibited high retention times, similar to our results. They identified these acylated anthocyanins as cyanidin-3-(6″-malonylglucoside) and cyanidin-3-(3″,6″-dimalonylglucoside).
Fig. 1.
Chromatograms of (a) standards and (b) raw blue maize. Peak A: cyanidin-3-galactoside; peak B: cyanidin-3-glucoside; peak C: pelargonidin-3-glucoside; peak D: malvidin-3-glucoside. Peak 2: cyanidin-3-glucoside; peak 4: pelargonidin-3-glucoside; peaks: 1, 3, 5, 6, 7, 8, 9, 10: not identified
The content of individual anthocyanins in blue maize flours is shown in Table 3. The cyanidin-3-glucoside content increased as the concentration of either calcium sources was increased. A significant difference (P < 0.05) was observed for the highest concentration of both calcium sources. The cyanidin-3-glucoside content was greater in all blue maize flours compared with raw blue maize. In addition, the highest cyanidin-3-glucoside content was found in EF prepared with 0.3 % Ca(OH)2 and NF prepared with Ca(OH)2. EFs prepared with Ca(OH)2 at different concentrations resulted in an increase in cyanidin-3-glucoside content from 8 % to 19 % compared with raw maize. Cortés-Gómez et al. (2006) reported changes in the stability of individual anthocyanins from performing nixtamalization after the fractionation process, finding cyanidin-3-glucoside retention values of 84 % for blue maize nixtamalized in 1.5 % calcium hydroxide for short time periods. However, in this study, the increase in the cyanidin 3-glucoside in the nixtamalized flour prepared with 1.0 % Ca(OH)2 was only 19.63 %. This result may be due to synergic effects of the alkaline pH and the longer cooking and steeping times used for the NFs that caused structural changes in the flavylium ions, thereby decreasing the concentration of total anthocyanins (Cortés-Gómez et al. 2006). The structural modifications to the anthocyanins present in blue maize may be a consequence of the loss of acyl groups from the acyl-type anthocyanins to when they become cyanidin-3-glucoside (Salinas-Moreno et al. 2003; Cortés-Gómez et al. 2006). An increase in cyanidin-3-glucoside content was also observed in EFs prepared with C6H10O6Ca, but it was lower than EFs prepared with Ca(OH)2 (Table 3).
Table 3.
Individuals anthocyanins content in extruded and nixtamalized blue maize flours with different calcium sources
| Flours | [Conc] (%) | Cy-3-gal (mg/100 g) | Cy-3-glu (mg/100 g) | Pe-3-glu (mg/100 g) | Ma-3-glu (mg/100 g) |
|---|---|---|---|---|---|
| EF Ca(OH)2 | 0.10 | nd | 8.97 ± 0.11bc | 0.74 ± 0.03bc | nd |
| 0.20 | nd | 9.17 ± 0.24bc | 0.81 ± 0.04ab | nd | |
| 0.30 | nd | 9.90 ± 0.17a | 0.92 ± 0.06a | nd | |
| EF C6H10O6Ca | 0.30 | nd | 8.52 ± 0.24cd | 0.78 ± 0.05ab | nd |
| 0.60 | nd | 9.01 ± 0.12bd | 0.80 ± 0.04ab | nd | |
| 0.90 | nd | 9.26 ± 0.18ab | 0.91 ± 0.005a | nd | |
| EF Control | 0.00 | nd | 8.55 ± 0.27cd | 0.66 ± 0.07bc | nd |
| NF Ca(OH)2 | 1.00 | nd | 9.93 ± 0.32a | 0.59 ± 0.08c | nd |
| NF C6H10O6Ca | 2.95 | nd | 8.56 ± 0.16cd | 0.61 ± 0.02c | nd |
| Blue maize | 0.00 | nd | 8.30 ± 0.25 | 3.42 ± 0.27 | nd |
Means values ± standard error of triplicate of two repetition treatments. Means by column and treatment with different letters shows significant difference, contrasts test (P < 0.05)
EF Extruded Flours, NF Nixtamalized Flours, Cy-3-gal Cyanidin-3-galactoside, Cy-3-glu Cyanidin-3-glucoside, Pe-3-glu Pelargonidin-3-glucoside, Ma-3-glu Malvidin-3-glucoside, nd no detectable
The content of pelargonidin-3-glucoside was not significantly affected (P < 0.05) by either of calcium sources or their concentrations. The proportion of this anthocyanin decreased in all treatments. Pelargonidin-3-glucoside displayed poor stability in extrusion and nixtamalization processes compared with the cyanidin-3-glucoside.
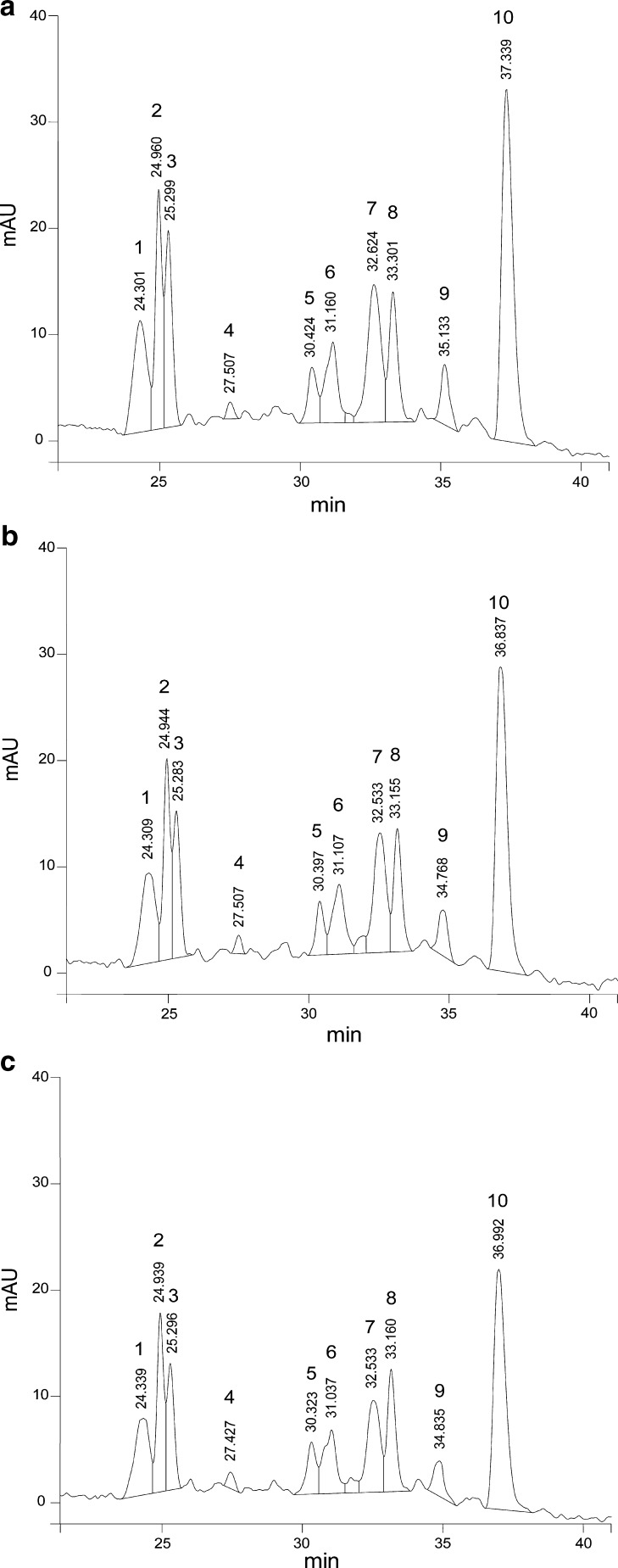
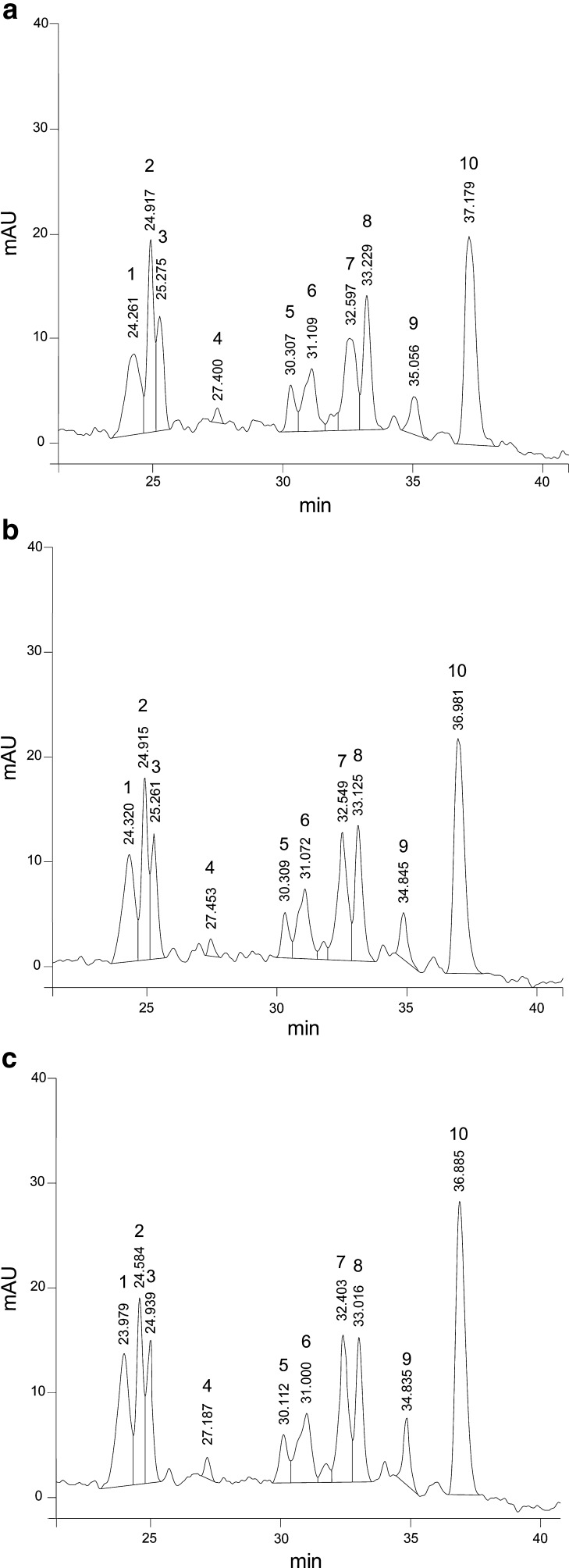
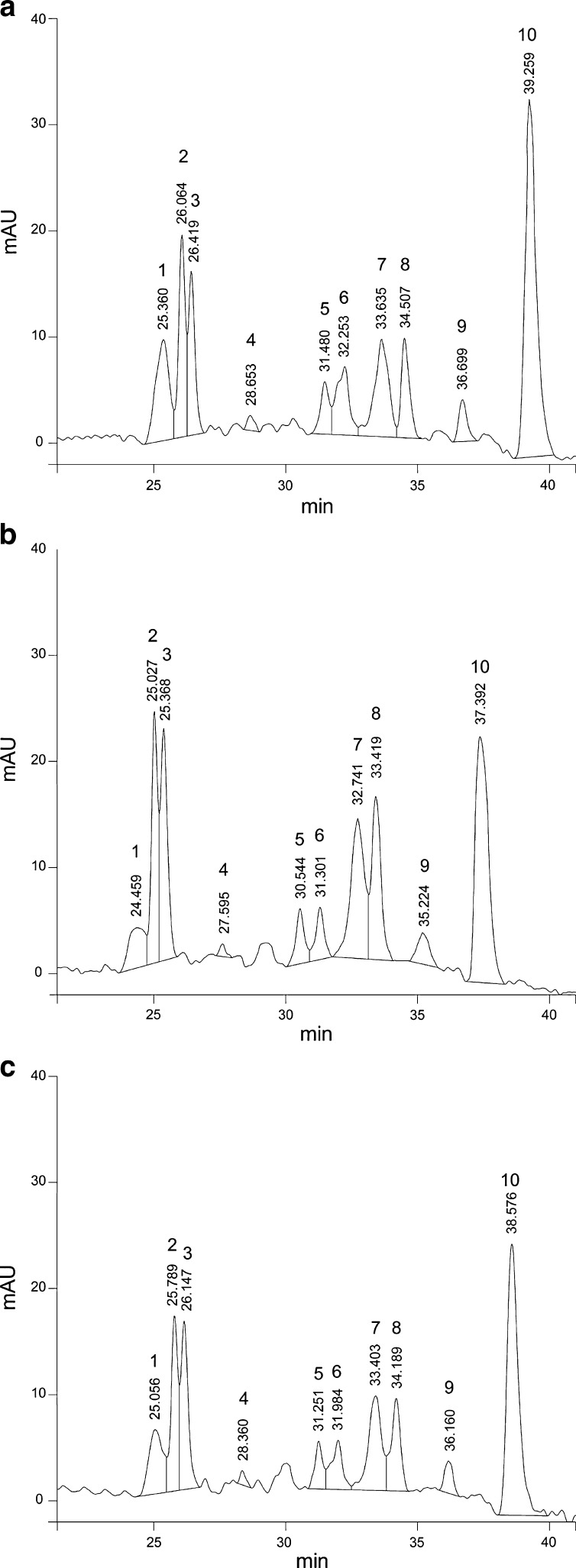
A general pattern was observed for unidentified anthocyanins (peaks 1, 3, 7, 9 and 10) and it decreased as the Ca(OH)2 concentration increased (Fig. 2) and increased as C6H10O6Ca concentration increased (Fig. 3) in EFs. While EF control (Fig. 4a) presented at higher retention compared with NFs with both calcium sources (Fig. 4b and c) showed a decrease especially on the peaks (peaks 1, 3, 7 and 10). The behavior of these unidentified anthocyanins respect to the concentration of both calcium sources was similar to that observed for the TA, TP and AA.
Fig. 2.
Chromatograms of EFs with Ca(OH)2. (a) 0.1 %, (b) 0.2 %, (c) 0.3 %. Peak 2: cyanidin-3-glucoside; peak 4: pelargonidin-3-glucoside; peaks:1,3,5,6,7,8,9,10: not identified
Fig. 3.
Chromatograms of EFs with C6H10O6Ca. (a) 0.3 %, (b) 0.6 %, (c) 0.9 %. Peak 2: cyanidin-3-glucoside; peak 4: pelargonidin-3-glucoside; peaks:1,3,5,6,7,8,9,10: not identified
Fig. 4.
Chromatograms of control flours. (a) EF without calcium source, (b) NF with Ca(OH)2, (c) NF with C6H10O6Ca. Peak 2: cyanidin-3-glucoside; peak 4: pelargonidin-3-glucoside; peaks:1,3,5,6,7,8,9,10: not identified
Conclusion
The extrusion process of blue maize using C6H10O6Ca as an alternate source of Ca(OH)2 was effective to improve the retention of antioxidants compounds in instant maize flours compared with the nixtamalization process. Ca(OH)2 concentration increases caused lower retention of antioxidants compounds and color, while that increases of C6H10O6Ca concentration showed a tendency to improve in the retention of this compounds in the extruded flours. This behavior was correlated to the pH value of each calcium source. Ca(OH)2 and C6H10O6Ca treatments caused increases of the cyanidin-3-glucoside concentration of both EFs and NFs compared with the raw maize, in contrast the pelargonidin-3-glucoside concentration decreased, showing poor stability for extrusion and nixtamalization process. In general, the extrusion process retained higher anthocyanins and antioxidants contents in the flours than traditional nixtamalization process. The extruded flours with calcium added with the highest antioxidants retention were: the EF prepared with 0.1 % Ca(OH)2 and the EF prepared with 0.9 % C6H10O6Ca.
Acknowledgments
The authors acknowledge the Universidad Autónoma de Chihuahua and the PromeP-SEP for supporting (Network Project.) this investigation. This paper is based on the Ms. Sc. thesis in Food Science and Technology of Miguel Ángel Sánchez-Madrigal.
References
- AACC (2000) American Association of Cereal Chemists. Approved Methods of AACC, 10th edition. Method 14–40. The Association, St. Paul, MN
- Abdel-Aal ESM, Hucl P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999;76:350–354. doi: 10.1094/CCHEM.1999.76.3.350. [DOI] [Google Scholar]
- Adom KK, Sorrels M, Liu RH. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J Agric Food Chem. 2005;53:2297–2306. doi: 10.1021/jf048456d. [DOI] [PubMed] [Google Scholar]
- Aguayo-Rojas J, Mora-Rochín S, Cuevas-Rodríguez EO, Serna-Saldívar SO, Gutiérrez-Uribe JA, Reyes-Moreno C, Milán-Carrillo J. Phytochemicals and antioxidant capacity of tortillas obtained after lime-cooking extrusion process of whole pigmented mexican maize. Plant Foods Hum Nutr. 2012;67:178–185. doi: 10.1007/s11130-012-0288-y. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm-WissTechnol /Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Brouillard R. Chemical structure of anthocyanins. In: Markakis P, editor. Anthocyanins as food colors. New York: Academic Press Inc; 1982. pp. 1–40. [Google Scholar]
- Cortés-Gómez A, Salinas-Moreno Y, San Martín-Martínez E, Martínez-Bustos F. Stability of anthocyanins of blue maize (Zea mays L.) after nixtamalization of separated pericarp-germ tip cap and endosperm fractions. J Cereal Sci. 2006;43:57–62. doi: 10.1016/j.jcs.2005.05.003. [DOI] [Google Scholar]
- De la Parra C, Serna-Saldívar SO, Hai-Lui R. Effect of processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas, and tortilla chips. J Agric Food Chem. 2007;55:4177–4183. doi: 10.1021/jf063487p. [DOI] [PubMed] [Google Scholar]
- De Pascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC. LC-MS analysis of anthocyanins from purple corn cob. J Sci Food Agric. 2002;82:1003–1006. doi: 10.1002/jsfa.1143. [DOI] [Google Scholar]
- Del Pozo-Insfran D, Serna-Saldívar SO, Brenes CH, Talcott ST. Polyphenolics and antioxidant capacity of white and blue corns processed into tortillas and chips. Cereal Chem. 2007;84:162–168. doi: 10.1094/CCHEM-84-2-0162. [DOI] [Google Scholar]
- Dykes L, Rooney LW, Waniska RD, Rooney WL. Phenolic compounds and antioxidant activity of sorghum grains of varying genotypes. J Agric Food Chem. 2005;53:6813–6818. doi: 10.1021/jf050419e. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Dorado R, Ayala-Rodríguez AE, Milán-Carrillo J, López-Cervantes J, Garzón-Tiznado JA, López-Valenzuela JA, Paredes-López O, Reyes-Moreno C. Technological and nutritional properties of flours and tortillas from nixtamalized and extruded quality protein maize (Zea mays L.) Cereal Chem. 2008;85:808–816. doi: 10.1094/CCHEM-85-6-0808. [DOI] [Google Scholar]
- He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- Hirt M, Leiter A, Beck SM, Schuchmann HP. Effect of extrusion cooking process parameters on the retention of bilberry anthocyanins in starch based food. J Food Eng. 2014;125:139–146. doi: 10.1016/j.jfoodeng.2013.10.034. [DOI] [Google Scholar]
- Kühnen S, Menel-Lemos PM, Campestrini LH, Bernardi-Ogliari J, Dias PF, Marashini M. Carotenoid and anthocyanin contents of grains of Brazilian maize landraces. J Sci Food Agric. 2011;91:1548–1553. doi: 10.1002/jsfa.4346. [DOI] [PubMed] [Google Scholar]
- López-Martínez LX, Parkin KL, García HS. Phase II-inducing, polyphenols content and antioxidant capacity of corn (Zea mays L.) from phenotypes of white, blue, red and purple colors processed into masa and tortillas. Plant Foods Hum Nutr. 2011;66:41–47. doi: 10.1007/s11130-011-0210-z. [DOI] [PubMed] [Google Scholar]
- Maya DC, De Figueroa JD, Garnica MG, Cuevas RA, Cortés R, Véles JJ, Martínez HE. Whole-grain corn tortilla prepared using an ecological nixtamalization process and its impact on the nutritional value. Int J Food Sci Technol. 2010;45:23–28. doi: 10.1111/j.1365-2621.2009.02095.x. [DOI] [Google Scholar]
- Mora-Rochín S, Gutiérrez-Uribe JA, Serna-Saldívar SO, Sánchez-Peña P, Reyes-Moreno C, Milán-Carrillo J. Phenolic content and antioxidant activity of tortillas produced from pigmented maize processed by conventional nixtamalization or extrusion cooking. J Cereal Sci. 2010;52:502–508. doi: 10.1016/j.jcs.2010.08.010. [DOI] [Google Scholar]
- Pedreschi R, Cisneros-Zeballos L. Phenolic profiles of Andean purple corn (Zea mays L.) Food Chem. 2007;100:956–963. doi: 10.1016/j.foodchem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Reyes-Moreno C, Milán-Carrillo J, Gutiérrez-Dorado R, Paredes-López O, Cuevas-Rodríguez EO, Garzón-Tiznado JA. Instant flour from quality protein maize (Zea mays L.). Optimization of extrusion process. Lebensm-Wiss Technol. 2003;36:685–695. doi: 10.1016/S0023-6438(03)00089-6. [DOI] [Google Scholar]
- Ruiz-Gutiérrez MG, Quintero-Ramos A, Meléndez-Pizarro CO, Talamás-Abbud R, Barnard J, Márquez-Meléndez R, Lardizábal-Gutiérrez D. Nixtamalization in two steps with different calcium salts and the relationship with chemical, texture and thermal properties in masa and tortillas. J Food Process Eng. 2012;35:772–783. doi: 10.1111/j.1745-4530.2010.00627.x. [DOI] [Google Scholar]
- Salinas-Moreno Y, Martínez-Bustos F, Soto-Hernández M, Ortega-Paczka R, Arellano-Vázquez JL. Efecto de la nixtamalización sobre las antocianinas del grano de maíces pigmentados. Agrociencia. 2003;37:617–628. [Google Scholar]
- Salinas-Moreno Y, Salas-Sánchez G, Rubio-Hernández D, Ramos-Lobato N. Characterization of anthocyanin extracts from maize kernels. J Chromatogr Sci. 2005;43:483–487. doi: 10.1093/chromsci/43.9.483. [DOI] [PubMed] [Google Scholar]
- Sánchez-Madrigal MA, Meléndez-Pizarro CO, Martínez-Bustos F, Ruiz-Gutiérrez MG, Quintero-Ramos A, Márquez-Meléndez R, Lardizábal-Gutiérrez D, Campos-Venegas K. Structural, functional, thermal and rheological properties of nixtamalised and extruded blue maize (Zea mays L.) flour with different calcium sources. Int J Food Sci Technol. 2014;49:578–586. doi: 10.1111/ijfs.12340. [DOI] [Google Scholar]
- SAS version 9.2 (2007) Statistical Analysis System. Institute Inc., Cary, NC. USA
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Tsuda T. Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res. 2012;56:159–170. doi: 10.1002/mnfr.201100526. [DOI] [PubMed] [Google Scholar]
- Urías-Peraldí M, Gutiérrez-Uribe JA, Preciado-Ortiz RE, Cruz-Morales AS, Serna-Saldívar SO, García-Lara S. Nutraceutical profiles of improved blue maize (Zea mays) hybrids for subtropical regions. Field Crop Res. 2013;141:69–76. doi: 10.1016/j.fcr.2012.11.008. [DOI] [Google Scholar]