Absract
The article is concerned with health benefits of two main physiologically active ingredients namely, Isoflavones and γ-Aminobutyric acid, with emphasis on their fitness for fortification of yoghurt to be consumed as a functional food. Isoflavones (ISO) are part of the diphenol compounds, called “phytoestrogens,” which are structurally and functionally similar to estradiol, the human estrogen, but much less potent. Because of this similarity, ISO were suggested to have preventive effects for many kinds of hormone-dependent diseases. In nature, ISO usually occur as glycosides and, once deconjugated by the intestinal microflora, the ISO can be absorbed into the blood. At present, it seems convincing their possible protective actions against various cancers, osteoporosis and menopausal symptoms and high levels of blood cholesterol as well as the epidemiological evidence. Γ-Aminobutyric acid (GABA), it is an amino acid that has long been reported to lower blood pressure by intravenous administration in experimental animals and in human subjects. GABA is present in many vegetables and fruits but not in dairy products. GABA was reported to lower blood pressure in people with mild hypertension. It was suggested that low-dose oral GABA has a hypotensive effect in spontaneously hypertensive. Yoghurt beyond its ability to be probiotic food via its culturing with the gut strains, it could further carry more healthy benefits when it was fortified with physiological active ingredients, especially GABA versus ISO preferring, whether, bacteriologically or biochemically, a fortification level of 50 mg ISO/kg or 200 mg GABA/kg.
Keywords: Probiotics, Prebiotics, Isoflavones, γ-Aminobutyric acid, Biochemical, Rheological properties
Introduction
Ever-growing consumer demand for convenience, combined with a healthy diet and preference for natural ingredients has led to a growth in functional beverage markets. Scientific and clinical evidence is also mounting to corroborate the consumer perception of health from fermented milks. Probiotics, prebiotics, synbiotics and associated ingredients also add an attractive dimension to cultured dairy products. Another potential growth area for fermented milks includes added-value products such as low calorie, reduced-fat varieties and those fortified with physiologically active ingredients including fibers, phytosterols, omega-3-fatty acids, whey based ingredients, antioxidant vitamins and isoflavones (ISO) those provide specific health benefits beyond basic nutrition. (Khurana and Kanawjia 2007).
Concerning physiologically active ingredients, isoflavones are functional ingredients of anther interest. Isoflavones are part of the diphenol compounds called “phytoestrogens,” which are structurally and functionally similar to estradiol, the human estrogen, but much less potent. Because of this similarity, isoflavones were suggested to have preventive effects for many kinds of hormone-dependent diseases. Isoflavones occur naturally in plants and mostly in soybeans. In nature, isoflavones usually occur as glycosides and once deconjugated by the intestinal microflora, the isoflavones can be absorbed into the blood (Mason 2001).
Regarding γ-aminobutyric acid (GABA), it is an amino acid that has long been reported to lower blood pressure by intravenous administration in experimental animals (Lacerda et al. 2003 and Stanton 1963) and in human subjects (Elliott and Hobbiger 1959). GABA presents in many vegetables and fruits but not in dairy products. However, the effect of dietary GABA has attracted little attention as a factor that may influence blood pressure.
The following literature is dealing in details with two physiological active ingredients namely ISO and GABA in relation to bacterial, biochemical, physical, rheological and organoleptical attributes of yoghurts fortified with any of them.
Isoflavones
Isoflavones are polyphenolic compounds that are capable of exerting estrogen-like effects. For this reason, they are classified as phytoestrogens—plant-derived compounds with estrogenic activity. Legumes, particularly soybeans, are the richest sources of isoflavones in the human diet. In soybeans, isoflavones are present as glycosides (bound to a sugar molecule). Fermentation or digestion of soybeans or soy products results in the release of the sugar molecule from the isoflavone glycoside, leaving an isoflavone aglycone. Soy isoflavone glycosides are called genistin, daidzin, and glycitin, while the aglycones are called genistein, daidzein, and glycitein (Chen et al. 2004). Unless otherwise indicated, quantities of isoflavones specified in this article refer to aglycones—not glycosides.
Chemical structure and biosynthesis
Isoflavones of nutritional interest are substituted derivatives of isoflavone, being related to the parent by the replacement of two or three hydrogen atoms with hydroxyl groups. The parent isoflavone is of no nutritional interest.
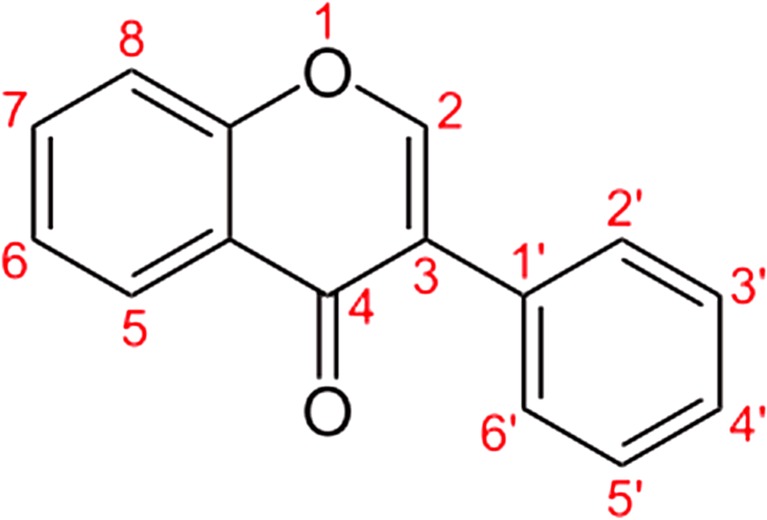
Isoflavone, numbering. Genistein (5-OH, 7-OH, 4′-OH) or daidzein (7-OH, 4′-OH) are e. g. members of the isoflavone family (Fig. 1).
Fig. 1.
The chemical structure of isoflavones
Isoflavone differs from flavone (2-phenyl-4H-1-benzopyr-4-one) in location of the phenyl group. Isoflavones are produced via a branch of the general phenylpropanoid pathway that produces flavonoid compounds in higher plants. Soybeans are the most common source of isoflavones in human food; the major isoflavones in soybean are genistein and daidzein. The phenylpropanoid pathway begins from the amino acid phenylalanine, and an intermediate of the pathway, naringenin, is sequentially converted into the isoflavone genistein by two legume-specific enzymes, isoflavone synthase, and a dehydratase. Similarly, another intermediate naringenin chalcone is converted to the isoflavone daidzein by sequential action of three legume-specific enzymes: chalcone reductase, type II chalcone isomerase, and isoflavone synthase.
Metabolism and bioavailability
The biological effects of soy isoflavones are strongly influenced by their metabolism, which is dependent on the activity of bacteria that colonize the human intestine (Rowland et al. 2003). For example, the soy isoflavone daidzein may be metabolized in the intestine to equol, a metabolite that has greater estrogenic activity than daidzein, and to other metabolites that are less estrogenic. Studies that measure urinary equol excretion after soy consumption indicate that only about 33 % of individuals from Western populations metabolize daidzein to equol (Setchell et al. 2002) Thus, individual differences in the metabolism of isoflavones could have important implications for the biological activities of these phytoestrogens.
Biological activities
Soy isoflavones and their metabolites have biological activities those are unrelated to their interactions with estrogen receptors (Barnes et al. 2000). Soy isoflavones are known to have weak estrogenic or hormone-like activity. Estrogens are signaling molecules that exert their effects by binding to estrogen receptors within cells (chemical structures of endogenous estrogens). The estrogen-receptor complex interacts with DNA to change the expression of estrogen-responsive genes. Estrogen receptors are present in numerous tissues other than those associated with reproduction including bone, liver, heart, and brain. Soy isoflavones and other phytoestrogens can bind to estrogen receptors, mimicking the effects of estrogen in some tissues and antagonizing (blocking) the effects of estrogen in others (Wang 2002).
By inhibiting the synthesis and activity of certain enzymes involved in estrogen metabolism, soy isoflavones may alter the biological activity of endogenous estrogens and androgens (Holzbeierlein et al. 2005). Soy isoflavones have also been found to inhibit tyrosine kinases and enzymes that play critical roles in the signaling pathways that stimulate cell proliferation (Akiyama et al. 1987).
Additionally, isoflavones can act as antioxidants in vitro (Ruiz-Larrea et al. 1997), but the extent to which they contribute to the antioxidant status of humans is not yet clear. Plasma F2-isoprostanes and biomarkers of lipid peroxidation in vivo were significantly lower after two weeks of daily consumption of soy protein containing 56 mg of isoflavones than after consumption of soy protein providing only 2 mg of isoflavones (Wiseman et al. 2000). However, daily supplementation with 50–100 mg of isolated soy isoflavones did not significantly alter plasma or urinary F2-isoprostane level (Djuric et al. 2001).
Diseases prevention
Cardiovascular disease
Serum cholesterol
There is limited evidence that soy protein containing isoflavones is more effective than soy protein without isoflavones in lowering LDL cholesterol (Zhan and Ho 2005), but the consumption of soy isoflavones alone (as supplements or extracts) does not appear to have favorable effects on serum lipid profile (Sacks et al. 2006).
Effects on arterial function
The preservation of normal arterial function plays an important role in cardiovascular disease prevention. The ability of arteries to dilate in response to nitric oxide produced by the endothelial cells that line their inner surface (endothelium-mediated vasodilation) is compromised in people at high risk for cardiovascular disease (Landmesser et al. 2004). However, most placebo-controlled trials found no significant improvement in endothelium-mediated vasodilation when postmenopausal women were supplemented with up to 80 mg/day of soy isoflavones (Katz et al. 2007) or up to 60 g/day of soy protein containing isoflavones (Evans et al. 2007). In placebo-controlled clinical trials, supplementation of postmenopausal women with 80 mg/day of a soy isoflavone extract for five weeks significantly decreased arterial stiffness as did supplementation of men and postmenopausal women with 40 g/day of soy protein providing 118 mg/day of soy isoflavones for 3 months (Teede et al. 2001). However, a recent randomized controlled, cross-over trial in hypertensive individuals found that supplementation with soy protein containing 118 mg/day of isoflavones for 6 months did not improve measures of arterial function including arterial stiffness (Teede et al. 2006).
Hormone-associated cancers
Breast cancer
Breast cancer incidence in Asia where average isoflavone intakes from soy foods range from 25 to 50 mg/day (Messina et al. 2006a), is lower than breast cancer rates in the Western countries where average isoflavone intakes in non-Asian women are less than 2 mg/day (Van Erp-Baart et al. 2003). However, a few studies suggest that a higher soy intake during adolescence may lower risk of developing breast cancer later in life (Wu et al. 2002). Breast cancer survivors in particular may experience more frequent and severe hot flushes related to therapies aimed to prevent breast cancer recurrence (Duffy and Cyr 2003). A recent prospective study in 5,042 female breast cancer survivors in China, who were followed for a median of 3.9 years, found that consumption of isoflavone-rich soy foods was significantly associated with a 29 % lower risk of death and a 32 % lower risk of cancer recurrence. In this study, soy isoflavone consumption was associated with a nonsignificant 21 % reduction in risk of death and a significant, 23 % reduction in risk of cancer recurrence (Shu et al. 2009).
Endometrial cancer
Because the development of endometrial (uterine) cancer is related to prolonged exposure to unopposed estrogens (estrogen not counterbalanced with the hormone progesterone), it has been suggested that high intakes of phytoestrogens with anti-estrogenic activity in uterine tissue could be protective against endometrial cancer (Horn-Ross et al. 2003). In support of this idea, three retrospective case–control studies found that women with endometrial cancer had lower intakes of soy isoflavones from foods compared to cancer-free control groups (Xu et al. 2004). However, supplementation of postmenopausal women with soy protein providing 120 mg/day of isoflavones for 6 months did not prevent endometrial hyperplasia induced by the administration of exogenous estradiol (Murray et al. 2003).
Prostate cancer
Mortality from prostate cancer is much higher in the U.S. than in Asian countries, such as Japan and China (Messina 2003). However, epidemiological studies do not provide consistent evidence that high intakes of soy foods are associated with reduced prostate cancer risk. The results of cell culture and animal studies suggest a potential role for soy isoflavones in limiting the progression of prostate cancer. Although soy isoflavone supplementation for up to 1 year did not significantly decrease serum concentration of prostate specific antigen (PSA) in men without confirmed prostate cancer (Adams et al. 2004). Soy isoflavone supplementation appeared to slow the rising serum PSA concentration associated with prostate tumor growth in two small studies of prostate cancer patients (Fischer et al. 2004). A trial of soy milk supplementation (141 mg/day isoflavones) in men with PSA recurrent prostate cancer found that PSA levels increased by an average of 20 % over a 12-months period compared to a 56 % yearly increase prior to the study. Messina et al. (2006b) reviewed that isoflavone supplementation in prostate cancer patients favorably affected PSA concentrations in four out of eight trials. Additionally, a recent meta-analysis of eight studies found that isoflavones consumption was associated with a reduction in risk of prostate cancer, but the association was not statistically significant (Yan and Spitznagel 2009).
Osteoporosis
Although hip fracture rates are generally lower among Asian populations consuming soy foods than among Western populations, it is not yet clear whether increasing soy isoflavone consumption in Western populations helps to prevent osteoporosis (Setchell and Lydeking-Olsen 2003). The results of short-term clinical trials (6 months or less) assessing the effects of increased soy intake on biochemical markers of bone formation and bone re-sorption are inconsistent. Some controlled trials in postmenopausal women have found that increasing intakes of soy foods, soy protein or soy isoflavones improves markers of bone re-sorption and formation, or attenuates bone loss (Ye et al. 2006). But other trials have found no significant benefit of increasing soy intake (Cheong et al. 2007). Two controlled clinical trials found that bone mineral density (BMD) losses over 6 months were significantly lower in postmenopausal women supplemented with soy protein containing isoflavones than in those supplemented with equal amounts of milk protein (Alekel et al. 2000). But two longer trials found that BMD loss did not differ significantly between postmenopausal women supplemented with soy protein containing isoflavones and those supplemented with milk protein (Arjmandi et al. 2005). A two-year clinical trial found that daily consumption of soy milk containing isoflavones decreased significantly BMD loss in the lumbar spine compared to daily consumption of soymilk without isoflavones, but other three studies found that BMD loss did not differ significantly between postmenopausal women taking soy protein supplements containing isoflavones and those taking soy protein supplements without or with negligible amounts of isoflavones (Newton et al. 2006). Loss of bone mineral content at the hip over 1 year was lower in Taiwanese women who took 80 mg/day of isolated soy isoflavones compared to placebo, but the difference was significantly only in those women who were at least four years past menopause, had lower body weights, or had lower calcium intakes (Chen et al. 2004). Another study in Taiwanese women found that those taking 100 mg/day of isolated soy isoflavones for 1 year experienced less bone loss compared to the control group, but women taking 200 mg/day of supplemental isoflavones did not experience any benefit (Huang et al. 2006). A randomized controlled trial in European postmenopausal women found that supplementation with isoflavone-enriched foods (110 mg/day of isoflavones) for 1 year had no significant effect on BMD (Brink et al. 2008). A recent placebo-controlled trial in postmenopausal women, aged >60 years, found that neither supplemental soy protein (18 g/day) nor isoflavones (105 mg/day), alone or in combination, affected significantly BMD over a one-year period (Ma et al. 2008). Some authors have proposed that the effect of soy isoflavones on bone health may be dependent on whether or not the individual produces the isoflavones metabolite, equol (Wu et al. 2007). This could possibly explain disparity results among clinical trials. Thus, while there is some evidence that isoflavone-rich diets have bone-sparing effects, it is not known whether increasing soy isoflavone intake appreciably decreases the risk of osteoporosis or osteoporotic fracture.
Cognitive decline
The results of several small clinical trials in postmenopausal women suggested that increasing soy isoflavone intake may result in modest improvements in performance on some cognitive tests for up to 6 months. Postmenopausal women given soy extracts, providing 60 mg/day of soy isoflavones for 6–12 weeks, performed better on cognitive tests of picture recall (short-term memory), learning rule reversals (mental flexibility), and a planning task compared to women given a placebo (File et al. 2005). In a long trial, postmenopausal women given supplements that provided 110 mg/day of soy isoflavones for 6 months performed better on a test of verbal fluency than women given placebos (Kritz-Silverstein et al. 2003). In a cross-over trial lasting 6 months, women receiving 60 mg/day of soy isoflavones experienced significant improvements in cognitive performance and overall mood compared to when the women were given a placebo (Casini et al. 2006). However, in larger placebo-controlled trials, postmenopausal women receiving 80 mg/day of isoflavones for 6 months or 99 mg/day of isoflavones for 1 year did not affect performance on a battery of cognitive function tests, including tests for memory, attention, verbal fluency, motor control, and dementia (Ho et al. 2007). Likewise, a review of eight trials, seven of which were conducted in postmenopausal women, found half reported that soy isoflavone treatment was associated with improvements in cognitive function (Zhao and Brint 2007).
Menopausal symptoms
Hot flushes (flashes) are the primary reason that women seek medical attention for menopausal symptoms (Tice et al. 2003). Concern over potential adverse effects of hormone replacement therapy has led to increase interest in the use of phytoestrogen supplements by women experiencing menopausal symptoms (Farquhar et al. 2009). In a study, one out of eight randomized controlled trials of soy foods reported a significant reduction in the frequency of hot flushes, while three out of five controlled trials of soy isoflavone extracts reported a significant reduction in hot flush frequency (Kreb et al. 2004). In general, any observed reductions were modest (10–20 %) compared to placebo. A systematic review and meta-analysis of 12 randomized controlled trials found that soy isoflavone supplementation was associated with a small reduction in the number of hot flushes; this analysis found that women with a higher number of daily flushes experienced the greatest benefit from isoflavone therapy (Howes et al. 2006). Interestingly, another study found that only women who produced the isoflavone metabolite and equol, which was detected in the urine, experienced improvements in menopausal symptoms like hot flushes following soy isoflavone supplementation (Jou et al. 2008).
Adverse effects of isoflavones
Safety for breast cancer survivors
The safety of high intakes of soy isoflavones and other phytoestrogens for breast cancer survivors is an area of considerable debate among scientists and clinicians (Duffy and Cyr 2003 and Messina and Loprinzi 2001). The results of cell culture and animal studies are conflicting, but some have found that soy isoflavones can stimulate the growth of estrogen receptor positive (ER+) breast cancer cells (Allred et al. 2001 and Ju et al. 2001). High intakes of the soy isoflavone (genistein) interfered with the ability of tamoxifen to inhibit the growth of ER + breast cancer cells implanted in mice (Ju et al. 2002), but it is not known if a similar effect would be seen in humans. Very limited data from clinical trials suggested that increased consumption of soy isoflavones (38–45 mg/day) can have estrogenic effects in human breast tissue (Hargreaves et al. 1999). However, a study in women with biopsy-confirmed breast cancer found that supplementation with 200 mg/day of soy isoflavones did not increase tumor growth over the next 2–6 weeks before surgery when compared to a control group that did not take soy isoflavones (Sartippour et al. 2004). Given the available data, some experts think that women with a history of breast cancer, particularly ER + breast cancer, should not increase their consumption of phytoestrogens, including soy isoflavones (Duffy and Cyr 2003). However, other experts argue that there is not enough evidence to discourage breast cancer survivors from consuming soy foods in moderation (Messina and Loprinzi 2001).
Thyroid function
In cell culture and animal studies, soy isoflavones have been found to inhibit the activity of thyroid peroxidase which is an enzyme required for thyroid hormone synthesis (Doerge and Sheehan 2002). However, high intakes of soy isoflavones do not appear to increase the risk of hypothyroidism as long as dietary iodine consumption is adequate (Messina and Redmond 2006). Since the addition of iodine to soy-based formulas in the 1960s, there have been no further reports of hypothyroidism in soy formula-fed infants (Chorazy et al. 1995). Several clinical trials, mostly in premenopausal and postmenopausal women with sufficient iodine intakes, have not found increased consumption of soy isoflavones to result in clinically significant changes in circulating thyroid hormone levels (Dillingham et al. 2007).
Gamma-amino butyric acid
Γ-amino butyric acid (GABA) is a non essential amino acid found in large quantities in the hypothalamus, the brain center that controls the pituitary gland and functioning. It is found in foods such as beans, dairy products, eggs, and brewer’s yeast. Because this is a substance naturally found in the human body and in many foods that are commonly eaten, it is a generally safe supplement for most individuals to use.
Structure and conformation
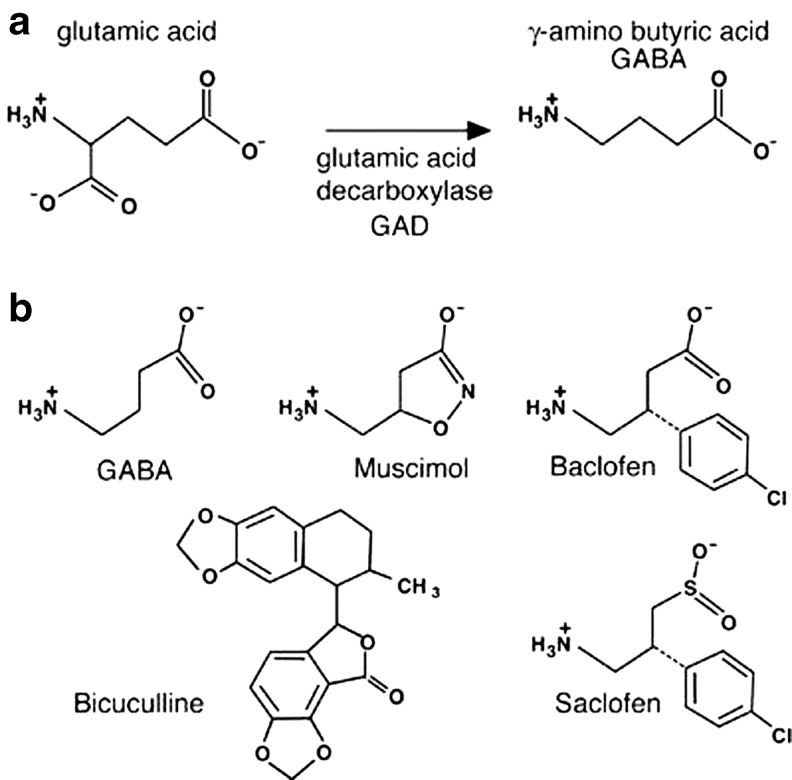
GABA is an amino acid neurotransmitter synthesized by decarboxylation of glutamate by the enzyme glutamic acid decarboxylase (Fig., 2). GABA had been long known to exist in plants and bacteria, where it serves a metabolic role in the Krebs cycle. There were prodigious amounts of GABA in the mammalian central nervous system 1 mg/g and GABA was virtually undetectable in other tissues. Thus, GABA did not act as a neurotransmitter in organisms that made it; and it was not found in organisms in which it acted. GABA no longer fulfilled the qualifications of a neurotransmitter and by 1960 it had been demoted to a mere metabolite (Edwards et al. 1999).
Fig. 2.
Chemical Structure of γ-amino butyric acid (GABA) and some its interactions
GABA’s role in the brain
GABA is made in brain cells from glutamate, and functions as an inhibitory neurotransmitter – meaning that it blocks nerve impulses. Glutamate acts as an excitatory neurotransmitter and when bound to adjacent cells encourages them to “fire” and send a nerve impulse. GABA does the opposite and tells the adjoining cells not to “fire” and not to send an impulse.
GABA is a substance that is one of many that transmits a signal across the synapses between brain cells. GABA, in particular, is found in high quantities in the hypothalamus, which means that it is largely responsible for our sleep cycles, body temperature, and the activities stimulation of the pituitary gland. This last factor is of most interest to those attempting to lose weight, look and feel younger, and add lean body mass. It is the pituitary gland that emits human growth hormone (HGH) into the body. HGH is which, in turn, providing high amounts of antiaging benefits to the body and the mind.
Without GABA, nerve cells fire too often and too easily. Anxiety disorders such as panic attacks, seizure disorders, and numerous other conditions including addiction, headaches, Parkinson’s syndrome, and cognitive impairment are all related to low GABA activity. GABA hinders the transmission of nerve impulses from one neuron to another. It has a calming or quieting influence.
GABA and human growth hormone
The relationship between GABA and HGH becomes a valuable relationship indeed. GABA is touted as increasing HGH levels and it is popular among body builders. The production and release of HGH that promotes growth in young people; it is also what keeps their skin clear, their memory sharp, and their bodies naturally lean. For adults, the natural release of HGH diminishes, and this is what is largely responsible for the signs of aging that develop over time. Therefore, it is an easy connection to draw that the use of GABA will lead to a decrease and even reversal of these signs.
Synthetic GABA can be used to stimulate the creation and secretion of HGH into the system, giving you all the benefits that come with it: increased energy, loss of body fat, a gain in the body’s lean body mass, and an overall youthful look and feeling. There is evidence that getting extra GABA into the brain increases HGH. Injections of GABA directly into the brain increase growth hormone in rats (Passariello et al. 1982).
Several studies support the notion that taking oral GABA increases HGH. Two of the studies were published almost 30 years ago. They used a small number of test subjects. Yet, they produced significant increases; HGH levels increased 500 % (Cavagnini et al. 1980a, b). No studies replicated this effect for years bringing the initial results into question. A study measured GABA and HGH in body builders. Three grams doses of GABA increased HGH levels, but only if taken just before exercise. Without exercise, the GABA had no effect on HGH (Powers et al. 2003). If GABA can raise HGH levels, some of it may cross the blood brain barrier, perhaps only after exhausting exercises.
The HGH studies raise some concerns. Oral GABA also affects the pancreas increasing insulin production. Of course with all the concern about Syndrome X and hyperinsulinemia, making more insulin might not desirable. Yet, a diabetic might find the insulin stimulating effect contributes to better blood sugar control. Besides increasing insulin and HGH, oral GABA increases also prolactin. Prolactin is the hormone that stimulates the breasts to produce milk. Although body builders want to build up their chest size, they probably do not want to do it this way. Although there is no research on taking GABA during pregnancy or nursing, pregnant or nursing mothers should not take this information to suggest that GABA might increase their milk supply. It might, but it also might stimulate early breast development and lactation in their infants.
Further health benefits of GABA
Several health benefits have been reviewed on the clinical use of amino acids as nutritional medicine helping for some symptoms treatment could be briefly summarized as following:
Anxiety
If oral GABA reaches the brain in any significant amount it should act as a tranquilizer. GABA as a neurotransmitter, blocks nerve impulses and slows neuronal transmission. Braverman and Pfeiffer (1987) described an anecdotal account of the successful treatment of a 40 year old woman suffering from anxiety with 800 mg of GABA/day.
Depression
There is a well proven tendency for depressed and bipolar patients to have lower levels of GABA in their blood plasma. These low levels are thought to reflect lower brain levels. Braverman and Pfeiffer (1987) suggested using GABA to treat depression. The theory is that oral GABA will bring up plasma levels. Unfortunately this theory is too simplistic and possibly dangerous.
The current theory of GABA and depression is that low plasma levels of GABA may identify an inheritable tendency for mood disorders such as depression or bipolar disease (Petty et al. 1993). Today’s view is that things which increase GABA in these people may trigger a depressive episode. It is not until time or treatment restores GABA to its former low level that these people feel better (Petty 1995).
Premenstrual syndrome
Women who become depressed with hormonal changes during their menstrual cycle have lower plasma GABA levels than women whose moods are unaffected by menstrual changes. Halbreich et al. (1996) suggested that it is this same inheritable tendency for low GABA levels that underlie their depressive tendencies and their premenstrual depression. More recent research suggests a more complicated interaction between sex hormones and GABA in the brain. In healthy women, brain GABA activity decreases through the menstrual cycle, especially the luteal phase. In women with premenstrual depression, brain GABA activity actually increases during the luteal phase. Giving GABA to women with premenstrual depression may aggravate their problem and drop their spirits.
Male contraceptive
Braverman and Pfeiffer (1987) suggested GABA as a possible male contraceptive because it decreases sperm motility but do not count on this. The reference they cite is referring to monosodium glutamate, a distant relative of GABA. Another studies reported that, GABA made sperm cells hyperactive (Calogero et al. 1996). In other words GABA might be useful for treating male infertility rather than as a contraceptive.
Seizures
It was mentioned Taurine’s apparent effect of suppressing seizures because it increases GABA effect in the brain. At this time, the research on Taurine and epileptic seizures is mixed. The effect of Taurine varies with the time it is administered, sometimes preventing and sometime precipitating seizures (Eppler et al. 1999).
Heart and blood health
GABA has been reported to reduce blood pressure (BP) by intravenous administration in experimental animals (Lacerda et al. 2003 and Stanton 1963) and in human subjects (Elliott and Hobbiger 1959). The BP-lowering effect of GABA is due to in part its ability to block peripheral ganglia (Stanton 1963). In spontaneously hypertensive rats, GABA has an antihypertensive effect, possibly through the inhibition of noradrenaline release from sympathetic nerve endings (Hayakawa et al. 2002).
The rostral ventrolateral medulla (RVLM) contains neurons involved in tonic and reflex control of arterial pressure. Electrolytic lesion or chemical inactivation of RVLM neurons by inhibitory amino acids such as glycine or GABA results in a fall of BP similar to that usually obtained in acute spinal animals (Granata et al. 1983). Lacerda et al. (2003) described the effects of GABA and anesthetics injected into the RVLM of conscious and urethane (1.2 g/kg) anesthetized Wistar rats. In conscious rats, bilateral microinjection of GABA (50 nmol/200 nl) induced a small, but significant decrease in blood pressure (from 130 to 110 mm Hg). A similar response was observed with sodium pentobarbital microinjection (24 nmol/200 nl).
Blood sugar and diabetes
Braverman and Pfeiffer (1987) suggested that 2–4 g of GABA may stimulate insulin production and lowering blood sugar levels. This idea is supported by the newer HGH studies which also see an increase in insulin levels with oral GABA.
Fortification application of physiologically active ingredients (PAI) in yoghurt making
Yoghurt fortification with isoflavones (ISO)
A novel ISO-yoghurt-like product of potential health benefits was developed by fermentation of cow’s milk fortified with 30 and 50 mg of soybean ISO per 100 ml of milk using yoghurt cultures. It was found that, addition of ISO to yoghurt milk increased the rate of acidity development and this increase was proportional to the ratio added. Improvements in body and texture as well as high palatability were noticed with addition of 30 mg ISO/100 ml milk while increasing the amount to 50 mg/100 ml resulted in lower sensory scores for the yoghurt (Ali et al. 2004).
Yoghurt fortification with γ-amino butyric acid (GABA)
A study carried out by Inoue et al. (2003) consisted of a 12-week period of daily intake of fermented milk containing GABA (FMG) or placebo (weeks 1–12) followed by 2 weeks of no intake (weeks 13 and 14). They found that, there was a significant decrease of peripheral blood pressure (BP) within 2 or 4 weeks and it remained decreased throughout the 12-week intake period. For the FMG recipients, the mean decrease after 12 weeks was 17.4 mm Hg in the systolic BP (SBP) and 7.2 mm Hg in the diastolic BP (DBP). They concluded that FMG may contribute to lowering BP in mildly hypertensive people.
Furthermore, Hayakawa et al. (2004) investigated the BP-lowering effects of GABA versus GABA-enriched fermented milk product (FMG) by low-dose oral administration to spontaneously hypertensive (SHR/Izm) and normotensive Wistar–Kyoto (WKY/Izm) rats. A single oral dose of GABA or FMG (5 ml/kg; 0·5 mg GABA/kg) significantly decreased the blood pressure of SHR/Izm from 4 to 8 h after administration, but it did not increase that of WKY/Izm rats. The hypotensive activity of GABA was dose-dependent from 0·05 to 5·00 mg/kg in SHR/Izm. During the chronic administration of experimental diets to SHR/Izm, a significantly slower increase in blood pressure with respect to the control group was observed at 1 or 2 weeks after the start of feeding with the GABA or FMG diet respectively and this difference was maintained throughout the period of feeding. They suggested that low-dose oral GABA has a hypotensive effect in SHR/Izm and that the hypotensive effect of FMG is due to GABA.
Conclusion: main differences between ISO and GABA containing yoghurts
As a conclusion, in a comparison between both of types of PAI on the several attributes of carbonated yoghurt, whether cultured with the ordinary bacterial starter culture (BSC) or by the gut bacterial strains in relation to the fortification with ISO or GABA, Fayed et al. (2012) found that, the yoghurt-milk fortification with any type of both PAI led to activate the bacterial growth of all strains used. This promotion of growth activation was more pronounced when GABA was used versus ISO for all strains except of Bifidobacterum sp., which exhibited more growth in the presence of the latter versus the former. The counts of all bacterial strains increased as the PAI fortification level progressed regardless their kind. Furthermore, all bacterial strains arrived their maximum counts at the 1st week of cold storage period (CSP) of carbonated yoghurt then trended to decline along CSP, but they stilled significantly higher (except Str. thermophilus) than those counted in fresh yoghurt either before or after the carbonation step. Moreover, Str.thermophilus achieved always counts in the ordinary yoghurt higher than those enumerated in the bio-one. The PAI, especially GABA, caused higher titratable acidity (TA%) and hence lower pH value in the resultant yoghurt. The TA% increased and pH value decreased as the fortification level with PAI was raised. BSC-yoghurt contained always higher TA% and hence lower pH value versus the bio one. The acetaldehyde (AC) and diacetyl (DA) contents of all yoghurts behaved trends similar to TA%. Rheologically, consistency coefficient and yield stress, of yoghurt were not influenced by the fortification with any type or level of PAI. However, the fortification with PAI, especially with GABA, caused a significant increment in the apparent viscosity of yoghurt. Bioyoghurt possessed rheological parameters lower than those of ordinary one. The sensory appearance as well as the consistency scours of yoghurt were not changed and were as good as the control among all factors studied while the statistical analysis confirmed that, the scores of the flavor and even of the overall quality were significantly negatively influenced by the fortification especially with GABA versus ISO. Moreover, the bioyoghurt gained flavor and total sensory scores higher than those of the ordinary one. The results suggested that, yoghurt beyond its ability to be probiotic food via its culturing with the gut bacterial strains, it could further carry more healthy benefits when it was fortified with physiological active ingredients, especially GABA versus ISO at any level studied.
References
- Adams KF, Chen C, Newton KM, Potter JD, Lampe JW. Soy isoflavones do not modulate prostate-specific antigen concentrations in older men in a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2004;13:644–648. [PubMed] [Google Scholar]
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Greenstein a specific inhibitor of tyrosine-specific protein kinesis. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Alekel DL, Germain AS, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000;72:844–852. doi: 10.1093/ajcn/72.3.844. [DOI] [PubMed] [Google Scholar]
- Ali AA, Roushdy IM, Awad RA. Development of soybean isoflavones yoghurt-like product of potential health benefits. Ann Agric Sci Moshtohor. 2004;42:129–138. [Google Scholar]
- Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–5050. [PubMed] [Google Scholar]
- Arjmandi BH, Lucas EA, Khalil DA, Devareddy L, Smith BJ, McDonald J, Arquitt AB, Payton ME, Mason C. One year soy protein supplementation has positive effects on bone formation markers but not bone density in postmenopausal women. Nutr J. 2005;4:8. doi: 10.1186/1475-2891-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Boersma B, Patel R, Kirk M, Darley-Usmar VM, Kim H, Xu J. Isoflavonoids and chronic disease: mechanisms of action. Biofactors. 2000;12:209–215. doi: 10.1002/biof.5520120133. [DOI] [PubMed] [Google Scholar]
- Braverman ER, Pfeiffer CC. The healing nutrients. New Canaan: Keats Pub Inc; 1987. [Google Scholar]
- Brink E, Coxam V, Robins S, Wahala K, Cassid A, Branca F. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr. 2008;87:761–770. doi: 10.1093/ajcn/87.3.761. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Hall J, Fishel S, Green S, Hunter A, D'Agata R. Effects of gamma-aminobutyric acid on human sperm motility and hyperactivation. Mol Hum Reprod. 1996;2:733–738. doi: 10.1093/molehr/2.10.733. [DOI] [PubMed] [Google Scholar]
- Casini ML, Marelli G, Papaleo E, Ferrari A, D'Ambrosio F, Unfer V. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: a randomized, double-blind, crossover, placebo-controlled study. Fertil Steril. 2006;85:972–978. doi: 10.1016/j.fertnstert.2005.09.048. [DOI] [PubMed] [Google Scholar]
- Cavagnini F, Benetti G, Invitti C, Ramella G, Pinto M, Lazza M, Dubini A, Marelli A, Muller EE. Effect of gamma-aminobutyric acid on growth hormone and prolactin secretion in man: influence of pimozide and domperidone. Acta Endocrinol (Copenh) 1980a;93:149–154. doi: 10.1210/jcem-51-4-789. [DOI] [PubMed] [Google Scholar]
- Cavagnini F, Invitti C, Pinto M, Maraschini C, Di Landro A, Dubini A, Marelli AJ. Effect of acute and repeated administration of gamma aminobutyric acid (GABA) on growth hormone and prolactin secretion in man. Clin Endocrinol Metab. 1980b;51:789–792. doi: 10.1210/jcem-51-4-789. [DOI] [PubMed] [Google Scholar]
- Chen YM, Ho SC, Lam SS, Ho SS, Woo JL. Beneficial effect of soy isoflavones on bone mineral content was modified by years since menopause, body weight, and calcium intake: a double-blind, randomized, controlled trial. Menopause. 2004;11:246–254. doi: 10.1097/01.GME.0000094394.59028.46. [DOI] [PubMed] [Google Scholar]
- Cheong JM, Martin BR, Jackson GS, Elmore D, McCabe GP, Nolan JR, Barnes S, Peacock M, Weaver CM. Soy isoflavones do not affect bone resorption in postmenopausal women: a dose–response study using a novel approach with 41Ca. J Clin Endocrinol Metab. 2007;92:577–582. doi: 10.1210/jc.2006-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorazy PA, Himelhoch S, Hopwood NJ, Greger NG, Postellon DC (1995) Persistent hypothyroidism in an infant receiving a soy formula: case report and review of the literature. Pediatrics 96:148–150 [PubMed]
- Dillingham BL, McVeigh BL, Lampe JW, Duncan AM. Soy protein isolates of varied isoflavone content do not influence serum thyroid hormones in healthy young men. Thyroid. 2007;17:131–137. doi: 10.1089/thy.2006.0206. [DOI] [PubMed] [Google Scholar]
- Djuric Z, Chen G, Doerge DR, Heilbrun LK, Kucuk O. Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Lett. 2001;172:1–6. doi: 10.1016/S0304-3835(01)00627-9. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Sheehan DM. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect. 2002;110(Suppl 3):349–353. doi: 10.1289/ehp.02110s3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy C, Cyr M. Phytoestrogens: potential benefits and implications for breast cancer survivors. J Womens Health (Larchmt) 2003;12:617–631. doi: 10.1089/154099903322404276. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, Krasne FB. Crustacean studies and the early history of GABA. Trends Neurol Sci. 1999;22:347. doi: 10.1016/S0166-2236(99)01449-6. [DOI] [PubMed] [Google Scholar]
- Elliott CAK, Hobbiger F. Gamma aminobutyric acid: circulatory and respiratory effects in different species: re-investigation of the anti-strychnine action in mice. J Physiol. 1959;146:70–84. doi: 10.1113/jphysiol.1959.sp006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppler B, Patterson TA, Zhou W, Millard WJ, Dawson R., Jr Kainic acid (KA)-induced seizures in Sprague–Dawley rats and the effect of dietary taurine (TAU) supplementation or deficiency. Amino Acids. 1999;16:133–147. doi: 10.1007/BF01321532. [DOI] [PubMed] [Google Scholar]
- Evans M, Njike VY, Hoxley M, Pearson M, Katz DL. Effect of soy isoflavone protein and soy lecithin on endothelial function in healthy postmenopausal women. Menopause. 2007;14:141–149. doi: 10.1097/01.gme.0000227404.83686.1b. [DOI] [PubMed] [Google Scholar]
- Farquhar C, Marjoribanks J, Lethaby A, Suckli JA, Lamberts Q. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2009;2:CD 004143. doi: 10.1002/14651858.CD004143.pub3. [DOI] [PubMed] [Google Scholar]
- Fayed AE, Ali AA, Hussein GA, Zein El-Dein HE. Production of carbonated stirred yoghurt fortified with some physiological active ingredients. J Biol Chem Environ Sci. 2012;7:157–189. [Google Scholar]
- File SE, Hartley DE, Elsabagh S, Duffy R, Wiseman H. Cognitive improvement after 6 weeks of soy supplements in postmenopausal women is limited to frontal lobe function. Menopause. 2005;12:193–201. doi: 10.1097/00042192-200512020-00014. [DOI] [PubMed] [Google Scholar]
- Fischer L, Mahoney C, Jeffcoat AR, Koch MA, Thomas BE, Valentine JL, StinchcombeT BJ, Crowell JA, Zeisel SH. Clinical characteristics and pharmacokinetics of purified soy isoflavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer. 2004;48:160–170. doi: 10.1207/s15327914nc4802_5. [DOI] [PubMed] [Google Scholar]
- Granata AR, Ruggiero DA, Park DJ, Reis DJ. Lesions of epinephrine neurons in the ventrolateral medulla abolish the vasopressor components of baroreflex and cardiopulmonary reflex. Hypertension. 1983;5:V80–V84. doi: 10.1161/01.HYP.5.6_Pt_3.V80. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Petty F, Yonkers K, Kramer GL, Rush AJ, Bibi KW. Low plasma gamma-aminobutyric acid levels during the late luteal phase of women with premenstrual dysphoric disorder. Am J Psychiatry. 1996;153:718–720. doi: 10.1176/ajp.153.5.718. [DOI] [PubMed] [Google Scholar]
- Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, Howell A, Bundred NJ. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84:4017–4024. doi: 10.1210/jcem.84.11.6152. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Kimura M, Kamata K. Mechanism underlying gamma-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. Eur J Pharmacol. 2002;438:107–113. doi: 10.1016/S0014-2999(02)01294-3. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Kimura M, Kasaha K, Matsumoto K, Sansawa H, Yamori Y. Effect of a g-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br J Nutr. 2004;92:411–417. doi: 10.1079/BJN20041221. [DOI] [PubMed] [Google Scholar]
- Ho SC, Chan AS, Ho YP, So EK, Sham A, Zee B, Woo JL. Effects of soy isoflavone supplementation on cognitive function in Chinese postmenopausal women: a double-blind, randomized, controlled trial. Menopause. 2007;14:489–499. doi: 10.1097/gme.0b013e318032b2d3. [DOI] [PubMed] [Google Scholar]
- Holzbeierlein JM, McIntosh J, Thrasher JB. The role of soy phytoestrogens in prostate cancer. Curr Opin Urol. 2005;15:17–22. doi: 10.1097/00042307-200501000-00005. [DOI] [PubMed] [Google Scholar]
- Horn-Ross PL, John EM, Canchola AJ, Stewart SL, Lee MM. Phytoestrogen intake and endometrial cancer risk. J Natl Cancer Inst. 2003;95:1158–1164. doi: 10.1093/jnci/djg015. [DOI] [PubMed] [Google Scholar]
- Howes LG, Howes JB, Knight DC. Isoflavone therapy for menopausal flushes: a systematic review and meta-analysis. Maturitas. 2006;55:203–211. doi: 10.1016/j.maturitas.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Huang HY, Yang HP, Yang HT, Yang TC, Shieh MJ, Huang SY. One-year soy isoflavone supplementation prevents early postmenopausal bone loss but without a dose-dependent effect. J Nutr Biochem. 2006;17:509–517. doi: 10.1016/j.jnutbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H. Blood-pressure-lowering effect of a novel fermented milk containing g-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr. 2003;57:490–495. doi: 10.1038/sj.ejcn.1601555. [DOI] [PubMed] [Google Scholar]
- Jou HJ, Wu SC, Chang FW, Ling PY, Chu KS, Wu WH. Effect of intestinal production of equol on menopausal symptoms in women treated with soy isoflavones. Int J Gynaecol Obstet. 2008;102:44–49. doi: 10.1016/j.ijgo.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Ju YH, Allred CD, Allred KF, Karko KL, Doerge DR, Helferich WG. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr. 2001;131:2957–2962. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- Ju YH, Doerge DR, Allred KF, Allred CD, Helferich WG. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002;62:2474–2477. [PubMed] [Google Scholar]
- Katz DL, Evans MA, Njike VY, Yanchou V, Hoxley ML, Nawaz H, Comerford BP, Sarrel PM. Raloxifene, soy phytoestrogens and endothelial function in postmenopausal women. Climacteric. 2007;10:500–507. doi: 10.1080/13697130701750123. [DOI] [PubMed] [Google Scholar]
- Khurana HK, Kanawjia SK. Recent trends in development of ferment milks. Curr Nutr Food Sci. 2007;9:91–108. doi: 10.2174/1573401310703010091. [DOI] [Google Scholar]
- Kreb EE, Ensrud KE, McDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Necol. 2004;104:824–836. doi: 10.1097/01.AOG.0000140688.71638.d3. [DOI] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Von Muhlen D, Barrett-Connor E, Bressel MA. Isoflavones and cognitive function in older women: the soy and postmenopausal health in aging (SOPHIA) study. Menopause. 2003;10:196–202. doi: 10.1097/00042192-200310030-00004. [DOI] [PubMed] [Google Scholar]
- Lacerda CE, Campos RR, Araujo CG, Andreatta-Van LS, Lopes OU, Guertzenstein PG. Cardiovascular responses to microinjections of GABA or anesthetics into the rostral ventrolateral medulla of conscious and anesthetized rats. Braz J Med Biol Res. 2003;36:1269–1277. doi: 10.1590/S0100-879X2003000900019. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis. Circulation. 2004;109:1127–1133. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2008;62:155–161. doi: 10.1038/sj.ejcn.1602748. [DOI] [PubMed] [Google Scholar]
- Mason P (2001) Isoflavones. Pharm J 266:16–19
- Messina MJ. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr Rev. 2003;61:117–131. doi: 10.1301/nr.2003.apr.117-131. [DOI] [PubMed] [Google Scholar]
- Messina MJ, Loprinzi CL. Soy for breast cancer survivors: a critical review of the literature. J Nutr. 2001;131(Suppl 11):3095S–3108S. doi: 10.1093/jn/131.11.3095S. [DOI] [PubMed] [Google Scholar]
- Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16:249–258. doi: 10.1089/thy.2006.16.249. [DOI] [PubMed] [Google Scholar]
- Messina M, Kucuk O, Lampe JW. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J AOAC Int. 2006a;89:1121–1134. [PubMed] [Google Scholar]
- Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006b;55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- Murray MJ, Meyer WR, Lessey BA, Oi RH, DeWire RE, Fritz MA. Soy protein isolate with isoflavones does not prevent estradiol-induced endometrial hyperplasia in postmenopausal women: a pilot trial. Menopause. 2003;10:456–464. doi: 10.1097/01.GME.0000063567.84134.D1. [DOI] [PubMed] [Google Scholar]
- Newton KM, LaCroix AZ, Levy L, Li SS, Qu P, Potter JD, Lampe JW. Soy protein and bone mineral density in older men and women: a randomized trial. Maturitas. 2006;55:270–277. doi: 10.1016/j.maturitas.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Passariello N, Giugliano D, Torella R, Sgambato S, Coppola L, Frascolla N. A possible role of gamma-aminobutyric acid in the control of the endocrine pancreas. J Clin Endocrinol Metab. 1982;54:1145–1149. doi: 10.1210/jcem-54-6-1145. [DOI] [PubMed] [Google Scholar]
- Petty F. GABA and mood disorders: a brief review and hypothesis. J Affect Disord. 1995;34:275–281. doi: 10.1016/0165-0327(95)00025-I. [DOI] [PubMed] [Google Scholar]
- Petty F, Kramer GL, Fulton M, Moeller FG, Rush AJ. Low plasma GABA is a trait-like marker for bipolar illness. Neuropsychopharmacology. 1993;9:125–132. doi: 10.1038/npp.1993.51. [DOI] [PubMed] [Google Scholar]
- Powers ME, Borst SE, McCoy SC, Conway R, Yarrow JF. The effects of gamma aminobutyric acid on growth hormone secretion at rest and following exercise. Med Sci Sports & Exerc. 2003;35(Suppl 1):S271. doi: 10.1097/00005768-200305001-01500. [DOI] [Google Scholar]
- Rowland I, Faughnan M, Hoey L, Wahala K, Williamson G, Cassidy A. Bioavailability of phyto-oestrogens. Br J Nutr. 2003;89(Suppl 1):S45–58. doi: 10.1079/BJN2002796. [DOI] [PubMed] [Google Scholar]
- Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res. 1997;26:63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- Sartippour MR, Rao JY, Apple S. A pilot clinical study of short-term isoflavone supplements in breast cancer patients. Nutr Cancer. 2004;49:59–65. doi: 10.1207/s15327914nc4901_8. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr. 2003;78(3 Suppl):593S–609S. doi: 10.1093/ajcn/78.3.593S. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-aclue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–2443. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton HC. Mode of action of gamma amino butyric acid on the cardiovascular system. Arch Int Pharmacodyn Ther. 1963;143:195–204. [PubMed] [Google Scholar]
- Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86:3053–3060. doi: 10.1210/jcem.86.7.7645. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Giannopoulos D, Dalais FS, Hodgson J, McGrath BP. Randomised, controlled, cross-over trial of soy protein with isoflavones on blood pressure and arterial function in hypertensive subjects. J Am Coll Nutr. 2006;25:533–540. doi: 10.1080/07315724.2006.10719569. [DOI] [PubMed] [Google Scholar]
- Tice JA, Ettinger B, Ensrud K, Wallace R, Blackwell T, Cummings SR. Phytoestrogen supplements for the treatment of hot flashes: the Isoflavone Clover Extract (ICE) Study: a randomized controlled trial. JAMA. 2003;290:207–214. doi: 10.1001/jama.290.2.207. [DOI] [PubMed] [Google Scholar]
- Van Erp-Baart MA, Brants HA, Kiely M, Mulligan A, Turrini A, Sermoneta C, Kilkkinen A, Valsta LM. Isoflavone intake in four different European countries: the VENUS approach. Br J Nutr. 2003;89(Suppl 1):S25–30. doi: 10.1079/BJN2002793. [DOI] [PubMed] [Google Scholar]
- Wang LQ. Mammalian phytoestrogens: enterodiol and enterolactone. J Chromatogr B Anal Technol Biomed Life Sci. 2002;777:289–309. doi: 10.1016/S1570-0232(02)00281-7. [DOI] [PubMed] [Google Scholar]
- Wiseman H, O'Reilly JD, Adlercreutz H, Mallet AI, Bowey EA, Rowland IR, Sanders TA. Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr. 2000;72:395–400. doi: 10.1093/ajcn/72.2.395. [DOI] [PubMed] [Google Scholar]
- Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- Wu J, Oka J, Ezaki J, Ohtomo T, Ueno T, Uchiyama S, Toda T, Uehara M, Ishimi Y. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women: a double-blind, randomized, controlled trial. Menopause. 2007;14:866–874. doi: 10.1097/gme.0b013e3180305299. [DOI] [PubMed] [Google Scholar]
- Xu WH, Zheng W, Xiang YB, Ruan ZX, Cheng JR, Dai Q, Gao YT, Shu XO. Soya food intake and risk of endometrial cancer among Chinese women in Shanghai: population based case–control study. BMJ. 2004;328:1285. doi: 10.1136/bmj.38093.646215.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. 2009;89:1155–1163. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- Ye YB, Tang XY, Verbruggen MA, Su YX. Soy isoflavones attenuate bone loss in early postmenopausal Chinese women: a single-blind randomized, placebo-controlled trial. Eur J Nutr. 2006;45:327–334. doi: 10.1007/s00394-006-0602-2. [DOI] [PubMed] [Google Scholar]
- Zhan S, Ho SC. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. 2005;81:397–408. doi: 10.1093/ajcn.81.2.397. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brint RD. WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert Rev Neurother. 2007;7:1549–1564. doi: 10.1586/14737175.7.11.1549. [DOI] [PubMed] [Google Scholar]