Abstract
Red-fleshed dragon fruit (Hylocereus polyrhizus) is rich in antioxidants. The aim of this study was to determine the effects of heat pasteurization, pH adjustment, ascorbic acid addition as well as storage under agitation and light or dark condition on betacyanin content in red-fleshed dragon fruit (Hylocereus polyrhizus) juice and concentrate. The concentrate was produced by concentrating clarified red-fleshed dragon fruit juice in a rotary evaporator at 40 °C. UV-Visible spectrophotometer was used for analyzing betacyanin content. Addition of 0.25 % ascorbic acid, pH 4.0, and pasteurization at 65 °C for 30 min were selected as the best processing conditions to retain betacyanin content in red-fleshed dragon fruit juice. Storage at the agitation speed of 220 rpm showed that the concentrated samples had higher betacyanin stability compared to juice, while both juice and concentrate had almost similar betacyanin stability when tested for storage in the presence of light. In summary, ascorbic acid stabilized betacyanin in both juice and concentrate at agitated or non-agitated conditions. In contrast, light degraded betacyanin in both juice and concentrate models.
Keywords: Betacyanin, Juice, Concentrate, Storage, Ascorbic acid, Dragon fruit
Introduction
Dragon fruit is part of the Cactaceae family and order of Caryophyllales (Rebecca et al. 2010). Originating in Central America and Mexico, this plant is now widely grown in countries like Taiwan, Vietnam and Malaysia (Nur ‘Aliaa et al. 2010). Two types of dragon fruit are commonly found in Malaysia; one with pink skin and white flesh (Hylocereus undatus) and another with pink skin and red flesh (Hylocereus polyrhizus) (Nur ‘Aliaa et al. 2010). The red-fleshed dragon fruit (H. polyrhizus) used in this study is either round or oval, with a slightly leafy fuchsia pink leathery skin when mature. It has an appealing deep purple/red coloured flesh, which is interspersed with tiny black seeds. The flesh is juicy; with a slightly sweet flavour.
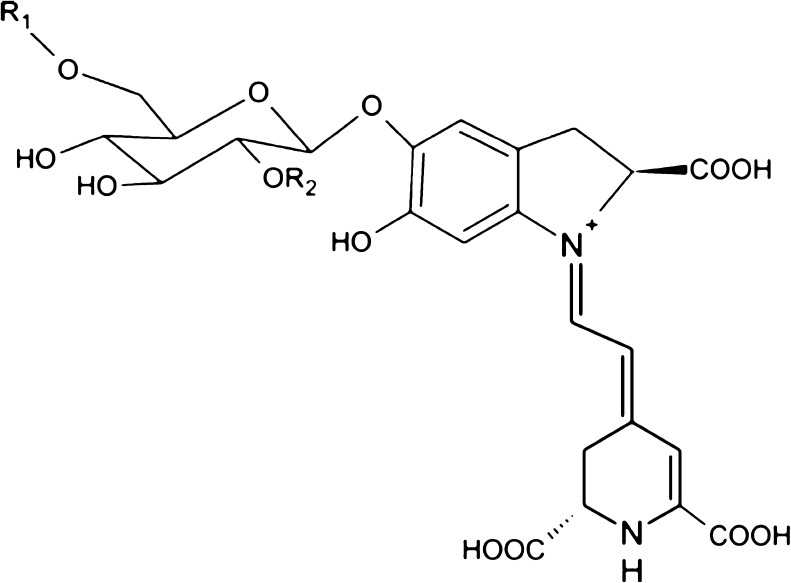
The colour of H. polyrhizus is contributed by betacyanin (Fig. 1), a compound from a set of water-soluble nitrogen-containing pigments known as betalains (Rebecca et al. 2010). Betalains play a vital role as the major antioxidant contributor in H. polyrhizus whereas non-betalainic phenolic compounds play a minor role (Esquivel et al. 2007). Betalains comprise of betacyanin (red-purple) and betaxanthins (yellow). However, betaxanthins are devoid in H. polyrhizus and only betacyanin can be found (Le Bellec et al. 2006).
Fig. 1.
Structure of betacyanin, R1 = H; R2 = H
The stability of betacyanin is affected by heat, oxygen, light, pH and moisture (Woo et al. 2011) as these factors are the main causes for discolouration of this pigment (Liu et al. 2008). The primary degradation products of betacyanin are the colourless cyclo-dopa 5-O-β-glucoside and bright yellow betalamic acid (Herbach et al. 2004).
Juice concentration reduces the volume of juice and water activity of the product (Seog et al. 2006). Juice concentration increases the relative solids concentration, enhances pigment stability and reduces the risk of microbial contamination (Castellar et al. 2006). Once juices are concentrated to a °brix value of >60, it is stable against yeast, mold and bacteria growth (Boulton et al. 1996). The concentrated products require less storage volume, thus reducing transportation and storage costs (Castellar et al. 2006). Since dragon fruit juice is high in moisture content, juice concentration offers an alternative to prolong the shelf life of the juice. No information is currently available on the stability of red-fleshed dragon fruit concentrate at various processing and storage conditions. Therefore, the objectives of this work were to investigate the effects of processing conditions on betacyanin stability using red-fleshed dragon fruit juice and concentrate as models. Betacyanin content for both juice and concentrate was determined under agitated or non-agitated condition and under light or dark during 5 weeks of storage at room temperature.
Materials and methods
Samples and chemicals
Hydrochloric acid 98 % (Fisher Chemicals, USA); sodium hydroxide 98 % (HmbG® Chemicals, Hamburg, Germany); ascorbic acid 99.7 % (Merck, Darmstadt, Germany); citric acid anhydrous (Euro Chemo Pharma Sdn. Bhd., Penang, Malaysia); di-sodium hydrogen phosphate (R&M Chemicals, Essex, U. K.).
Preparation of juice and juice concentrate
Fruit sample
Red-fleshed dragon fruits were obtained from Great Sun Pitaya Sdn. Bhd. (the orchard is located at Telok Panglima Garang, Kuala Langat, Selangor). Fresh fruits were stored at 4 °C and allowed to mature for 1 week before use to ensure all fruits had similar maturity.
Filtration
Dragon fruit pulp was treated as discussed by Nur ‘Aliaa et al. (2009). The treated pulp was centrifuged (Hettich Zentrifugen Universal 320R, Germany) at 3000 g for 10 min, and the supernatant was collected. The juice was then filtered through a filter paper (CHMLAB GROUP, Barcelona, Spain) with a pore size of 11 μm using a Buchner funnel attached to an oil-less vacuum pump (Rocker 300, Rocker Scientific Company Limited, Taiwan).
Juice concentration
Juice concentration was modified from Schweiggert et al. (2009) and Woo et al. (2011). Filtered juice was concentrated to a total soluble solids of 60 ± 5 °brix in a rotary evaporator (EYELA, model N-1100S-WD, Fisher Scientific, USA) with a water bath (EYELA OSB-2100, Fisher Scientific, USA) at 40 °C attached to an aspirator (EYELA, model A-1000S, Fisher Scientific, USA) followed by frozen storage at -80 °C until further analysis. Juice concentrates were stored in freezer bags made from polyethylene (PE).
Studies on processing conditions
Effect of heating conditions on betacyanin content
Juice samples with 0 % ascorbic acid and a fixed pH 5 (using 1 M HCl and 1 M NaOH) were subjected to various heating conditions. For this experiment, temperatures of 65 °C, 70 °C, 75 °C, 80 °C, 85 °C, 90 °C and 95 °C were tested each for 10, 20 and 30 min because these are the common juice pasteurization temperatures and times (Herbach et al. 2007; Walkowiak-Tomczak 2007; de Bruijin et al. 2003; Okoth et al. 2000). Juice samples were placed into test tubes with cap and immersed into water baths for the given time-temperature regimes. After heating, the test tubes were immediately cooled in an ice-water bath. Betacyanin content for each treated sample was assessed.
Effect of pH adjustment on betacyanin content
Juice samples were adjusted to pH 3, 4, 5, 6 and 7 using 1 M HCl and 1 M NaOH, as modified from Woo et al. (2011). These juice samples were then subjected to heat treatment at 65 °C for 30 min. After cooling, betacyanin content for each treated sample was assessed.
Effect of ascorbic acid addition on betacyanin content
The addition of ascorbic acid was modified from Woo et al. (2011). Different percentages of ascorbic acid (0.25 %, 0.50 %, 0.75 %, 1.00 %, 1.25 % and 1.50 % (w/w)) were added to juice samples. Sample without addition of ascorbic acid was prepared as control. Samples were then adjusted to pH 4, and subjected to heat treatment at 65 °C for 30 min. After cooling, betacyanin content for each treated sample was assessed.
Studies on storage conditions
Effect of agitation on betacyanin stability during storage
Study on the effect of agitation on betacyanin content was modified from Walkowiak-Tomczak (2007). Agitation is an important parameter to determine the stability of juice as agitation may occur during the transportation of juice. Juice and concentrate samples were pretreated with the optimized processing conditions for red-fleshed dragon fruit juice (0.25 % ascorbic acid, adjusted to pH 4, and pasteurized at 65 °C for 30 min). Vials of 10.5 mL capacity were used for sample storage. The agitated sample was prepared by storing 5 mL juice/concentrate in a vial and the vials were incubated in an incubator shaker (ZHWY 200B, Labwit, Shanghai, China) at room temperature with continuous shaking at 220 rpm. Non-agitated juice/concentrate was left at room temperature. Weekly determination of betacyanin content for a total of 5 weeks was carried out. Five weeks were arbitarily chosen in this study.
Effect of light on betacyanin stability during storage
Effect of light on betacyanin content was conducted according to a method modified from Herbach et al. (2007). Juice or concentrate samples were pretreated with the optimized processing conditions for red-fleshed dragon fruit juice (0.25 % ascorbic acid, adjusted to pH 4 and pasteurized at 65 °C for 30 min). Juice or concentrate sample (10 mL) was stored in clear 10.5 mL vials. Samples that were stored under light conditions were placed in a laminar flow cabinet (model AHC-4D1, Esco, Malaysia) with a built-in warm white 5000 k electronically ballasted lighting. Samples stored in the dark were wrapped in aluminum foil and kept in the dark. All the samples were stored at room temperature. Samples were taken out weekly from the same vial for a total of 5 weeks for analysis of betacyanin content.
Determination of betacyanin content
Dragon fruit juice was diluted with McIlvaine buffer (pH 6.5) until a maximum absorption of 1.00 ± 0.05 was reached (Herbach et al. 2007). McIlvaine buffer was prepared from 0.1 M citric acid (30 mL) and 0.2 M sodium phosphate dibasic (70 mL) (Jamilah et al. 2011). The obtained dilution (40×) was then retained for all processing and storage samples (Herbach et al. 2007). Hence for each sample, 0.1 mL sample was added with 3.9 mL McIlvaine buffer in a plastic cuvette prior to spectrophotometric analysis. For blank, 4.0 mL McIlvaine buffer was used.
Betacyanin content (Bc) was expressed as the following equation
| 1 |
A: absorption value at λmax (537 nm) corrected by the absorption at 600 nm (correction for impurities); F: dilution factor; MW: molecular weight of betanin (550 g/mol); ε: molar extinction coefficient of betanin (60,000 L/mol cm); and l: path length of the cuvette (1 cm). In this study, all determinations were performed in triplicate using a UV-Vis spectrophotometer (Shimadzu 1240, Japan).
Betacyanin content (photometrical) was calculated as modified by Schweiggert et al. (2009):
| 2 |
where Bc0 represents the initial betacyanin content and Bc1 represents the final betacyanin content. Since F was held constant throughout the experiment and MW, ε and l are all constants, only the corrected absorbance (A) was used to calculate the proportion of betacyanin retained.
Statistical analysis
Analysis was conducted in triplicates (n = 3) and was reported as mean ± standard deviation. Variance was analyzed using Statistics Package for the Social Sciences (SPSS) version 16.0.
Results and discussion
Effects of processing conditions on betacyanin content
Effect of heating conditions on betacyanin content
There was no significant difference (p > 0.05) for betacyanin content with increasing temperatures at 65 °C, 70 °C, 75 °C and 80 °C for10, 20 and 30 min, respectively (Table 1). At 85 °C, no significant difference (p > 0.05) in betacyanin content of juice was observed for 10 and 20 min heating (Table 1). At 30 min heating, a significant reduction of betacyanin content was observed at 85 °C compared to lower temperatures (Table 1). The current study shows that betacyanin is heat sensitive as previously reported by Woo et al. (2011). Since betacyanin pigment is heat sensitive, the lowest temperature of 65 °C was selected for subsequent studies. Betacyanin content after heated at 65 °C, 70 °C, 75 °C and 80 °C for 20 and 30 min showed no significant difference (p > 0.05) (Table 1). Since the longer heating time of 30 min did not lead to a lower betacyanin content compared to 20 min, heating time of 30 min was thus chosen for preservative purposes in subsequent studies. A similar heating time at 65 °C has been previously reported for apple juice (de Bruijin et al. 2003).
Table 1.
Proportion of betacyanin retained (mean ± standard deviation, n = 3) in the red-fleshed dragon fruit juice sample after subjected to different pasteurization time and temperature regimes
| Time (min) | 0 | 10 | 20 | 30 |
|---|---|---|---|---|
| Temperature (°C) | ||||
| 65 | 1.00 ± 0.00aA | 0.83 ± 0.05aB | 0.77 ± 0.05aBC | 0.69 ± 0.04aC |
| 70 | 1.00 ± 0.00aA | 0.81 ± 0.07aB | 0.70 ± 0.06aBC | 0.61 ± 0.06aC |
| 75 | 1.00 ± 0.00aA | 0.76 ± 0.02abB | 0.70 ± 0.06aBC | 0.61 ± 0.07aC |
| 80 | 1.00 ± 0.00aA | 0.75 ± 0.06abB | 0.62 ± 0.07abBC | 0.57 ± 0.06aC |
| 85 | 1.00 ± 0.00aA | 0.67 ± 0.03bcB | 0.51 ± 0.05bcC | 0.41 ± 0.02bD |
| 90 | 1.00 ± 0.00aA | 0.55 ± 0.04cB | 0.41 ± 0.03cC | 0.32 ± 0.03bD |
| 95 | 1.00 ± 0.00aA | 0.54 ± 0.06cB | 0.39 ± 0.04cC | 0.28 ± 0.06bC |
a, b, c Means in the same column with different superscript letters are significantly different (p ≤ 0.05)
A, B, C, D Means in the same row with different superscript letters are significantly different (p ≤ 0.05)
Effect of pH on betacyanin content
Red-fleshed dragon fruit has an original pH of approximately pH 5. Woo et al. (2011) examined the stability of betacyanin between pH 3, 5 and 7; and found that pH 5 gave a slightly better content of betacyanin. pH 4–6 was reported as the optimal pH for betanin stability by other authors (Vaillant et al. 2005). Herbach et al. (2006) reported that the best betacyanin stability results were obtained when red-fleshed dragon fruit juice was heated at pH 4 compared to pH 6. Woo et al. (2011) further reiterated that betacyanin favours acidic pH region and are stable between pH 3 to 7 whilst beyond this range, it would be readily degraded. In the current study, betacyanin content was significantly higher at pH 5 compared to pH 7 (p ≤ 0.05) (Table 2), in accordance to that of Woo et al. (2011). No significant difference was observed at pH 3, 4 and 6 (p > 0.05) (Table 2). Since there are limited types of yeasts, molds and bacteria that are able to grow in low pH juices of pH 3.3–4.0 (Bull et al. 2004), pH 4 was selected for subsequent studies for preservation purposes. Under acidic conditions, decarboxylation of betanin was observed especially when heated at approximately pH 3–4 (Huang and von Elbe 1987). Dehydrogenation to the yellow neobetanin was also observed at low pH values (Azeredo 2009). Acidic conditions encourage recondensation of betalamic acid with cyclo-dopa 5-O-β-glucoside to regenerate betacyanin (Azeredo 2009; Schwartz and von Elbe 1983).
Table 2.
Proportion of betacyanin retained (mean ± standard deviation, n = 3) after pH adjustment and pasteurization
| pH | Proportion of betacyanin retained |
|---|---|
| 3 | 0.67 ± 0.06ab |
| 4 | 0.69 ± 0.06ab |
| 5 | 0.78 ± 0.03a |
| 6 | 0.69 ± 0.04ab |
| 7 | 0.61 ± 0.04b |
a, b Means with different superscript letters are significantly different (p ≤ 0.05)
Effect of ascorbic acid on betacyanin content
Ascorbic acid enhances betacyanin stability by removing oxygen from the surrounding environment; as oxygen is a factor that accelerates the degradation of betacyanin (Attoe and von Elbe 1982). A previous study has shown that ascorbic acid is added to fruit juice to enhance its nutritional quality (Ozkan et al. 2004). In this study, red-fleshed dragon fruit juice added with 0.25 % ascorbic acid prior to thermal pasteurization gave the highest betacyanin content (p ≤ 0.05) (Table 3). Since low level of ascorbic acid ranging from 7.0 to 11.4 mg 100 g−1 was found in red-fleshed dragon fruit juice (Nerd et al. 1999), the addition of ascorbic acid into red-fleshed dragon fruit juice in the current study appears to be promising. The significant (p ≤ 0.05) decrease in betacyanin content as ascorbic acid was added at 0.50–1.5 % (Table 3) could be due to the pro-oxidative effect of ascorbic acid at a concentration of 1000 mg/kg as previously reported in beet juice by Pasch and von Elbe (1979). The concentration variation of the prooxidative effect of ascorbic acid could be due to the difference in betacyanin concentration and initial ascorbic acid content between red-fleshed dragon fruit juice and concentrate in the current study and beet juice as previously reported.
Table 3.
Proportion of betacyanin retained (mean ± standard deviation, n = 3) after red-fleshed dragon fruit juice samples were added with ascorbic acid, and subjected to thermal pasteurization
| Ascorbic acid added (% w/w) | Proportion of betacyanin retained |
|---|---|
| 0.00 | 0.81 ± 0.02bc |
| 0.25 | 0.85 ± 0.01a |
| 0.50 | 0.82 ± 0.01b |
| 0.75 | 0.81 ± 0.01b |
| 1.00 | 0.79 ± 0.02bc |
| 1.25 | 0.79 ± 0.01bc |
| 1.50 | 0.77 ± 0.01c |
a, b, c Means with different superscript letters are significantly different (p ≤ 0.05)
Effect of storage conditions on betacyanin content in juice and concentrates
Effect of ascorbic acid and agitation on betacyanin content during storage
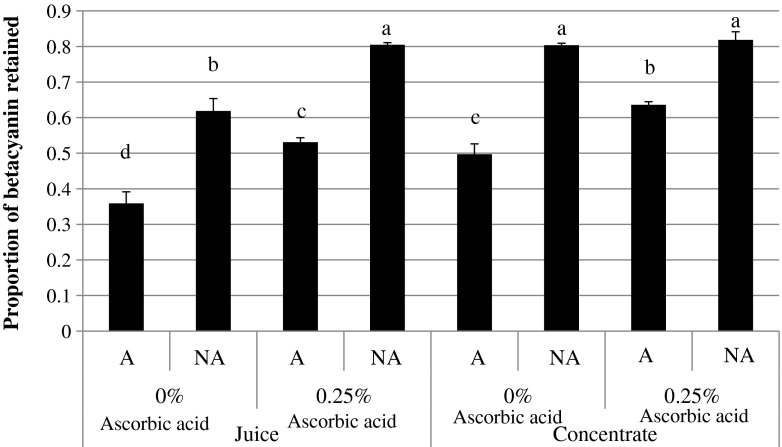
Agitation resulted in a significantly lower betacyanin content (p ≤ 0.05) in both juice and concentrate samples (Fig. 2). Oxidation impacts colour stability of the red-fleshed dragon fruit juice as betacyanins are sensitive to oxidation (Reynoso et al. 1997). An increased in the stability of betanin pigments were reported when oxygen was removed (Huang and von Elbe 1987). The concentrate samples generally had a significantly higher betacyanin content (p ≤ 0.05) compared to juice (Fig. 2). Concentration lowers the water activity, thus reducing the mobility of reactants and solubility of oxygen (Delgado-Vargas et al. 2000). As a result, concentrate samples were more stable in betacyanin content compared to juice samples.
Fig. 2.
Effect of ascorbic acid and agitation conditions on the proportion of betacyanin retained (mean ± standard deviation, n = 3) after 5 weeks storage.a, b, c, d Means with different superscript letters are significantly different (p ≤ 0.05). A agitated at 220 rpm, NA non agitated
The addition of ascorbic acid to juice samples has a stabilizing effect in retaining betacyanin (Fig. 2). As for concentrate samples, ascorbic acid helps to stabilize betacyanin at the agitated condition; whilst under non-agitated condition, betacyanin in the concentrate sample was relatively stable even in the absence of ascorbic acid (Fig. 2). Betacyanin content of both juice and concentrate samples added with 0.25 % ascorbic acid and stored under non-agitated condition were higher than those stored at the agitaion speed of 220 rpm (p ≤ 0.05) (Fig. 2). According to Reynoso et al. (1997), ascorbic acid has been used to counteract oxidation since it acts as an oxygen scavenger in a closed system.
Effect of ascorbic acid and light availability on betacyanin content during storage
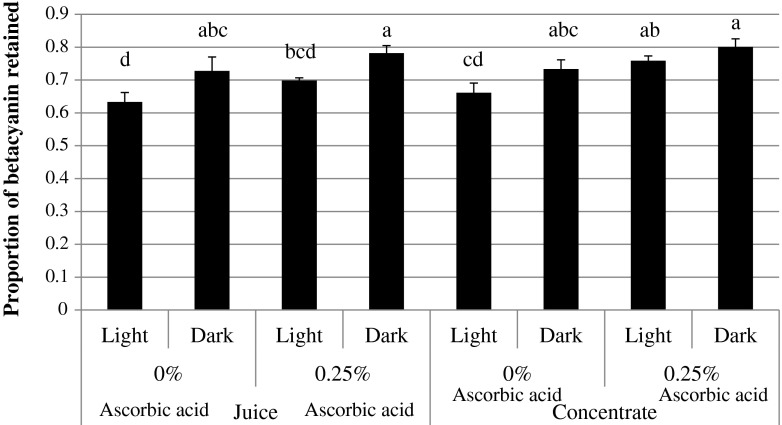
Juice sample stored under dark conditions had a better betacyanin content compared to storage under light conditions (Fig. 3). Stability of betalain is influenced by light (UV or visible) because it excites the π electron of betalain chromophores to a higher energy state (π*), resulting in lower activation energy and thus higher reactivity of the molecule (Attoe and von Elbe 1981; Jackman and Smith 1996). Unlike juice samples, betacyanin content of concentrate sample was not significant different (p > 0.05) between storage under light or dark (Fig. 3). Attoe and von Elbe (1981) reported that as pigment concentration increased, the influence of light on the degradation of betanin was negligible. This could possibly explain the negligible effect of light on the degradation of betacyanin in the concentrated sample. This postulation, however, needs to be confirmed by further studies.
Fig. 3.
Effect of ascorbic acid and light availability on the proportion of betacyanin retained (mean ± standard deviation, n = 3) after 5 weeks storage.a, b, c, d Means with different superscript letters are significantly different (p ≤ 0.05)
The current study shows that ascorbic acid appeared to have a stabilizing effect on illuminated concentrate samples as significantly higher betacyanin content (p ≤ 0.05) was noted (Fig. 3). Similar effect has been reported in several previous studies where the presence of 0.1 % (w/v) ascorbic acid managed to preserve the red hue in red beet and garambullo pigments under light conditions (Reynoso et al. 1997). For concentrates stored under dark condition, however, no stabilizing effect was observed when ascorbic acid was added to the sample compared to that of control (p > 0.05) (Fig. 3). Addition of ascorbic acid did not show a stabilizing effect for juice samples stored in the light or dark (Fig. 3).
Conclusion
The highest betacyanin content was observed when 0.25 % (w/w) ascorbic acid was added to red-fleshed dragon fruit juice and the juice was adjusted to pH 4 and pasteurized at 65 °C for 30 min. Betacyanin juice was found to deteriorate at the agitation speed of 220 rpm. The addition of 0.25 % (w/w) ascorbic acid helped to stabilize betacyanin in both juice and concentrate stored under agitated or non-agitated conditions. Light was also found to degrade betacyanin. In juice samples, dark storage resulted in higher betacyanin content. There was no difference in betacyanin content of concentrate samples stored in dark or light. The addition of 0.25 % (w/w) ascorbic acid shows a stabilizing effect on betacyanin of concentrate samples that were stored in light.
Acknowledgments
The authors thank Monash University Malaysia for financial support during the duration of this study and Great Sun Pitaya Sdn. Bhd. for sponsoring red-fleshed dragon fruit.
References
- Attoe EL, von Elbe JH. Photochemical degradation of betanine and selected anthocyanins. J Food Sci. 1981;46:1934–1937. doi: 10.1111/j.1365-2621.1981.tb04522.x. [DOI] [Google Scholar]
- Attoe EL, von Elbe JH. Degradation kinetics of betanine in solutions as influenced by oxygen. J Agric Food Chem. 1982;30:708–712. doi: 10.1021/jf00112a021. [DOI] [Google Scholar]
- Azeredo H. Betalains: properties, sources, applications, and stability—a review. Int J Food Sci Technol. 2009;44:2365–2376. doi: 10.1111/j.1365-2621.2007.01668.x. [DOI] [Google Scholar]
- Boulton RB, Singleton VL, Bisson LF, Kunkee RE. Preparation of musts and juice. In: Boulton RB, Singleton VL, Bisson LF, Kunkee RE, editors. Principles and practices of winemaking. New York: Chapman & Hall; 1996. pp. 65–101. [Google Scholar]
- Bull MK, Zerdin K, Howe E, Goicoechea D, Paramanandhan P, Stockman R, Sellahewa J, Szabo EA, Johnson RL, Stewart CM. The effect of high pressure processing on the microbial, physical and chemical properties of Valencia and Navel orange juice. Innov Food Sci Emerg Technol. 2004;5:135–149. doi: 10.1016/j.ifset.2003.11.005. [DOI] [Google Scholar]
- Castellar MR, Obon JM, Fernandez-Lopez JA. The isolation and properties of a concentrated red-purple betacyanin food colourant from Opuntia stricta fruits. J Sci Food Agric. 2006;86:122–128. doi: 10.1002/jsfa.2285. [DOI] [Google Scholar]
- de Bruijin JPF, Venegas A, Martinez JA, Borquez R. Ultrafiltration performance of Carbosep membranes for the clarificaiton of apple juice. LWT Food Sci Technol. 2003;36:397–406. doi: 10.1016/S0023-6438(03)00015-X. [DOI] [Google Scholar]
- Delgado-Vargas F, Jimenez AR, Paredes-Lopez O. Natural pigments: carotenoids, anthocyanins, and betalains - characteristics, biosynthesis, processing, and stability. Crit Rev Food Sci. 2000;40:173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- Esquivel P, Stintzing F, Carle R. Phenolic compound profiles and their corresponding antioxidant capacity of purple pitaya (Hylocereus sp.) genotypes. Z Naturforsch C. 2007;62:636–644. doi: 10.1515/znc-2007-9-1003. [DOI] [PubMed] [Google Scholar]
- Herbach KM, Stintzing FC, Carle R. Thermal degradation of betacyanins in juices from purple pitaya [Hylocereus polyrhizus (Weber) Britton & Rose] monitored by high-performance liquid chromatography-tandem mass spectrometric analyses. Eur Food Res Technol. 2004;219:377–385. doi: 10.1007/s00217-004-0948-8. [DOI] [Google Scholar]
- Herbach KM, Stintzing FC, Carle R. Betalain stability and degradation - Structural and chromatic aspects. J Food Sci. 2006;71:R41–R50. doi: 10.1111/j.1750-3841.2006.00022.x. [DOI] [Google Scholar]
- Herbach KM, Maier C, Stintzing FC, Carle R. Effects of processing and storage on juice colour and betacyanin stability of purple pitaya (Hylocereus polyrhizus) juice. Eur Food Res Technol. 2007;224:649–658. doi: 10.1007/s00217-006-0354-5. [DOI] [Google Scholar]
- Huang AS, von Elbe JH. Effect of pH on the degradation and regeneration of betanine. J Food Sci. 1987;52:1689–1693. doi: 10.1111/j.1365-2621.1987.tb05907.x. [DOI] [Google Scholar]
- Jackman RI, Smith JL. Anthocyanins and betalain. In: Hendry CF, Houghton JD, editors. Natural food colorants. London: Blackie Academic and Professional; 1996. pp. 244–309. [Google Scholar]
- Jamilah B, Shu CE, Kharidah M, Dzulkifly MA, Noranizan A. Physico-chemical characteristics of red pitaya (Hylocereus polyrhizus) peel. Int Food Res J. 2011;18:279–286. [Google Scholar]
- Le Bellec F, Vaillant F, Imbert E. Pitahaya (Hylocereus spp.): a new fruit crop, a market with a future. Fruits. 2006;61:237–250. doi: 10.1051/fruits:2006021. [DOI] [Google Scholar]
- Liu X, Gao Y, Xu H, Wang Q, Yang B. Impact of high-pressure carbon dioxide combined with thermal treatment on degradation of red beet (Beta vulgaris L.) pigments. J Agric Food Chem. 2008;56:6480–6487. doi: 10.1021/jf800727q. [DOI] [PubMed] [Google Scholar]
- Nerd A, Gutman F, Mizrahi Y. Ripening and postharvest behaviour of fruits of two Hylocereus species (Cactaceae) Postharvest Biol Technol. 1999;17:39–45. doi: 10.1016/S0925-5214(99)00035-6. [DOI] [Google Scholar]
- Nur ‘Aliaa AR, Siti Mazlina MK, Taip FS. Effects of commercial pectinases application on selected properties of red pitaya juice. J Food Process Eng. 2009;34:1523–1534. doi: 10.1111/j.1745-4530.2009.00388.x. [DOI] [Google Scholar]
- Nur ‘Aliaa AR, Siti Mazlina MK, Taip FS, Liew Abdullah AG. Response surface optimization for clarification of white pitaya juice using a commercial enzyme. J Food Process Eng. 2010;33:333–347. doi: 10.1111/j.1745-4530.2008.00277.x. [DOI] [Google Scholar]
- Okoth MW, Kaahwa AR, Imungi JK. The effect of homogenization, stabilizer and amylase on cloudiness of passion fruit juice. Food Control. 2000;11:305–311. doi: 10.1016/S0956-7135(99)00107-3. [DOI] [Google Scholar]
- Ozkan M, Kirca A, Cemeroglu B. Effects of hydrogen peroxide on the stability of ascorbic acid during storage in various fruit juices. Food Chem. 2004;88:591–597. doi: 10.1016/j.foodchem.2004.02.011. [DOI] [Google Scholar]
- Pasch JH, von Elbe JH. Betanine stability in buffered solutions containing organic acids, metal cations, antioxidants, or sequestrants. J Food Sci. 1979;44:72–74. doi: 10.1111/j.1365-2621.1979.tb10007.x. [DOI] [Google Scholar]
- Rebecca OPS, Boyce AN, Chandran S. Pigment identification and antioxidant properties of red dragon fruit (Hylocereus polyrhizus) Afr J Biotechnol. 2010;9:1450–1454. [Google Scholar]
- Reynoso R, Garcia FA, Morales D, de Mejia EG. Stability of betalain pigments from Cactacea fruit. J Agric Food Chem. 1997;45:2884–2889. doi: 10.1021/jf960804r. [DOI] [Google Scholar]
- Schwartz SJ, von Elbe JH. Identification of betanin degradation products. Eur Food Res Technol. 1983;176:448–453. doi: 10.1007/BF01042560. [DOI] [PubMed] [Google Scholar]
- Schweiggert RM, Villalobos-Gutierrez MG, Esquivel P, Carle R. Development and optimization of low temperature enzyme-assisted liquefaction for the production of colouring foodstuff form purple pitaya (Hylocereus sp. [Weber] Britton & Rose) Eur Food Res Technol. 2009;230:269–280. doi: 10.1007/s00217-009-1167-0. [DOI] [Google Scholar]
- Seog EJ, Sohn KS, Lee JH. Color characteristics of concentrated single and blend juices as influenced by concentration methods. J Food Technol. 2006;4:143–146. [Google Scholar]
- Vaillant F, Perez A, Davila I, Dornier M, Reynes M. Colourant and antioxidant properties of red-purple pitahaya (Hylocereus sp.) Fruits. 2005;60:1–10. doi: 10.1051/fruits:2005007. [DOI] [Google Scholar]
- Walkowiak-Tomczak D. Changes in antioxidant activity of black chokeberry juice concentration solutions during storage. Acta Sci Pol Technol Aliment. 2007;6:49–55. [Google Scholar]
- Woo K, Ngou F, Ngo L, Soong W, Tang P. Stability of betalain pigment from red dragon fruit (Hylocereus polyrhizus) Am J Food Technol. 2011;6:140–148. doi: 10.3923/ajft.2011.140.148. [DOI] [Google Scholar]