Abstract
Basella rubra fruit juice with a total soluble solids content of 5 to 9 0Brix was fermented using the wine yeast Saccharomyces cerevisiae. An 87.5 % of conversion of fermentable sugar was achieved. The TSS (0Brix) reduced from 0.60 0Brix to 0.17 0Brix (71.67 % decrease in TSS) upon performing fermentation of fruit juice water extract with Saccharomyces cerevisiae strain 2. There was 8 folds reduction in pigment quality as evidenced from fermentation. Besides, the potential increase of phenolics, thanks to a higher content of total betalains in general and betacyanins in particular when fermentation was carried out with S. cerevisiae strain 3. The DPPH (2, 2 -diphenyl-1-picrylhydrazyl hydrate) free radical scavenging potential (IC50) of fermented juice (1.9 mg.ml−1) was significant over control (2.4 mg.ml−1) extracts of B. rubra. The reducing power of fermented extracts was significantly high compared to control samples. The multiple antioxidant activity of fermented extract was also evident by significant reducing power assay when compared to its control samples.
Keywords: Betalains, Phenolics, TSS (total soluble solids), Reducing power, Hue values
Introduction
Consumer’s preference to natural pigments over synthetic dyes for various purposes has gained importance in view of various adverse effects attributed to synthetic dyes rampant usage. (Downham and Collins 2000). This has created great attention for their usefulness in food and cosmetic industries. Betalains are water soluble nitrogen containing natural pigments, which comprise red violet betacyanins and yellow betaxanthins (Lin et al. 2010; Khan et al. 2012). Betalains are good colouring agents than anthocyanins in acidic and natural food products. Among the natural pigments, betalains have recently re-gained interest in food science (Delgado-Vargas et al. 2000; Stintzing and Carle 2005) in view of their vibrant red-violet shades in food matrix. As a source of betalains, red beet root powder or juice concentrates have found a wide commercial use among natural food colorants (Turker et al. 2001). The beet root pigments, composed of mainly betanin and vulgaxanthin, give a red to red violet colour when added to food stuffs. There has been a growing interest on the stability of these betalain pigments in terms of pH, temperature and water activity etc. Other than beet root, recently, the potential of Cactus pear for natural colouring was demonstrated (Castellar et al. 2003; Stintzing et al. 2005; Moßhammer et al. 2005), but lack of sustainable production systems for Cactus pears is a drawback. Hence, alternate and cheap sources of betalains are a good choice and warranted. Under this context, B. rubra (Basellaceae) which is native to India and popularly known as Malabar spinach, a twiner that bears dark purple berries is a good choice and sporadic reports indicates the presence of betalains group pigments (Turker et al. 2001). In oriental countries including India and also in some parts of Africa B. rubra is considered as a leafy vegetable for making various recipes (Palada and Crossman 1999), and its pharmaceutical principles were investigated (Sonkar et al. 2012). The plant is used for its medicinal properties in Unani -ancient Indian medical system (Nayampalli et al. 1982), and in Chinese traditional medicine to treat constipation, used as a diuretic, a toxicide an anti-inflammatory (Toshiyuki et al. 2001) and to enhance fertility in women (Mensah et al. 2008). Recently Lin et al. (2010) had reported gomphrenins I belongs to betalains group (36.1 mg.100 g−1 FW) from B. alba fruits. The main advantage of water soluble pigments from fruits is that they get from the tissue into the juice. Besides pigments, other useful water soluble substances are also extracted, which leads to increase in the total soluble solid content of the juice mainly due to total soluble solids that hamper pigment quality and quantity (Khan et al. 2012). Under this context, increasing the betalains pigment content of the extract is having implications for their effective use to give desired hue to the food matrix. One of the approaches has been the fermentation of the juice with yeast as shown in Cactus spp. (Turker et al. 2001). In this study, the B. rubra fruits were chosen as a betalains source to investigate the effects of fermentation on stability of betalains.
Materials and methods
Sample preparation
The ripened fruits (dark violet), from 3 months old B. rubra twine were collected from green house maintained plants. The herbarium specimen of this plant was deposited at Herbarium deposition centre of Department of Botany, University of Mysore, Mysore (Ref. No.02-08-05-13). Manually deseeded fruit pulp of 25 g was extracted with 250 ml of water and centrifuged at 8000 rpm at 4 °C for 15 min in order to obtain clear B. rubra fruit juice for fermentation.
Microorganisms
Three different strains of S. cerevisiae (1, 2 and 3) were collected from Food Microbiology Department of this Institute.
Inoculum preparation
Respective strains of S. cerevisiae cells were grown in YPD broth (Yeast-10 g.l−1, Peptone-20 g.l−1, Dextrose-20 g.l−1) at 30 °C overnight in an orbital shaker at 150 rpm and the culture was centrifuged. The supernatant was discarded and the cell pellets were suspended in known volume of water, prior to inoculation (Turker et al. 2001).
Fermentation
The clear fruit juice water extract of B. rubra was equally divided into 25, 150 ml conical flasks and pre counted innoculum of S. cerevisiae cells were added (approximately 500 cells.flask−1) and incubated at 30 °C, in an orbital shaker maintained at 150 rpm for 3 h & 6 h. As the pigment is light sensitive the flasks were rapped with aluminum foil. The data was collected after 3 h and 6 h incubation (Turker et al. 2001).
Storage of the pigment extract
The fermented juice extract was collected and centrifuged at 12,000 rpm for 10 min to remove the cells grown. Then, the clear fermented juice was concentrated in a rotavapor under pressure 150 mbar at 40 °C, followed by freeze drying at −110 °C with 0.05 hPa and stored at 4 °C till its further analysis and kinetic measurements (Turker et al. 2001).
Analysis of fermented fruit juice
Total carbohydrates content
The total carbohydrates in all samples were determined by using a method of Sadasivam and Manickam (1992). In brief, 100 mg of the sample was weighed into each boiling tube. The sample was hydrolyzed by boiling with 5 ml of 2.5 N Hydrochloric acid for 3 h, cooled to room temperature and neutralized with sodium carbonate. The volume was made up to 10 ml and centrifuged. The supernatant was collected and 0.1 ml aliquots were taken for analysis. Standards were prepared by pipetting out 0.1 ml to 1 ml of the working standard (0.1 mg.ml−1 of Glucose). The volume was made up to 1 ml with distilled water in all the tubes including the sample tubes. 4 ml of anthrone reagent was added and heated for 8 min in boiling water bath and cooled rapidly. The green color developed was read at 630 nm. By using the standard graph plotted, the amount of carbohydrate present in the sample was calculated.
Total soluble solids
Total soluble solids (0Brix) of fermented fruit juice extract were determined using a digital refractometer RX- 500 (Atago Co. Ltd, Tokyo, Japan).
Colour measurement
Colour measurement analysis was also carried out by using a method of Kunnuka and Pranee (2011), by measuring L, a* and b* values using colour measurement instrument (Master color data Hunters lab 10*/D 65).
Estimation of total betalains content
The total betalains in the fermented fruit juice extract was carried out by Spectrophotometric method of Castellanos-Santiago and Yahia (2008). The absorbance of fruit extract was measured at 477 nm, 535 nm, 600 nm. Measurements were made in triplicates. For quantitative analysis, the betalain content was calculated according to literature (Khan et al. 2012).
Where A is the absorption value at the absorption maxima of 535 nm and 477 nm for betacyanins and betaxanthins, respectively, DF is the dilution factor, V is the solution volume (mL), Wd is the pulp weight (g), and L is the path length (1 cm) of the cuvette. The molecular weight (MW) and the molar extinction coefficient (ε) of betanin [MW = 550 g/mol and 339 g/mol; ε = 60000 L/(mol cm) and 48000 L/(mol cm) in/H2O] were applied in order to quantify the betacyanins and quantitative equivalents of the major betaxanthins respectively.
Antioxidant activity by 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH) Radical Inhibition Assay
The antioxidant activity was determined by the DPPH radical-scavenging method of Khan et al. (2012). Gallic acid and ascorbic acid (each of 100 μg.ml−1) were taken as standards. Samples (fermented extract) at 1–5 mg.ml−1 concentration were taken in different test tubes. The volume of the samples was made up with distilled water in such a way that the volume was equal to that of the extract with highest concentration. Accordingly, DPPH was added to make up the total volume to 2 ml. The contents of the test tubes were thoroughly mixed and a reaction time of 15 min was allowed. The absorbance was measured at 517 nm with methanol as blank.
From the absorbance, % inhibition or % scavenging activity is calculated using this formula,
Where, OD control is the absorbance of DPPH solution against methanol as blank. From the % inhibition to concentration plot, IC50 value for the standards and samples were estimated. The antioxidant property of the fermented extract was compared and analyzed using IC50 value.
Reducing power assay
The reducing power assay was determined by the method of Oyaizu (1986). Fermented extract crude samples (10–40 mg.ml−1) and ascorbic acid (standard) of different concentration ranging from 100 and 500 μg.ml−1 dissolved in distilled water were taken in different test tubes. This was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide [K3Fe(CN)6] (1 % (w/v)) and then the mixture was incubated for 30 min at 50 °C. Later, 2.5 ml of 10 % trichloroacetic acid (TCA) was added to the mixture, which was centrifuged at 3000 rpm for 10 min. Finally, 2.5 ml of the upper layer solution was taken and mixed with 2.5 ml of distilled water and 0.5 ml of 0.1 % ferric chloride. Absorbance was measured at 700 nm.
Statistical analysis
Three parallel experiments were carried out for all the analyses. All the results are presented in the form of mean ± S.D of three replicates. Microcal Origin 6.0 software (M/s Microcal Software, Inc., Northampton, MA, USA) was used to calculate IC50. One way ANOVA was performed by using Microsoft Excel program of Windows 7 software. Values with P < 0.05 were considered significant. Error bar (%) is with the specified values for graphs.
Results and discussion
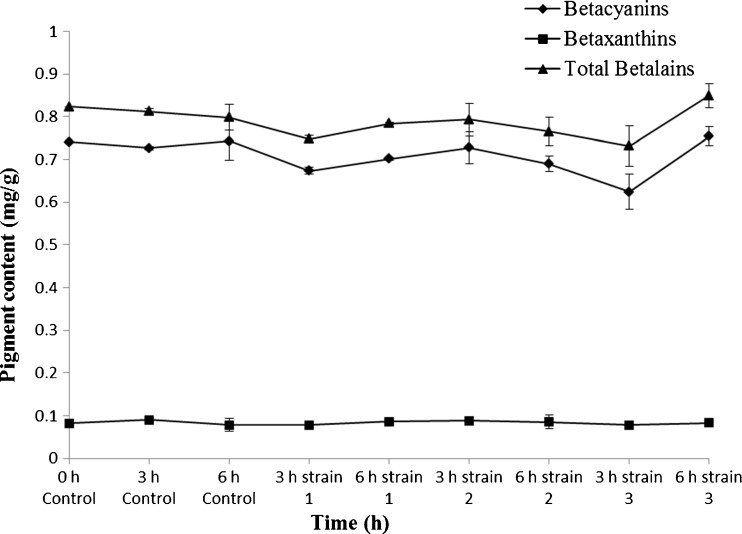
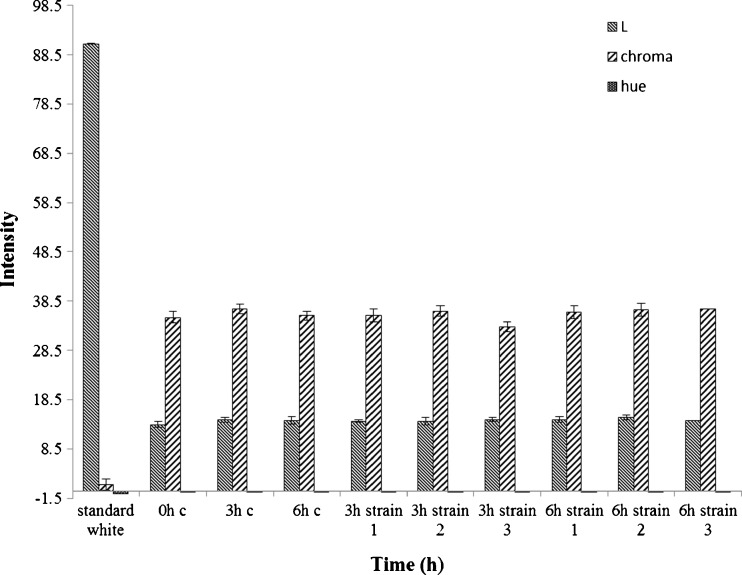
In this study B. rubra fruits were chosen as betalains source and for the first time these pigments were partially purified by fermentation. The B. rubra fruit extract (0.58–0.60 0Brix) contains 0.172–0.180 % of total carbohydrates and 0.052–0.094 % of total phenolics on fresh weight basis. Three different strains of S. cerevisiae were chosen for fermentation. B. rubra fruit juice containing red-violet betalain pigment was fermented to decrease the total soluble solids, the aim being to end up with a higher pigment concentration. The B. rubra fruit juice prepared for fermentation had 165.34 mg of total pigment content in terms of betalains (Fig. 1). After 3–6 h incubation the change in pigment content was insignificant. Upon fermentation by using different strains of S. cerevisiae there was a marginal decrease in total betalains content and it was 9 %, 5 % and 11 % for S. cerevisiae strains 1, 2 and 3 respectively after 3 h fermentation compared to 3 h incubated control (Fig. 1). Similarly, upon 6 h fermentation with yeast, there was a reduction in pigment content of 1.6 % and 4.06 % for strain 1 and 2 respectively. However, there was 6.44 % increase in pigment content for strain 3 upon 6 h fermentation compared to 6 h incubated control. During this, 3–6 h fermentation process overall, the change in total pigment content was on par with changes in betacyanin and betaxanthin content, wherein, an increase in betacyanins content was evident from 3 h to 6 h fermentation, and a reverse for betaxanthin content was noticed for strain 1 and 3. The changes in pigment content of B. rubra juice was in concomitant with L, a* and b* values during fermentation by yeast (Figs. 1 and 2).
Fig. 1.
Total betalains (betacyanins: betaxanthins) content of B. rubra fermented fruit juice extract. Values are mean ± S.D of three replicates and significant at P < 0.05
Fig. 2.
Color intensity (Hunter’s lab) of B. rubra fermented fruit extract. Values are mean ± S.D of three replicates and significant at P < 0.05
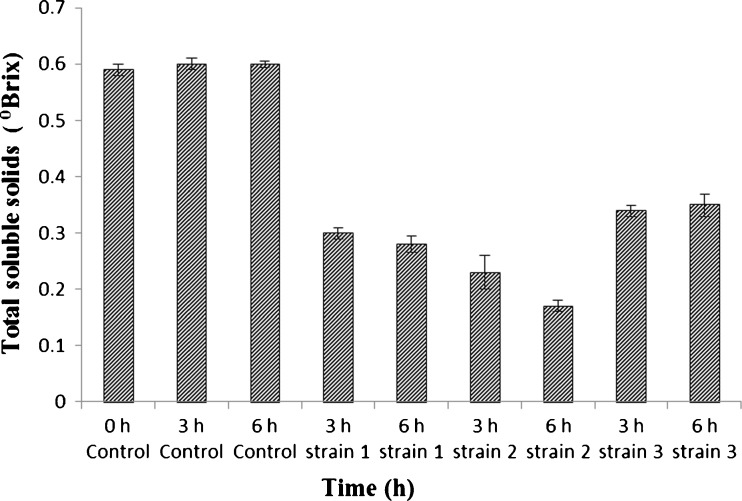
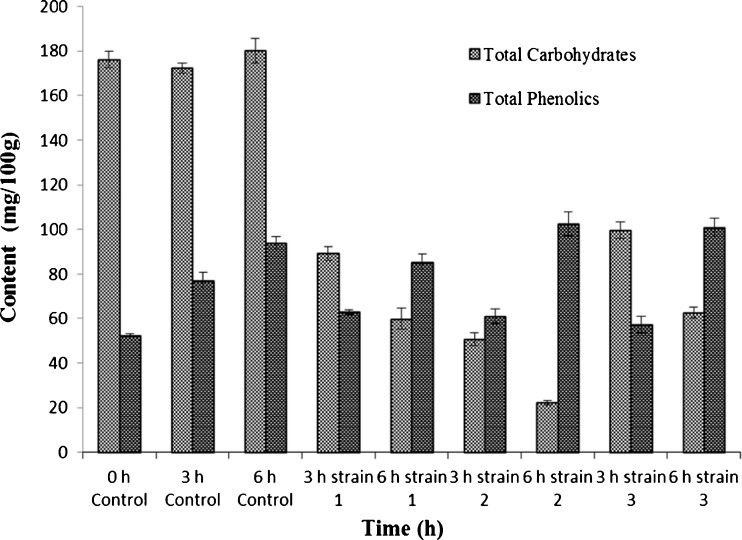
A glance at Fig. 3 indicates the influence of fermentation on total soluble solids and carbohydrates consumption during the fermentations of B. rubra juice. The B. rubra fruit juice prepared for fermentation had a total soluble content of 0.59 0Brix. Its fermentation pattern followed in terms of total solid reduction and also carbohydrates reduction. The whole fermentation process was carried out in dark to protect the pigment from exposure to light. The total soluble solids of the B. rubra fruit juice was decreased from 0.60 0Brix to 0.17 0Brix and it varied with yeast strain used and also fermentation time (Fig. 3). There was a reduction of 50 %, 62 % and 44 % in total solids content for S. cerevisiae 1, 2 and 3 respectively after 3 h fermentation and 54 %, 72 % and 42 % reduction after 6 h fermentation compared to 3–6 h controls (Fig. 3). Similarly, 48 %, 71 % and 43 % fall in total carbohydrates was evident for S. cerevisiae 1, 2 and 3 respectively (Fig. 4), upon 3 h fermentation compared to 3 h control samples. A similar trend for 6 h fermented samples was noticed wherein, 67 %, 88 % and 66 % decline in total carbohydrates was evident when S. cerevisiae 1, 2 and 3 were used respectively compared to 6 h control sample (Fig. 4). A similar fermentation pattern and sugar conversion was also observed for Cactus spp. (Turker et al. 2001). The other betalains producing plant such as Cactus pear pulp contains 0.006 to 0.114 % pigment (Castellar et al. 2003; Stintzing et al. 2005) wherein, betaxanthin:betacyanin ratios varying between 0 and 11.7 resulting in different colour shades (Odoux and Dominguez-Lopez 1996; Butera et al. 2002; Stintzing et al. 2005). In the present study the total pigment content of B. rubra fruit extracts upon fermentation was 0.160 to 0.180 % with betaxanthin:betacyanin ratios varying between 0 and 8 resulting in different colour shades as evident from colour measuring values. This clearly shows the efficiency of fermentation on pigment stability. As pigment dilution takes place due to poor tinctorial value, large quantity of pigment has to be added to obtain desired hue of the product. Normally pigment rich concentrates contains fermentable carbohydrates, proteins, and ash besides pigment (Nemzer et al. 2011). As the market potential of fruit juice based natural colours are considered fair due to their acceptable colour, nutritional constituents, and also for their utilization as a functional additive in view of antioxidant activity, hence though B. rubra fruits this dual benefit through food colouring appears to be more promising for this still not much explored betalains source.
Fig. 3.
Total soluble solids (0Brix) of B. rubra fermented fruit extract. Values are mean ± S.D of three replicates and significant at P < 0.05
Fig. 4.
Total carbohydrates and total phenolics content in B. rubra fermented fruit extract. Values are mean ± S.D of three replicates and significant at P < 0.05
Due to the broader range of colour shades as demonstrated through colour measuring values in the present study, and also due to the absence of any off flavour, B. rubra fruits appears to be an advantageous alternative to red beet which is the only commercially exploited betalain source, so far. Moreover, with a high colouring power at pH 5.0 to neutral pH where most of the anthocyanin-based preparations fail, B. rubra concentrates may be suitable for colouring appropriate milk products such as yoghurts, ice creams, and also fruit preparations. However, studies pertaining to the production of tailor-made hues may be a further promising feature for industrial applications.
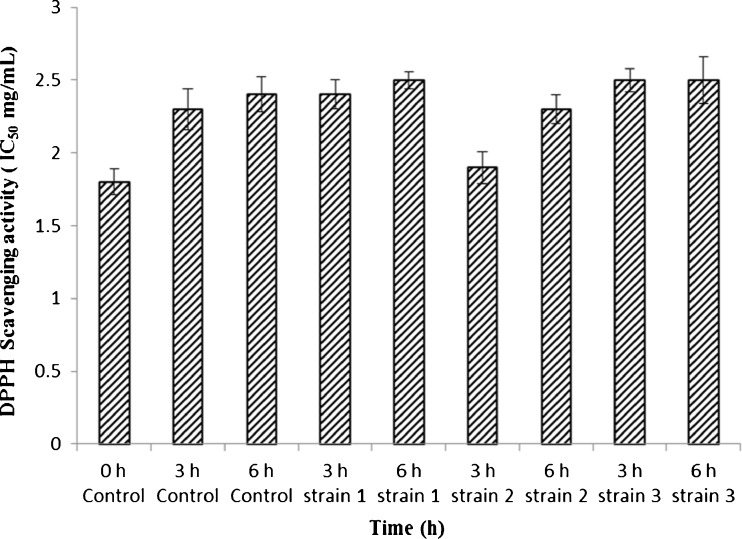
The free radical scavenging activity of fermented fruit extract and control samples were determined by the DPPH assay (Fig. 5) wherein, the DPPH radical is reduced by antioxidant compound to its α, α-diphenyl, β-picryl hydrazine derivative which involves discoloration of violet colour that is measured at 517 nm. DPPH is a nitrogen centered free radical, mostly used to study the in vitro natural antioxidant activity with short time. All the extracts were capable of scavenging the DPPH free radical, which decolourized with the increasing concentration of fruit juice. The fermented juice (IC50 1.9 mg.ml−1) was significant over control (2.4 mg.ml−1) extracts of B. rubra (Fig. 5). The reason for high activity B. rubra fruit extract may be due to the presence of partially purified betalains and higher content of phenolic compounds. Prathapan et al (2011) reported that phenolic compounds in plants are used as powerful in vitro antioxidants because of their ability to donate hydrogen or electron. In this study, gallic acid and ascorbic acid were used as standard compounds which showed IC50 values of 2.3 μg.ml−1 and 4.3 μg.ml−1 respectively.
Fig. 5.
DPPH scavenging activity (IC50) of B. rubra fermented fruit extract. Values are mean ± S.D of three replicates and significant at P < 0.05
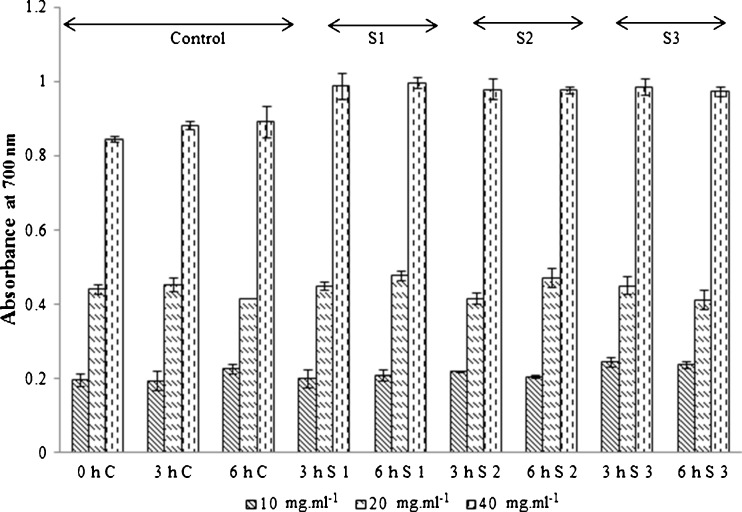
The reducing power of a compound may serve as an indicator of its potential antioxidant activity. It is necessary to determine the reducing power of the fermented fruit extract and control to elucidate their antioxidant activity. The presence of antioxidants in the extract causes a reduction of the Fe3+ to its ferrous form which results in a change of the yellow colour of the reaction mixture to Prussian blue depending on the reducing (or electron donating) power of fermented juice extract. The extent of reduction is explained in terms of absorbance values at 700 nm for the increasing fermented juice concentration ranging from 10 to 40 mg.ml−1. All the extracts exhibited increasing reducing activity with increasing concentration (Fig. 6). In the present study, all the fermented extracts exhibited higher levels of reducing power compared to control samples. Increase in concentration of the sample shows higher absorbance which in turn indicates good reducing capacity. Thus, the result of fermentation indicates that the concentration of sugar has reduced by 8 folds. However, pigment quality has increased and the pigment displays good antioxidant property. This is in agreement with similar studies on orange juice (Escudero-Lopez et al. 2013). The increase in ORAC and DPPH values could be due to enhancement of phenolics and also due to the increase in extractable pigment. It is recognized that the consumption of fruits and vegetables, which contain various antioxidant compounds like polyphenols, carotenoids and ascorbic acid, intensifies and enhances the antioxidant defense system (Park et al. 2003). Under, this context, the data obtained in this study would having implications in considering fermented B. rubra juice as a source of bioactive compounds, which is in line of recent reports on orange (Escudero-Lopez et al. 2013).
Fig. 6.
Reducing power assay of B. rubra control and fermented fruit extract. S1, S2, S3 indicates strains 1, 2 and 3. Values are mean ± S.D of three replicates and significant at P < 0.05
At a technological level, this study presents a novelty. Furthermore, the potential drink of fermented B. rubra fruit juice would presume to provide the consumer with a new potentially healthful beverage, however further studies on this aspect are warranted.
Acknowledgments
The authors are thankful to Department of Biotechnology, New Delhi for financial assistance and Director, CSIR-CFTRI, Mysore for encouragement.
References
- Butera D, Tesoriere L, Di Gaudio F, Bongiorno A, Allegra M, Pintaudi AM, Kohen R, Livrea MA. Antioxidant activities of Sicilian prickly pear (Opuntia ficus indicus) fruit extracts and reducing properties of its betalains: betanin and indiaxanthin. J Agric Food Chem. 2002;50:6895–6901. doi: 10.1021/jf025696p. [DOI] [PubMed] [Google Scholar]
- Castellanos-Santiago E, Yahia EM. Identification and Quantification of Betalains from the fruits of 10 Mexican prickly pear cultivars by high performance liquid chromatography and electron spray ionization mass spectrometry. J Agric Food Chem. 2008;56:5758–5764. doi: 10.1021/jf800362t. [DOI] [PubMed] [Google Scholar]
- Castellar MR, Obon JM, Alacid M, Fernandez-Lopez JA. Color properties and stability of betacyanins from Opuntia fruits. J Agric Food Chem. 2003;51:2772–2776. doi: 10.1021/jf021045h. [DOI] [PubMed] [Google Scholar]
- Delgado-Vargas F, Jimenez AR, Paredes-Lopez O. Natural pigment, carotenoids, anthocyanin, and betalains. Characteristics, biosynthesis processing and stability. Crit Rev Food Sci Nutr. 2000;40:173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- Downham A, Collins P. Colouring our foods in the last and next millennium. Int J Food Sci Technol. 2000;35:5–22. doi: 10.1046/j.1365-2621.2000.00373.x. [DOI] [Google Scholar]
- Escudero-Lopez B, Cerrillo I, Herrero-Martin G, Hornero-Mendez D, Gil-Izquierdo A, Medina S, Ferreres F, Berna G, Martin F, Fernandez-Pachon MS. Fermented orange juice: source of higher carotenoid and flavanone content. J Agric Food Chem. 2013 doi: 10.1021/jf401240p. [DOI] [PubMed] [Google Scholar]
- Khan MI, Harsha PSC, Giridhar P, Ravishankar GA. Pigment Identification, nutritional composition, bioactivity, and in vitro cell cytotoxicity of Rivina humilis L. berries, potential source of betalains. Food Sci Technol. 2012;47:315–323. doi: 10.1111/j.1365-2621.2011.02841.x. [DOI] [Google Scholar]
- Kunnuka S, Pranee A. Influence of enzyme treatment on bioactive compounds and colour stability of betacyanin in flesh and peel of red dragon fruit Hylocereus polyrhizus (Weber) Britton and Rose. Int Food Res J. 2011;18(4):1437–1448. [Google Scholar]
- Lin SM, Lin BH, Hsieh WM, Ko HJ, Lin CD, Chen LG, Robin YY, Chiou Structural identification of Bioactivities of Red- Violet pigments present in Basella alba fruits. J Agric Food Chem. 2010;58:10364–10372. doi: 10.1021/jf1017719. [DOI] [PubMed] [Google Scholar]
- Mensah JK, Okoli RI, Ohaju-Obodo JO, Eifediyi K. Phytochemical, nutritional and medical properties of some leafy vegetables consumed by Edo people of Nigeria. Afr J Biotechnol. 2008;7(14):2304–2309. [Google Scholar]
- Moßhammer MR, Stintzing FC, Carle R. Development of a process for the production of a betalain-based colouring food stuff from cactus pear. Innov Food Sci Emerg Technol. 2005;6:221–231. doi: 10.1016/j.ifset.2005.02.001. [DOI] [Google Scholar]
- Nayampalli S, Ainapure SS, Nadkarni PM. Study of anti allergic acid Bronchodilator effects of Tinospora cordifolia. Indian J Pharm. 1982;14:64–66. [Google Scholar]
- Nemzer B, Pietrzkowski Z, Spornac A, Stalicac P, Thresher W, Michalowski T, Wybraniecc S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011;127:42–53. doi: 10.1016/j.foodchem.2010.12.081. [DOI] [Google Scholar]
- Odoux E, Dominguez-Lopez A. Le figuier de Barbarie: une source industrielle de bétalaines? Fruits. 1996;51:61–78. [Google Scholar]
- Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Palada MC, Crossman SMA. Evaluation of tropical leaf vegetables in the Virgin Islands. In: Janick J, editor. Perspectives on new crops and new uses. Alexandria: ASHS Press; 1999. pp. 388–393. [Google Scholar]
- Park YK, Park E, Kim JS, Kang MH. Daily grape juice consumption reduces oxidative DNA damage and plasma free radical levels in healthy Koreans. Mutat Res. 2003;529:77–86. doi: 10.1016/S0027-5107(03)00109-X. [DOI] [PubMed] [Google Scholar]
- Prathapan A, Singh MK, Anusree SS, Soban Kumar DR, Sundaresan A, Raghu KG. Antiperoxidative, free radical scavenging and metal chelating activity of Boerhaavia diffusa L. J Food Biochem. 2011;35:1548–1554. doi: 10.1111/j.1745-4514.2010.00477.x. [DOI] [Google Scholar]
- Sadasivam S, Manickam A. Biochemical methods. 2. New Delhi: New Age International; 1992. [Google Scholar]
- Sonkar DS, Gupta R, Shubhini A, Saraf Effect of Basella rubra L. leaf extract on haematological parameters and amylase activity. Pharmacol Commun. 2012;2:10–12. doi: 10.5530/pc.2012.3.3. [DOI] [Google Scholar]
- Stintzing FC, Carle R. Cactus stems (Opuntia spp.): a review on their chemistry, technology, and uses. Mol Nutr Food Res. 2005;49:175–194. doi: 10.1002/mnfr.200400071. [DOI] [PubMed] [Google Scholar]
- Stintzing FC, Herbach KM, Moßhammer MR, Carle R, Yi WE, Sellappan S, Akoh CC, Bunch R, Felker P. Color, betalain pattern and antioxidant properties of cactus pear (Opuntia spp.) clones. J Agric Food Chem. 2005;53:442–451. doi: 10.1021/jf048751y. [DOI] [PubMed] [Google Scholar]
- Toshiyuki M, Kazuhiro H, Masayuki Y. Structures of new oleanane-type triterpene oligoglycosides, basella saponins A, B, C, and D, from the fresh aerial parts of Basella rubra L. Chem Pharm Bull. 2001;49(6):776–779. doi: 10.1248/cpb.49.776. [DOI] [PubMed] [Google Scholar]
- Turker N, Coskumer Y, Ekiz HI, Aksay J, Karababa E. The effects of fermentation on the thermo stability of the yellow orange pigments extracted from cactus pear (Opuntia Ficus – Indica) Eur Food Res Technol. 2001;212:213–216. doi: 10.1007/s002170000247. [DOI] [Google Scholar]