Abstract
Low-sugar bilberry jams without added herbs and those with added mentha (1 %) and lemon balm (1 %) were examined for levels of selected physico-chemical indicators, antioxidant activity, colour and texture. Jams were obtained by two methods: cooked in an open pan and cooked in a vacuum evaporator. 100 g fresh mass contained 0.076–0.481 mg HMF, 5.8–7.1 mg vitamin C, 176–232 mg total polyphenols, 122–156 mg total flavonoids, 73–96 mg total anthocyanins, with antioxidant activity per 1 g of 405–575 μM Trolox (ABTS), 71–89 μM Trolox (DPPH) and 120–176 μM Fe2+ (FRAP). Jams cooked in a vacuum evaporator had higher levels of the indicators examined, better colour and worse texture. Jams with added herbs generally showed higher levels of all indicators, but their colour and texture were slightly worse. Storing jams for 8 months caused a reduction in antioxidant constituents of 7–20 % along with a deterioration of colour and texture.
Keywords: Bilberry, Jam, Processing and storage, Antioxidants, Colour, Texture
Introduction
Bilberries are a good source not only of polyphenols but also of vitamin C, which also shows antioxidant activity. However, the processing and subsequent storage of bilberry fruits may substantially reduce their antioxidant properties, mainly as a result of changes in polyphenolic constituents, especially anthocyanins (Lee and Wrolstad 2004).
Biological activity is mainly associated with the content of polyphenols, especially flavonoids and anthocyanins (Kalt et al. 1999). Such constituents, considered both individually and synergistically, can reduce the risk of cardiovascular disease, cancer, inflammation, obesity and diabetes (Seeram 2008). Among berry fruits, bilberry (Vaccinium myrtillus L.) shows the highest antioxidant properties. Food chemists are interested in bilberry due to its potential as a source of biologically active non-nutrient compounds, that is, as a functional food (Witzell et al. 2003). Anthocyanins are probably the largest group of phenolic compounds in the human diet. They are used therapeutically for their ability to cure diabetic rethinopathy and vision disorders (Cacace and Mazza 2003). Although more than 550 anthocyanins have been identified, only a few, including cyanidin, delphinidin, pelargonidin, peonidin, malvidin, and petunidin, are in the land plant group. They are located mostly in the flowers and skins of fruits, especially berry fruits (Kong et al. 2003). Due to their high anthocyanin content, some Vaccinium species, particularly Vaccinium myrtillus, are used in the treatment of vascular disease and in ophthalmology (Kalt and Dufour 1997).
Preserving fruit in the form of jams is an important sector of the food processing industry since jams are a convenient way of consuming fruit throughout the year. However, the antioxidant and sensory properties of the final product depends on the quality of the raw material, method of production and storage conditions, among other factors (Brownmiller et al. 2008). Fruit is heated during jam production, which can significantly affect polyphenol content (García-Viguera et al. 1999b). Moreover, the quality of food products and their attractiveness is also determined by colour. Anthocyanins, whose presence in bilberries is well attested, are responsible for their red, violet and blue colour (Moyer et al. 2002). Little is known, however, about colour retention in bilberry jams.
Texture is an important factor in the appreciation of food products (Mojet and Köster 2005). Texture seems to be of varying importance in different fruit and vegetable products (Beveridge 2002). The high temperatures reached during thermal processing often cause detrimental changes in the processed product, which may include undesirable changes in nutritional aspects and texture (van Buggenhout et al. 2009).
The aim of this paper was to determine the level of selected physico-chemical indicators, antioxidant activity, colour, and texture in low-sugar bilberry jams with added herbs. Jams were produced by means of two methods: the vacuum method (cooked in a vacuum evaporator) and cooked in an open pan. Final products were evaluated immediately after production and after 8 months of storage.
Materials and methods
Material
The experimental material consisted of low-sugar jams obtained from bilberry (Vaccinium myrtillus L.) without added herbs as well as those with added mentha (1 %) and lemon balm (1 %) with a refractometric extract of 35 %. Jams produced from frozen bilberries (Vaccinium myrtillus L.) were obtained by means of two methods: cooked in an open pan and vacuum. The remaining semi-products were: fresh herbs (mentha and lemon balm), sugar, citrus-apple pectin (NECJ-A2, Pektowin Jasło Poland), and citric acid.
Production of bilberry jams and storage
Jams were produced from bilberries in three variants: without added herbs (variant I); with 1 % addition of mentha (variant II); and with 1 % addition of lemon balm (variant III). Fruit comprised 50 % of jam mass.
Jam preparation by the cooking in an open pan
Using this method, 500 g of fruit was cooked in an open pan with 307 g of sugar and 172 ml of water at approx. 103 °C for 20 min until exceeding the assumed refractometric extract by 4–5 units. Afterwards, chopped leaves of mentha (10 g) or lemon balm (10 g) were added along with previously prepared 4 % pectin solution (250 g). The whole batch was again cooked for several minutes, finally adding citric acid. The overall cooking time was 30 ± 3 min.
Jam preparation by the vacuum method
With this method, 500 g of fruit, 307 g of sugar, and 172 ml of water were cooked in a round bottomed flask coupled to a vacuum pump in a Laborota 4000 evaporator (Heidolph, Germany). Cooking was carried out at 68–70 °C until exceeding the assumed refractometric extract by 4–5 units. Chopped leaves of mentha (10 g) or lemon balm (10 g) were then added together with previously prepared 4 % pectin solution (250 g). The whole batch was again cooked under vacuum for several minutes and finally citric acid was added. The overall cooking time did not exceed 40 min.
The jams obtained were pasteurized in glass jars (0.2 L) at 82–85 °C for 12 min and then cooled to room temperature (20 ± 2 °C). Jams were chill-stored at 8–10 °C prior to evaluation.
Evaluation of physico-chemical indicators and antioxidant activity
Dry matter content was determined by means of the weight method (AOAC 1995); total refractometric extract by the refractometric method (AOAC 1995); total acidity by the titratable method (AOAC 1995); and active acidity (pH) using the potentiometric method (AOAC 1995).
The content of 5-hydroxymethyl-2-furaldehyde (HMF) was determined by means of high performance liquid chromatography (HPLC) using a modified version of the Kukurova et al. (2006) procedure. Detection was performed using Merck HITACHI high performance liquid chromatography (HPLC) apparatus equipped with a UV–VIS (L-7420) detector. A monolithic column (Onyx Monolithic C 18 100 × 4.6 mm) with pre-column was employed for analysis. Measurements were carried out at a wavelength of 283 nm. Mobile phase was water with acetonitrile (9:1). Isocratic elution was performed at a flow rate of 0.8 ml/min. An external HMF standard (Dr. Ehrenstorfer GmbH) in water was used to identity HMF, the quantity of which was then found from the standard curve (y = 0.00000257x + 0.02660214) plotted for the HMF standard.
Vitamin C content, as the sum of ascorbic and dehydroascorbic acid, was determined using high performance liquid chromatography (HPLC). A sample was prepared for analysis according to the EN 14130 standard (PCS 2003) standard. Prior to application to an HPLC column, the samples were purified using Sep-Pak C18 micro-columns and then centrifuged. A monolithic column (Onyx Monolithic C 18 100 × 4.6 mm) was employed for HPLC analysis. Detection was conducted at a wavelength of 254 nm using a UV–VIS (L-7420) detector. Metaphosphoric acid (0.1 %) was used as an eluent.
In order to determine total polyphenols, total flavonoids, and antioxidant activity, the sample was extracted by the use of 80 % methanol acidified with HCl (0.5 %). Polyphenols were determined by the Folin-Ciocalteu method (Singleton et al. 1999). Briefly, 0.125 ml of Folin-Ciocalteu reagent and 0.25 ml of 25 % sodium carbonate were added to the extract previously diluted with deionised water. After 60 min, the absorbance at 765 nm was measured. Polyphenol content was found from a standard curve plotted for (+)- catechin.
Total flavonoid content was measured by aluminium chloride assay (Zhishen et al. 1999; Ardestani and Yazdanparast 2007). Briefly, after appropriate dilution of the extract with deionised water, NaNO2, AlCl3 and NaOH were added; the sample was then thoroughly vortex mixed and placed in darkness for 15 min. Afterwards, the absorbance at 510 nm was measured. Total flavonoid content was calculated from the standard curve plotted for (+)-catechin.
Total anthocyanins and degradation index were determined by means of the spectroscopic method (Giusti and Wrolstad 2001). Samples were prepared for analysis according to the procedure described by Plessi et al. (2007). Anthocyanin content, expressed as cyanidin-3-glucoside equivalent.
Antioxidant activity was determined by means of three spectrophotometric methods: as scavenging activity against DPPH (1.1-diphenyl-2-picrylhydrazyl) free radical (Pekkarinen et al. 1999); applying ABTS (2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) cation radical (Re et al. 1999); and by the ferric reducing antioxidant power (FRAP) method (Benzie and Strain 1996).
A Hitachi U-2900 double beam spectrophotometer (Hitachi Europe Ltd) was used to analyse total polyphenols, total flavonoids, total anthocyanins and antioxidant activity.
Colour evaluation
Colour was determined instrumentally using the CIE (L*a*b*) system. Reflectance measurement was performed in a Petri dish (65 mm in diameter) at a measurement angle of 10° and measurement diameter of 30 mm using a Konica Minolta CM-3500d. Measurement of each jam was carried out in four replications.
Texture analysis
Jam texture was analyzed according to the procedure given by Genovese et al. (2010) using a EZTest texturometer (Schimadzu, Tokyo, Japan) coupled with a computer equipped with RheoMeter v. 2.04 software. The compressing force applied was 20 N. The probe used (20 mm in diameter) moved at a rate 60 mm/min to a penetration depth of 20 mm. Four measurement replications were performed for each jam.
The following texture parameters were established
F3 (N) - gel strength (a point in the initial stage of penetration where little deformation has occurred, in this case 3 mm)
FR (N) - rupture force (the rupture point of the gel)
D (mm) - gel brittleness (the distance that the probe penetrates before this break occurs)
F20 (N) – force required for probe penetration to a depth of 20 mm;
E (N mm) - energy of penetration (area under the first pick)
A (N mm) – adhesiveness (area under the negative region of the curve).
Statistical analysis
The results obtained in the present work have been expressed in fresh mass. In order to show whether differences between the jams compared was significant, the results of compositional analyses were subjected to Snedecor F and Student’s t tests using the Statistica ver. 7.0 computer program. Next, the least significant difference (LSD) was calculated at the probability level p = 0.05.
Results and discussion
The jam recipes were established on the basis of the physico-chemical composition of bilberries. No significant differences in the fundamental physico-chemical compounds were found between the various samples of jam either before or after storage; thus, Table 1 shows their ranges only.
Table 1.
Basic physico-chemical composition of frozen bilberries and bilberry jams
| Sample* | Dry matter (g/kg) | Extract (g/kg) | Total acidity as citric acid (g/kg) | Active acidity (pH) |
|---|---|---|---|---|
| Frozen fruits** | 133 | 86 | 12.5 | 3.03 |
| Jams before and after 8 months of storage | ||||
| I–III | 353–359 | 348–353 | 6.7–7.3 | 3.16–3.26 |
*Sample: I – jam from bilberry without herbs, II – jam from bilberry with mentha (1 %), III – jam from bilberry with lemon balm (1 %)
**Fruit comprised 50 % jam mass
Hydroxymethylfurfural is acknowledged as a deterioration indicator for several products containing carbohydrates (Rattanathanalerk et al. 2005). This aldehyde is formed from the dehydration of hexoses in an acid medium or as a result of Maillard reactions and caramelization. There is virtually no HMF in fresh fruit; however, its occurrence in food results from the application of high temperatures during thermal treatment. Several authors claim that HMF reduces the nutritive value of many foodstuffs (Rattanathanalerk et al. 2005). However, its toxicity or mutagenicity is still a controversial issue (Janzowski et al. 2000).
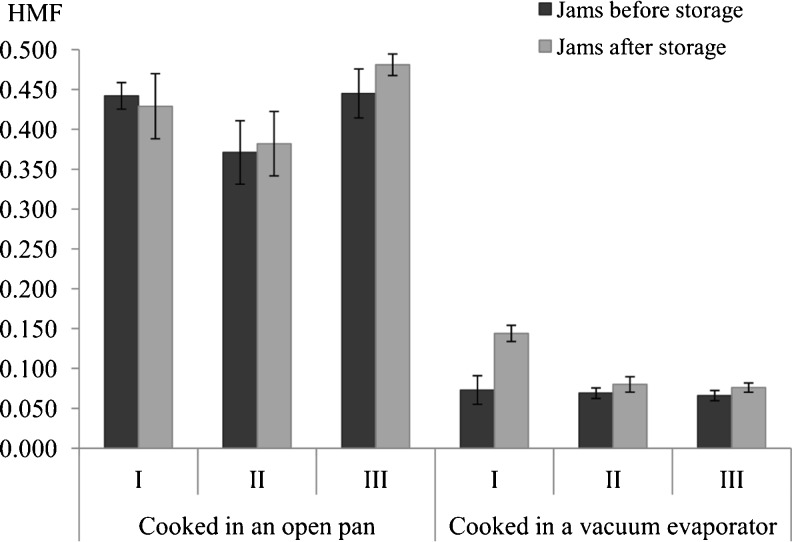
HMF content in jams immediately after production was within the range 0.006–0.445 mg/100 g (Fig. 1). Average HMF levels in an open pan cooked jams were 5 times higher than in those produced by vacuum evaporation. After storage, the HMF level in an open pan cooked jams remained unchanged, whereas that in the vacuum evaporated product increased by 15–16 %. Adding herbs to jams had no effect on the level of this indicator. Moreover, the level of HMF did not exceed 5 mg/100 g in any of the jams analyzed, which, according to Steber and Klostermeyer (1987), may be an indication that the process of cooking was properly performed.
Fig. 1.
Content of HMF in bilberry jams, mg/100 g of fresh matter Values are mean ± SD, n = 4, sample: I – jam from bilberry without herbs, II – jam from bilberry with mentha (1 %), III – jam from bilberry with lemon balm (1 %), LSD (p < 0.05) = 0.035
Bilberries are a rich source of antioxidants, chiefly due to their high levels of phenolic compounds. They are one of the more important berry fruits in terms of health-promoting properties (Smith 2002). The bilberries used in this study had relatively low amounts of vitamin C (16.8 mg/100 g). Taking into account the fact that fruit mass comprised 50 % of the finished product, technological treatment reduced the level of vitamin C by 26–31 % in an open pan cooked jams and by 15–25 % in the vacuum evaporated product (Table 2). Vitamin C loss during jam production has been reported by numerous authors for various fruit species (Ajenifujah-Solebo and Aina 2011). Such losses may be as much as 63 % compared with fresh fruit (Jawaheer et al. 2003).
Table 2.
The level of constituents with antioxidant properties and degradation index of anthocyanins in frozen bilberries and bilberry jams
| Sample* | Vitamin C (mg/100 g) | Total polyphenols (mg/100 g) | Total flavonoids (mg/100 g) | Total anthocyanins (mg/100 g) | Anthocyanin degradation index | |
|---|---|---|---|---|---|---|
| Frozen fruits | 16.8 ± 0.2 | 495 ± 20 | 342 ± 11 | 205 ± 13 | 1.00 ± 0.03 | |
| Jams before storage | ||||||
| Cooked in an open pan | I | 5.8 ± 0.2 | 176 ± 12 | 122 ± 8 | 73 ± 7 | 1.44 ± 0.10 |
| II | 6.2 ± 0.2 | 192 ± 19 | 134 ± 4 | 83 ± 3 | 1.41 ± 0.02 | |
| III | 6.0 ± 0.3 | 190 ± 20 | 137 ± 7 | 86 ± 4 | 1.43 ± 0.02 | |
| Cooked in a vacuum evaporator | I | 6.3 ± 0.3 | 213 ± 14 | 142 ± 3 | 88 ± 12 | 1.09 ± 0.01 |
| II | 6.9 ± 0.2 | 229 ± 16 | 153 ± 5 | 94 ± 5 | 1.08 ± 0.02 | |
| III | 7.1 ± 0.3 | 232 ± 15 | 156 ± 6 | 96 ± 8 | 1.07 ± 0.01 | |
| Jams after 8 months of storage | ||||||
| Cooked in an open pan | I | 5.0 ± 0.2 | 142 ± 8 | 98 ± 5 | 58 ± 5 | 1.46 ± 0.05 |
| II | 5.7 ± 0.5 | 173 ± 14 | 114 ± 4 | 68 ± 4 | 1.42 ± 0.04 | |
| III | 5.4 ± 0.2 | 170 ± 12 | 117 ± 4 | 69 ± 4 | 1.44 ± 0.07 | |
| Cooked in a vacuum evaporator | I | 5.8 ± 0.2 | 181 ± 12 | 127 ± 4 | 79 ± 6 | 1.10 ± 0.01 |
| II | 6.5 ± 0.2 | 209 ± 10 | 139 ± 6 | 87 ± 3 | 1.09 ± 0.02 | |
| III | 6.8 ± 0.2 | 214 ± 14 | 141 ± 5 | 89 ± 3 | 1.08 ± 0.02 | |
| LSD p = 0.05 | 0.40 | 23.6 | 15.2 | 7.4 | 0.06 | |
Values are mean ± SD, n = 4
*Sample: See Table 1
After storage, vitamin C content in the jams examined decreased by 4–14 %. Vacuum evaporated jams contained 14–26 % higher levels of vitamin C than jams prepared in an open pan. Vitamin C content was also affected by the addition of herbs; jams cooked with mentha or lemon balm contained 12–14 % and 8–17 % respectively more vitamin C than those prepared without herbs.
100 g frozen bilberry fruit contained 495 mg total polyphenols, giving 248 mg total polyphenols in 100 g of the jam mix. This level was not affected in vacuum evaporated products; however, in an open pan cooked products it decreased by 6–14 % (Table 2). Losses of total polyphenols during the cooking of jams in an open pan (with access to the air and at a higher temperature) have also been reported by other authors (Amakura et al. 2000). After storage, an 8–19 % reduction of these substances was noted compared with non-stored jams. It was also found that the addition of herbs had a beneficial effect on polyphenol levels; jams with mentha or lemon balm contained 15–22 % and 18–20 % respectively more total polyphenols than those without added herbs.
Bilberries also contain compounds with antibacterial properties including flavonoids (Burdulis et al. 2009), whose content in the fruit used to make the jams was 342 mg/100 g, giving 171 mg flavonoids per 100 g jam mix. This level fell by 8–17 % and 20–29 % in evaporator cooked and in an open pan cooked jams respectively. There was further flavonoid loss during storage. After storage, flavonoid levels fell further, by 15–20 % and 9–11 %, respectively in vacuum evaporated and in an open pan cooked products compared with non-stored jam. The addition of herbs had a significant effect only on an open pan cooked jams. A decrease in flavonoids during cooking in an open pan of strawberry jams was also observed by Häkkinen et al. (2000). The cell structure of fruit is damaged during processing, making fruit more susceptible to non-enzymic oxidation, which in turn may be one of the main reasons for the loss of phenolic substances (Patras et al. 2011).
Anthocyanins, pigments belonging to the flavonoid group, not only give fruits their dark blue colour but also perform several therapeutic functions (Cacace and Mazza 2003). In jams made with 50 % fruit, the anthocyanin content in the raw fruit was 103 mg, which was reduced by 7–15 % and 17–29 % respectively following vacuum evaporation and in an open pan cooking (Table 2). According to García-Viguera and Zafrilla (2001), anthocyanin loss due to cooking ranges from 10 to 80 %, which has also been confirmed by the findings of Kim and Padilla-Zakour (2004). Anthocyanin reduction in jam production is probably due to complexation with other compounds (Da Silva Pinto et al. 2007), a process involving sugars, metal ions, hydrogen peroxide, and ascorbic acid (Clifford and Scalbert 2000).
The present study found that the cooking method had an effect on anthocyanin content, which, prior to storage, was significantly (12–21 %) higher in vacuum evaporated jam than in an open pan cooked product. A similar dependency was observed after storage. It was also shown that, regardless of the cooking method, the addition of herbs improved anthocyanin retention: products with mentha and lemon balm contained 10–20 % more anthocyanins than those without. The degradation index for anthocyanins increased with increasing degradation of these constituents (Table 2), and across all the jams evaluated was highest in an open pan cooked samples, both non-stored and after 8 months’ storage.
During the production process, radical scavenging and antioxidant activities measured using DPPH, FRAP, and ABTS increased by 9–34 %, 4–53 %, and 12–59 % respectively in jams produced with 50 % fruit (Table 3). Furthermore, the level of such activity was always higher in jams cooked by evaporation. A number of authors attribute increases in antioxidant activity after thermal treatment to the release of bound phenolic compounds resulting from damage to the cell walls of the raw material, which in turn leads to the formation of new constituents with increased antioxidant activity (Tomaino et al. 2005). Amakura et al. (2000) studied the effect of jam processing on DPPH radical scavenging activity in nine types of berry and found that jams exhibited stronger activity than unprocessed berries. Similarly, Šavikin et al. (2009) showed an increase in antioxidant activity in blackcurrant jams.
Table 3.
Radical scavenging and antioxidant activity of frozen bilberries and bilberry jams
| Sample* | ABTS (μM Trolox/g) | DPPH (μM Trolox/g) | FRAP (μM Fe2+/g) | |
|---|---|---|---|---|
| Frozen fruits | 722 ± 41 | 130 ± 11 | 230 ± 18 | |
| Jams before storage | ||||
| Cooked in an open pan | I | 405 ± 7 | 71 ± 4 | 120 ± 7 |
| II | 458 ± 19 | 74 ± 5 | 160 ± 7 | |
| III | 452 ± 7 | 74 ± 3 | 150 ± 6 | |
| Cooked in a vacuum evaporator | I | 498 ± 9 | 76 ± 6 | 134 ± 3 |
| II | 575 ± 10 | 89 ± 4 | 176 ± 6 | |
| III | 570 ± 14 | 87 ± 5 | 171 ± 8 | |
| Jams after 8 months of storage | ||||
| Cooked in an open pan | I | 361 ± 11 | 63 ± 3 | 102 ± 6 |
| II | 420 ± 21 | 66 ± 3 | 139 ± 7 | |
| III | 410 ± 13 | 65 ± 2 | 144 ± 10 | |
| Cooked in a vacuum evaporator | I | 459 ± 27 | 69 ± 3 | 120 ± 3 |
| II | 545 ± 23 | 82 ± 3 | 164 ± 5 | |
| III | 550 ± 25 | 80 ± 3 | 165 ± 3 | |
| LSD p = 0.05 | 27.0 | 5.2 | 8.9 | |
Values are mean ± SD, n = 4
*Sample: See Table 1
In comparison with the non-stored products, 8-month storage of the jams investigated led to a reduction in their radical scavenging and antioxidant activity by 4–11 % (ABTS), 8–12 % (DPPH), and 4–15 % (FRAP) (Table 3). Depending on the analytical method, antioxidant activity was 5–37 % higher in jams with added mentha and 3–41 % higher in those with lemon balm compared to jams without added herbs.
Since consumers tend to look for products with strong colours and natural appearance, colour is a crucial factor determining food quality. The colour of the jams obtained from berry fruits is determined by the content of anthocyanin pigments as well as the presence of substances of greyish brown colouration, which are formed during degradation of anthocyanins and during non-enzymatic browning (Kim and Padilla-Zakour 2004). Suutarinen et al. (2002) noted significant reduction in colour parameters as a result of the process applied in bilberry jam production. García-Viguera et al. (1999a) also reported that the degradation and loss of red colour in strawberry jam could be due to Maillard and non-enzymatic browning, ascorbic acid degradation and polymerization of anthocyanins with other phenolics. Instrumental colour measurements make it possible to evaluate the extent to which these pigments change.
According to Czapski and Walkowiak-Tomczak (2005), the longer the time and the higher the temperature of cooking, the greater the degradation of anthocyanins and changes in colour parameters. Average L*, a* and b* values in bilberry jams cooked by the evaporation method were respectively 39 %, 48 %, and 44 % lower than in an open pan cooked jams (Table 4). Thus, the jams cooked in a vacuum exhibited lower brightness than those cooked in an open pan.
Table 4.
Colour parameters of frozen bilberries and bilberry jams
| Sample* | L* | a* | b* | C* | h o | |
|---|---|---|---|---|---|---|
| Frozen fruits | 3.25 ± 0.11 | 7.12 ± 0.12 | 1.90 ± 0.17 | 7.72 ± 0.16 | 16.25 ± 0.80 | |
| Jams before storage | ||||||
| Cooked in an open pan | I | 1.82 ± 0.17 | 3.12 ± 0.35 | 0.79 ± 0.09 | 3.22 ± 0.35 | 10.71 ± 0.42 |
| II | 1.47 ± 0.10 | 2.39 ± 0.16 | 0.67 ± 0.09 | 2.48 ± 0.18 | 15.68 ± 1.27 | |
| III | 1.52 ± 0.07 | 2.85 ± 0.12 | 0.87 ± 0.11 | 2.38 ± 0.15 | 16.88 ± 1.41 | |
| Cooked in a vacuum evaporator | I | 1.03 ± 0.07 | 1.56 ± 0.19 | 0.53 ± 0.07 | 1.65 ± 0.19 | 15.95 ± 1.53 |
| II | 0.82 ± 0.04 | 1.20 ± 0.03 | 0.35 ± 0.04 | 1.55 ± 0.04 | 17.19 ± 0.95 | |
| III | 0.87 ± 0.12 | 1.58 ± 0.20 | 0.43 ± 0.10 | 1.60 ± 0.22 | 17.09 ± 1.43 | |
| Jams after 8 months of storage | ||||||
| Cooked in an open pan | I | 2.82 ± 0.11 | 2.84 ± 0.24 | 0.60 ± 0.06 | 2.88 ± 0.20 | 9.48 ± 0.13 |
| II | 2.43 ± 0.23 | 2.05 ± 0.08 | 0.56 ± 0.07 | 2.00 ± 0.10 | 15.08 ± 0.28 | |
| III | 2.42 ± 0.22 | 2.37 ± 0.35 | 0.69 ± 0.15 | 2.47 ± 0.23 | 16.22 ± 0.88 | |
| Cooked in a vacuum evaporator | I | 1.38 ± 0.19 | 0.92 ± 0.12 | 0.27 ± 0.008 | 1.45 ± 0.11 | 15.70 ± 0.99 |
| II | 0.87 ± 0.09 | 0.93 ± 0.13 | 0.31 ± 0.09 | 1.12 ± 0.38 | 15.96 ± 0.09 | |
| III | 1.19 ± 0.16 | 1.00 ± 0.09 | 0.35 ± 0.14 | 1.26 ± 0.39 | 16.41 ± 1.62 | |
| LSD p = 0.05 | 0.179 | 0.258 | 0.122 | 0.305 | 1.439 | |
Values are mean ± SD, n = 4
*Sample: See Table 1
Colour lightness in jams obtained from bilberries without added herbs was 1.03–1.82 units (Table 4), whereas in jams with added mentha and lemon balm these values were 16–20 % lower, the decrease probably being due to 1 % addition of green leaves. This factor also affected the proportion of red (a*) and yellow (b*) colour in jams. The low values for these parameters in the samples containing herbs confirm the low proportion of red and yellow colours in these products. Therefore, the addition of either herb resulted in colour orientation towards green and blue. Moreover, jams with these herbs showed significantly lower colour saturation than those without (23 % for mentha and 26 % lemon balm), but only in an open pan cooked samples; however, colour shade was 46 % and 58 % higher respectively.
After 8 months of storage, the colour of the jams changed compared to non-stored products: a*, b* and C* values decreased by 9–41 %, 11–49 %, and 4–42 % respectively, while lightness, expressed as a rise in L* value, increased by 6–65 % accompanied by slight changes in colour saturation. Brightening of bilberry jams during storage of was also observed by Ścibisz et al. (2011), who suggested that this phenomenon could be caused by transformation of red flavylium cation into colourless and yellow chalcones.
Jam structure is determined by the equilibrium between the pectin, sugar and acid contents present in the fruit, which are fundamental components of soluble solid contents (Pilgrim et al. 1991). Food processing critically affects the pectin structure–function relationship and thus the texture of fruit and vegetable products depending on the processing technique applied and the intensity of that process (safety and/or quality) (van Buggenhout et al. 2009).
The gels in non-stored jams were characterized by hardness of 0.72–1.81 N; the force required to rupture a gel was within the range 0.87–2.37 N depending on the type of product (Table 5). The addition of herbs caused a reduction in the aforementioned parameters of 12–20 % and 6–18 % respectively. The force (F20) and energy of penetration (E) values were also lower in such products. Furthermore, jams cooked in an open pan were harder than those cooked in the evaporator, on average by 109 %. In all the jams examined, significant differences were also found in the level of gel brittleness (D). Products, both in an open pan cooked and vacuum cooked, to which mentha and lemon balm were added showed lower brittleness compared to those without these additives. Adhesiveness is an important parameter for food products. It enables the degree of adhesion of food on the teeth to be predicted (Besbes et al. 2009). A decline in hardness observed in jams with herbs was accompanied by an increase in adhesiveness, regardless of the cooking method.
Table 5.
Texture parameters in bilberry jams
| Sample* | F3** (N) | FR (N) | D (mm) | F20 (N) | E (N mm) | A (N mm) | |
|---|---|---|---|---|---|---|---|
| Jams before storage | |||||||
| Cooked in an open pan | I | 1.81 ± 0.13 | 2.37 ± 0.11 | 5.28 ± 0.31 | 2.45 ± 0.11 | 42.80 ± 2.00 | −6.29 ± 0.30 |
| II | 1.59 ± 0.12 | 2.22 ± 0.09 | 6.30 ± 0.62 | 3.02 ± 0.09 | 41.41 ± 2.61 | −7.09 ± 1.10 | |
| III | 1.49 ± 0.08 | 2.17 ± 0.13 | 5.47 ± 0.40 | 2.12 ± 0.12 | 38.53 ± 0.76 | −6.21 ± 0.41 | |
| Cooked in a vacuum evaporator | I | 0.89 ± 0.04 | 1.06 ± 0.13 | 3.77 ± 0.06 | 1.66 ± 0.12 | 24.65 ± 0.65 | −5.55 ± 0.37 |
| II | 0.73 ± 0.09 | 0.95 ± 0.02 | 5.50 ± 0.64 | 1.75 ± 0.14 | 23.28 ± 1.32 | −6.20 ± 0.70 | |
| III | 0.72 ± 0.04 | 0.87 ± 0.03 | 4.77 ± 0.28 | 1.54 ± 0.08 | 27.55 ± 1.56 | −6.59 ± 0.60 | |
| Jams after 8 months of storage | |||||||
| Cooked in an open pan | I | 1.03 ± 0.09 | 1.67 ± 0.09 | 5.25 ± 0.56 | 1.81 ± 0.13 | 30.84 ± 1.08 | −3.56 ± 0.05 |
| II | 0.84 ± 0.14 | 2.68 ± 0.15 | 8.73 ± 0.77 | 2.48 ± 0.24 | 43.78 ± 2.89 | −2.46 ± 0.46 | |
| III | 0.72 ± 0.04 | 1.67 ± 0.09 | 7.90 ± 0.56 | 1.54 ± 0.03 | 27.55 ± 1.03 | −2.67 ± 0.39 | |
| Cooked in a vacuum evaporator | I | 0.69 ± 0.11 | 0.89 ± 0.01 | 4.45 ± 0.25 | 1.48 ± 0.14 | 20.11 ± 1.95 | −3.13 ± 0.61 |
| II | 0.64 ± 0.04 | 0.70 ± 0.03 | 4.60 ± 0.10 | 1.55 ± 0.10 | 20.11 ± 1.58 | −2.55 ± 0.01 | |
| III | 0.53 ± 0.07 | 0.70 ± 0.06 | 5.32 ± 0.23 | 1.21 ± 0.18 | 17.96 ± 2.37 | −2.13 ± 0.36 | |
| LSD p = 0.05 | 0.150 | 0.149 | 0.745 | 0.218 | 2.940 | 0.666 | |
Carbonell et al. (1991) noted that differences in texture depend on the species of fruit. For example, according to Rababah et al. (2011), in an open pan cooked strawberry jams showed hardness at the level 0.62 N, while Besbes et al. (2009) found a maximum value of (1.40 N) for this parameter in date jams. Thus, the results obtained in this work corresponded to these findings.
Suutarinen et al. (2002) reported that strawberry jam firmness was not significantly changed because cross linking between carboxyl groups of adjacent polyuronide chains via calcium ions made the cell wall less accessible to enzymes in the fruit that cause softening or to cell wall degradation. However, Kopjar et al. (2009) and Rababah et al. (2011) showed that storage time affected the texture of strawberry jams. 8-months’ storage of the jams investigated resulted in deterioration of the texture (Table 5), probably due to the presence of acids in fruits, which could contribute to partial depolymerization of pectins and a loosening of their consistency. The hardness of bilberry jams fell to 0.53–1.03 N; however, in an open pan cooked jams were on average 39 % harder than those cooked in the evaporator. Moreover, jams without the addition of herbs were on average 21 % harder than those with herbs. A decline was also observed in the remaining texture parameters of jams, resulting in a decrease in brittleness and adhesiveness.
Conclusions
After 8 months of storage, 100 g fresh mass of the bilberry jams examined contained 5.9 mg vitamin C, 182 mg total polyphenols, 123 mg total flavonoids and 75 mg total anthocyanins. Total antioxidant activity per 1 g at average levels was 458 μM Trolox (ABTS), 71 μM Trolox (DPPH) and 139 μM Fe2+ (FRAP). These products may therefore be regarded as a good source of antioxidants in the diet. Jams with added herbs exhibited 3–41 % higher antioxidant activity than those without. Moreover, the addition of herbs resulted in better retention of antioxidant properties during storage but caused colour and texture deterioration. Jams cooked by the vacuum method showed 30 % (ABTS), 18 % (DPPH), and 17 % (FRAP) higher average antioxidant activity than those obtained using the method of cooking in an open pan. It was also found that cooking in an evaporator had a beneficial effect on colour; however, this was accompanied by a deterioration of texture.
References
- Ajenifujah-Solebo SO, Aina JO. Physico-chemical properties and sensory evaluation of jam made from black-plum fruit (Vitex doniana) Afr J Food Agric Nutr Dev. 2011;11(4772):4784. [Google Scholar]
- Amakura Y, Umino Y, Tsuji S, Tonogai Y. Influence of jam processing on the radical scavenging activity and phenolic content in berries. J Agric Food Chem. 2000;48:6292–6297. doi: 10.1021/jf000849z. [DOI] [PubMed] [Google Scholar]
- AOAC . Official Methods of Analysis, 16th edn (edited by AOAC) Arlington, USA: AOAC; 1995. [Google Scholar]
- Ardestani A, Yazdanparast R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007;104:21–29. doi: 10.1016/j.foodchem.2006.10.066. [DOI] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Besbes S, Drira L, Blecker C, Deroanne C, Attia H. Adding value to hard date (Phoenix dactylifera L.): compositional, functional and sensory characteristics of date jam. Food Chem. 2009;112:406–411. doi: 10.1016/j.foodchem.2008.05.093. [DOI] [Google Scholar]
- Beveridge T. Opalescent and cloudy fruit juices: formation and particle stability. Crit Rev Food Sci Nutr. 2002;42:317–337. doi: 10.1080/10408690290825556. [DOI] [PubMed] [Google Scholar]
- Brownmiller C, Howard L, Prior R. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry products. J Food Sci. 2008;73:H72. doi: 10.1111/j.1750-3841.2008.00761.x. [DOI] [PubMed] [Google Scholar]
- Burdulis D, Šarkinas A, Jasutiené I, Stackevičienė E, Nikolajevas L, Janulis V. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta Pol Pharm. 2009;66:399–408. [PubMed] [Google Scholar]
- Cacace J, Mazza G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J Food Sci. 2003;68:240–248. doi: 10.1111/j.1365-2621.2003.tb14146.x. [DOI] [Google Scholar]
- Carbonell E, Costell E, Durán L. Fruit content influence on gel strength of strawberry and peach jams. J Food Sci. 1991;56:1384–1387. doi: 10.1111/j.1365-2621.1991.tb04780.x. [DOI] [Google Scholar]
- Clifford MN, Scalbert A. Ellagitannins - nature, occurrence and dietary burden. J Sci Food Agr. 2000;80:1118–1125. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1118::AID-JSFA570>3.0.CO;2-9. [DOI] [Google Scholar]
- Czapski J, Walkowiak-Tomczak D. Zmiany parametrów barwy roztworów antocyjanów w czasie ogrzewania. Inż Rol. 2005;9:27–33. [Google Scholar]
- Da Silva Pinto M, Lajolo FM, Genovese MI. Bioactive compounds and antioxidant capacity of strawberry jams. Plant Foods Hum Nutr. 2007;62:127–131. doi: 10.1007/s11130-007-0052-x. [DOI] [PubMed] [Google Scholar]
- García-Viguera C, Zafrilla P. Changes in anthocyanins during food processing: influence on color. In: Ames JM, Hofmann T, editors. Chemistry and physiology of selected colorants. Washignton (DC): American Chemical Society; 2001. pp. 56–65. [Google Scholar]
- García-Viguera C, Zafrilla P, Romero F, Abellan P, Artes F, Tomas-Barberan F. Color stability of strawberry jam as affected by cultivar and storage temperature. J Food Science. 1999;64:243–247. doi: 10.1111/j.1365-2621.1999.tb15874.x. [DOI] [Google Scholar]
- García-Viguera C, Zafrilla P, Tomas-Barberan F. Influence of processing and storage conditions in strawberry jam color. Food Sci Technol Int. 1999;5:487–492. doi: 10.1177/108201329900500606. [DOI] [Google Scholar]
- Genovese DB, Ye A, Singh H. High methoxyl pectin/apple particles composite gels: effect of particle size and particle concentration on mechanical properties and gel structure. J Texture Stud. 2010;41:171–189. doi: 10.1111/j.1745-4603.2010.00220.x. [DOI] [Google Scholar]
- Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr Protoc Food Analyt Chem. 2001;F1(2):1–F1.2.13. [Google Scholar]
- Häkkinen SH, Kärenlampi SO, Mykkänen HM, Heinonen IM, Törrönen AR. Ellagic acid content in berries: Influence of domestic processing and storage. Eur Food Res Technol. 2000;212:75–80. doi: 10.1007/s002170000184. [DOI] [PubMed] [Google Scholar]
- Janzowski C, Glaab V, Samimi E, Schlatter J, Eisenbrand G. 5-Hydroxymethylfurfural: assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem Toxicol. 2000;38:801–809. doi: 10.1016/S0278-6915(00)00070-3. [DOI] [PubMed] [Google Scholar]
- Jawaheer B, Goburdhun D, Ruggoo A. Effect of processing and storage of guava into jam and juice on the ascorbic acid content. Plant Foods Hum Nutr. 2003;58:1–12. doi: 10.1023/B:QUAL.0000041161.05123.66. [DOI] [Google Scholar]
- Kalt W, Dufour D. Health functionality of blueberries. HortTechnology. 1997;7:216–221. [Google Scholar]
- Kalt W, Forney CF, Martin A, Prior RL. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J Agric Food Chem. 1999;47:4638–4644. doi: 10.1021/jf990266t. [DOI] [PubMed] [Google Scholar]
- Kim D, Padilla-Zakour O. Jam processing effect on phenolics and antioxidant capacity in anthocyanin-rich fruits: cherry, plum, and raspberry. J Food Science. 2004;69:S395. doi: 10.1111/j.1365-2621.2004.tb09956.x. [DOI] [Google Scholar]
- Kong J, Chia L, Goh N, Chia T, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/S0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- Kopjar M, Piližota V, Tiban N, Đubarić D, Babić J, Ačkar Đ, Sajdl M. Strawberry jams: influence of different pectins on color and textural properties. Czech J Food Sci. 2009;27:20–28. [Google Scholar]
- Kukurova K, Karovičová J, Greif G, Kohajdová Z, Lehkoživová J. Determination of 5-hydroxymethylfurfural after Winkler and by the HPLC method for authentication of honey. Chem Pap. 2006;60:186–191. doi: 10.2478/s11696-006-0034-8. [DOI] [Google Scholar]
- Lee J, Wrolstad R. Extraction of anthocyanins and polyphenolics from blueberry processing waste. J Food Sci. 2004;69:564–573. doi: 10.1111/j.1365-2621.2004.tb13651.x. [DOI] [Google Scholar]
- Mojet J, Köster E. Sensory memory and food texture. Food Qual Prefer. 2005;16:251–266. doi: 10.1016/j.foodqual.2004.04.017. [DOI] [Google Scholar]
- Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium Rubus and Ribes. J Agric Food Chem. 2002;50:519–525. doi: 10.1021/jf011062r. [DOI] [PubMed] [Google Scholar]
- Patras A, Brunton NP, Tiwari BK, Butler F. Stability and degradation kinetics of bioactive compounds and colour in strawberry jam during storage. Food Bioprocess Technol. 2011;4:1245–1252. doi: 10.1007/s11947-009-0226-7. [DOI] [Google Scholar]
- PCS . Standard EN 14130: Foodstuff-Determination of vitamin C by HPLC. Warsaw: European Committee for Standardization, Technical Committee CEN/TC 275; 2003. [Google Scholar]
- Pekkarinen SS, Heinonen IM, Hopia AI. Flavonoids quercetin, myricetin, kaemferol and (+) catechin and antioxidants in methyl linoleate. J Sci Food Agr. 1999;79:499–506. doi: 10.1002/(SICI)1097-0010(19990315)79:4<499::AID-JSFA204>3.0.CO;2-U. [DOI] [Google Scholar]
- Pilgrim GW, Walter RH, Oakenfull DG. Jam, jellies and preserves. In: Walter RH, editor. The chemistry and technology of pectin. California: Academic; 1991. pp. 23–50. [Google Scholar]
- Plessi M, Bertelli D, Albasini A. Distribution of metals and phenolic compounds as a criterion to evaluate variety of berries and related jams. Food Chem. 2007;100:419–427. doi: 10.1016/j.foodchem.2005.09.018. [DOI] [Google Scholar]
- Rababah TM, Al-u’datt MH, Al-Mahasneh MA, Feng H, Alothman AM, Almajwal A, Yang W, Kilani I, Alhamad MN, Ereifej K, Abu-Darwish M. Effect of storage on the physico-chemical properties, total phenolic, anthocyanin, and antioxidant capacity of strawberry jam. J Food Agri Environ. 2011;9:101–105. [Google Scholar]
- Rattanathanalerk M, Chiewchan N, Srichumpoung W. Effect of thermal processing on the quality loss of pineapple juice. J Food Eng. 2005;66:259–265. doi: 10.1016/j.jfoodeng.2004.03.016. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Šavikin K, Zdunić G, Janković T, Tasić S, Menković N, Stević T, Đorđević B. Phenolic content and radical scavenging capacity of berries and related jams from certificated area in Serbia. Plant Foods Hum Nutr. 2009;64:212–217. doi: 10.1007/s11130-009-0123-2. [DOI] [PubMed] [Google Scholar]
- Ścibisz I, Gasik A, Mitek M, Cendrowski A. Wpływ warunków przechowywania na barwę dżemów z owoców kolorowych. Żywność: Nauka, Technologia, Jakość. 2011;74:99–111. [Google Scholar]
- Seeram NP. Berry fruits for cancer prevention: current status and future prospects. J Agric Food Chem. 2008;56:630–635. doi: 10.1021/jf072504n. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Smith MA. Vaccinium species (small-fruited berries): in vitro culture and the production of food colorants and phytochemicals. In: Nagata T, Ebizuka Y, editors. Biotechnology in Agriculture and Forestry. Medicinal and Aromatic Plants XII. Berlin: Springer Verlag; 2002. pp. 328–344. [Google Scholar]
- Steber F, Klostermeyer H (1987) Heat treatment of fruit preparations and jams, and monitoring its efficacy. Die Molkereizeitung “Welt der Milch” 41:289-290, 292-295
- Suutarinen J, Honkapaa K, Heinio R, Autio K, Mustranta A, Karppinen S, Klutamo T, Llukkonen-Lllja H, Mokkila M. Effects of calcium chloride-based prefreezing treatments on the quality factors of strawberry jams. J Food Science. 2002;67:884–894. doi: 10.1111/j.1365-2621.2002.tb10694.x. [DOI] [Google Scholar]
- Tomaino A, Cimino F, Zimbalatti V, Venuti V, Sulfaro V, de Pasquale A, Saija A. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem. 2005;89:549–554. doi: 10.1016/j.foodchem.2004.03.011. [DOI] [Google Scholar]
- van Buggenhout S, Sila D, Duvetter T, van Loey A, Hendrickx M. Pectins in processed fruits and vegetables: Part III-Texture engineering. Compr Rev Food Sci Food Saf. 2009;8:105–117. doi: 10.1111/j.1541-4337.2009.00072.x. [DOI] [Google Scholar]
- Witzell J, Gref R, Näsholm T. Plant-part specific and temporal variation in phenolic compounds of boreal bilberry (Vaccinium myrtillus) plants. Biochem Syst Ecol. 2003;31:115–127. doi: 10.1016/S0305-1978(02)00141-2. [DOI] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]