Abstract
Oral administration of specific egg yolk immunoglobulin (IgY) is effective against a number of gastrointestinal pathogens. However, the activity of orally administered IgY is reduced rapidly, since IgY is sensitive to pepsin and low pH. In this study, hydrogels containing acrylamide and acrylic acid were synthesized and used to encapsulate IgY. The capacity of these structures to load, protect and release IgY and the interaction between IgY and hydrogels by FTIR spectroscopy were studied. The particle size and swelling percentage of hydrogels were highly dependent on the pH of the buffer solution. As expected, pH-sensitive hydrogels had a high IgY loading percentage (99.2 ± 12.9 mg IgY/mg hydrogel) at pH 7.4. It means that each gel piece incorporated approximately 8.4 ± 1.1 mg IgY. The results showed that the hydrogels could efficiently incorporate IgY and retain it inside the polymer network at pH <2.2. However, IgY was slowly released at basic pH and a high percentage remained inside. The IR spectra show that IgY interacts with the hydrogel in its network with extended hydrogen bonds. The present study demonstrates that hydrogels particles can efficiently incorporate the IgY but cannot show a controlled and sustained release of IgY in simulated intestinal fluid probably due to hydrophobic interactions with the polymer network. The stability of IgY in simulated gastric fluid was greatly improved by encapsulation in hydrogels. This approach provides information about a novelty method for delivery of IgY for the prevention and control of enteric diseases.
Keywords: Egg yolk immunoglobulins, pH-sensitive hydrogels, Delivery, FT-IR spectroscopy
Introduction
Egg yolk immunoglobulin (IgY) is actively transported from the serum of the hen to the yolk, providing passive immunity to the embryo (Rose et al. 1974). IgY technology is a highly innovative and growing biotechnology area, offering numerous advantages, including low cost, effectiveness, efficiency and the minimization of animal suffering (Schade et al. 2007).
IgY was previously studied against different intestinal infectious pathogens such as Escherichia coli (Bellingeri et al. 2013; Feng et al. 2013), Salmonella spp. (Chalghoumi et al. 2009), Pseudomonas aeruginosa (Nilsson et al. 2007) and rotavirus (Vega et al. 2012) and has shown to have characteristics that make it an efficient alternative traditional treatments (Kovacs-Nolan and Mine 2012). However, the activity of orally administered IgY may be reduced rapidly, even destroyed completely, under gastric conditions since IgY is sensitive to pepsin and low pH. Since the primary target site of IgY is in the small intestine, it is necessary to find an effective method to protect IgY against peptic digestion and acidity during the gastric passage.
Different microencapsulation techniques for the protection of IgY from gastric inactivation have been developed, such as chitosan-alginate microcapsules (Li et al. 2007), liposomes (Chang et al. 2002; Shimizu et al. 1993), pH-sensitive methacrylic acid copolymer (Kovacs-Nolan and Mine 2005), (poly(D,L-lactide-co-glycolide) microspheres (Torche et al. 2006) and multiple emulsification (Cho et al. 2005).
Polyacrylamide hydrogels have been extensively exploited for biomedical applications due to their versatility and excellent biocompatibility (Hoffman 2002; Yang 2008; Jaiswal et al. 2013). There are several publications dealing with the high carcinogenic and toxicity of the monomer of acrylamide, however, after the polymerization it becomes not toxic (Andersen 2005). Several In vitro studies have revealed that polyacrylamide gels are biocompatibles (Karadaǧ et al. 1996; Risbud and Bhonde 2000). Polyacrylamide gels also exhibit excellent biocompatibility In vivo (Gin et al. 1990; Saraydin et al. 2004; Wenger et al. 2011). Polyacrylamide hydrogels with pH-sensitive swelling have been used with excellent results as carriers in drug delivery research (Gupta et al. 2002).
The objective of this research was to develop a novel drug delivery system with pH-sensitive swelling and drug release properties for localized IgY delivery in the intestine. This study focused on the properties of IgY-hydrogels composites. Physical characteristics, the loading capacity for IgY, and release profiles of these hydrogels were investigated.
Materials and methods
pH-sensitive hydrogel synthesis
Acrylamide (AAm, Aldrich) and acrylic acid (AAc, Aldrich) were used as monomers. The crosslinker used was N,N’-metylenbisacrylamide (BAAm, Roth). The redox initiator system used was made of ammonium persulfate (APS, Roth) and tetramethylethylenediamine (TEMED, Merck). Monomers and crosslinker were dissolved in distilled water, and then the solution was bubbled with nitrogen for 15 min. After that, a solution containing APS (0.001 gr/ml) and TEMED (10 μl/ml) was added and the reaction mix was sealed. The free radical polymerization of the hydrogels was carried out in a tuberculin syringes at room temperature (22 °C) for 3 h. The extreme of the syringe was cut and the gel was expulsed and sectioned into similar pieces (~5 mm). The resulting gels were washed several times with distilled water during 1 week to remove all the unreacted monomers. The pH of the water was measured to verify that unreacted monomers were eliminated. Then, gels were dried at room temperature until they reached constant weight. Three different types of hydrogels were prepared (Table 1).
Table 1.
Properties of different hydrogels used in this study
| Hydrogel | AAm (M) | AAc (M) | BAAm (M) | Rate monomer/crosslinker | Crosslinker percentage (%) |
|---|---|---|---|---|---|
| A | 0.70 | 1.40 | 0.01 | 262.50 | 0.38 |
| B | 1.40 | 1.40 | 0.02 | 175.00 | 0.57 |
| C | 2.80 | 1.40 | 0.03 | 131.25 | 0.76 |
M molarity, AAm acrylamide, AAc acrylic acid, BAAm, N,N’-metylenbisacrylamide
Characterization of pH-sensitive hydrogels
For the pH dependent swelling studies, hydrogels in triplicate were incubated in buffer solutions ranging from pH 2.2 to 10 at room temperature for 24 h. For pH 2.2 buffer solution 0.2 M KCl/0.2 M HCl buffer was used, NaOH/KH2PO4/Na2HPO4 buffer for pH 7.4 and 0.1 M NaHCO3/NaOH buffer for pH 10. The pH values were checked precisely by a pH-meter. Periodically, the hydrogels were withdrawn from the buffer solution, measured and weighed after removal of excessive surface water by lightly blotting with a filter paper. The following parameters were calculated:
Particle size was measured using a manual caliper taking the average of five measurements.
Swelling percentage = [(Ws − Wd)/Ws] * 100
where Ws represents the weight of the swollen state of the sample and Wd is the weight of dry sample. The geometric mean of particle size in each pH was compared with a non parametric Kruskal-Wallis test. A p-value < 0.05 was considered to be significant. Statistical analyses were performed using Infostat software (Di Rienzo et al. 2008).
Isolation and precipitation of IgY
The water soluble fraction (WSF) of egg yolk was isolated as described Akita and Nakai (1992) with minor modifications. Briefly, egg yolk was separated from egg white and the yolk membrane was punched. Yolk without membrane was transferred into a graduated cylinder and mixed with six volumes of cold acidified distilled water (pH 2.5 adjusted with 0.1 M HCl). The mixture was then adjusted to pH 5.0–5.2 and incubated at 4 °C for 12 h. Following centrifugation at 12,000 xg and 4 °C for 20 min, the supernatant was considered as WSF. Sodium sulfate was added to the WSF to 18 % saturation and the mixture was stirred at 25 °C for 30–60 min, and then centrifuged at 2,000 xg at 25 °C for 30 min. The pellet, which contained crude IgY, was resuspended in PBS, dialyzed against 0.15 M NaCl to remove residual ammonium sulfate and PBS buffer salts, and finally lyophilized to obtain the IgY powder.
IgY incorporation to pH-sensitive hydrogels
IgY content incorporated into hydrogels was assayed by immersing pre-weighed dry hydrogels (10 mg) in 5 mL of IgY solution (10 mg IgY/ml, pH = 10) for 72 h.
IgY loading was obtained by the following equation:
where Whydrogel-IgY represents the weight of the dried state of the hydrogel containing IgY and Whydrogel is the weight of dry hydrogel before the IgY incorporation.
In vitro study of IgY release
The release of IgY from the hydrogels was studied by incubating 50 mg of hydrogel-IgY in 50 mL of simulated gastric fluid (SGF) without pepsin, at 37 °C, with shaking. Simulated gastric fluid consisted of 0.03 M NaCl, at pH 1.2. After 2 h, the hydrogels were filtered and transferred to 50 mL of simulated intestinal fluid (SIF, 0.05 M KH2PO4, pH 6.8) without pancreatin and were incubated at 37 °C with shaking for 3 h. At desired intervals of time, 200 μL aliquots were removed and replaced with the same amount of fresh medium. Protein concentration was assayed using the Bradford (1976). The cumulative release percentage (%) was calculated.
Interaction between IgY and hydrogels
The interaction between IgY and hydrogels was determined with Fourier transform infrared (FT-IR) spectroscopy. The characteristic absorption peaks were detected using a FTIR Nicolet Impact 400 spectrometer. The samples were mixed with KBr (rate 1:3). Before the spectrum was obtained, this mixture was mortared and pressed for 15 min at 15 tn/cm2 under dynamic vacuum.
In vitro stability of IgY to simulated gastric conditions
The stability of IgY to simulated gastric conditions was evaluated using SGF (3.2 mg/mL pepsin in 0.03 M NaCl). Encapsulated and non-encapsulated IgY were measured for antibody activity after 2 h of incubation in SGF. Non-encapsulated was neutralized with 2 M Tris–HCl (pH 8.0). The microcapsules were filtered, and IgY was released in SIF. Antibody activity was defined as the ability of the anti-ETEC IgY to bind to ETEC and was determined by ELISA. Activities were expressed as a percent relative to an untreated positive control (100 % activity).
Results
Characterization of pH-sensitive hydrogels
Particle size
Table 2 shows the length and diameter of hydrogels incubated in buffers with different pH. Type B hydrogels (0.57 % of crosslinker) have the smallest diameter and length when the particles were incubated for 24 h in a solution with pH 2.2.
Table 2.
Length, diameter, and swelling percentage of hydrogels incubated for 24 h in buffers with different pH
| Hydrogel | pH 2.2 | pH 7.4 | pH 10 | |
|---|---|---|---|---|
| Length (mm) | A | 3.0 ± 0.3b | 5.3 ± 0.6b | 6.3 ± 1.2a |
| B | 1.8 ± 0.6a | 4.3 ± 0.6a | 5.7 ± 0.8a | |
| C | 3.2 ± 0.6b | 5.7 ± 1.5b | 6.0 ± 1.0a | |
| Diameter (mm) | A | 4.6 ± 0.1 | 10.3 ± 1.2 | 11.5 ± 0.9a |
| B | 3.5 ± 0.2a | 9.3 ± 0.6a | 11.8 ± 0.3a | |
| C | 6.3 ± 0.6 | 10.0 ± 0.1 | 11.5 ± 0.1a | |
| Swelling percentage (%) | A | 66.8 ± 1.5b | 99.3 ± 0.1a | 99.5 ± 0.0a |
| B | 57.1 ± 0.8a | 99.2 ± 0.1a | 99.3 ± 0.1a | |
| C | 89.6 ± 9.5c | 99.6 ± 0.1a | 99.6 ± 0.0a |
Data are represented as means ± standard deviation (SD). Different letters indicate significant difference (p < 0.05). Kruskal-Wallis Test, Infostat, 2009
Swelling percentage
Swelling percentage of hydrogels incubated in buffer solution with different pH was evaluated. As it can be seen, the type B hydrogels had the less swelling percentage when it is incubated at pH 2.2, because of that the following assays were do with this type of hydrogels. The swelling percentage markedly increased in high pH solutions (Table 2). The presence of negative charges due to the formation of the carboxylate groups at pH >4.5 produces the incorporation of solvated counter-ions inside the gel. It enhances the swelling of the hydrogel at basic pH compared with neutral or acid conditions.
Incorporation of IgY into the hydrogel
The percentage of IgY retained inside the type B hydrogels when a piece was swelled in a solution of basic pH was assessed. As expected, pH-sensitive hydrogels had a high IgY loading percentage (99.2 ± 12.9 mg IgY/mg hydrogel) at pH 7.4. It means that each gel piece incorporated approximately 8.4 ± 1.1 mg IgY.
In vitro study of IgY release
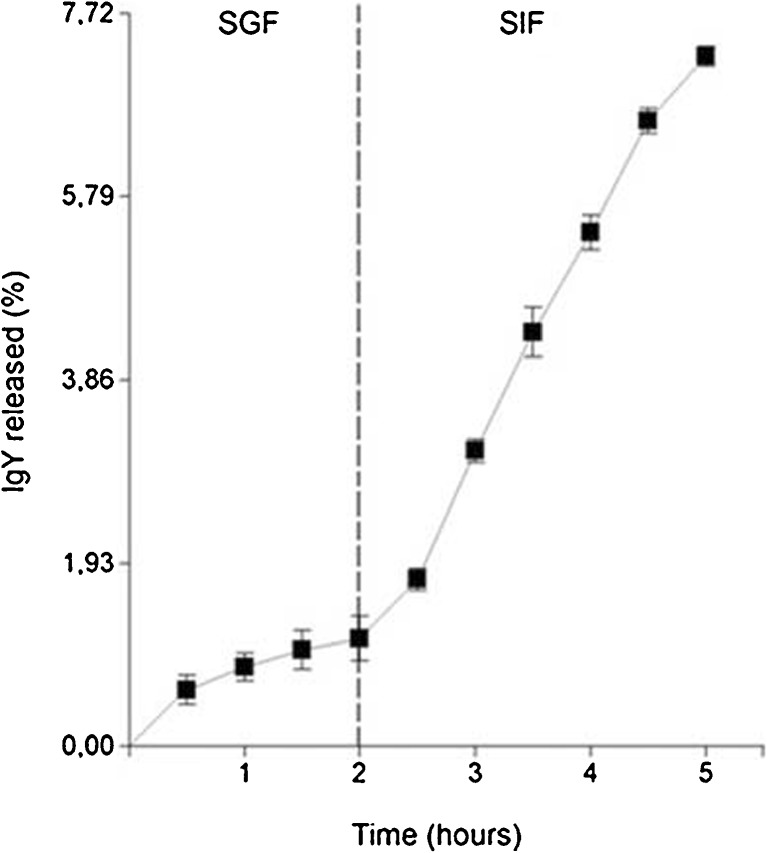
The type B hydrogel has high retention ratio of IgY when it is incubated in SGF but also in SIF. Of the 8.43 mg of IgY incorporated into the gel 0.096 ± 0.019 mg of IgY (~1.13 %) were released in SGF and 0.613 ± 0.008 mg of IgY (~7.27 %) were released in SIF (Fig. 1).
Fig. 1.
IgY release from the hydrogels; samples were first incubated in simulated gastric fluid (SGF) and then were transferred to simulated intestinal fluid (SIF). Data are presented as mean ± SD (n=3)
Interaction between hydrogels and IgY
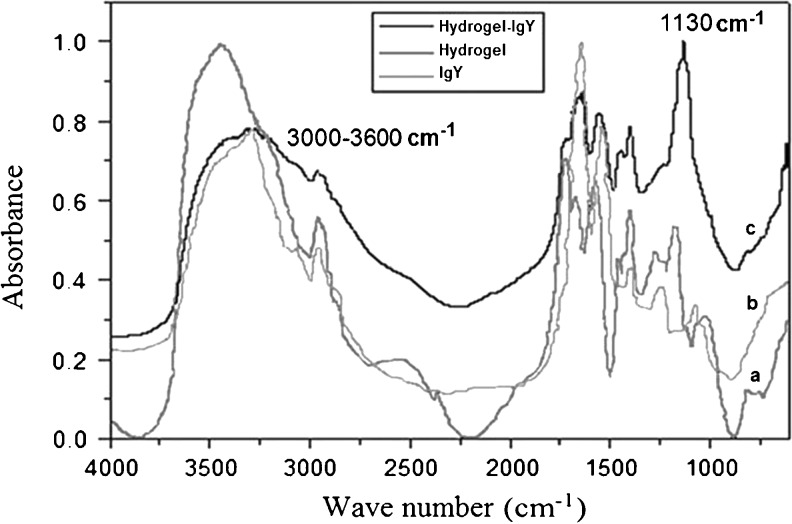
The type of interaction between IgY and type B hydrogels was studied by FT-IR spectroscopy (Fig. 2).
Fig. 2.
The FT-IR spectra of: a hydrogel, b IgY, and c hydrogel-IgY
In the FT-IR spectrum of hydrogel it is possible to observe the broad band at 3,437 cm−1 corresponding to N-H stretching of the secondary amides. Also, bands at 2,974 and 2,881 cm−1 are assigned to the –CH3 symmetric and asymmetric stretching. The band at 1,670 cm−1 is due to the C = O stretching vibration of the amide I band. The amide II band that appears at 1,576 cm−1 is attributed to the N-H bending motion.
Figure 2 of the IR spectra also shows a broaden peak at 3,000–3,600 cm−1 for hydrogel-IgY compared to the respective peaks of hydrogel alone and IgY alone and an intensive peak at 1,130 cm−1 for hydrogel-IgY disproportional higher compared to the respective peaks of IgY alone and to hydrogel alone. All the above are evident that the immunoglobulin interacts with the hydrogel in its network with extended hydrogen bonds.
In vitro stability of IgY to simulated gastric conditions
The stability of IgY in SGF was improved by the incorporation into the hydrogels (Fig. 3). Non-encapsulated IgY was highly hydrolyzed after the incubation in SGF, with a remanent antibody activity of 14.33 %. The activity of the IgY incorporated to the hydrogels was significantly higher (87 %).
Fig. 3.
In vitro stability of non-encapsulated and microencapsulated IgY to simulated gastric conditions. The retaining IgY activity was measured by ELISA and was expressed as % activity relative to an untreated sample. Data are presented as mean ± SD (n = 3)
Discussion
Oral administration of IgY has proved successful for treatment of a variety of gastro-intestinal infections (Mine and Kovacs-Nolan 2002). However, inactivation of IgY with the gastric fluids may reduce the therapeutic value of IgY. An ideal oral delivery system for IgY should have a high loading capacity, protect IgY from gastric inactivation, and rapid sustain release in the small intestine. In the present study, hydrogel particles were evaluated as a method of encapsulation for IgY.
The equilibrium swelling values for the hydrogels used in this study were pH responsive, showing less swelling at low pH and greater swelling at basic pH. This is because more hydrogen bonds are present at low pH and more electrostatic repulsion at high pH. This swelling is due to the ionization/deionization of carboxyl groups. At a low pH (pka > pH), the carboxyl groups are non-ionized; therefore, the mesh is in a collapsed state. At high pH values (pka < pH), carboxyl groups repel each other, causing the swelling of the system (Molina et al. 2010). These results agree with reported swelling data of similar hydrogels (Zhang et al. 2005).
A high percentage of IgY was retained inside the hydrogels when a piece was swelled in a solution of IgY at basic pH. As expected, pH-sensitive hydrogels retain a significant amount of IgY at high pH, when the matrix has negative charges. More than 80 % of the IgY was retained inside the hydrogel when it was contacted with an excess of solution. These results agree with Dhakar et al. (2010) using chitosan, poly vinylpyrrolidone, and polyacrylic acid as polymers and as crosslinking agents glutaraldehyde and N,N’-methylenebisacrylamide.
Depending on the composition of hydrogel (type of polymer, type of drug and additives), geometry (size and shape), preparation technique and environmental conditions during drug release, one or more of the following physical and chemical phenomena affect the drug release kinetics (Zarzycki et al. 2010). In this study, at low pH range of the simulated gastric conditions, the hydrogels have a low equilibrium degree of swelling. The degree of swelling increases with the pH of simulated intestinal conditions. In the SIF, the gels have reached a high degree of swelling but cannot release all the IgY from the hydrogel. Because of that, the interaction between IgY and polymers network was studied by FT-IR spectroscopy. The IgY spectrum shows to be similar to that found in a study by Wang et al. (2012) on the stability of modified IgY. The FTIR spectrum of a murine IgG2a monoclonal antibody studied by Picquart and Haro-Poniatowski (1999) also shows notable similarity. The comparison of the spectrum of pure polymer, IgY and the composite hydrogel-IgY, shows that the IgY interacts with the hydrogel in its network with extended hydrogen bonds.
The stability of IgY under various processing and physiological conditions is an important consideration for passive immunotherapy applications. After evaluate the stability of IgY in SGF, it was found that non-encapsulated IgY was extremely sensitive to gastric conditions and maintained only 14 % of its biological activity while the IgY protected by the hydrogel retained a 85 % of its activity. These results are in concordance with Li et al. (2009) who found that the stability of IgY in simulated gastric fluid was greatly improved by encapsulation in chitosan–alginate microcapsules, and greater than 70 % of IgY activity was retained after 2 h exposure to simulated gastric fluid. Similarly, Cho et al. (2005) found that the residual IgY activity of microencapsulated IgY decreased 20 % after 30 min in SGF but non-encapsulated IgY showed an 80 to 90 % reduction in residual IgY activity. Kovacs-Nolan and Mine (2005) reported that microencapsulated IgY retain an 95 % of it activity after 6 h of incubation in SGF.
Conclusions
The present study demonstrates that hydrogel particles can efficiently incorporate and protect the IgY from simulated gastric conditions but cannot show a complete release of IgY in SIF probably due to hydrophobic interactions with the polymer network. Additional study will be needed before encapsulation of IgY with pH-sensitive hydrogels can be applied under commercial conditions.
Acknowledgments
The authors wish to thank CONICET. This work was supported by grants from Ministerio de Ciencia y Tecnología de Córdoba (MinCyT), Agencia Nacional de Promoción Científica y Tecnológica (FONCYT) and Secretaría de Ciencia y Técnica de la Universidad Nacional de Río Cuarto (SECYT-UNRC).
References
- Akita EM, Nakai S. Immunoglobulins from egg yolk: isolation and purification. J Food Sci. 1992;57:629–634. doi: 10.1111/j.1365-2621.1992.tb08058.x. [DOI] [Google Scholar]
- Andersen FA. Cosmetic ingredient review expert panel. Amended final report on the safety assessment of polyacrylamide and acrylamide residues in cosmetics. Int J Toxicol. 2005;24(2):21–50. doi: 10.1080/10915810590953842. [DOI] [PubMed] [Google Scholar]
- Bellingeri RV, Busso L, Alustiza F, Picco N, Molinero D, Vivas A. Characterization of egg yolk immunoglobulin (IgY) against enterotoxigenic escherichia coli and evaluation of its effects on bovine intestinal cells. Afr J Microbiol Res. 2013;7(5):398–405. [Google Scholar]
- Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chalghoumi R, Thewis A, Beckers Y, Marcq C, Portetelle D, Schneider YJ. Adhesion and growth inhibitory effect of chicken egg yolk antibody (IgY) on salmonella enterica serovars enteritidis and typhimurium in vitro. Foodborne Pathog Dis. 2009;6:593–604. doi: 10.1089/fpd.2008.0258. [DOI] [PubMed] [Google Scholar]
- Chang HM, Lee YC, Chen CC, Tu YY. Microencapsulation protects immunoglobulin in yolk (IgY) specific against Helicobacter pylori urease. J Food Sci. 2002;67:15–20. doi: 10.1111/j.1365-2621.2002.tb11351.x. [DOI] [Google Scholar]
- Cho YH, Lee JJ, Park IB, Huh CS, Baek YJ, Park J. Protective effect of microencapsulation consisting of multiple emulsification and heat gelation processes on immunoglobulin in yolk. J Food Sci. 2005;70:148–151. doi: 10.1111/j.1365-2621.2005.tb07088.x. [DOI] [Google Scholar]
- Dhakar RC, Gupta AK, Maurya SD, Singh RD. pH-sensitive interpenetrating hydrogel for eradication of Helicobacter pylori. Int J Pharm Sci Nanotechnol. 2010;3:924–932. [Google Scholar]
- Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2008) InfoStat, versión 2008, Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina
- Feng Y, Liu W, Shi D. Effectiveness of egg yolk antibody against Shiga toxin II variant toxicity in vitro and in vivo. Curr Microbiol. 2013;67:448–453. doi: 10.1007/s00284-013-0384-8. [DOI] [PubMed] [Google Scholar]
- Gin H, Dupuy B, Bonnemaison-Bourignon D, Bordenave L, Bareille R, Latapie MJ, Baquey C, Bezian JH, Ducassou D. Biocompatibility of polyacrylamide microcapsules implanted in peritoneal cavity or spleen of the rat. Effect on various inflammatory reactions in vitro. Biomater Artif Cells Artif Organs. 1990;18(1):25–42. doi: 10.3109/10731199009117287. [DOI] [PubMed] [Google Scholar]
- Gupta P, Vermani K, Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today. 2002;7:569–579. doi: 10.1016/S1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- Hoffman AS. Hydrogels in biomedical applications. Adv Drug Deliv Rev. 2002;43:3–12. doi: 10.1016/S0169-409X(01)00239-3. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, Lale S, Ramesh NG, Koul V. Synthesis and characterization of positively charged interpenetrating double-network hydrogel matrices for biomedical applications. React Funct Polym. 2013;73(11):1493–1499. doi: 10.1016/j.reactfunctpolym.2013.07.003. [DOI] [Google Scholar]
- Karadaǧ E, Saraydin D, Çetinkaya S, Güven O. In vitro swelling studies and preliminary biocompatibility evaluation of acrylamide-based hydrogels. Biomaterials. 1996;17(1):67–70. doi: 10.1016/0142-9612(96)80757-5. [DOI] [PubMed] [Google Scholar]
- Kovacs-Nolan J, Mine Y. Microencapsulation for the gastric passage and controlled intestinal release of immunoglobulin Y. J Immunol Methods. 2005;296:199–209. doi: 10.1016/j.jim.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Kovacs-Nolan J, Mine Y. Egg yolk antibodies for passive immunity. Annu Rev Food Sci Technol. 2012;3:163–182. doi: 10.1146/annurev-food-022811-101137. [DOI] [PubMed] [Google Scholar]
- Li XY, Jin LJ, McAllister TA, Stanford K, Xu JY, Lu YN, Zhen YH, Sun YX, Xu YP. Chitosan–alginate microcapsules for oral delivery of egg yolk immunoglobulin (IgY) J Agric Food Chem. 2007;55:2911–2917. doi: 10.1021/jf062900q. [DOI] [PubMed] [Google Scholar]
- Li XY, Jin LJ, Uzonna JE, Li SY, Liu JJ, Li HQ, Lu YN, Zhen YH, Xu YP. Chitosan–alginate microcapsules for oral delivery of egg yolk immunoglobulin (IgY): in vivo evaluation in a pig model of enteric colibacillosis. Vet Immunol Immunopathol. 2009;129:132–136. doi: 10.1016/j.vetimm.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Mine Y, Kovacs-Nolan J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a review. J Med Food. 2002;5:159–169. doi: 10.1089/10966200260398198. [DOI] [PubMed] [Google Scholar]
- Molina MA, Rivarola CR, Barbero CA. Evidence of hydrophobic interactions controlling mobile ions release from smart hydrogels. Mol Cryst Liquid Cryst. 2010;521:265–271. doi: 10.1080/15421401003722732. [DOI] [Google Scholar]
- Nilsson E, Kollberg H, Johannesson M, Wejåker P, Carlander D, Larsson A (2007) More than 10 Years’ continuous oral treatment with specific immunoglobulin Y for the prevention of pseudomonas aeruginosa infections: a case report. J Med Food 375–378 [DOI] [PubMed]
- Picquart M, Haro-Poniatowski E. Study of a murine IgG2a monoclonal antibody by vibrational spectroscopies. Rev Mex Fis. 1999;45:459–465. [Google Scholar]
- Risbud MV, Bhonde RR (2000) Polyacrylamide-Chitosan Hydrogels: in vitro biocompatibility and sustained antibiotic release studies. Drug Deliv 7(2):69–75 [DOI] [PubMed]
- Rose ME, Orlans E, Buttress N. Immunoglobulin classes in the hen’s egg: their segregation in yolk and white. Eur J Immunol. 1974;4:521–523. doi: 10.1002/eji.1830040715. [DOI] [PubMed] [Google Scholar]
- Saraydin D, Unver-Saraydin S, Karadag E, Koptagel E, Guven O. In vivo biocompatibility of radiation crosslinked acrylamide copolymers. Nucl Instrum Meth Phys Res Sect B. 2004;217(2):281–292. doi: 10.1016/j.nimb.2003.09.035. [DOI] [Google Scholar]
- Schade R, Zhang X, Terzolo H (2007) Use of IgY Antibodies in Human and Veterinary Medicine. In: Huopalahti R, López-Fandiño R, Anton M & Schade R (Eds.) Bioactive Egg Compounds. Springer, pp. 213–219
- Shimizu M, Miwa HK, Goto A. Encapsulation of chicken egg yolk immunoglobulin G (IgY) by liposomes. Biosci Biotechnol Biochem. 1993;57:1445–1449. doi: 10.1271/bbb.57.1445. [DOI] [PubMed] [Google Scholar]
- Torche AM, Le Dimna M, Le Corre P, Mesplede A, Le Gal S, Cariolet R, Le Potier MF. Immune responses after local administration of IgY loaded-PLGA microspheres in gut-associated lymphoid tissue in pigs. Vet Immunol Immunopathol. 2006;109:209–217. doi: 10.1016/j.vetimm.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Vega CG, Bok M, Vlasova AN, Chattha KS, Fernández FM, Wigdorovitz A, Parreño VG. IgY antibodies protect against human rotavirus induced diarrhea in the neonatal gnotobiotic piglet disease model. PLoS ONE. 2012;7(8):e42788. doi: 10.1371/journal.pone.0042788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ma M, Huang Q, Shi X. Study on the stability of chicken Egg yolk immunoglobulin (IgY) modified with mPEG. Spectrosc Spectr Anal. 2012;32:2501–2507. [PubMed] [Google Scholar]
- Wenger Y, Schneider RJ, II, Reddy GR, Kopelman R, Jolliet O, Philbert MA. Tissue distribution and pharmacokinetics of stable polyacrylamide nanoparticles following intravenous injection in the rat. Toxicol Appl Pharmacol. 2011;251(3):181–190. doi: 10.1016/j.taap.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TH. Recent applications of polyacrylamide as biomaterials. Recent Patents Mater Sci. 2008;1(1):29–40. doi: 10.2174/1874464810801010029. [DOI] [Google Scholar]
- Zarzycki R, Modrzejewska Z, Nawrotek K. Drug release from hydrogel matrices. Chemia i Inżynieria Ekologiczna S/Ecol Chem Eng S. 2010;17(2):117–136. [Google Scholar]
- Zhang YX, Wu FP, Li MZ, Wang EJ. pH switching on-off semi-IPN hydrogel based on cross-linked poly(acrylamide-co-acrylic acid) and linear polyallyamine. Polymer. 2005;46:7695–700. doi: 10.1016/j.polymer.2005.05.121. [DOI] [Google Scholar]