Abstract
Fish skin, a by-product from fish processing industries, still contains a significant amount of protein-rich material. Gelatin was extracted from Nile tilapia skin with the yield 20.77 ± 0.80 % wet weight. Gelatin was then separately hydrolyzed by proteases, including bromelain, papain, trypsin, flavourzyme, alcalase and neutrase. Low molecular weight gelatin hydrolysate (<10 kDa) has a great potential as an antioxidant agent. Flavourzyme hydrolysate has potent activity on ABTS radical scavenging (1,413.61 ± 88.74 μg trolox/mg protein) and also inhibits the oxidation of linoleic acid at a high level (59.74 ± 16.57 % inhibition). The greatest reducing power is in alcalase hydrolysate (4.951 ± 1.577 mM trolox/mg protein). While, bromelain hydrolysate has the highest ferrous ion chelating activity (86.895 ± 0.061 %). Evaluation of the angiotensin-I-converting enzyme’s inhibitory activity indicates that all hydrolysates have great potency as an antihypertensive agent. All studied tilapia skin gelatin hydrolysates contain potent antioxidant and anti-hypertensive effects.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-014-1581-6) contains supplementary material, which is available to authorized users.
Keywords: Nile tilapia skin, Gelatin hydrolysate, ACE inhibitory activity, Antioxidant effect
Introduction
Hypertension or high blood pressure, a serious condition of cardiovascular disease, becomes a common health problem in the world (Zhou et al. 2013). It is estimated that one third of the Western population suffers from this condition (Hernández-Ledesma et al. 2011). Hypertension is also associated with myocardial infarction, coronary heart disease, stroke, kidney failure, heart failure and vascular dementia (Sharp et al. 2011). The vital system that controls blood pressure is the renin-angiotensin system (RAS). In this system, the main regulators are renin and an angiotensin-I-converting enzyme (ACE) (Fernández-Musoles et al. 2013; He et al. 2013). Many researchers have studied the enzymatic hydrolysates of various marine by-products for their anti-hypertensive activity, such as Hoki skin (Mendis et al. 2005), squid skin (Alemán et al. 2011b) and Pacific cod skin (Himaya et al. 2012).
Nile tilapia, Oreochromis niloticus, is one of the popular species grown in freshwater aquaculture. Hence, the demand of tilapia in all forms is increasing dramatically in the global market (Fitzsimmons 2004). The main tilapia producers in Asia are China, the Philippines, Thailand and Taiwan (El-Sayed 2006). However, the more tilapia processing industries increase, the more by-products are produced. More than 60 % of the by-products generated from fish processing industry are waste. This includes skin, head, fins and bones (Dekkers et al. 2011) and these by-products still contain a significant amount of protein-rich material (Hsu 2010). Fish skin is a rich source of collagen and gelatin. After being heated to 45 °C or higher, collagen can convert to gelatin (Gόmez-Guillén et al. 2011). Enzymatic hydrolysis of fish gelatin results in production of bioactive peptides without organic solvents or toxic chemical residuals (Vercruysse et al. 2005). These peptides commonly consist of 2–20 amino acids. The composition and structure also affect their bioactivity, including their inhibitory effect against angiotensin-I-converting enzymes (ACE) and their antioxidant, immunomodulatory and antimicrobial activities (Raghavan and Kristinsson 2009; Thiansilakul et al. 2007; Zhang et al. 2008).
This study focused on comparison of antioxidant and anti-hypertensive activities of bioactive peptides prepared from tilapia skin gelatin that was hydrolyzed by different proteases.
Materials and methods
Materials
Nile tilapia (Oreochromis niloticus) skin was obtained from a farm under Charoen Pokphand Group in Doi Tao district, Chiang Mai, Thailand. Alcalase (30079), bromelain (B4882), papain (P4762), flavourzyme, protease from Aspergillus oryzae (P6110), neutrase, protease from Bacillus amyloliquifaciems (P1236), trypsin (T8003), ACE from rabbit lung (A6778) and hippuryl-histidyl-leucine (859052), as a substrate of ACE, were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Other reagents used in this study were of standard laboratory grade.
Gelatin extraction and hydrolysis
The tilapia skin was washed with tap water and then cut into small squares (1 × 1 cm) by knife. The gelatin extraction method was slightly modified from that of Songchotikunpan et al. (2008). To remove noncollagenous proteins, the small pieces of tilapia skin were soaked in 0.8 M NaOH, with the skin:solution ratio of 1:7 (w/v), for 2 h. This solution was changed when it became dark. These alkaline treated skins were then washed with tap water approximately 1 h. The treated skins were then soaked in 0.5 % (v/v) HCl for 1 h, with the skin:solution ratio of 1:7 (w/v), and then washed with tap water until a neutral pH of the wash water was obtained. Gelatin was extracted from washed fish skins added with distilled water (1:2 w/v) by heating at 60 °C for 1.5 h. The resulting gelatin solution was then filtered through double layer cheese cloth and lyophilized. The freeze-dried gelatin was dissolved in 0.1 M sodium phosphate buffer and hydrolyzed separately by six different protease enzymes under the previously reported conditions: alcalase (pH 7.0, 50 °C), bromelain (pH 7.0, 55 °C), flavourzyme (pH 7.0, 50 °C), neutrase (pH 8.0, 50 °C), papain (pH 6.0, 37 °C) and trypsin (pH 8.0, 37 °C) (Song et al. 2008; Zhao et al. 2009; Alemán et al. 2011a; Li-Chan et al. 2012). The ratio for the enzyme:substrate was 1:100 (w/w). After incubation for 4 h, the mixtures were boiled for 10 min to deactivate the enzymes and determined their degree of hydrolysis (DH).
Determination of degree of hydrolysis
The DH of each hydrolysate was determined by the Benjakul and Morrissey (1997) method. A 0.2125 M phosphate buffer (2.0 mL, pH 8.2) was mixed with aliquot of each sample (125 μL), followed by a 0.01 % 2,4,6-Trinitrobenzenesulfonic acid (TNBS) solution (1.0 mL). The mixtures were incubated at 50 °C for 30 min in the dark and 0.1 M sodium sulfite (2.0 mL) was added to stop the reaction. Then, the mixtures were cooled for 15 min. Absorbance was measured at 420 nm using a spectrophotometer (Genesys 20, Thermo Fischer Scientific, Massachusetts, United States).
Fractionation of gelatin hydrolysate
Gelatin hydrolysate was fractionated by using Amicon® Ultra-15 centrifugal filter devices with MWCO 10,000 Da. These devices were centrifuged at 3,000 × g for 30 min and the filtrates were collected to determine their antioxidant and anti-hypertensive activities.
Determination of antioxidant activities
ABTS radical scavenging assay
This assay is based on the reduction of the 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid), ABTS, radical cation by antioxidants (Arts et al. 2004). Briefly, 7 mM of ABTS solution was mixed with 2.45 mM potassium persulfate in the ratio of 1:1 (v/v) and kept in the dark for 16–19 h. The ABTS˙ solution was then diluted with Milli-Q water to give an A734 around 0.700 ± 0.200 before further use. Then, 980 μL of ABTS˙ was added to 20 μL of sample and left at room temperature for 10 min in the dark. The absorbance was measured at 734 nm.
Ferric reducing antioxidant power (FRAP) assay
The FRAP method was slightly modified from that described by Griffin and Bhagooli (2004). The working FRAP reagent was freshly prepared by mixing 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ), FeCl2 and 300 mM acetate buffer pH 3.6 in the ratio of 1:1:10 and incubated at 37 °C for 30 min. Sample (100 μL) was mixed with 900 μL of FRAP reagent and kept in the dark for 10 min. The absorbance was measured at 593 nm.
Ferrous ion chelating activity assay
The principle of this method is based on the ability of test samples to trap the Fe2+ ion. This assay was slightly modified from the method described by Benjakul et al. (2005). A 200 μL sample was mixed with 800 μL of distilled water and then 100 μL of 20 mM FeSO4 and 200 μL of 5 mM ferrozine were added. After leaving at room temperature for 20 min, the ferrous ion-ferrozine complex formed was measured the absorbance at 562 nm. The chelating activity percentage was calculated from this formula:
Inhibition of lipid peroxidation in linoleic acid system
This method was slightly modified from that of Yang et al. (2009). The system mixture was composed of 0.5 mL of sample, 2.5 mL of 2 mg/mL linoleic acid in absolute ethanol, and 2 mL of 0.2 M potassium phosphate buffer pH 7.0. The mixture was incubated at 37 °C for 5 days in the dark. After that, 0.1 mL aliquot was added to 4.7 mL of 75 % ethanol and followed by 0.1 mL of 30 % ammonium thiocyanate and 0.1 mL of 20 mM FeCl2. The absorbance was measured at 500 nm after 3 min, and the inhibition percentage was calculated using the following formula:
Measurement of ACE inhibitory activity
The ACE inhibitory activity was evaluated by the modified method of Park et al. (2003). A solution containing the sample and 25 mU/mL ACE (50 μL each) was pre-incubated at 37 °C for 10 min. Then 100 μL of substrate (6 mM hippuryl-histidyl-leucine (HHL) in 50 mM Tris with 300 mM NaCl) was added and incubated further for 30 min. HCl (1.0 M, 200 μL) was added to stop reaction. Ethyl acetate (600 μL) was added to extract hippuric acid, followed by centrifugation at 4,000 × g for 15 min. The supernatant (200 μL) was transferred to a test tube and evaporated at 95 °C to remove the ethyl acetate. Distilled water (1.0 mL) was added to dissolve the hippuric acid and the absorbance was determined at 228 nm using a UV/VIS spectrophotometer (Lambda25, Perkin Elmer, Massachusetts, United States). The ACE inhibition was calculated from this equation:
Statistical analysis
All data are averages of triplicates. Standard deviation was also calculated. Significant differences (p < 0.05) were calculated using the SPSS statistic program (Version 17.0) with one-way ANOVA analysis.
Results and discussions
Gelatin extraction and degree of hydrolysis
The yield of tilapia skin gelatin was 20.77 ± 0.80 % wet weight basis which was higher than that previously reported by Zhuang and Sun (2011). Six proteases, alcalase, bromelain, flavourzyme, neutrase, papain and trypsin, were chosen to hydrolyze the gelatin. After 4 h of hydrolysis, the highest DH was observed in papain (59.55 ± 0.014 %) followed by flavourzyme (56.61 ± 0.011 %), bromelain (50.59 ± 0.024 %), and trypsin (44.55 ± 0.010 %). The hydrolysates from neutrase and alcalase had the lowest DH among the six hydrolysates, these being 15.71 ± 0.016 and 16.05 ± 0.014 %, respectively. Ovissipour et al. (2013) reported the DH of hydrolyzed proteins from a whole anchovy sprat (Clupeonella engrauliformis) with alcalase, flavourzyme, papain and bromelain. Those were 55.8 ± 0.56, 30.5 ± 2.1, 49.74 ± 2.6 and 43.05 ± 2.7 %, respectively. The difference of DH for each enzyme may result from the different amino acid sequences of gelatin in tilapia skin and anchovy sprat.
Antioxidant activities of each hydrolysate
Each hydrolysate fraction (MW < 10 kDa) was tested for its antioxidant activities by ABTS, lipid peroxidation methods, FRAP, and ferrous ion chelating. These four methods form two groups on the basis of their oxidizing reagents: organic radical producers (ABTS and Lipid peroxidation) and metal ions (FRAP and ferrous ion chelating). Another difference among these tests is the reaction procedure. Lipid peroxidation assay determines the delay of radical generation, as well as the radical scavenging ability. The other three assays analyze the ability to reduce radical cations or ferric ions. For ABTS, the highest activity occurred in flavourzyme, and trypsin. Papain and neutrase had the lowest ABTS activity. No ABTS activity was observed in the alcalase hydrolysate within 10 min (Table 1). Alemán et al. (2011a, b) determined the antioxidant activity of squid and tuna skin hydrolysates and found that the ABTS activity decreased in the case of alcalase, collagenase, trypsin and pepsin hydrolysates of both squid and tuna. Ovissipour et al. (2013) reported that the whole anchovy sprat protein hydrolysate with alcalase had the greatest ABTS activity, while flavourzyme showed the lowest activity among all hydrolysates. This difference may arise from the different DH of alcalase in each study and from the different starting materials. In our study, alcalase hydrolysate from tilapia skin had a DH of 16.05 ± 0.014 % compared to those of alcalase hydrolysate from squid and anchovy sprat, which were 30.9 ± 0.6 and 55.8 ± 0.56 %, respectively (Alemán et al. 2011a; Ovissipour et al. 2013).
Table 1.
Antioxidative properties of tilapia skin gelatin hydrolysates by ABTS and inhibition of lipid peroxidation methods
| Hydrolysate | ABTS | Lipid peroxidation |
|---|---|---|
| (μg trolox/mg protein) | (%inhibition) | |
| Bromelain | 1,072.35 ± 29.54a | 56.61 ± 13.26a,b |
| Flavourzyme | 1,413.61 ± 88.74b | 59.74 ± 16.57a,b |
| Trypsin | 1,316.42 ± 43.93b | 56.71 ± 7.16a,b |
| Alcalase | ND | 29.22 ± 8.26c |
| Papain | 256.02 ± 121.86c | 41.56 ± 1.22b,c |
| Neutrase | 130.25 ± 17.37c | 29.01 ± 14.08c |
| Non-hydrolyzed | 10.65 ± 1.30d | 7.12 ± 4.18e |
ND not detectable
Different letters represent significant differences (p < 0.05)
In the linoleic acid model system, the hydrolysates that exhibited more than 50 % lipid peroxidation inhibition were flavourzyme (59.74 ± 16.57 %), trypsin (56.71 ± 7.16 %), and bromelain (56.61 ± 13.26 %), which were not significantly different (p > 0.05) from the positive control, 4 mM trolox (70.30 ± 7.27 %). While, alcalase and neutrase hydrolysates had low levels of inhibiting activity (less than 50 %). The inhibition of non-hydrolyzed gelatin was 7.12 ± 4.18 %. Yang et al. (2009) reported that tilapia retorted skin gelatin hydrolysate could reduce the peroxidation rates of linoleic acid and had an inhibitory effect on lipid peroxidation (77.3 %) similar to 10 ppm BHA.
Iron is an important ion because it has an ability to generate free radicals from Fenton reaction. Fenton reaction is the reaction between ferrous salts and peroxides that produce hydroxyl radicals. The attack of hydroxyl radicals can result in an initiation of lipid peroxidation in human body. Minimization of the Fe2+ concentration in the Fenton reaction provides protection against oxidative damage (Borah et al. 2011). FRAP and ferrous ion chelating results are presented in Table 2. In the FRAP assay, the highest reducing power was observed from alcalase hydrolysate and the lowest was noticed in bromelain. Non-hydrolyzed gelatin was negligible in reducing FRAP reagent. In Arca subcrenata case, the neutrase hydrolysate displayed the most reductive ability and alcalase was the second best (Song et al. 2008). Alemán et al. (2011b) reported that the highest FRAP values occurred in squid skin gelatin hydrolyzed with alcalase and in tuna skin gelatin hydrolyzed with trypsin. Conversely, anchovy sprat hydrolysate prepared by bromelain exhibited the highest reducing power and the bottom three was observed in flavourzyme, alcalase and papain hydrolysates, respectively (Ovissipour et al. 2013).
Table 2.
Ferric reducing antioxidant power and iron chelating activity of different gelatin hydrolysates from nile tilapia skin
| Hydrolysate | FRAP | Ferrous ion chelating |
|---|---|---|
| (mM trolox/mg protein) | (%chelating activity) | |
| Bromelain | 2.840 ± 3.237a | 86.895 ± 0.061a |
| Flavourzyme | 4.266 ± 6.120a,b | 85.092 ± 0.118a |
| Trypsin | 3.469 ± 2.829a,b | 83.607 ± 0.035a |
| Alcalase | 4.951 ± 1.577b | 77.275 ± 0.048a |
| Papain | 3.477 ± 1.396a,b | 15.783 ± 0.016b |
| Neutrase | 4.146 ± 1.133a,b | 83.426 ± 0.014a |
| Non-hydrolyzed | 0.008 ± 0.001c | 8.338 ± 0.681c |
Different letters represent significant differences (p < 0.05)
All gelatin hydrolysates manifested the great ferrous chelation, being higher than 75 %. However, the papain hydrolysate having only 15.783 ± 0.016 % of ferrous chelation (p < 0.05). In whole anchovy sprat hydrolysates, the maximum percentage occurred in bromelain hydrolysate (52.5 ± 1.0 %), followed by those in papain (51.1 ± 1.2 %), promod (48.1 ± 1.4 %), alcalase (40.4 ± 1.4 %), protamax (30.9 ± 0.8 %), and flavourzyme (19.9 ± 1.0 %) (Ovissipour et al. 2013).
These results suggested that hydrolysates catalyzed by different enzymes exhibited distinct levels of antioxidant activity. These different levels of antioxidant activity may contribute from the amino acid sequences in the gelatin peptides. The bioactive mechanisms of such peptides are not well understood and only a few studies have been made concerning the structure-activity relationship (Schmelzer et al. 2007, Hernández-Ledesma et al. 2011). Some studies have indicated which protease should be chosen to produce the desired fragment according to the effect required (Tavano 2013). In addition, most researchers agree that the presence of different amino acids in the peptides play an important role in the antioxidant properties, so that no single antioxidant mechanism can represent the overall antioxidant activity of the peptides. For instance, hydrophobic amino acid-rich peptides are expected to inhibit lipid peroxidation, acting as proton donors to the hydrophobic peroxyl radicals and as metal ion chelators. In this sense, peptides containing histidine in their sequences have been reported to act as metal ion chelators, perhaps because of their ring structure characteristic (Alemán et al. 2011b).
Antihypertensive effect of gelatin hydrolysates
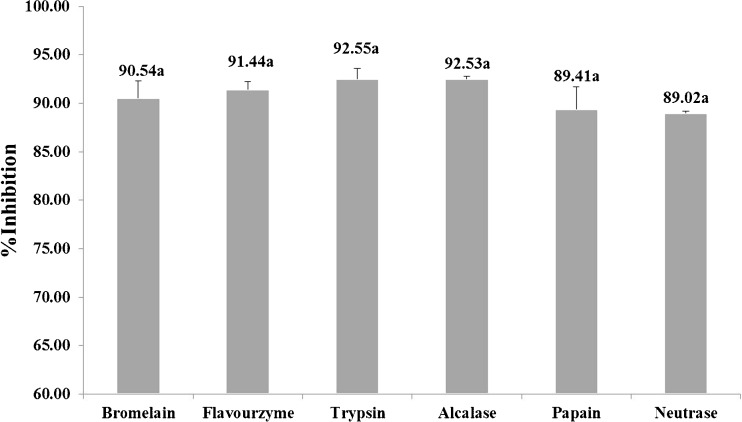
The gelatin hydrolysates were evaluated for their anti-hypertensive activity. All hydrolysates exhibited great anti-hypertensive activity, ranging from 89.02–92.55 % (Fig. 1). The inhibition percentages of the six enzyme hydrolysates were not statistically different. No inhibition activity was noticed in non-hydrolyzed gelatin. It was previously reported that hydrolysate from squid skin gelatin and porcine skin collagen could effectively lower blood pressure in vivo (Ichimaru et al. 2009; Lin et al. 2012). Zhao et al. (2009) hydrolyzed sea cucumber body wall protein sequentially with bromelain and alcalase and found that protein hydrolysate with a molecular weight lower than 2 kDa had high ACE inhibition activity. Vo et al. (2011) evaluated the ACE inhibitory activities of Nile tilapia gelatin using alcalase, pronase E, pepsin and trypsin. High ACE inhibitory activity also occurred in the alcalase hydrolysate, being higher than other specific (pepsin and trypsin) and nonspecific (pronase E) proteases.
Fig. 1.
Antihypertensive effect of from tilapia skin gelatin hydrolysates from different proteases. Same letter above each bar indicates statistically insignificant difference (p < 0.05)
Tavano (2013) suggested that peptides that possess an ACE inhibitory effect usually are small fragments composed of 2 to 12 amino acids. Many studies indicate that tripeptide residues are an essential part of competitive binding at the active site of ACE. In addition, the hydrophobic, or positively charged, amino acids at C-terminal are commonly found in the most effective ACE inhibitory peptides. In this context, this helps explaining why anti-hypertensive peptide production is usually prepared by pepsin or trypsin hydrolysis. Pepsin favors the cleavage between hydrophobic residues, whereas, Arg- and Lys- cleavage generally occur with trypsin hydrolysis. However, the mechanisms involved in the ACE inhibitory effect should be further investigated.
Conclusion
The results of this study suggest that trypsin and flavourzyme hydrolysate with a molecular weight lower than 10 kDa has potent ACE inhibitory activity and is a potent antioxidative agent. Bromelain, flavourzyme and trypsin hydrolysates exhibited great antioxidant properties in all assays. Both trypsin and flavourzyme hydrolysates have great anti-hypertensive activity. The gelatin hydrolysate from tilapia skin could be useful as a potential functional anti-hypertensive agent. Nevertheless, the structure of tilapia skin peptides must be studied further for elucidating their effects on anti-hypertensive activity.
Electronic supplementary material
(PDF 401 kb)
(PDF 485 kb)
(PDF 748 kb)
Acknowledgments
The authors thank the National Research Council of Thailand and the Graduate School of Chiang Mai University for the financial support.
Conflict of Interest
All listed authors have nothing to disclose.
Author Contributions
All experiments were mainly carried out by SC, a Ph.D. candidate, under supervision of HN. While SJ, NR and NS are co-supervisors of SC. The primary supporting grant (National Research Council of Thailand) was given to SJ.
References
- Alemán A, Giménez B, Montero P, Gόmez-Guillén MC. Antioxidant activity of several marine skin gelatins. LWT – food. Sci Technol. 2011;44:407–413. [Google Scholar]
- Alemán A, Pérez-Santín E, Bordenave-Juchereau S, Arnaudin I, Gómez-Guillén MC, Montero P. Squid gelatin hydrolysates with antihypertensive, anticancer and antioxidant activity. Food Res Int. 2011;44:1044–1051. doi: 10.1016/j.foodres.2011.03.010. [DOI] [Google Scholar]
- Arts MJTJ, Haenen GRMM, Voss HP, Bast A. Antioxidant capacity of reaction products limits the applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) assay. Food Chem Toxicol. 2004;42:45–49. doi: 10.1016/j.fct.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Benjakul S, Morrissey MT. Protein hydrolysates from pacific whiting solid wastes. J Agric Food Chem. 1997;45:3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Phongkanpai V, Tanaka M. Antioxidative activity of caramelisation products and their preventive effect on lipid oxidation in fish mince. Food Chem. 2005;90:231–239. doi: 10.1016/j.foodchem.2004.03.045. [DOI] [Google Scholar]
- Borah A, Yadav RNS, Unni BG. In vitro antioxidant and free radical scavenging activity of Alternanthera sessilis. Int J Pharm Sci Res. 2011;2:1502–1506. [Google Scholar]
- Dekkers E, Raghavan S, Kristinsson HG, Marshall MR. Oxidative stability of mahi mahi red muscle dipped in tilapia protein hydrolysates. Food Chem. 2011;124:640–645. doi: 10.1016/j.foodchem.2010.06.088. [DOI] [Google Scholar]
- El-Sayed AM. Tilapia culture. Oxford: CABI publishing; 2006. pp. 1–24. [Google Scholar]
- Fernández-Musoles R, Salom JB, Martínez-Maqueda D, Lόpez-Díez JJ, Recio I, Manzanares P. Antihypertensive effects of lactoferrin hydrolyzates: inhibition of angiotensin and endothelin-converting enzyme. Food Chem. 2013;139:994–1000. doi: 10.1016/j.foodchem.2012.12.049. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons KM (2004) Development of new products and markets for the global tilapia trade. Proceedings of ISTA 6. Manila, Philippine, pp 624–633
- Griffin SP, Bhagooli R. Measuring antioxidant potential in corals using the FRAP assay. J Exp Mar Biol Ecol. 2004;302:201–211. doi: 10.1016/j.jembe.2003.10.008. [DOI] [Google Scholar]
- Gόmez-Guillén MC, Giménez B, López-Caballero ME, Montero MP. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll. 2011;25:1813–1827. doi: 10.1016/j.foodhyd.2011.02.007. [DOI] [Google Scholar]
- He R, Alashi A, Malomo SA, Girgih AT, Chao D, Ju X, Aluko RE. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013;141:153–159. doi: 10.1016/j.foodchem.2013.02.087. [DOI] [PubMed] [Google Scholar]
- Hernández-Ledesma B, Contreras MM, Recio I. Antihypertensive peptides: production, bioavailability and incorporation into foods. Adv Colloid Interf Sci. 2011;165:23–25. doi: 10.1016/j.cis.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Himaya SWA, Ngo DH, Ryu B, Kim SK. An active peptide purified from gastrointestinal enzyme hydrolysate of pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012;132:1872–1882. doi: 10.1016/j.foodchem.2011.12.020. [DOI] [Google Scholar]
- Hsu K. Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem. 2010;122:42–48. doi: 10.1016/j.foodchem.2010.02.013. [DOI] [Google Scholar]
- Ichimaru T, Yamanaka A, Otsuka T, Yamashita E, Maruyaman S. Antihypertensive effect of enzymatic hydrolysate of collagen and gly-pro in spontaneously hypertensive rats. Biosci Biotechnol Biochem. 2009;73:2317–2319. doi: 10.1271/bbb.90197. [DOI] [PubMed] [Google Scholar]
- Li-Chan ECY, Hunag SL, Jao CL, Ho KP, Hsu KC. Peptides derived from atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J Agric Food Chem. 2012;60:973–978. doi: 10.1021/jf204720q. [DOI] [PubMed] [Google Scholar]
- Lin L, Lv S, Li B. Angiotensin-I-converting enzyme (ACE) inhibitory and antihypertensive properties of squid skin gelatin hydrolysates. Food Chem. 2012;131:225–230. doi: 10.1016/j.foodchem.2011.08.064. [DOI] [Google Scholar]
- Mendis E, Rajapakse N, Kim SK. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J Agric Food Chem. 2005;53:581–587. doi: 10.1021/jf048877v. [DOI] [PubMed] [Google Scholar]
- Ovissipour M, Rasco B, Shiroodi SG, Modanlow M, Gholami S, Nemati M. Antioxidant activity of protein hydrolysates from whole anchovy sprat (Clupeonella engrauliformis) prepared using endogenous enzymes and commercial proteases. J Sci Food Agric. 2013;93:1718–1726. doi: 10.1002/jsfa.5957. [DOI] [PubMed] [Google Scholar]
- Park PJ, Je JY, Kim SK. Angiotensin I converting enzyme (ACE) inhibitory activity of hetero-chitooligosaccharides prepared from partially different deacetylated chitosans. J Agric Food Chem. 2003;51:4930–4934. doi: 10.1021/jf0340557. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Kristinsson HG. ACE-inhibitory activity of tilapia protein hydrolysates. Food Chem. 2009;117:582–588. doi: 10.1016/j.foodchem.2009.04.058. [DOI] [Google Scholar]
- Schmelzer CEH, Schöps R, Reynell L, Ulbrich-Hofmann R, Neubert RHH, Raith K. Peptic digestion of β-casein: time course and fate of possible bioactive peptides. J Chromatogr A. 2007;1166:108–115. doi: 10.1016/j.chroma.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Sharp SI, Aarsland D, Day S, Sonnesyn H, Ballard C, Syst A. Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatr. 2011;26:661–669. doi: 10.1002/gps.2572. [DOI] [PubMed] [Google Scholar]
- Song L, Li T, Yu R, Yan C, Ren S, Zhao Y. Antioxidant activities of hydrolysates of Arca subcrenata prepared with three proteases. Mar Drugs. 2008;6:607–619. doi: 10.3390/md6040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songchotikunpan P, Tattiyakul J, Supaphol P. Extraction and electrospinning of gelatin from fish skin. Int J Biol Macromol. 2008;42:247–255. doi: 10.1016/j.ijbiomac.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Tavano OL. Protein hydrolysis using proteases: an important tool for food biotechnology. J Mol Catal B Enz. 2013;90:1–11. doi: 10.1016/j.molcatb.2013.01.011. [DOI] [Google Scholar]
- Thiansilakul Y, Benjakul S, Shahidi F. Antioxidative activity of protein hydrolysate from round scad muscle using alcalase and flavourzyme. J Food Biochem. 2007;31:266–287. doi: 10.1111/j.1745-4514.2007.00111.x. [DOI] [Google Scholar]
- Vercruysse L, Van CJ, Smagghie G. ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein, a review. J Agric Food Chem. 2005;53:8106–8115. doi: 10.1021/jf0508908. [DOI] [PubMed] [Google Scholar]
- Vo TS, Ngo DH, Kim JA, Ryu BM, Kim SK. An antihypertensive peptide from tilapia gelatin diminishes free radical formation in murine microglial cells. J Agric Food Chem. 2011;59:12193–12197. doi: 10.1021/jf202837g. [DOI] [PubMed] [Google Scholar]
- Yang JI, Liang WS, Chow CJ, Siebert KJ. Process for the production of tilapia retorted skin gelatin hydrolysates with optimized antioxidative properties. Process Biochem. 2009;44:1152–1157. doi: 10.1016/j.procbio.2009.06.013. [DOI] [Google Scholar]
- Zhang YX, Zou AH, Manchu RG, Zhou YC, Wang SF. Purification and antimicrobial activity of antimicrobial protein from brown-spotted grouper, Epinephelus fario. J Zool Syst Evol Res. 2008;29:627–632. [Google Scholar]
- Zhao Y, Li B, Dong S, Liu Z, Zhao X, Wang J, Zeng M. A novel ACE inhibitory peptide isolated from Acaudina molpadioidea hydrolysate. Pept. 2009;30:1028–1033. doi: 10.1016/j.peptides.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Zhou P, Yang C, Ren Y, Wang C, Tian F. What are the ideal properties for functional food peptides with antihypertensive effect? a computational peptidology approach. Food Chem. 2013;1141:2967–2973. doi: 10.1016/j.foodchem.2013.05.140. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Sun L. Preparation of reactive oxygen scavenging peptides from tilapia (Oreochromis niloticus) skin gelatin: optimization using response surface methodology. J Food Sci. 2011;76:483–489. doi: 10.1111/j.1750-3841.2011.02108.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 401 kb)
(PDF 485 kb)
(PDF 748 kb)