Abstract
Purpose
Cockroach feces are known to be rich in IgE-reactive components. Various protease allergens were identified by proteomic analysis of German cockroach fecal extract in a previous study. In this study, we characterized a novel allergen, a chymotrypsin-like serine protease.
Methods
A cDNA sequence homologous to chymotrypsin was obtained by analysis of German cockroach expressed sequence tag (EST) clones. The recombinant chymotrypsins from the German cockroach and house dust mite (Der f 6) were expressed in Escherichia coli using the pEXP5NT/TOPO vector system, and their allergenicity was investigated by ELISA.
Results
The deduced amino acid sequence of German cockroach chymotrypsin showed 32.7 to 43.1% identity with mite group 3 (trypsin) and group 6 (chymotrypsin) allergens. Sera from 8 of 28 German cockroach allergy subjects (28.6%) showed IgE binding to the recombinant protein. IgE binding to the recombinant cockroach chymotrypsin was inhibited by house dust mite chymotrypsin Der f 6, while it minimally inhibited the German cockroach whole body extract.
Conclusions
A novel allergen homologous to chymotrypsin was identified from the German cockroach and was cross-reactive with Der f 6.
Keywords: Allergens, chymotrypsin, German cockroach
INTRODUCTION
Proteases in allergen extracts are thought to be important factors in primary sensitization, not only to the protease itself but also to bystander allergens. They also may exacerbate allergic disorders by damaging epithelial barrier function.1 Abolishment of the proinflammatory effects of German cockroach extract by serine protease inhibitors suggests the important role of proteases in inflammation of airway epithelial cells.2
However, none of the German cockroach allergens listed in International Union of Immunological Society (IUIS) allergen nomenclature have been shown to have serine protease activity. Recently, we found that the IgE binding proteins detected by proteomics from German cockroach fecal extract were mainly digestive enzymes such as amylase, midgut carboxy peptidase A, astacin-like metalloprotease, trypsin, and chymotrypsin.3
Chymotrypsin-like protease was first described as an allergen from Aspergillus fumigatus.4 Chymotrypsin-like serine proteases were also identified as allergens from house dust mites5,6,7; however, only a small amount of mite chymotrypsin was found in the extract, suggesting a limited role in mite allergenicity.8 Molecular cloning of mite chymotrypsin was performed for Dermatophagoides pteronyssinus (Der p 6) in 19969 and for D. farinae (Der f 6).10 α-chymotrypsin activity was not detected from German cockroach extract by ApiZym assay (bioMerieux, Marcy l'Etoile, France), although various protease activities were detected.11 Much stronger gelatinolytic activity was also detected from cockroach extracts compared to house dust mite extracts, as measured by zymography.
In this study, we identified a chymotrypsin-like clone by expressed sequence tag (EST) analysis and produced its recombinant protein and evaluated its allergenicity using ELISA.
MATERIALS AND METHODS
Expressed sequence tag analysis
A cDNA library for the German cockroach was constructed using a Lambda ZAP II XR library construction kit (Stratagene, La Jolla, CA, USA). The phage library was converted to a phagemid library by mass excision and was transformed into Escherichia coli. The transformed colonies were randomly selected and prepared as plasmid DNA. Subsequently, the DNA sequences of the inserts were determined at Macrogen (Daegeon, Korea). All sequences were analyzed with the non-redundant database at the National Center for Biotechnology Information using the Basic Local Alignment Search Tool X (BLASTX). Clones showing homology with known allergens were summarized in Table 1.
Table 1.
Allergen homologues obtained from German cockroach EST analysis
| Biochemical identity | Allergen | Isoforms | Clone number | Clone | |
|---|---|---|---|---|---|
| Midgut microvilli protein | Bla g 1 | 1.01 | 1 | 3 | 04-C01 |
| 1.02 | 1 | 02-E03 | |||
| (1.03) | 1 | 05-E01 | |||
| Hexamerin | Bla g 3 | Isoform 1 | 21 | 48 | 01-A10, 01-F06, 03-E01, 02-F12, 02-H01, 03-G04, 03-G10, 03-G12, 04-B08, 04-H02, 05-A05, 06-A10, 07-C09, 07-C11, 07-D07, 11-D04, 11-D08, 09-C08, 09-H05, 10-G02 |
| Isoform 2 | 7 | 03-D03, 03-E04, 04-H05, 05-D12, 05-E09, 07-H08, 11-G07 | |||
| 7 | 01-H01, 06-C08, 06-H09, 07-H11, 10-F04, 11-B10, 11-C02 | ||||
| 4 | 06-G11, 07-C08, 07-D01, 10-D11 | ||||
| 9 | 01-B12 01-E08, 01-F12, 02-B07, 03-H09, 04-F06, 07-H10, 09-B04, 10-H12 | ||||
| Glutathione S-transferase | Bla g 5 | Sigma class | 4 | 12 | 02-C02, 02-F03, 03-B03, 10-G10 |
| Delta class | 3 | 02-C11, 02-H08, 03-E05 | |||
| Theta class | 4 | 05-H11, 11-F02, 01-E05, 07-F03 | |||
| Omega class | 1 | 09-D09 | |||
| Myosin light chain | Bla g 8 | 23 | 23 | 01-C01, 01-F04, 02-A07, 02-C09, 03-G11, 04-B02, 04-C11, 04-E07, 04-E12, 04-F08, 05-E03, 06-A08, 07-D02, 07-D06, 07-G05, 07-G06, 08-D08, 08-F06, 08-G06, 10-B04, 10-H03, 11-E01, 11-E03 | |
| Arginine kinase | (Bla g 9) | 8 | 8 | 01-B07, 02-B10, 02-D02, 04-H09, 06-A11, 08-F08, 10-A04, 11-C10 | |
| Trypsin | (Bla g 10) | 9 | 9 | 01-G04, 02-G07, 10-B09, 10-C10, 11-H04, 11-H09, 04-A02, 05-B08, 01-G02 | |
| α-amylase | Bla g 11 | 13 | 13 | 01-D01, 05-B03, 02-F09, 03-C02, 04-G11, 09-D03, 11-D01, 02-B06, 04-C09, 07-H06, 08-B07, 06-B06, 06G12 | |
| Chymotrypsin | (Bla g 12) | 3 | 3 | 04-H06, 09-B12, 11-D12 | |
*The deduced amino acid sequences from 1,177 clones were analyzed. A total of 64 clones were shown to be β-actin. Clones with full-length sequence were underlined. Brackets indicate allergens which are not officially listed in IUIS.
Expression of recombinant proteins
For the expression of recombinant proteins, the coding region, which includes an 11-amino acid prosequence and the mature protein, was amplified using the phagemid (BgEST04H06) as a template with oligonucleotide primers (forward: 5'-GCTGAACTTGCTGCCCTAGAC-3', reverse: 5'-TTAAACGGCATTTTGGTTGAT-3'). Simultaneously, recombinant Der f 6, a chymotrypsin from D. farinae, was also amplified by reverse-transcriptase PCR with oligonucleotide primers (forward: 5'-GATGCACGATTTCCACGC-3', reverse: 5'-TCAAACAATGTTTTTTGT-3'). PCR products were cloned into the pEXP-5NT vector/TOPO vector (Invitrogen Life Technologies, Carlsbad, CA, USA). The orientation of the inserts was confirmed by PCR using T7 primer annealing to the vector and reverse primer annealing to the insert. The resultant sequences should contain an additional 22 amino acids (MSGSHHHHHHGSSGENLYFQSL) at the N-terminus. DNA sequences were determined at Solgent (Daejeon, Korea) and then were transformed into E. coli BL21 (DE3).
Expression of the recombinant proteins was induced by adding 1 mM of isopropyl-1-thio-β-galactopyranoside when bacteria were grown to an absorbance of 0.6 at 600 nm. Recombinant proteins were purified under denaturing conditions (6 M urea) using Ni-nitrilotriacetic acid-agarose (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Proteins were dialyzed against refolding buffer (0.1 M Tris, pH 8.0, 0.4 M L-Arginine, 0.5 mM oxidized glutathione, 5 mM reduced glutathione) and their concentration was determined by Bradford assay (Bio-Rad, Hercules, CA, USA). Proteins were analyzed by 12% polyacrylamide gel containing sodium dodecyl sulfate under reducing conditions.
Serum samples
Serum samples were collected from patients at the Allergy-Asthma Clinic at Severance Hospital, Seoul, Korea. Patient consent was obtained before blood collection. Sera from allergic patients (25 males and 3 females, mean age 23 years, ranging from 3 to 57 years) with ImmunoCAP (Phadia, Uppsala, Sweden) higher than 0.7 kU/L to the German cockroach were chosen (Table 2). Diagnosis of German cockroach allergy was based on case history and skin test. Seventeen control sera from individuals with no history of allergic symptoms and negative for German cockroach allergy on ImmunoCAP assay were included. This study was approved by the institutional review board (4-2009-0717).
Table 2.
Clinical features of the enrolled subjects
| Subject | Gender | Age | Symptom/diagnosis | Sensitization profile | i6 specific IgE (Class) | Total IgE |
|---|---|---|---|---|---|---|
| *S01 | M | 28 | Allergic rhinitis | i6 | 9.45 (3) | 926 |
| S02 | M | 37 | Allergic rhinitis, Chronic urticaria | i6 | 8.05 (3) | class 3 |
| S03 | M | 8 | Allergic rhinitis, Asthma | d1, d2, i6 | 1.74 (2) | 1994 |
| S04 | M | 53 | Allergic rhinitis, Asthma | i6 | 21.0 (4) | 1114 |
| S05 | M | 27 | Allergic rhinitis, Asthma | i6 | 7.79 (3) | 403 |
| S06 | M | 41 | Allergic rhinitis, Atopic dermatitis | i6 | 6.68 (3) | 3769 |
| S07 | M | 45 | Allergic rhinitis, Allergic conjunctivitis | i6 | 4.19 (3) | 503 |
| S08 | M | 7 | Allergic rhinitis, Asthma | m6, d2, i6 | 1.58 (2) | 234 |
| S09 | M | 13 | Asthma | d1, d2, i6 | 1.72 (2) | 1494 |
| S10 | M | 7 | Allergic rhinitis | d1, d2, i6, f1, f2 | 2.62 (2) | 2311 |
| S11 | M | 27 | Allergic rhinitis, Asthma, Chronic urticaria | i6 | 4.71 (3) | 305 |
| S12 | F | 34 | Anaphylaxis | i6 | 1.66 (2) | ND |
| S13 | M | 57 | Allergic rhinitis | i6 | 13.0 (3) | ND |
| S14 | M | 31 | Allergic rhinitis, Allergic conjunctivitis, Asthma | i6 | 2.48 (2) | 845 |
| S15 | M | 6 | Allergic rhinitis | d1, d2, i6, f207 | 6.98 (3) | 771 |
| S16 | M | 7 | Allergic rhinitis, Asthma | d1, i6, f13, f2 | 3.11 (3) | 415 |
| S17 | M | 38 | Allergic rhinitis, Asthma | ND | 1.95 (2) | ND |
| S18 | F | 17 | Allergic rhinitis | d1, d2, i6 | 2.76 (2) | 443 |
| S19 | M | 28 | Allergic rhinitis, Allergic conjunctivitis, Atopic dermatitis | i6 | 1.45 (2) | 2726 |
| S20 | M | 8 | Allergic rhinitis, Sinusitis | d1, i6, f13, f2 | 16.1 (3) | 389 |
| S21 | M | 14 | Asthma, acute attack | d1, d2, i6 | 6.02 (3) | 474 |
| S22 | M | 14 | Allergic rhinitis | d1, d2, i6 | 3.63 (3) | 406 |
| S23 | F | 10 | Allergic rhinitis, Atopic dermatitis | d1, d2, i6, f1, f2 | 2.31 (2) | 3266 |
| S24 | M | 49 | Asthma, Chronic urticaria | ND | 1.22 (2) | ND |
| S25 | M | 25 | Allergic rhinitis, Asthma | i6 | 2.17 (2) | 685 |
| S26 | M | 9 | Allergic rhinitis | d1, d2, i6 | 2.09 (2) | 3600 |
| S27 | M | 3 | Asthma | d2, e5, f1 | 4.98 | 81.4 |
| S28 | M | 16 | Allergic rhinitis | i6 | 2.34 | 224 |
*The positives to the recombinant chymotrypsin are printed in bold. ND, not determined. d1, Dermatophagoides pteronyssinus; d2, D. farinae; i6, German cockroach; e5, Dog dander; m6, Alternaria alternata; f1, Egg white; f2, Milk; f13, Peanut; f207, Clam.
Enzyme-linked immunosorbent assay
Serum IgE specific to recombinant allergen was detected by ELISA. Purified proteins (2 µg/mL) were coated in 0.05 M carbonated buffer (pH 9.6) overnight at 4℃. After blocking with 3% skim milk in phosphate-buffered saline containing 0.05% Tween 20 (PBST), serum samples (1:4 diluted in PBST containing 1% bovine serum albumin) were incubated for one hour. IgE antibodies were probed by incubating with biotinylated goat anti-human IgE (1:1,000) (Vector, Burlingame, CA, USA) for an hour, followed by incubation with streptavidin-peroxidase conjugate (1:1,000) (Sigma-Aldrich, St. Louis, MO, USA) for 30 minutes. Color was developed using 3,3',5,5'-tetramethyl-benzidine (TMB, Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA) as a substrate. After stopping the enzyme reaction with 0.5 M H2SO4, the absorbance at 450 nm was measured. The cutoff value was determined by mean absorbance plus 2 SDs for the negative controls.
For inhibition analysis, 10 µg/mL of recombinant chymotrypsin from the German cockroach (rBg04H06) was coated onto microtiter ELISA plates. Serum samples (diluted 1:4) from three subjects with positive reactions to rBg04H06 were incubated with 5 serially diluted antigens (rBg04H06 and rDer f 6) starting with an inhibitor concentration of 10 µg/mL. The inhibitor mixtures were incubated at room temperature for 2 hours and overnight at 4℃. IgE antibodies were detected as described above.
RESULTS
Analysis of allergen homologous molecules from the EST database
DNA sequences of 1,226 clones were determined. A total of 1,177 clones showing valid, readable amino acid sequences were obtained. A BLASTX search of 119 clones showed strong homology with the previously known allergens (Table 1). Bla g 3 (48) was the most frequently identified allergen-like clone, followed by Bla g 8 (23), Bla g 11 (13), glutathione S-transferases (12), trypsin (9), arginine kinase (8), Bla g 1 (3) and chymotrypsin (3).
Homology with chymotrypsin-like allergens
Three clones in the EST database showed homology with chymotrypsins, and only one of them (BgEST04H06) contained the full-length sequence information of an open reading frame. The complete sequence was found to be 1,096 nucleotides, which included 12 bp of 5'-noncoding region, 758 bp of open reading frame, and 338 bp of 3'-untranslated region (Fig. 1). A protein of 252 amino acids with an estimated molecular mass of 25.8 kDa and an isoelectric point (pI) of 8.07 was conceptually translated. The N-terminus of mature chymotrypsin from the German cockroach was thought to start from Val30 based on sequence alignment with mite chymotrypsins. The mature form had a calculated molecular mass of 22.8 kDa and pI of 8.5.
Fig. 1.
The nucleotide and deduced amino acid sequences of chymotrypsin-like serine protease from the German cockroach. The full-length sequence encoded 252 amino acids with an estimated molecular mass of 25.8 kDa (22.8 kDa for the mature protein) and a pI of 8.07 (8.487 for the mature protein). Underlining indicates the sequences where oligonucleotides anneal.
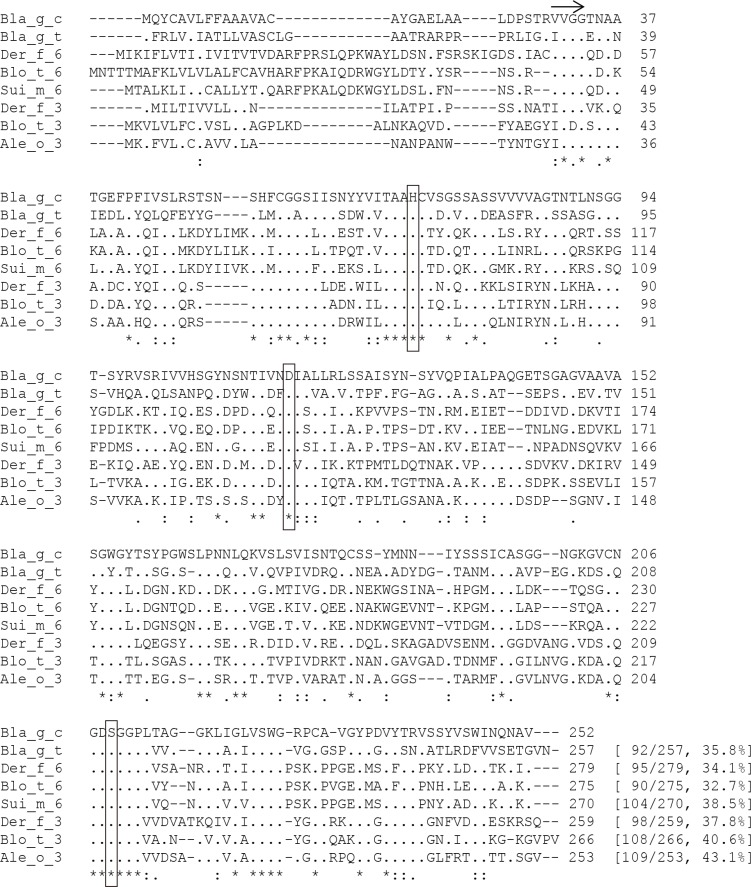
The translated amino acid sequence of the German cockroach putative chymotrypsin showed 32.7 to 43.1% identity with mite chymotrypsin and trypsin allergens (Fig. 2). In particular, it exhibited 35.8% identity with German cockroach trypsin. Interestingly, it shares 32.7 to 38.5% identity with mite chymotrypsins (34.1% with Der f 6, 32.7% with Der p 6, and 38.5% with Blo t 6) and 37.8 to 43.1% with mite trypsins (37.8% to Der f 3, 40.6% to Blo t 3, and 43.1% to Ale o 3). Amino acids known to comprise the catalytic triad (His81, Asp116, Ser209) are well conserved. This sequence was deposited at the GenBank under accession no. KJ801816.
Fig. 2.
Multiple sequence alignment of the German cockroach chymotrypsin-like serine protease with allergenic chymotrypsins and trypsins from dust mites. The amino acids that comprise the catalytic triad are shown open boxed. An arrow indicates the putative N-terminus of mature proteins. *, identical; :, highly conserved; ., less conserved.
Expression of recombinant proteins
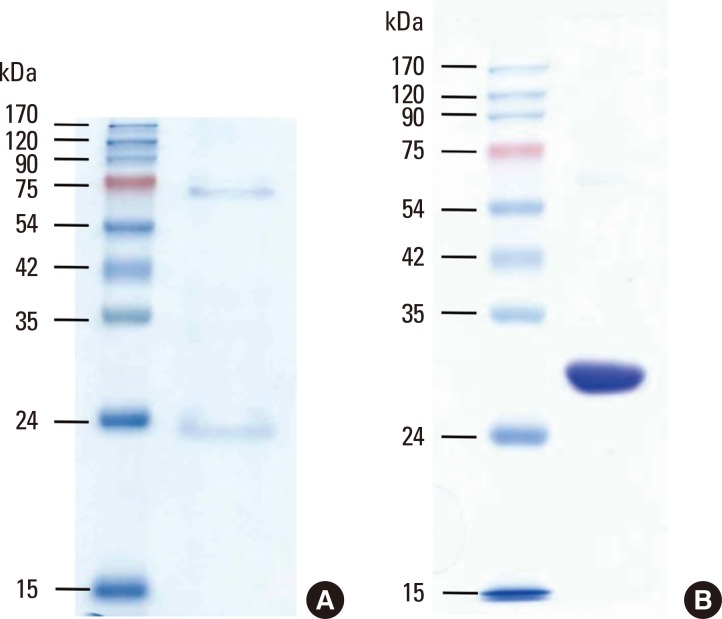
Recombinant proteins with a short N-terminal fusion protein containing 6-histidine were expressed in E. coli and purified using a Ni-resin. The yields of chymotrypsin from the German cockroach and D. farinae were 2.27 and 9.41 mg/liter of bacteria culture, respectively. A protein band matching the postulated molecular weight was detected by SDS-PAGE (Fig. 3).
Fig. 3.
Purification of recombinant proteins. Recombinant chymotrypsins from the German cockroach (A) and Dermatophagoides farinae (B) were purified using Ni-NTA agarose, and 20 µg of protein was separated onto 15% SDS-PAGE gel under reducing conditions.
Allergenicity of the German cockroach recombinant chymotrypsin
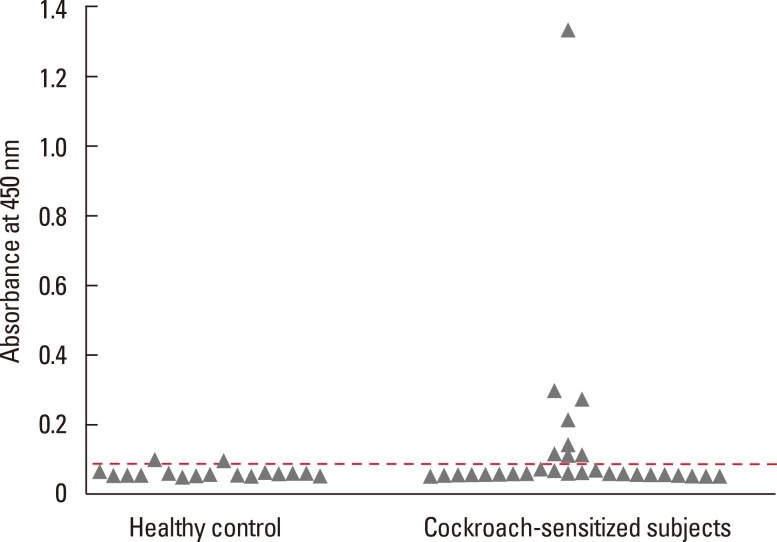
IgE binding reactivity of the recombinant protein was tested by ELISA. Eight of 28 (28.6%) were considered rBg04H06-positive (Fig. 4). Inhibition analysis was performed using serum samples from three patients who showed strong IgE reactivity to the recombinant protein. However, rBg04H06 was not able to inhibit IgE reactivity to cockroach extract (data not shown).
Fig. 4.
IgE reactivity of human sera against recombinant chymotrypsin. The horizontal line indicates the cutoff value.
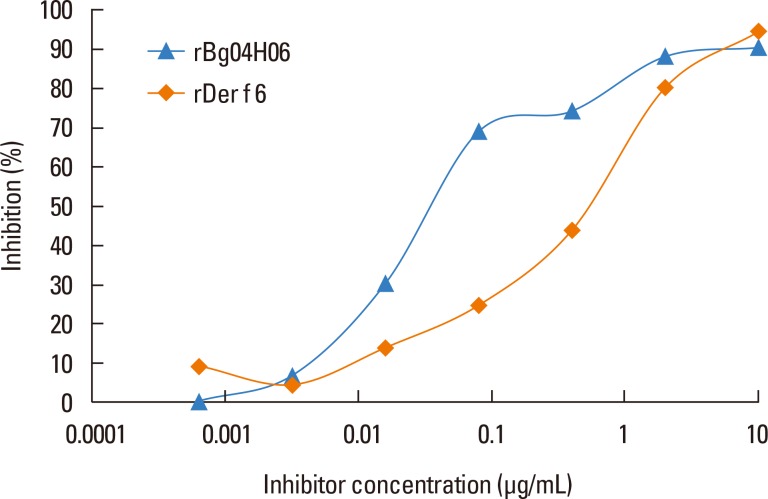
In order to investigate the cross-reactivity between rBg04H06 and rDer f 6, inhibition analysis was performed. rDer f 6 inhibited a maximum of 90.5% of the rBg04H06-specific IgE reactivity, while rBg04H06 inhibited 94.5% of the IgE reactivity (Fig. 5). However, rBg04H06 was able to inhibit 30.3 to 74.3% of the IgE reactivity while rDer f 6 inhibited 13.8 to 43.8% IgE reactivity at inhibitor concentrations from 0.016 to 0.4 µg/mL.
Fig. 5.
ELISA inhibition. IgE reactivity to recombinant cockroach chymotrypsin was assessed with a pool of serum samples preabsorbed with various quantities of recombinant chymotrypsins from Blattella germanica and Dermatophagoides farinae.
DISCUSSION
About 1,500 proteins have been described as allergens.12 Various bioinformatics methods have been developed based on allergen databases.13,14,15,16,17 EST analysis is a powerful tool for identifying candidate allergens homologous to allergens in the database.18 Moreover, information on the expression levels of allergens could be provided by EST analysis, as the number of each clone present should reflect its expression level and concentration in the allergen extract. For example, the allergenic potency of German cockroach extract is thought to be influenced by Bla g 3 and α-amylase because their concentration is very high in the extract.19 The number of Bla g 3 clones was highest (48) in EST analysis, followed by Bla g 8 (23) and α-amylase (13) (Table 1). In 2005, Chung et al.20 described the analysis of EST clones prepared from German cockroach midgut, and 154 clones showed significant homology with other database-registered genes among 363 ESTs generated from 465 clones. Among 154 clones analyzed, four allergen homologs were identified (4 clones of Bla g 1, 2 clones of amylase, 4 clones of trypsin and 1 clone of chymotrypsin). Bla g 3 was not identified since it is mainly expressed in hemolymph, not in midgut.21 Moreover, expression levels of Bla g 1 and Bla g 2 could be affected by the culture conditions of cockroach, as their expression is known to be influenced by foods and insecticides.22,23 A larger-scale EST analysis and investigation of mRNA expression is necessary to better understand allergen expression.
In this study, 6 groups of allergens (Bla g 1, Bla g 3, Bla g 5, Bla g 8, and α-amylase), listed in IUIS, and three novel German cockroach candidate allergens (arginine kinase, trypsin, delta class GST, and chymotrypsin) were identified. Expression levels of Bla g 2 and Bla g 4 are not thought to be high, as they were not detected by analysis of two EST databases. We first analyzed the chymotrypsin for its allergenicity because it has not yet been described. IgE reactivities of arginine kinase,24 trypsin,25 and delta class GST26 were previously described, at least partially.
The putative chymotrypsin identified in this study shares 32.7 to 43.1% identity to allergenic chymotrypsins (Fig. 2). We did not observe gelatinolytic activity using zymography (Data not shown); however, it showed strong cross-reactivity (Fig. 5) with Der f 6 although it was recognized by only 28.6% of the sera tested (Fig. 4). A chymotrypsin identified by proteome analysis of fecal extract had a pI of approximately 4.63; however, the cloned chymotrypsin had a pI of 8.5. This finding implies the presence of isoallergens. Cockroach chymotrypsin, due to its low abundance, does not seem to be a determinant of the extract's potency.
In conclusion, we were able to identify various allergen homologues by analyzing German cockroach EST clones and to compare their expression levels. Furthermore, a novel chymotrypsin-like cockroach allergen was identified, and its allergenicity was reported using recombinant allergen. This cockroach allergen was highly cross-reactive with Der f 6, a mite chymotrypsin. Recombinant allergens could be helpful for the diagnosis of cockroach allergy by distinguishing genuine sensitization from cross-reaction. Continued efforts to characterize cockroach allergens by the analysis of ESTs and proteomes are needed.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (A092076).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Matsumura Y. Role of allergen source-derived proteases in sensitization via airway epithelial cells. J Allergy (Cairo) 2012;2012:903659. doi: 10.1155/2012/903659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhat RK, Page K, Tan A, Hershenson MB. German cockroach extract increases bronchial epithelial cell interleukin-8 expression. Clin Exp Allergy. 2003;33:35–42. doi: 10.1046/j.1365-2222.2002.01481.x. [DOI] [PubMed] [Google Scholar]
- 3.Jeong KY, Kim CR, Park J, Han IS, Park JW, Yong TS. Identification of novel allergenic components from German cockroach fecal extract by a proteomic approach. Int Arch Allergy Immunol. 2013;161:315–324. doi: 10.1159/000347034. [DOI] [PubMed] [Google Scholar]
- 4.Robinson BW, Venaille TJ, Mendis AH, McAleer R. Allergens as proteases: an Aspergillus fumigatus proteinase directly induces human epithelial cell detachment. J Allergy Clin Immunol. 1990;86:726–731. doi: 10.1016/s0091-6749(05)80176-9. [DOI] [PubMed] [Google Scholar]
- 5.Stewart GA, Lake FR, Thompson PJ. Faecally derived hydrolytic enzymes from Dermatophagoides pteronyssinus: physicochemical characterisation of potential allergens. Int Arch Allergy Appl Immunol. 1991;95:248–256. doi: 10.1159/000235437. [DOI] [PubMed] [Google Scholar]
- 6.Stewart GA, Bird CH, Krska KD, Colloff MJ, Thompson PJ. A comparative study of allergenic and potentially allergenic enzymes from Dermatophagoides pteronyssinus, D. farinae and Euroglyphus maynei. Exp Appl Acarol. 1992;16:165–180. doi: 10.1007/BF01201499. [DOI] [PubMed] [Google Scholar]
- 7.Yasueda H, Mita H, Akiyama K, Shida T, Ando T, Sugiyama S, et al. Allergens from Dermatophagoides mites with chymotryptic activity. Clin Exp Allergy. 1993;23:384–390. doi: 10.1111/j.1365-2222.1993.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 8.Stewart GA, Kollinger MR, King CM, Thompson PJ. A comparative study of three serine proteases from Dermatophagoides pteronyssinus and D. farinae. Allergy. 1994;49:553–560. doi: 10.1111/j.1398-9995.1994.tb01128.x. [DOI] [PubMed] [Google Scholar]
- 9.Bennett BJ, Thomas WR. Cloning and sequencing of the group 6 allergen of Dermatophagoides pteronyssinus. Clin Exp Allergy. 1996;26:1150–1154. [PubMed] [Google Scholar]
- 10.Kawamoto S, Mizuguchi Y, Morimoto K, Aki T, Shigeta S, Yasueda H, et al. Cloning and expression of Der f 6, a serine protease allergen from the house dust mite, Dermatophagoides farinae. Biochim Biophys Acta. 1999;1454:201–207. [PubMed] [Google Scholar]
- 11.Jeong KY, Kim C, Yong TS. Enzymatic activities of allergen extracts from three species of dust mites and cockroaches commonly found in Korean home. Korean J Parasitol. 2010;48:151–155. doi: 10.3347/kjp.2010.48.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman RE. Practical and predictive bioinformatics methods for the identification of potentially cross-reactive protein matches. Mol Nutr Food Res. 2006;50:655–660. doi: 10.1002/mnfr.200500277. [DOI] [PubMed] [Google Scholar]
- 13.Stadler MB, Stadler BM. Allergenicity prediction by protein sequence. FASEB J. 2003;17:1141–1143. doi: 10.1096/fj.02-1052fje. [DOI] [PubMed] [Google Scholar]
- 14.Fiers MW, Kleter GA, Nijland H, Peijnenburg AA, Nap JP, van Ham RC. Allermatch, a webtool for the prediction of potential allergenicity according to current FAO/WHO Codex alimentarius guidelines. BMC Bioinformatics. 2004;5:133. doi: 10.1186/1471-2105-5-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang ZH, Koh JL, Zhang GL, Choo KH, Tammi MT, Tong JC. AllerTool: a web server for predicting allergenicity and allergic cross-reactivity in proteins. Bioinformatics. 2007;23:504–506. doi: 10.1093/bioinformatics/btl621. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Yu Y, Zhao Y, Zhang D, Li J. Evaluation and integration of existing methods for computational prediction of allergens. BMC Bioinformatics. 2013;14(Suppl 4):S1. doi: 10.1186/1471-2105-14-S4-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mari A, Rasi C, Palazzo P, Scala E. Allergen databases: current status and perspectives. Curr Allergy Asthma Rep. 2009;9:376–383. doi: 10.1007/s11882-009-0055-9. [DOI] [PubMed] [Google Scholar]
- 18.Angus AC, Ong ST, Chew FT. Sequence tag catalogs of dust mite-expressed genomes: utility in allergen and acarologic studies. Am J Pharmacogenomics. 2004;4:357–369. doi: 10.2165/00129785-200404060-00003. [DOI] [PubMed] [Google Scholar]
- 19.Jeong KY, Lee JH, Kim EJ, Lee JS, Cho SH, Hong SJ, et al. Current status of standardization of inhalant allergen extracts in Korea. Allergy Asthma Immunol Res. 2014;6:196–200. doi: 10.4168/aair.2014.6.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung HS, Yu TH, Kim BJ, Kim SM, Kim JY, Yu HS, et al. Expressed sequence tags analysis of Blattella germanica. Korean J Parasitol. 2005;43:149–156. doi: 10.3347/kjp.2005.43.4.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khurana T, Collison M, Chew FT, Slater JE. Bla g 3: a novel allergen of German cockroach identified using cockroach-specific avian single-chain variable fragment antibody. Ann Allergy Asthma Immunol. 2014;112:140–145.e1. doi: 10.1016/j.anai.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Chuang JG, Su SN, Chiang BL, Lee HJ, Chow LP. Proteome mining for novel IgE-binding proteins from the German cockroach (Blattella germanica) and allergen profiling of patients. Proteomics. 2010;10:3854–3867. doi: 10.1002/pmic.201000348. [DOI] [PubMed] [Google Scholar]
- 23.Ock MS, Kim BJ, Kim SM, Byun KH. Cloning and expression of trypsin-encoding cDNA from Blattella germanica and its possibility as an allergen. Korean J Parasitol. 2005;43:101–110. doi: 10.3347/kjp.2005.43.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong KY, Jeong KJ, Yi MH, Lee H, Hong CS, Yong TS. Allergenicity of sigma and delta class glutathione S-transferases from the German cockroach. Int Arch Allergy Immunol. 2009;148:59–64. doi: 10.1159/000151506. [DOI] [PubMed] [Google Scholar]
- 25.Gore JC, Schal C. Expression, production and excretion of Bla g 1, a major human allergen, in relation to food intake in the German cockroach, Blattella germanica. Med Vet Entomol. 2005;19:127–134. doi: 10.1111/j.0269-283X.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YC, Perzanowski MS, Chew GL. Sub-lethal exposure of cockroaches to boric acid pesticide contributes to increased Bla g 2 excretion. Allergy. 2005;60:965–968. doi: 10.1111/j.1398-9995.2005.00814.x. [DOI] [PubMed] [Google Scholar]