Abstract
A mutant library of Cordyceps militaris was constructed by improved Agrobacterium tumefaciens-mediated transformation and screened for degradation features. Six mutants with altered characters in in vitro and in vivo fruiting body production, and cordycepin formation were found to contain a single copy T-DNA. T-DNA flanking sequences of these mutants were identified by thermal asymmetric interlaced-PCR approach. ATP-dependent helicase, cytochrome oxidase subunit I and ubiquitin-like activating enzyme were involved in in vitro fruiting body production, serine/threonine phosphatase involved in in vivo fruiting body production, while glucose-methanol-choline oxidoreductase and telomerase reverse transcriptase involved in cordycepin formation. These genes were analyzed by bioinformatics methods, and their molecular function and biology process were speculated by Gene Ontology (GO) analysis. The results provided useful information for the control of culture degeneration in commercial production of C. militaris.
Keywords: Agrobacterium tumefaciens, Cordycepin, Cordyceps militaris, Degeneration, Fruiting body
Cordyceps militaris is an insect-born fungus with abundant active constituents and a multitude of pharmacological activities [1, 2]. Methods for commercial production of fruiting bodies of this fungus have been established in artificial media [3, 4] or with insects, such as silkworm Bombyx mori pupae [5], millworm Tenebrio molitor pupae [6] and greater wax moth Galleria mellonella larvae [7]. Usually, four pivotal growth periods are identified during cultivating C. militaris fruiting bodies, including mycelial culture, color induction, stromal stimulation, and fruiting body production [4, 8]. For the better commercialization of this medical fungus, several improvements are of great importance, including prevention of the culture loss during in vitro or in vivo fruiting body production, increase of the active constituents (particularly cordycepin) and enhancement of the fungal pathogenicity to the target insects.
Fungal degeneration is detrimental to the culture of this fungus. Detection of the culture degeneration in the early stage may avoid great economic loss. Apart from the reports of phenotypic variation in the degenerative fungal cultures with Metarhizium anisopliae [9], little information is available to explain the culture instability of C. militaris at the molecular level.
As an entomopathogenic fungus, C. militaris not only infects different insect hosts, but also produces fruiting bodies from its hosts. Infection ability is very important in this process. The critical genes involved in the fungal pathogenicity were identified, including adhesin MAD1 [10], Subtilisin-like (Pr1), trypsin-like (Pr2), and chitinases (CHI1) from M. anisoplia [11, 12], and two serine protease genes (csp1 and csp2) from Ophiocordyceps sinensis [13]. But no reports are available on the molecular control of C. militaris growth in the infected insect hosts.
Cordycepin (3'-deoxyadenosine), a nucleoside derivative, is a major bioactive compound found in Cordyceps species [14]. High production of cordycepin in Cordyceps cultures significantly increases the commercial value of this fungus. Methods to increase the cordycepcin production by optimizing the culture media and conditions were reported recently [15, 16]. However, the genes involved in the cordycepin production in C. militaris remain unknown.
Agrobacterium tumefaciens-mediated transformation (ATMT) was used for insertional mutagenesis in C. militaris [4]. In this study, attempts were made to identify the genes involved in in vitro and in vivo fruiting body production, and cordycepin formation from C. militaris mutant library.
MATERIALS AND METHODS
Fungal strains and media
A laboratory and commercial JM4 strain of C. militaris from Guangdong Entomological Institute was used. Potato dextrose agar supplemented with 10% peptone (PPDA) was prepared for culturing C. militaris. The stock culture of this strain was maintained on PPDA plates at 4℃. Escherichia coli strain DH5α was used as a host for the propagation of plasmid DNA. A. tumefaciens strain AGL-1 (provided by Prof. Zide Jiang from South China Agricultural University, China) was maintained at 28℃ on Luria-Bertani medium.
Fungal transformation
A random T-DNA insertion library of C. militaris JM4 was constructed by ATMT method as described previously, with A. tumefaciens strain AGL-1 carrying the binary vector pATMT1 with hyg gene under Aspergillus nidulans trpC promoter [4]. Mutants with altered characters in in vitro and in vivo fruiting body production, and cordycepin formation were screened. The copy number of T-DNA in transformants was determined by Southern analysis using a PCR-amplified digoxigenin-labeled hyg gene probe, and DIG High Prime DNA labeling and Detection Starter Kit (Roche, Basel, Switzerland).
Cultures for fruiting body production
In vitro fruiting body production of C. militaris was performed with an artificial medium containing 20 g rice, 0.5 g powder of silkworm pupae and 25-mL nutrient solution (glucose 20 g, KH2PO4 2 g, MgSO4 1 g, ammonium citrate 1 g, peptone 5 g, vitamin B1 20 mg, and 1,000-mL distilled water), according to the method by Zheng et al. [4]. The phenotypic characters of transformants, including mycelial growth and color in PPDA, together with the stromal and fruiting body formation on the artificial medium, were observed. The fresh weight of fruiting bodies from each bottle was recorded.
In vivo fruiting body production of C. militaris in the infected G. mellonella larvae was also performed according to the method by Han et al. [7]. The mycelia and conidia (at least 106 conidia/mL) for infecting insect larvae were prepared by taking a patch of C. militaris with hypha body from PPDA plates (1 cm × 1 cm) into 6-mL shaking sterile water. One milliliter of the suspension was introduced into a 100-mL culture bottle with two layers of filter paper and one larva of greater wax moth G. mellonella. The bottle was loosely capped and sealed by parafilm, placed at 12~14℃ for 2 wk and then at 20℃ for fruiting body formation. The infected larvae were recorded every two days. Three replicates were established for each treatment. All the experiments were repeated twice.
Cordycepin assay
Mutants were checked for cordycepin formation by thin layer chromatography (TLC). Cordycepin was extracted according to Haddad et al. [17]. Briefly, dried fruiting body (30 mg) of each mutant was mixed with 1-mL of 50% ethanol for 6 hr in the dark at 25℃. The extracts were centrifuged (3,000 rpm, 10 min, 4℃) and the supernatant was used directly for TLC analysis or stored in a refrigerator (4℃) until use.
TLC analyses were performed according to Li et al. [18] by using aluminum sheets (5 × 20 cm) precoated with silica gel 60 F254 (layer thickness 0.2 mm). The TLC plates were developed in a horizontal developing chamber. The solvent system includes chloroform/ethyl acetate/isopropanol/water/strong ammonia-water (80/20/60/3/2, v/v/v/v). Chromatographic development of plates was performed at room temperature. After development, the plates were air-dried for 20 min. Separated compounds on the plates were visualized using long-wavelength 254 nm UV illumination, then the photographs of the plates were taken by a Coolpix 990 digital camera (Nikon, Tokyo, Japan). The compounds spots on photographs of the plates were analyzed by Quantity One 4.6.2 program (Bio-Rad, Hercules, CA, USA). Standard cordycepin for TLC analyses was purchased from Sigma Chemical (St. Louis, MO, USA). There were three replicates for each mutant.
Identification of T-DNA flanking sequences and full-length genes
Thermal asymmetric interlaced-PCR (TAIL-PCR) was employed to obtain genomic DNA sequences of C. militaris flanking inserted T-DNA from the selected transformants, using a Genome Walking Kit (Takara, China). The PCR products were ligated into pMD19-T vector (Takara). The plasmid DNA was transformed into Escherichia coli DH5α. Colony PCR was used to validate positive clones which were subsequently sequenced by Invitrogen Trading (Shanghai) Co. Ltd. (Shanghai, China).
Full-length genes were obtained by 5'- and 3'-rapid amplification of cDNA ends (RACE) and TAIL-PCR. Briefly, total RNA was extracted using the TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA). RNA yield was estimated spectrophotometrically, and the integrity of RNA was confirmed via the detection of discrete 18S and 28S ribosomal RNA bands after agarose gel electrophoresis. With gene-specific primers designed to flanking sequences, two partially overlapping cDNA fragments were generated from total RNA of C. militaris using RACE (SMARTer RACE cDNA Amplification Kit; Clontech, Palo Alto, CA, USA). The PCR products were sequenced and confirmed as above. If the amplification of cDNA ends of some genes were not obtained by RACE method, TAIL-PCR was also applied to gain the upstream and downstream sequences of the T-DNA flanking fragment.
Bioinformatics analysis of sequences
DNA sequences from RACE PCR or TAIL-PCR were sequenced and assembled respectively. The sequences were compared with those available at the GenBank databases using BLASTn and BLASTx. Searches for potential open reading frames (ORF) were carried out using ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). Classification of sequences was performed under Gene Ontology (GO) criteria. For a general approach, the BLAST2GO program (http://www.blast2go.de/) was used for a similarity search.
RESULTS
Fungal transformation
Four hundered to 600 T-DNA-tagged C. militaris mutants per 105 conidia were generated by Agrobacterium tumefaciens-mediated fungal transformation. 34 mutants with degradation features were obtained. Southern blot analysis showed that the mutants contained 1 or 2 copy of T-DNA and more than 64% mutants contained a single copy. Six mutants with a single T-DNA copy involved in altered phenotypes, cordycepin metabolic disorder or decreased entomopathogenic ability were characterized.
Mutants and genes involved in abnormal in vitro fruiting body production
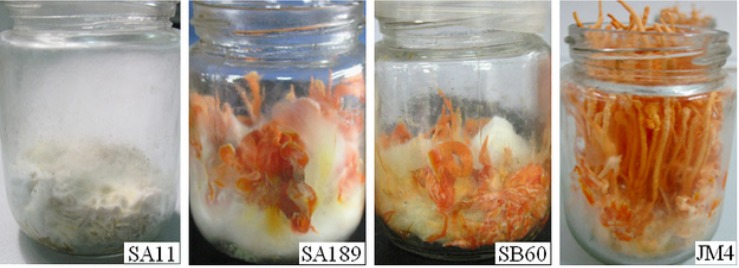
Fresh weight decline of the fruiting bodies is one of the signs of C. militaris degradation. Average fresh weight of the fruiting bodies from 600 transformants was determined to isolate the degenerated mutants [4]. Two mutants (SA189 and SB60) produced poor fruiting bodies, compared to wild-type C. militaris JM4. Moreover, mutant SA11 was found without color change after light induction (Fig. 1).
Fig. 1. In vitro fruiting body production of the insertional mutants of Cordyceps militaris in the artificial medium containing 20 g rice, 0.5 g powder of silkworm pupae and 25-mL nutrient solution (glucose 20 g, KH2PO4 2 g, MgSO4 1 g, ammonium citrate 1 g, peptone 5 g, vitamin B1 20 mg, and 1,000-mL distilled water). Mutant SA11, no color production; mutant SA189 and SB60, poor fruiting body production; JM4, wild-type strain.
The T-DNA flanking and full-length sequences of SA11, SA189, and SB60 were obtained by TAIL-PCR or RACE methods (Table 1)
Table 1. Homology analysis (BLASTx) of T-DNA-insertion sites in the mutants of Cordyceps militaris.
The assembled T-DNA flanking sequence from SA189 was homologous to ATP-dependent helicase gene from M.anisopliae (EFZ02692.1) and Penicillium marneffei (XP_002144718.1) (Table 1). The putative ATP-dependent helicase of C. militaris contained a DEXDc (DEAD-like helicases superfamily) domain and a HELICc (helicase superfamily c-terminal) domain, predicted in SMART database (Simple Modular Architecture Research Tool, http://smart.emblheidelberg.de/).
The T-DNA flanking sequence of SA60 was homologous to ubiquitin-like activating enzyme E1 gene from Verticillium alboatrum (XP_003009099.1). Reverse transcription-PCR was also carried out to confirm the cDNA sequence of ubiquitin-like activating enzyme gene in C. militaris (data not shown). The full-length sequences of ubiquitin-like activating enzyme E1 gene (accession No. JQ680978) between genomic DNA (1,804 bp) and cDNA (1,689 bp) were aligned and compared (Table 1).
From the T-DNA flanking sequence of mutant SA11, the full-length cytochrome oxidase subunit I gene (cox1, accession No. JQ680973) of C. militaris was obtained by TAIL-PCR, containing a 1,593 bp coding region of cox1 (CRcox1) interrupted by a 1,050 bp group I intron coding a LAGLIDADG endonuclease. The transcriptional analysis and molecular characterization of this gene was reported in our previous paper [4].
Mutants and genes involved in in vivo fruiting body production.
From 600 transformants, no mutant was detected to completely lose infection capacity to G. mellonella larvae, while 8 mutants were found with different infection outputs. Mutant SA125 was one of the mutants showing abnormal fruiting body production (no stromal production or no sclerotia formation) in infected G. mellonella larvae (Fig. 2). The mutant appeared not to fully use the cadavers as nutrients for development (Fig. 2). The full-length T-DNA flanking sequence of SA125 (accession No. JQ680975) was predicted to be homologous to serine/threonine phosphatase gene from M. anisopliae (EFY99037.1) (Table 1).
Fig. 2. In vivo fruiting body production of the insertional mutants of Cordyceps militaris in the infected Galleria mellonella larvae. Mutant SA125, no stromal production or sclerotia formation; JM4, wild-type strain.

Mutants and genes involved in cordycepin deficiency
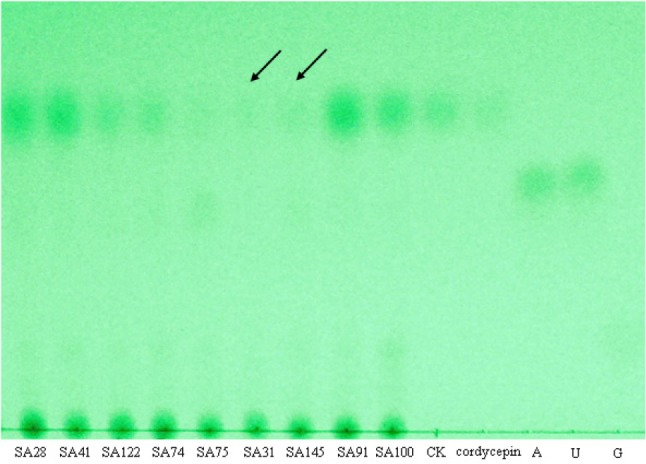
The cordycepin production of 600 mutants was determined by TLC for semi-quantitative analysis. The cordycepin contents of most mutants and wild type JM4 were higher than the standard concentration (0.5 mg/mL). However, the cordycepin contents of five mutants were much lower than that of wild type JM4. Mutant SA31 and SA145 were two of them showing cordycepin deficiency (Fig. 3).
Fig. 3. Cordycepin production determined by thin layer chromatography in the insertional mutants of Cordyceps militaris. Among mutants SA28, SA41, SA74, SA75, SA31, SA91, SA100, SA122, and SA145, the cordycepin contents of the mutants SA31 and SA145 were significantly lower than that of wild type JM4. The standard cordycepin, adenoside, uridine and guanine, were marked as cordycepin, A, U, and G. CK, wild type JM4.
The assembled T-DNA flanking sequences from SA31 and SA145 were predicted to be homologous to telomerase reverse transcriptase gene from M. anisopliae (EFZ00131.1) and glucose-methanol-choline oxidoreductase gene from M. anisopliae (EFY96931.1), respectively.
GO analysis
GO database was used to analyse the putative biological processes and molecular functions of the identified genes. Three genes (ATP-dependent helicase, ubiquitin-like activating enzyme E1 and cytochrome c oxidase subunit I) included the following five categories: metabolic process (GO:0008152), aerobic respiration (GO:0009060), electron transport chain (GO:0022900) and oxidation-reduction process (GO:0055114). Regarding molecular functions, they included the following categories: DNA binding (GO:0003677), ATP binding (GO:0005524), helicase activity (GO:0004386), catalytic activity (GO:0003824), protein binding (GO:0005515), zinc ion binding (GO:0008270), ligase activity (GO:0016874), metal ion binding (GO:0046872), cytochrome-c oxidase activity (GO:0004129), iron ion binding (GO:0005506), electron carrier activity (GO:0009055), and oxidoreductase activity (GO:0016491). The biological processes of the gene (glucose-methanol-choline oxidoreductase) related to the cordycepin production included RNA-dependent DNA replication (GO:0006278) and alcohol metabolic process (GO:0006066); while its molecular functions included DNA binding (GO:0003677), telomeric template RNA reverse transcriptase activity (GO:0003721), RNA binding (GO:0003723), RNA-directed DNA polymerase activity (GO:0003964), flavin adenine dinucleotide binding (GO:0050660), and oxidoreductase activity (acting on CH-OH group of donors) (GO:0016614). The serine/threonine phosphatase gene, influencing C. militaris fruiting body production in infected G. mellifera larvae, possessed the molecular functions of phosphoprotein phosphatase activity (GO:0004721) and hydrolase activity (GO:0016787).
DISCUSSION
From C. militaris mutant library, several genes involved in mutated characters, such as abnormal fruiting body production in artificial medium and infected insects, or lower cordycepin formation, were successfully identified. The results would provide useful information for the effective control of culture degeneration in commercial production of C. militaris.
Fungal culture degeneration is usually irreversible and inheritable, and can result in great commercial losses [19, 20]. In C. militaris, culture degeneration was reflected with poor fruiting body production. The degenerate strains usually showed significantly lower protease activity, chitinase activity, dehydrogenase activity, oxidative stress, decolorization activity or/and infection activity to Galleria larvae [21]. In the present study, three genes (cytochrome c oxidase subunit I, ATP-dependent helicase and ubiquitin-like activating enzyme E1) were identified to be involved in the poor fruiting body production in the artificial medium.
Cytochrome c oxidase subunit I, encoded by mitochondrial DNA (mtDNA), was the terminal component of the mitochondrial respiratory chain, transfers electrons from reduced cytochrome c to molecular oxygen. Fungi degeneration was closely related to mitochondria, including mitochondrial DNA alterations [9], mtDNA glycation [21] and decreasing activation of dehydrogenase in mitochondria [22]. It was conceivable that the disruption of cytochrome c oxidase subunit I with a group I intron [4] might affect the respiration and growth of C. militaris mutant. ATP-dependent helicase was implicated in many cellular processes, including translation initiation [23], and pre-mRNA splicing [24]. Several putative RNA helicases were found in ribosome biogenesis in Saccharomyce cerevisiae [25, 26]. The mutation of ATP-dependent RNA helicase caused a severe slow-growth phenotype in S. cerevisiae [25]. Here the ATP-dependent helicase mutant caused no fruiting body production of C. militaris. C. militaris also contained a 1,689 bp gene encoding putative ubiquitin-like activating enzyme E1, an essential gene with extensive sequence similarity to the E1 genes in other fungi. A ubiquitin-protein ligase (E3) specifically attached ubiquitin to the ε-amino group of a lysine residue in the target protein [27]. Ubiquitin-like proteins were signaling messengers that control many cellular functions, such as cell proliferation, apoptosis, the cell cycle and DNA repair [28]. It was interesting that ubiquitin-like activating enzyme E1 in C. militaris also controlled the fruiting body production.
No stromal production or sclerotia formation was found in G. mellonella larval cadavers infected by mutant SA125. A 1,347-bp serine/threonine phosphatase gene was involved in this mutated phenotype. Serine/threonine phosphatases usually control key biological pathways including early embryonic development, cell proliferation, cell death, circadian rhythm and cancer [28]. It was reported that the kinase homologue involved in fungal pathogenesis was required for full virulence in disparate hosts [29]. Although serine/threonine phosphatase gene did not influence the pathogenic ability of this fungus against G. mellonella larvae, it was involved in the fruiting body production in the infected insect larvae.
Cordycepin contents in mutant SA31 and SA145 were scarcely detected by TLC and obviously lower than that in wild type JM4. Two genes, encoding telomerase reverse transcriptase and glucose-methanol-choline oxidoreductase were involved in the cordycepin production in C. militaris. The study of telomerase reverse transcriptase mainly focused on human disease. Most telomerase reverse transcriptase gene variants reduced telomerase enzymatic activity in vitro. Loss-of-function telomerase gene variants associated with short telomeres were risk factors for sporadic cirrhosis [30]. The expression of telomerase reverse transcriptase in different parts of human body was found associated with cancer [31, 32]. Glucose-methanol-choline oxidoreductase played an important role of cyanohydrin formation and fungal degradation of lignin [33, 34]. It could also oxidize phenolic and nonphenolic benzyl alcohols in Bjerkandera [35]. Fungal pyranose oxidase, belonging to glucose-methanol-choline oxidoreductase family, was a flavoenzyme whose preferred substrate among several monosaccharides was D-glucose [36]. Cordycepin might involve in flavin adenine dinucleotide binding and oxidoreductase activity (acting on CH-OH group of donors) according to the gene GO analysis.
ACKNOWLEDGEMENTS
The work was supported by the Guangdong Province-Chinese Academy of Sciences Comprehensive Strategic Cooperation Project (2009B091300015), Research Project of Guangdong Province (2010A040301012) and Young Scientist Fund of Guangdong Academy of Sciences (qnjj201301).
References
- 1.Won SY, Park EH. Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J Ethnopharmacol. 2005;96:555–561. doi: 10.1016/j.jep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Tuli HS, Sharma AK, Sandhu SS, Kashyap D. Cordycepin: a bioactive metabolite with therapeutic potential. Life Sci. 2013;93:863–869. doi: 10.1016/j.lfs.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 3.Xie CY, Gu ZX, Fan GJ, Gu FR, Han YB, Chen ZG. Production of cordycepin and mycelia by submerged fermentation of Cordyceps militaris in mixture natural culture. Appl Biochem Biotechnol. 2009;158:483–492. doi: 10.1007/s12010-009-8567-2. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Z, Huang C, Cao L, Xie C, Han R. Agrobacterium tumefaciens-mediated transformation as a tool for insertional mutagenesis in medicinal fungus Cordyceps militaris. Fungal Biol. 2011;115:265–274. doi: 10.1016/j.funbio.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Hong IP, Kang PD, Kim KY, Nam SH, Lee MY, Choi YS, Kim NS, Kim HK, Lee KG, Humber RA. Fruit body formation on silkworm by Cordyceps militaris. Mycobiology. 2010;38:128–132. doi: 10.4489/MYCO.2010.38.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li TH, Lin QY, Song B, Huang H, Zhong YJ, Shen YH. The cultivation methold of Cordyceps militaris fruiting body by infecting Tenebrio molitor pupae. No.200510101348.0. China Patent. 2005 Beijing: China Government.
- 7.Han RC, Liu XF, Cao L, Chen JH. The cultivation methold of Cordyceps militaris fruiting body by infecting Gallerai mellifera larvae. No. 200610123355.5. China Patent. 2006 Beijing: China Government.
- 8.Lu JM, Zeng ZJ, He HQ. Culture technique of Cordyceps militaris on artificial media. Guangdong Agric Sci. 2005;2:88–89. [Google Scholar]
- 9.Wang C, Butt TM, St. Leger RJ. Colony sectorization of Metarhizium anisopliae is a sign of ageing. Microbiology. 2005;151(Pt 10):3223–3236. doi: 10.1099/mic.0.28148-0. [DOI] [PubMed] [Google Scholar]
- 10.Wang CS, St. Leger RJ. The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot Cell. 2007;6:808–816. doi: 10.1128/EC.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St. Leger RJ, Bidochka MJ, Roberts DW. Isoforms of the cuticle-degrading Pr1 proteinase and production of a metalloproteinase by Metarhizium anisopliae. Arch Biochem Biophys. 1994;313:1–7. doi: 10.1006/abbi.1994.1350. [DOI] [PubMed] [Google Scholar]
- 12.Tiago PV, Fungaro MH, Furlaneto MC. Cuticle-degrading proteases from the entomopathogen Metarhizium flavoviride and their distribution in secreted and intracellular fractions. Lett Appl Microbiol. 2002;34:91–94. doi: 10.1046/j.1472-765x.2002.01064.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Liu X, Wang M. Cloning, expression, and characterization of two novel cuticle-degrading serine proteases from the entomopathogenic fungus Cordyceps sinensis. Res Microbiol. 2008;159:462–469. doi: 10.1016/j.resmic.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Mao XB, Zhong JJ. Hyperproduction of cordycepin by two-stage dissolved oxygen control in submerged cultivation of medicinal mushroom Cordyceps militaris in bioreactors. Biotechnol Prog. 2004;20:1408–1413. doi: 10.1021/bp049765r. [DOI] [PubMed] [Google Scholar]
- 15.Jiapeng T, Yiting L, Li Z. Optimization of fermentation conditions and purification of cordycepin from Cordyceps militaris. Prep Biochem Biotechnol. 2014;44:90–106. doi: 10.1080/10826068.2013.833111. [DOI] [PubMed] [Google Scholar]
- 16.Kang C, Wen TC, Kang JC, Meng ZB, Li GR, Hyde KD. Optimization of large-scale culture conditions for the production of cordycepin with Cordyceps militaris by liquid static culture. Sci World J. 2014;2014:510627. doi: 10.1155/2014/510627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad R, Milagre HM, Catharino RR, Eberlin MN. Easy ambient sonic-spray ionization mass spectrometry combined with thin-layer chromatography. Anal Chem. 2008;80:2744–2750. doi: 10.1021/ac702216q. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Liu AY, Liang ZQ. Biological activity and determination method of cordycepin. Acta Edulis Fungi. 2002;9:57–62. [Google Scholar]
- 19.Li A, Begin M, Kokurewicz K, Bowden C, Horgen PA. Inheritance of strain instability (sectoring) in the commercial button mushroom, Agaricus bisporus. Appl Environ Microbiol. 1994;60:2384–2388. doi: 10.1128/aem.60.7.2384-2388.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan MJ, Bridge PD, Smith D, Jeffries P. Phenotypic degeneration occurs during sector formation in Metarhizium anisopliae. J Appl Microbiol. 2002;93:163–168. doi: 10.1046/j.1365-2672.2002.01682.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin QQ, Qiu XH, Zheng ZL, Xie CH, Xu ZF, Han RC. Characteristics of the degenerate strains of Cordyceps militaris. Mycosystema. 2010;29:670–677. [Google Scholar]
- 22.Li L, Pischetsrieder M, St. Leger RJ, Wang C. Associated links among mtDNA glycation, oxidative stress and colony sectorization in Metarhizium anisopliae. Fungal Genet Biol. 2008;45:1300–1306. doi: 10.1016/j.fgb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Pause A, Sonenberg N. Helicases and RNA unwinding in translation. Curr Opin Struct Biol. 1993;3:953–959. [Google Scholar]
- 24.Rymond BC, Rosbash M. Yeast pre-mRNA splicing. In: Jones EW, Pringle JR, Broach JR, editors. The molecular and cellular biology of the yeast Saccharomyces: gene expression. New York: Cold Spring Harbor; 1992. pp. 143–192. [Google Scholar]
- 25.Daugeron MC, Linder P. Dbp7p, a putative ATP-dependent RNA helicase from Saccharomyces cerevisiae, is required for 60S ribosomal subunit assembly. RNA. 1998;4:566–581. doi: 10.1017/s1355838298980190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kressler D, de la Cruz J, Rojo M, Linder P. Dbp6p is an essential putative ATP-dependent RNA helicase required for 60S-ribosomal-subunit assembly in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:1855–1865. doi: 10.1128/mcb.18.4.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoeller D, Hecker CM, Dikic I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat Rev Cancer. 2006;6:776–788. doi: 10.1038/nrc1994. [DOI] [PubMed] [Google Scholar]
- 28.Gallego M, Virshup DM. Protein serine/threonine phosphatases: life, death, and sleeping. Curr Opin Cell Biol. 2005;17:197–202. doi: 10.1016/j.ceb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Mylonakis E, Idnurm A, Moreno R, El Khoury J, Rottman JB, Ausubel FM, Heitman J, Calderwood SB. Cryptococcus neoformans Kin1 protein kinase homologue, identified through a Caenorhabditis elegans screen, promotes virulence in mammals. Mol Microbiol. 2004;54:407–419. doi: 10.1111/j.1365-2958.2004.04310.x. [DOI] [PubMed] [Google Scholar]
- 30.Calado RT, Brudno J, Mehta P, Kovacs JJ, Wu C, Zago MA, Chanock SJ, Boyer TD, Young NS. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology. 2011;53:1600–1607. doi: 10.1002/hep.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Zhang B, Wong N, Lo AW, To KF, Chan AW, Ng MH, Ho CY, Cheng SH, Lai PB, et al. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res. 2011;71:4138–4149. doi: 10.1158/0008-5472.CAN-10-4274. [DOI] [PubMed] [Google Scholar]
- 32.Porika M, Tippani R, Bollam SR, Panuganti SD, Thamidala C, Abbagani S. Serum human telomerase reverse transcriptase: a novel biomarker for breast cancer diagnosis. Int J Clin Oncol. 2011;16:617–622. doi: 10.1007/s10147-011-0230-6. [DOI] [PubMed] [Google Scholar]
- 33.Dreveny I, Andryushkova AS, Glieder A, Gruber K, Kratky C. Substrate binding in the FAD-dependent hydroxynitrile lyase from almond provides insight into the mechanism of cyanohydrin formation and explains the absence of dehydrogenation activity. Biochemistry. 2009;48:3370–3377. doi: 10.1021/bi802162s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira P, Hernandez-Ortega A, Herguedas B, Martínez AT, Medina M. Aryl-alcohol oxidase involved in lignin degradation: a mechanistic study based on steady and pre-steady state kinetics and primary and solvent isotope effects with two alcohol substrates. J Biol Chem. 2009;284:24840–24847. doi: 10.1074/jbc.M109.011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero E, Ferreira P, Martínez AT, Martínez MJ. New oxidase from Bjerkandera arthroconidial anamorph that oxidizes both phenolic and nonphenolic benzyl alcohols. Biochim Biophys Acta. 2009;1794:689–697. doi: 10.1016/j.bbapap.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Albrecht M, Lengauer T. Pyranose oxidase identified as a member of the GMC oxidoreductase family. Bioinformatics. 2003;19:1216–1220. doi: 10.1093/bioinformatics/btg140. [DOI] [PubMed] [Google Scholar]


