Abstract
The present study is the first report on the isolation of Penicillium menonorum from rhizosphere soil in Korea and its identification based on morphological characteristics and internal transcribed spacer gene sequence. The fungal isolate was named KNU-3 and was found to exhibit plant growth-promoting (PGP) activity through indole acetic acid (IAA) and siderophore production, as well as P solubilization. KNU-3 produced 9.7 mg/L IAA and solubilized 408 mg of Ca3PO4/L, and inoculation with the isolate significantly (p < 0.05) increased the dry biomass of cucumber roots (57%) and shoots (52%). Chlorophyll, starch, protein, and P contents were increased by 16%, 45%, 22%, and 14%, respectively, compared to plants grown in uninoculated soil. The fungus also increased soil dehydrogenase (30%) and acid phosphatase (19%) activities. These results demonstrate that the isolate KNU-3 has potential PGP attributes, and therefore it can be considered as a new fungus to enhance soil fertility and promote plant growth. Moreover, the discovery of PGP ability and traits of this fungus will open new aspects of research and investigations. In this study, plant growth promotion by P. menonorum KNU-3 is reported for the first time in Korea after its original description.
Keywords: Fungi, Molecular diversity, Morphology, Penicillium menonorum, Sequence analysis
The growing world population and the increasing demand for crop production have led to excessive use of pesticides and inorganic fertilizers worldwide. The application of chemical fertilizers causes an increase in crop yield, but large quantities of these chemicals are deposited in the soil, thereby posing a potential health hazard and affecting the microbial soil population. Furthermore, the fertilizers are highly costly and therefore are associated with an increased cost of production. Current trends in agriculture are focused on the reduction of the use of inorganic fertilizers, and the use of plant growth-promoting (PGP) microorganisms for alternative ways to improve a more sustainable agriculture [1, 2]. PGP inoculants have been employed as biofertilizers, as supplements to chemical fertilizers, in the production of field, vegetable, and forage crops [2, 3]. Among the PGP microorganisms, fungi are important components of the soil microbiota and typically constitute more of the soil biomass than bacteria [4].
Rhizosphere- and root-associated fungi have been known to benefit plants and are hence referred to as plant growth-promoting fungi. Penicillium species are widely distributed in nature across diverse soil environments such as cultivated soil, forest soil, desert soil, beach soil, and marine habitats [5, 6]. Penicillium species have received significant attention in the production of bioactive compounds, including mycotoxins, antibiotics, herbicides, antioxidants, insecticides, and anticancer compounds [7], as well as extracellular enzymes and crops [8]. Recent studies have reported that some species of Penicillium promote plant growth by one of several different mechanisms, such as the production of PGP phytohormones (gibberellins, auxin, cytokinin, and siderophores), solubilization of minerals, and antagonism to phytopathogens [9, 10, 11]. Previous studies have revealed that inoculation of soil with PGP Penicillium species significantly promotes the growth, P uptake, and yield of several important crops, including wheat [9], sesame [11], and pomegranate [3].
Approximately 100 Penicillium species have been recorded in Korea [12]. Many of these species were isolated from soil and marine habitats [6], and some were identified as harmful pathogens that can cause spoilage and decay of plant products [13]. Other species were characterized as plant growth promoters by producing secondary metabolites and colonizing plant roots and/or soils. In the present study, we report the isolation of Penicillium menonorum from crop field soil for the first time in Korea. Furthermore, we report its PGP attributes and its positive effect on the growth enhancement of cucumber plants.
MATERIALS AND METHODS
Fungus isolation and identification.
Soil samples were collected (0~15 cm depth) from crop field soil at Jeongseon (37°22'45'' N, 128°39'53'' E), Gangwon-do, Korea. Fungi were isolated by conventional dilution and the growth medium was supplemented with 100 µg chloramphenicol (bacteriostat) per mL potato dextrose agar (PDA; Difco Laboratories, Detroit, MI, USA). After incubating the plates at 27℃ for 3~15 days, fungal isolates were tentatively identified based on the differing morphology upon subculturing to the genus level based on taxonomic keys [14]. Pure cultures were maintained on PDA slants and stored at 4℃. A pure culture of Penicillium sp. KNU-3 showing a definitive similarity match for P. menonorum was identified based on its morphological description provided by Peterson et al. [15], and selected for further internal transcribed spacer (ITS) gene sequence analysis.
Molecular identification.
Genomic DNA of the strain KNU-3 was extracted using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The ITS regions, including the 5.8S, were amplified with the primers ITS1 and ITS4 [16]. The amplified PCR product (619 bp) was purified using a QIAquick PCR Purification Kit (Qiagen, Valencia, CA, USA) following the manufacturer's recommendations. The PCR product was sequenced with an ABI Prism 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA). The sequence was compared with reference ITS1~ITS4 rDNA sequences in GenBank using BLAST analysis ( http://www.ncbi.nlm.nih.gob/blast). The nucleotide sequence reported here has been deposited at NCBI-GenBank (accession No. KJ921605). The sequences of closely related strains were aligned using MultAlin program. Phylogenetic analysis was carried out by the neighbor-joining method using MEGA software [17] with the Kimura 2-parameter model. The robustness of the tree was evaluated by 1,000 bootstrap replications.
Screening for PGP properties.
The fungus KNU-3 was further screened for PGP traits that could be associated with its ability to promote plant growth. Production of the phytohormone indoleacetic acid (IAA) in the presence of tryptophan was estimated as described by Babu et al. [18]. Briefly, plugs of the fungus with a diameter of 4 mm were inoculated into potato dextrose broth (PDB; diluted 1 : 7) supplemented with 100 mg/L of filter-sterilized tryptophan (Sigma-Aldrich, St. Louis, MO, USA) and incubated for 7 days on a rotary shaker (170 rpm) at 28 ± 2℃. The supernatants were obtained by centrifugation at 12,000 rpm for 10 min. One milliliter of the supernatant was mixed with 2 mL of Salkowsky's reagent (50 mL 35% perchloric acid, 1 mL 0.5M FeCl3), incubated at room temperature for 20 min, and the absorbance was read at 535 nm. IAA was estimated using a standard curve prepared by serial dilution of 100 µg/mL IAA solution in PDB (diluted 1 : 7). Fungal 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity was estimated [19]. Siderophore production was determined using modified Chrome azurol S (CAS) agar medium [20].
Quantitative estimation of phosphate solubilization was conducted in Pikovskya's broth [21]. One milliliter homogenized mycelium suspension grown for 7 days was inoculated to 100-mL broth in 250-mL conical flasks. Flasks were incubated on a rotary shaker at 130 rpm and 30℃ for 8 days. Experiments were conducted in triplicate and the uninoculated broth served as the control. Samples were collected at prescribed time intervals (2, 4, 6, and 8 days) and the mixtures were centrifuged at 12,000 rpm for 5min. Five milliliters of supernatant were immediately filtered through a 0.2-µm membrane, and soluble P in the culture filtrate was determined [18]. Periodic pH estimation of the culture filtrate was performed using a digital pH meter.
Pot experiment.
Effects of KNU-3 on cucumber growth were studied in earthen pots (50 cm diameter, 20 cm height) containing 2 kg of soil (60% field soil and 40% commercial soil, w/w). The dried soil had the following composition: loamy sand (78.3% sand, 7.2% silt, and 14.5% clay), 0.36% total organic C, 2.47 mg/kg available N, 0.97 mg/kg available P, 8.9 mg/kg available K, and 8.27 mg/kg available Mg. The pH of the dry soil was 5.1. Cucumber (Cucumis sativus L.) seeds were disinfected by dipping in 2% NaOCl solution for 5 min and then washed four times with sterile double-distilled water. Three seeds were sown (3.0 cm deep) in each pot with or without 10 g of fungus inoculum (10-mL fungus suspension mixed with 10 g autoclaved commercial soil). The fungus inoculum was applied near the seeds during seeding. Treatments were replicated 12 times in a completely randomized block design. After 1 wk, the seedlings were thinned to two per pot. Seedlings were grown in an open environment, using tap water to maintain 70% of soil water-holding capacity. After 4 wk, plants were removed from the pots; roots and shoots were separated, and repeatedly washed in deionized water. The leaf chlorophyll content was measured using a SPAD-502 chlorophyll meter (Konica Minolata, Tokyo, Japan). The total soluble protein content in the leaf was estimated according to the Bradford method [22] and starch was estimated by using phenol sulfuric acid reagent [23]. Shoot and root dry weights were measured after oven drying at 60℃ to a constant weight. The P concentration in dried leaves (dried at 65℃) was measured [24]. Soil available P [25] and the acid phosphatase [26] and dehydrogenase [27] enzyme activities were estimated.
Statistical analysis.
Data were subjected to an analysis of variance (ANOVA) and means were compared using Tukey's test at p < 0.05. Statistical analyses were performed using SigmaPlot (Systat Software Inc., San Jose, CA, USA).
Results
Fungus isolation and identification.
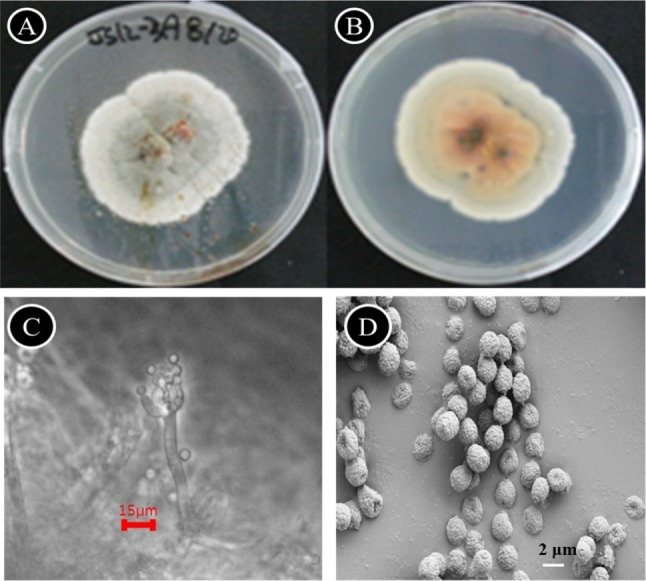
A large number of different microfungi were isolated and tentatively identified based on phenotypic data (data not shown). Among these fungi, the Penicillium sp. KNU-3 showed distinct morphology and was selected and studied in detail. The fungus colonies on PDA (Fig. 1A) attained 14~18 mm (maximum, 20 mm) diameters after 7 days growth at 25℃. The vegetative hyphae were velutinous-silky, radially sulcate peripherally, and centrally raised by approximately 2~3 mm. Sporulation was moderate, the central region was pale bluish to dark gray, the peripheral area was white, and a clear rosy exudate was observed in the center. No sclerotia or ascomata appeared. The colony reverse was yellow with a yellow-brown or reddish-brown center. Conidiophores (Fig. 1C) were simple, arising from basal and aerial hyphae, smooth-walled, hyaline, 5~15 (~20) × 1.5~2.0 µm, nonvesiculate, with an apical whorl of (1~) 2~5 fields 5~7 (~9) by 2.5~3.5-µm-bearing conidia. Conidia were spherical to subspherical, (2~) 2.5~3.5 µm (Fig. 1D), with rugose surface. In order to confirm the morphological results, the isolate KNU-3 was further identified through analysis of the rDNA ITS region.
Fig. 1. Penicillium menonorum KNU-3. A, Colonies grown 7 d at 25℃ on potato dextrose agar showing a pale blue-gray color in the central area where the fungus is sporulating; B, Colony showing white in reverse; C, Conidiophores, phialides, and conidia; D, Conidia (roughened conidia) (scale bars: C = 15 µm, D = 2 µm).
Molecular identification.
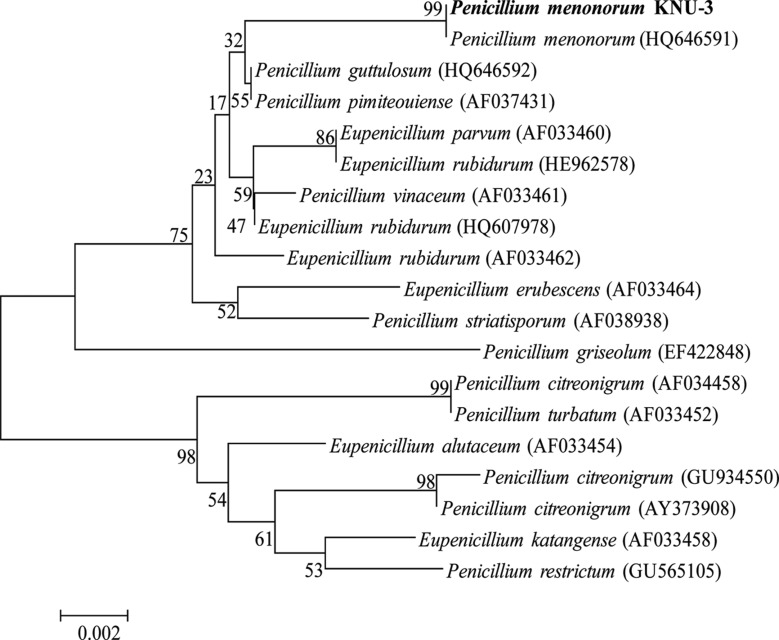
The ITS sequence of the P. menonorum KNU-3 isolate was 100% identical to the culture collection NRRL: 50410 of P. menonorum S. W. Peterson sp. nov (GenBank accession No. HQ646591). The phylogenetic analysis of KNU-3 was carried out by distance tree construction. The ITS1 sequence of KNU-3 was aligned with sequences of available P. menonorum strains and its nearest species through the BLAST sequence using MultAlin program, and a neighbor-joining tree was constructed from the aligned sequences. The phylogenetic analysis revealed that the isolate was grouped with a reference isolate of P. menonorum with 99% bootstrap value support (Fig. 2), and was distinct from other Penicillium spp., which were grouped in distinct clades. These results indicate that the isolate KNU-3 is closely related to P. menonorum. Based on morphological properties, sequence homology, and phylogenetic analysis, the isolate KNU-3 was thus identified as P. menonorum.
Fig. 2. Neighbor-joining phylogenetic analysis of the Penicillium menonorum partial 18S-ITS1-5.8S-ITS2-28S rDNA region sequence obtained from crop field soil in Korea. The sequence obtained in this study is shown in boldface. Numerical values on branches are the bootstrap values as percentage of bootstrap replication from 1,000 replicate analysis. The scale represents substitution per site.
PGP traits.
P. menonorum KNU-3 was able to produce siderophores in the plate-based assay as evidenced by the formation of a yellow zone on the CAS agar medium plate (Supplementary Fig. 1A). While the fungal isolate produced a significant amount of IAA (9.7 mg/L), no ACC deaminase activity was detected. As shown in Table 1, P-solubilization increased from day 2 to day 6 with a value ranging from 198 to 408 mg/L (Supplementary Fig. 1B). The pH of the medium decreased up to 6 days ranging from 6.2 to 5.1 with an increase of soluble P. After 5 days of incubation, soluble P decreased slowly with a concomitant slight pH increase of the medium (Table 1).
Table 1. Amount of P solubilization and change of pH in liquid culture medium supplemented with tri-calcium phosphate (TCP) and inoculated with Penicillium menonorum KNU-3a.
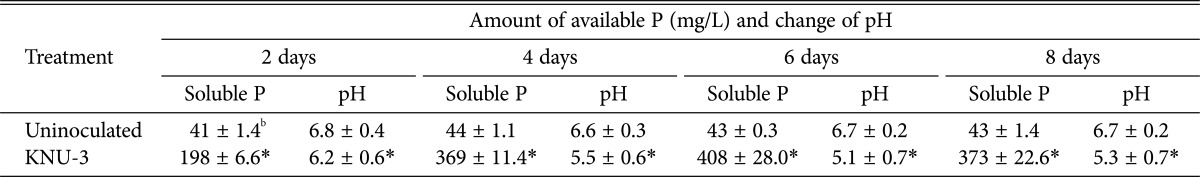
aThe experiment were performed twice with three replicates for each treatment.
bMean and standard deviation. An asterisk (*) indicates a significant difference between uninoculated liquid culture medium and liquid culture medium inoculated with KNU-3 (p < 0.05).
Plant growth promotion and soil enzyme activity.
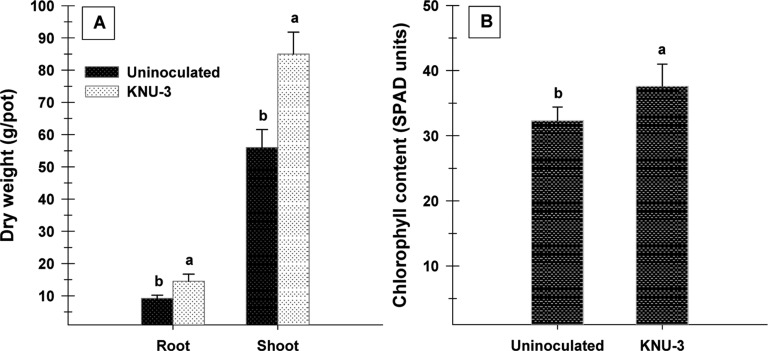
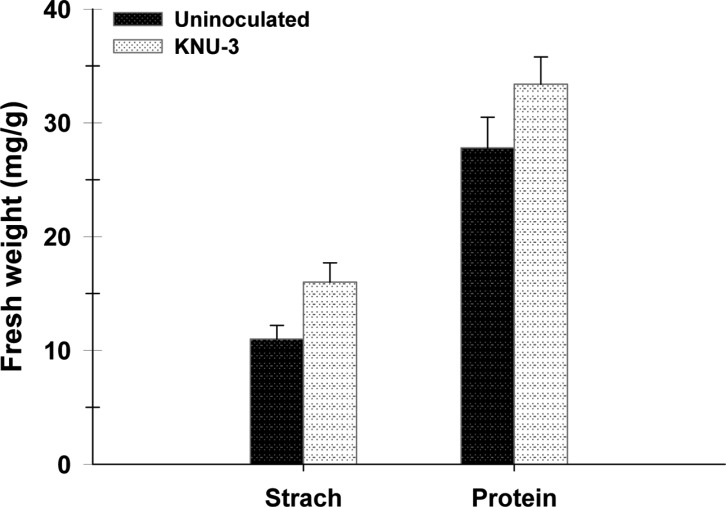
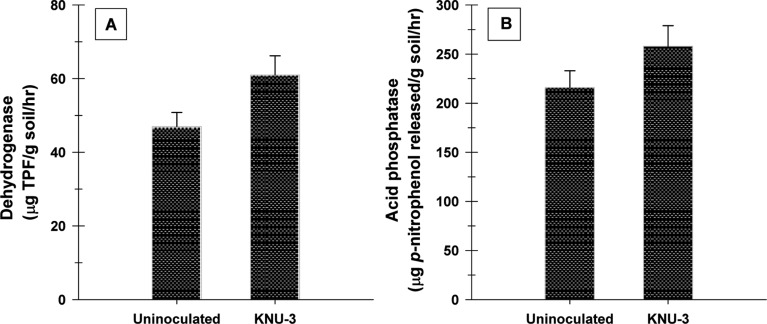
The isolate promoted root and shoot growth of cucumber plants, with a concomitant increase in the dry biomass of the shoots and roots compared with the uninoculated controls (Fig. 3A). Inoculation also resulted in a significantly higher total chlorophyll content (16%) (Fig. 3B) as well as an accumulation of total starch (45%) and protein content (22%) (Fig. 4). KNU-3 inoculation significantly increased the total P content (510 mg/L) in plant shoots compared to uninoculated controls (460 mg/L). Soil inoculation with KNU-3 resulted in significantly enhanced levels of organic carbon (3.9%) and available P (3.4mg/L) of the soil compared to that of uninoculated soil organic carbon (4.6%) and available P (2.9mg/L). The enzyme activity of dehydrogenase gives an indication of an active microbial population in the soil. Inoculation with KNU-3 induced a significant increase in dehydrogenase activity (30%) compared with uninoculated soil (Fig. 5A). In addition, a significant elevated acid phosphatase activity (19%) was observed in soil inoculated with KNU-3 compared to the uninoculated soil (Fig. 5B).
Fig. 3. Dry biomass (A) and leaf chlorophyll content (B) of cucumber plants grown in uninoculated soil and in soil inoculated with Penicillium menonorum KNU-3. Error bars indicate standard deviations of the means (n = 12). Bars with the same letter are not significantly different at p < 0.05 according to Tukey's test.
Fig. 4. Starch and protein content in leaves of cucumber plants grown in uninoculated soil and in soil inoculated with Penicillium menonorum KNU-3. Error bars indicate standard deviations of the means (n = 12). Bars with the same letter are not significantly different at p < 0.05 according to Tukey's test.
Fig. 5. Dehydrogenase (A) and acid phosphatase (B) activities in uninoculated soil and in soil inoculated with Penicillium menonorum KNU-3. Error bars indicate standard deviations of the means (n = 12). Bars with the same letter are not significantly different at p < 0.05 according to Tukey's test. TPF, triphenylformazan.
Discussion
Numerous microfungi were isolated in the present study to investigate the fungal community in Jeongseon, Gangwon-do, Korea. Among these isolates, one Penicillium sp. showed definitive similarity matches with P. menonorum. In order to confirm the morphological results, molecular identification of the isolate was carried out based on ITS sequence analysis. The ITS sequence of the isolate was compared to the GenBank database sequences by using the NCBI-BLAST search program, and it was found to be identical to the type strain NRRL: 50410 of P. menonorum S.W. Peterson sp. nov. In addition, the phylogenetic tree revealed that the isolate was grouped in a distinct clade together with P. menonorum with bootstrap support value of 100% (Fig. 2). Morphological and molecular characteristics of the isolate were in agreement with the description by Peterson et al. [15]. P. menonorum S.W. Peterson sp. nov. is a phenotypically and genotypically broadly circumscribed new species that was first isolated from garden soil in southern California by Peterson et al. [15], and P. menonorum isolation from Panax ginseng has been reported in China [28]. Nevertheless, to our knowledge, this study presents the first record of P. menonorum in Korea.
Many microbial-based agricultural techniques have been developed to exploit potential mechanisms for the enhancement of crop production in different soils. Although members of the genus Penicillium are common opportunistic pathogens in the soil environment, some Penicillium strains associated with the different plant rhizospheres are able to solubilize inorganic insoluble P salts and promote plant growth in the soil. Thus, the growth-promoting capacity of P. menonorum KNU-3 was evaluated by assaying for IAA and siderophore production, ACC deaminase activity, and P solubilization. IAA secreted by microorganisms promotes root growth through a direct stimulatory effect on plant cell elongation or cell division [29]. In the present study, P. menonorum KNU-3 was positive for both IAA and siderophore production. Other researchers have made similar observations for the IAA production by Penicillium sp. [11]. Siderophores are important metabolites released by the PGP microorganisms that directly alleviate iron deficiency by increasing the supply of iron to the plant and indirectly depriving fungal pathogens of iron [30, 31]. Kang et al. [32] reported that siderophores are also able to chelate Mg2+, Ca2+, and Al3+ from a soil solution. In this study, the isolate showed both IAA production and the presence of siderophores, which may provide an explanation for its PGP activity. Several studies have demonstrated that production of siderophores, other secondary metabolites, and lytic enzymes by Penicillium spp. were most effective in promoting plant growth and controlling plant root pathogens.
The inoculation of P-solubilizing fungi in P-deficient soil has been reported to increase the available P content in the soil and the P uptake in plants. Thus, the efficiency of P. menonorum for its phosphate-solubilizing activity was quantitatively estimated at different time intervals in liquid medium during the in vitro study. The level of P availability in the culture filtrate was significantly increased by decreasing the pH of the medium up to the 5th day (Fig. 2). However, a significant drop in soluble P levels was observed on later days (Fig. 2). This could be due to the depletion of nutrients in the culture medium [32], limiting the availability of the soluble form of P, which has an inhibitory effect on further P solubilization [33]. In addition, the formation of an organo-phosphate compound induced by the release of organic metabolites might have reduced the amount of available P [34]. In this study, phosphate solubilization was accompanied by a decrease in the pH of the medium. The results of this study are in agreement with other studies on the effect of pH on the solubilization of the P source.
The decrease in pH indicates the production of organic acids, which chelate cations through their carboxylic acid group and convert it into the soluble form. Nonetheless, phosphate-solubilizing microorganisms solubilize inorganic phosphates by several mechanisms, including the production of organic acids [35], polysaccharides [36], and phosphatase enzymes (mainly acid phosphatases) [37]. Periodic estimation of available P in the medium revealed the potential of the isolate to release P from insoluble phosphate sources from agricultural soil. In addition to P solubilization, KNU-3 also produced other secondary metabolites such as IAA and siderophores. Evidence pointing to plant growth promotion by P-solubilizing microorganisms through the production of IAA and siderophores renders the P-solubilizing microorganisms more suitable as biofertilizers [29].
Based on plant growth promoting traits, a pot experiment was conducted to test the effect of KNU-3 inoculation on concurrent plant growth promotion in the cucumber plants. Soil inoculation with KNU-3 significantly increased the dry biomass by 53%. It is expected that inoculation with KNU-3 exhibiting PGP traits consequently promotes the growth promotion of the soil-plant system. Inoculation resulted in significantly higher chlorophyll contents of cucumber plants. Our results agree with a previous report of significant increases in chlorophyll content in leaves of pomegranate [3] after inoculation with P. pinophilum. Rasouli-Sadaghiani et al. [38] suggested that the enhanced chlorophyll content may be correlated with higher iron acquisition, and that this can be considered as a marker of iron availability to the plant system.
Both starch and protein contents are important measurements for plant health and fruit yield. In this study, the P. menonorum inoculation significantly increased the starch and protein contents of the plants compared with the control plants. The main mechanisms potentially involved are the mobilization of insoluble sources of P-bearing minerals and subsequent enhancement of uptake and production of siderophores/plant growth regulators that enhance photosynthesis rate and plant growth, which results in higher starch and protein contents in cucumber plants. The beneficial effects of certain Penicillium spp. in terms of plant growth promotion and biological control have been reported by many researchers. Recently, Radhakrishnan et al. [11] reported enhancement of shoot length, root length, and fresh and dry seedling weight of sesame plants under salt stress when co-cultivated with Penicillium sp.. Soil inoculation with P. pinophilum was found to increase N, P, and K uptake, which resulted in improved growth, significantly higher leaf area index, and an elevated photosynthetic rate of pomegranate plant [3]. However, to the best of our knowledge, this is the first study to demonstrate that P. menonorum can increase chlorophyll, starch, and protein contents, as well as the yield of cucumber plants.
The organic content in soil samples has been considered one of the key determinants driving the microbial community structure [39]. The increased amount of organic carbon in KNU-3-inoculated soil indicates decomposition of organic wastes, organic matter formation, and nutrient cycling by KNU-3 or other microorganisms in the rhizospheric soil. Because P in soil is likely to be fixed, it is usually present in trace amounts and is relatively unavailable to plants. The increase in P uptake by cucumber plants and higher concentrations of available P in soil reported in this study might be due to solubilization of insoluble P present in the soil by phosphate-solubilizing P. menonorum, thereby enhancing the P uptake. It has been reported that P-solubilizing Penicillium spp. are capable of solubilizing the unavailable forms of P in P-bearing minerals in soil through the production and excretion of organic acids, phosphatase, and phytase enzymes [3, 40].
Soil enzymes are important catalyzers of several reactions required for life processes of microorganisms in soils and the stabilization of soil structure, the decomposition of organic wastes, organic matter formation, and nutrient cycling [41]. Among the soil enzymes, dehydrogenase and acid phosphatase play an important role in oxidative metabolism, and thus in determining the metabolic activity of microorganisms and P-mineralization in soils, respectively. In this study, dehydrogenase and acid phosphatase activities were increased in KNU-3-inoculated treatments compared with the non-inoculated control treatment. These results confirm earlier reports demonstrating greater P uptake by plants, mobilization of insoluble P, dehydrogenase, and acid phosphatase activity in soil when inoculated with Penicillium pinophilum [3]. The enhanced enzymatic activities in soil is associated with an increase in the availability of nutrients to the plants, which in turn have a positive influence on soil fertility [42]. KNU-3 was capable of increasing the organic carbon, available P, and enzyme activities, which are all beneficial characteristics to improve crop productivity in sustainable agriculture.
The present study has established that P. menonorum exhibits not only plant growth promoting attributes in vitro, but also has a positive effect on the growth of cucumber plants than the non-inoculated plants in in vivo. This is the first report of P. menonorum isolated from crop field soil in Korea. Furthermore, it is the first time we report plant growth promoting attributes of P. menonorum and its positive effect on the growth enhancement of cucumber plants in in vitro. Summarizing the discussion above, it can be concluded that the P. menonorum species can be used as a biofertilizer after the conduction of further extensive field trials.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Biological Resources (NIBR) under the Ministry of Environment, Republic of Korea, for the project on survey and excavation of Korean indigenous fungal species and University-Industry Cooperation Foundation of Kangwon National University, Korea. We would like to express our sincere thanks to Hongcheon Agriculture Technology Center for providing the soil sample.
ELECTRONIC SUPPLEMENTARY MATERIAL
Supplementary data including one figure can be found with this article online at http://www.mycobiology.or.kr/src/sm/mb-43-49-s001.pdf.
Supplementary Material
Characterization of plant growth promoting Penicillium menonorum: A, CAS agar plate detection of siderophores produced by KNU-3; B, P-solubilization in control vs. KNU-3 in Pikovskaya medium.
References
- 1.Smit E, Leeflang P, Gommans S, Van den Broek J, Van Mil S, Wernars K. Diversity and seasonal fluctuations of the dominant members of the bacterial soil community in a wheat field as determined by cultivation and molecular methods. Appl Environ Microbiol. 2001;67:2284–2291. doi: 10.1128/AEM.67.5.2284-2291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pešaković M, Karaklajić-Stajić Ž, Milenković S, Mitrović O. Biofertilizer affecting yield related characteristics of strawberry (Fragaria × ananassa Duch.) and soil micro-organisms. Sci Hortic. 2013;150:238–243. [Google Scholar]
- 3.Maity A, Pal RK, Chandra R, Singh NV. Penicillium pinophilum: a novel microorganism for nutrient management in pomegranate (Punica granatum L.) Sci Hortic. 2014;169:111–117. [Google Scholar]
- 4.Malviya J, Singh K, Joshi V. Effect of phosphate solubilizing fungi on growth and nutrient uptake of ground nut (Arachis hypogaea) plants. Adv Biores. 2011;2:110–113. [Google Scholar]
- 5.Christensen M, Frisvad JC, Tuthill DE. Penicillium species diversity in soil and some taxonomic and ecological notes. In: Samson RA, Pitt JI, editors. Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Amsterdam: Harwood Academic Publishers; 2000. pp. 309–320. [Google Scholar]
- 6.Park MS, Fong JJ, Oh SY, Kwon KK, Sohn JH, Lim YW. Marine-derived Penicillium in Korea: diversity, enzyme activity, and antifungal properties. Antonie Van Leeuwenhoek. 2014;106:331–345. doi: 10.1007/s10482-014-0205-5. [DOI] [PubMed] [Google Scholar]
- 7.Frisvad JC, Samson RA. Polyphasic taxonomy of Penicillium subgenus Penicillium: a guide to identification of the food and air-borne terverticillate Penicillia and their mycotoxins. Stud Mycol. 2004;49:1–174. [Google Scholar]
- 8.Tiwari KL, Jadhav SK, Fatima A. Culture condition for the production of thermostable amylase by Penicillium rugulosum. Glob J Biotechnol Biochem. 2007;2:21–24. [Google Scholar]
- 9.Wakelin SA, Anstis ST, Warren RA, Ryder MH. The role of pathogen suppression on the growth promotion of wheat by Penicillium radicum. Aust Plant Pathol. 2006;35:253–258. [Google Scholar]
- 10.Nath R, Sharma GD, Barooah M. Efficiency of tricalcium phosphate solubilization by two different endophytic Penicillium sp. isolated from tea (Camellia sinensis L.) Eur J Exp Biol. 2012;2:1354–1358. [Google Scholar]
- 11.Radhakrishnan R, Shim KB, Lee BW, Hwang CD, Pae SB, Park CH, Kim SU, Lee CK, Baek IY. IAA producing Penicillium sp. NICS01 triggers plant growth and suppresses Fusarium sp.-induced oxidative stress in sesame (Sesamum indicum L.) J Microbiol Biotechnol. 2013;23:856–863. doi: 10.4014/jmb.1209.09045. [DOI] [PubMed] [Google Scholar]
- 12.Min YJ, Park MS, Fong JJ, Quan Y, Jung S, Lim YW. Diversity and saline resistance of endophytic fungi associated with Pines thunbergii in coastal shelterbelts of Korea. J Microbiol Biotechnol. 2014;24:324–333. doi: 10.4014/jmb.1310.10041. [DOI] [PubMed] [Google Scholar]
- 13.Korean Society of Plant Pathology. List of plant diseases in Korea. 5th ed. Suwon: Korean Society of Plant Pathology; 2009. [Google Scholar]
- 14.Pitt JI, Hocking AD. Fungi and food spoilage. 3rd ed. New York: Springer-Dordrecht; 2009. pp. 194–173. [Google Scholar]
- 15.Peterson SW, Orchard SS, Menon S. Penicillium menonorum, a new species related to P. pimiteouiense. IMA Fungus. 2011;2:121–125. doi: 10.5598/imafungus.2011.02.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govinda Rajulu MB, Thirunavukkarasu N, Babu AG, Aggarwal A, Suryanarayanan TS, Reddy MS. Endophytic Xylariaceae from the forests of Western Ghats, southern India: distribution and biological activities. Mycology. 2013;4:29–37. [Google Scholar]
- 17.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 18.Babu AG, Shim J, Bang KS, Shea PJ, Oh BT. Trichoderma virens PDR-28: a heavy metal-tolerant and plant growth-promoting fungus for remediation and bioenergy crop production on mine tailing soil. J Environ Manage. 2014;132:129–134. doi: 10.1016/j.jenvman.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Gravel V, Antoun H, Tweddell RJ. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA) Soil Biol Biochem. 2007;39:1968–1977. [Google Scholar]
- 20.Milagres AM, Machuca A, Napoleão D. Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J Microbiol Methods. 1999;37:1–6. doi: 10.1016/s0167-7012(99)00028-7. [DOI] [PubMed] [Google Scholar]
- 21.Pikovskaya RI. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya. 1948;17:362–370. [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 24.Murphy J, Riley JP. A modified single method for the determination of phosphate in natural waters. Anal Chem Acta. 1962;27:31–36. [Google Scholar]
- 25.Olsen SR, Cole CV, Watanabe FS, Dean LA. Estimation of available phosphorous in soils by extraction with sodium bicarbonate. USDA Circ. 1954;939:1–19. [Google Scholar]
- 26.Tabatabai MA, Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem. 1969;1:301–307. [Google Scholar]
- 27.Casida LE, Jr, Klein DA, Santoro T. Soil dehydrogenase activity. Soil Sci. 1964;98:371–376. [Google Scholar]
- 28.Wu H, Yang HY, You XL, Li YH. Diversity of endophytic fungi from roots of Panax ginseng and their saponin yield capacities. SpringerPlus. 2013;2:107. doi: 10.1186/2193-1801-2-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Y, Prasad MN, Rajkumar M, Freitas H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv. 2011;29:248–258. doi: 10.1016/j.biotechadv.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Glick BR, Patten CL, Holguin G, Penrose DM. Biochemical and genetic mechanisms used by plant growth promoting bacteria. London: Imperial College Press; 1999. pp. 1–13. [Google Scholar]
- 31.Ahmad F, Ahmad I, Khan MS. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res. 2008;163:173–181. doi: 10.1016/j.micres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Kang SC, Ha CG, Lee TG, Maheshwari DK. Solubilization of insoluble inorganic phosphates by a soil-inhabiting fungus Fomitopsis sp. PS 102. Curr Sci. 2002;82:439–442. [Google Scholar]
- 33.Varsha-Narsian J, Thakkar J, Patel HH. Inorganic phosphate solubilization by some yeast. Indian J Microbiol. 1994;35:113–118. [Google Scholar]
- 34.Illmer P, Barbato A, Schinner F. Solubilization of hardly-soluble AlPO4 with P-solubilizing microorganisms. Soil Biol Biochem. 1995;27:265–270. [Google Scholar]
- 35.Vazquez P, Holguin G, Puente ME, Lopez-Cortes A, Bashan Y. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol Fertil Soils. 2000;30:460–468. [Google Scholar]
- 36.Goenadi DH, Sisweto, Sugiarto Y. Bioactivation of poorly soluble phosphate rocks with a phosphorus-solubilizing fungus. Soil Sci Soc Am J. 2000;64:927–932. [Google Scholar]
- 37.Rodríguez H, Rossolini GM, González T, Li J, Glick BR. Isolation of a gene from Burkholderia cepacia IS-16 encoding a protein that facilitates phosphatase activity. Curr Microbiol. 2000;40:362–366. doi: 10.1007/s002840010071. [DOI] [PubMed] [Google Scholar]
- 38.Rasouli-Sadaghiani M, Hassani A, Barin M, Danesh YR, Sefidkon F. Effects of arbuscular mycorrhizal (AM) fungi on growth, essential oil production and nutrients uptake in basil. J Med Plants Res. 2010;4:2222–2228. [Google Scholar]
- 39.Zhou J, Xia B, Treves DS, Wu LY, Marsh TL, O'Neill RV, Palumbo AV, Tiedje JM. Spatial and resource factors influencing high microbial diversity in soil. Appl Environ Microbiol. 2002;68:326–334. doi: 10.1128/AEM.68.1.326-334.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yadav BK, Tarafdar JC. Penicillium purpurogenum, unique P mobilizers in arid agro-ecosystems. Arid Land Res Manag. 2011;25:87–99. [Google Scholar]
- 41.Dick RP, Breakwell DP, Turco RF. Soil enzyme activities and biodiversity measurements and integrative microbial indicators. In: Doran JW, Jones AJ, editors. Methods of assessing soil quality. Madison: Soil Science Society of America Publication; 1996. pp. 247–271. [Google Scholar]
- 42.García C, Hernandez T, Costa F. Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun Soil Sci Plant Anal. 1997;28:123–134. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of plant growth promoting Penicillium menonorum: A, CAS agar plate detection of siderophores produced by KNU-3; B, P-solubilization in control vs. KNU-3 in Pikovskaya medium.