Abstract
Phytophthora diseases have become a major impediment in the citrus production in Thailand. In this study, an isolate of Phytophthora denominated as PHY02 was proven to be causal pathogen of root rot of Pomelo (Citrus maxima) in Thailand. The isolate PHY02 was morphologically characterized and identified as Phytophthora palmivora based on molecular analysis of an internal transcribed spacer rDNA sequence. This work also presents in vitro evaluations of the capacities of Chaetomium spp. to control the P. palmivora PHY02. As antagonists, Chaetomium globosum CG05, Chaetomium cupreum CC3003, Chaetomium lucknowense CL01 inhibited 50~61% mycelial growth, degraded mycelia and reduced 92~99% sporangial production of P. palmivora PHY02 in bi-culture test after 30 days. Fungal metabolites from Chaetomium spp. were tested against PHY02. Results showed that, methanol extract of C. globosum CG05 expressed strongest inhibitory effects on mycelial growth and sporangium formation of P. palmivora PHY02 with effective dose ED50 values of 26.5 µg/mL and 2.3 µg/mL, respectively. It is interesting that C. lucknowense is reported for the first time as an effective antagonist against a species of Phytophthora.
Keywords: Biological control, Chaetomium, Phytophthora palmivora, Pomelo
Citrus are important fruit crops, being widely and commercially grown in Southeast Asia. However, under prevailing wet climatic conditions, Phytophthora is a major impediment in the citrus production and has caused annual loss of about 6~12% of yield in Thailand alone [1].
Phytophthora palmivora (Butl.) is a ubiquitous and prominent plant pathogen with a wide host range, which infects various important crops in Southeast Asia [1, 2]. On citrus, this fungal-like organism has been reported to infect almost every part of the plant at any stage of its growth [3]. The spread and pathogencity were reported to be faster and more aggressive to roots of citrus than P. parasitica and P. citrophthora [4, 5]. Since Phytophthora and other oomycetous organisms have different biochemical pathways compared to the true fungi, they are insensitive to many fungicides [6]. In addition, the resistance of Phytophthora species to an important group of fungicides such as phenylamides (metalaxyl and related compounds) has become a serious problem in their chemical control [2]. In order to develop eco-friendly management of Phytophthora diseases and to reduce the costly applications and harm of fungicides, screening of bio-control agents against citrus Phytophthora has become a vital research aspect and is being carried out all over the world [3].
Chaetomium Kunze is a large genus of saprophytic ascomycetes with more than 350 species [7]. Relying on lytic enzymes, they decompose cellulose and other organic materials [8, 9]. Some Chaetomium species are reported to act as antagonists against various plant pathogens. Even commercial bio-product has also been developed from potent strains of Chaetomium spp. [10]. Furthermore, over 200 metabolites with a wide range of bioactivities have been found from the genus Chaetomium and many of which exhibited antifungal activity against plant pathogens [7]. However, there is a significant limitation of research on the abilities of Chaetomium species and their metabolites to control the Phytophthora.
Recently, we have isolated a single species of Phytophthora (denominated as PHY02) from roots of pomelo (Citrus maxima) in orchards having serious root rot problems in Chang chen Sao province, Thailand. This study reports characteristics and identification of this isolate as well its in vitro bio-control using Chaetomium species as antagonists and their metabolites.
MATERIALS AND METHODS
Characteristics and identification
The Phytophthora (PHY02) was cultured on potato dextrose agar (PDA; potato infusion from 200 g/L, 20 g/L dextrose, and 15 g/L agar), cornmeal agar (CMA [Hardy Diagnostics Co., Santa Maria, CA, USA]: 2 g/L corn meal infusion from solids, 15 g/L agar) and V8 juice agar (V8A; 200 mL/L V8 juice [Cambell soup Co., Camden, NJ, USA], 3 g/L CaCO3, 20 g/L agar, 800 mL/L water) for morphological study. Sporangia and other structures of PHY02 were observed under a light microscope (Olympus CH40; Olympus Optical Co. Ltd., Tokyo, Japan) then taken photos and measured by a camera (Moticam 2000; Motic Incorporation Ltd., Hong Kong, China) using enclosed software MoticPlus 2.0.
To identify the pathogen at molecular level, universal primers ITS 5 (5'-GGAAGTAAAAGTCGTAACAAGG) and ITS 4 (5'-TCCTCCGCTTATTGATATGC) were used to amplify an internal transcribed spacer (ITS) rDNA region of isolate PHY02, with previously described PCR conditions [11], then was directly sequenced with the same primers at First BASE Laboratories Sdn Bhd (Selangnor, Malaysia). The full-length ITS rDNA nucleotide sequence of PHY02 was used as a query to search the GenBank DNA database of Phytophthora Database (http://www.phytophthoradb.org/blast/) using BLAST tool. Reference ITS rDNA sequences of related taxa were also downloaded from the Phytophthora Database. Subsequently, the sequences of PHY02 and related taxa were aligned and performed phylogenetic analysis using MEGA ver. 5.2. Finally, phylogenetic tree was constructed using the neighbor-joining method with 1,000 bootstrap replications [12].
Pathogenicity test
Pathogenicity was proved by artificial inoculation PHY02 into roots of pomelo seedlings (Citrus maxima) var. Khao nam Pueng (the same affected variety where PHY02 was obtained).
Root inoculation was done using the infested soil method of Zitko et al. [4]. Six-month-old pomelo seedlings were thoroughly washed to be free of potting mix and then planted in plastic tubes (10 × 15 cm) containing sterilized clay soil-sand (1 : 1) with 5 chlamydospores of PHY02 per cubic centimeter. Controls were prepared by planting the seedlings in same size tubes, but containing sterilized clay soil-sand (1 : 1) only. All pots including the controls were maintained in the green house at temperature of about 25~30℃ and flooded with water for 24 hr each week. After 6 wk, the plants were carefully removed from plastic tubes, the soil was washed out and rating was done based on degree of the root rot symptoms. The pathogen then was re-isolated from newly infected roots symptom and morphology characteristics were compared with the isolate PHY02.
Fungal isolates
C. globosum strain CG05, C. cupreum strain CC3003, and C. lucknowense strain CL01 (from collection of Dr. Soytong K., KMITL, Thailand) were used as antagonists and produced antagonistic substances. The isolate PHY02 was represented as the target control in this study.
Bi-culture test
A mycelial disc of PHY02 (5mm diameter) was placed singly (as controls) or oppositely to a mycelial disc of each above antagonist on 9-cm-diameter Petri dishes, which contained PDA. After incubation at 25℃ for 30 days, data were collected as colony diameter and number of sporangia produced by PHY02 in both bi-culture and control plates. Numbers of sporangia were counted by using haemacytometer. Data were computed in a form of inhibition percentage of mycelial growth and sporangial production of the pathogen by using the formula below:
% Inhibition = 100 × (A-B)/A
, where A = colony diameter or numbers of sporangia of PHY02 in control plates; B = colony diameter or numbers of sporangia of PHY02 in bi-culture plates.
Finally, variance and the treatment means were analyzed and compared by using Duncan's multiple range tests at 0.05.
In vitro test of crude extracts from Chaetiomium spp. to inhibit P. palmivora PHY02
C. globosum CG05, C. lucknowense CL01, and C. cupreum CC3003 were separately cultured in potato dextrose broth (500 plates per antagonist) at room temperature in 45 days. Fungal biomass of each antagonist was separately collected as fresh biomass, then was dried out at room temperature. Subsequently, the extraction of dried biomass from each antagonist was performed by the method described by Kanokmedhakul et al. [13]. Each dried biomass was ground and extracted with hexane (1 : 1 v/v) and incubated by shaking for 72 hr at room temperature. The filtrate was separated out the marc by filtration through filter paper (Whatman No. 4). The hexane filtrate was performed through rotary vacuum evaporator to yield crude hexane extract. The marc from hexane extraction was further extracted with ethyl acetate and followed with methanol using the same procedure as hexane to yield crude ethyl acetate and methanol extract.
The three different crude extracts of each antagonist were conducted in a factorial experiment to test for inhibition of mycelial growth and sporangium formation of P. palmivora PHY02 by using poisonous food method. A mycelial disc (5 mm in diameter) of PHY02 placed on PDA plates (5 cm in diameter), which contained different concentrations of each crude extract (0, 10, 50, 100, 500, and 1,000 µg/mL). To obtain the desired crude extract concentrations, stock crude extract was weighted then dissolved in 2% dimethyl sulfoxide and added to molten PDA before autoclaving at 121℃ (15 psi) for 20 min. After incubating at 25℃ for 10 days, colony diameter and numbers of sporangia production of the pathogen were collected and then expressed as inhibition percentage using the same formula above. Effective dose ED50 values on mycelial growth and sporangial production were calculated by probit analysis using the software SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA).
RESULTS
Pathogenicity of Phytophthora PHY02
After 6 wk of inoculation with chlamydospores of PHY02 on roots, the pomelo seedlings had an average of 47.6% root tips rotted and produced very few new roots (data not shown). Cortex of feeder roots turned soft and was sloughed to leave only steles, which were similar to symptoms of root rot in the affected orchards in Chang chen Sao, Thailand (Fig. 1). Meanwhile, non-inoculated seedlings produced abundantly new roots and no symptom of rot was observed. A single species of Phytophthora was re-isolated from disease symptoms on newly infected roots, which all had identical morphology characteristics with the isolated PHY02. As a result, PHY02 was proven to be the causal pathogen of the pomelo root rot in Chang chen Sao, Thailand.
Fig. 1. Symptoms caused by Phytophthora palmivora PHY02 on root of pomelo in pathogenicty test (INO, inoculated; NINO, non-inoculated).

Characteristics and identification of Phytophthora PHY02
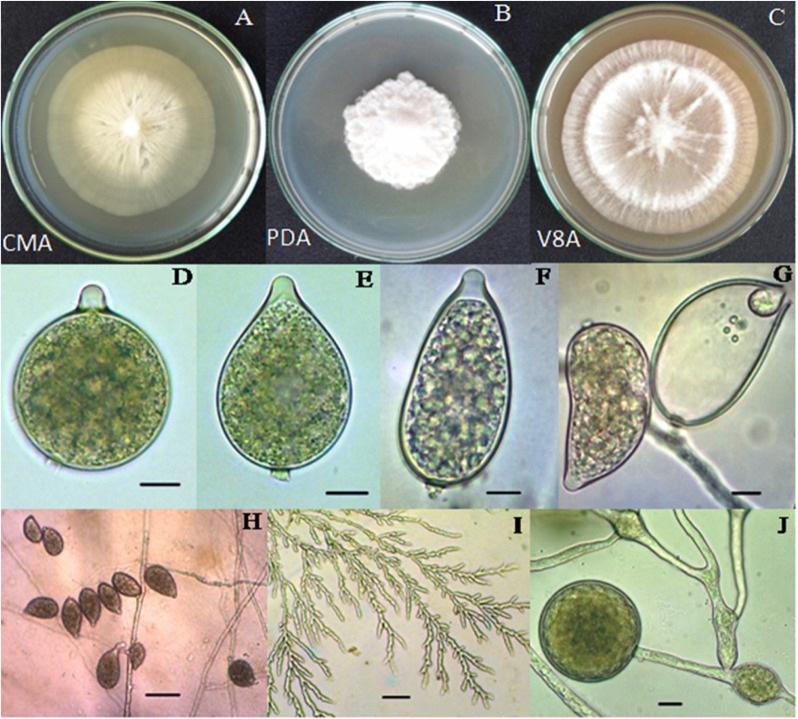
Colonies of PHY02 showed stellate pattern, with aerial mycelia on V8A and PDA whereas nearly no aerial mycelium on CMA (Fig. 2). The isolate PHY02 produced lumpy-branching hyphae with hyphal swellings. Zoospores were directly released from sporangia when flooded in distilled water. Sporangia produced abundantly when grown on PDA and V8A after 3~5 days, occurred in groups on sympodium or irregularly, were papillate and caducous with short pedicels up to 6 µm long (mean 3.3). Sporangial shape varied from ellipsoid, ovoid, pyriform, obpyriform to near spherical, and mean of sizes were 53.8 × 33.3 µm with a length to breadth ratio of 1.2~2.2 (mean 1.6) (Table 1). Most of chlamydospores were globose in shape, produced abundantly from mycelia when incubated in dark. No sexual organ was observed in cultures of this isolate since it was a heterothallic species.
Fig. 2. Morphological characteristics of Phytophthora palmivora PHY02. A~C, Colony types of PHY02 at 7 days grown on different media; D~F, Different shapes of zoosporangia; G, Zoospore release directly from sporangium; H, Occurrence of sporangia on sympodium; I, Lumpy-branching mycelia; J, Chlamydospore and swelling hyphae (scale bars: D~G, J = 10 µm, H, I = 50 µm). CMA, cornmeal agar; PDA, potato dextrose agar; V8A, V8 juice agar.
Table 1. Characteristics of Phytophthora palmivora PHY02 from citrus maxima.
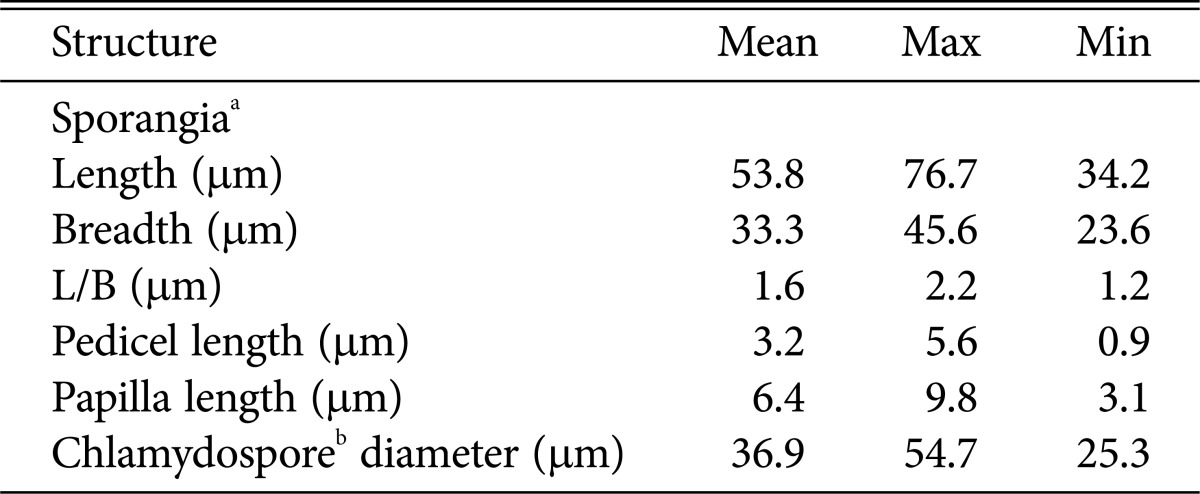
aData collected from 200 separate sporangia.
bData collected from 150 separate chlamydospores.
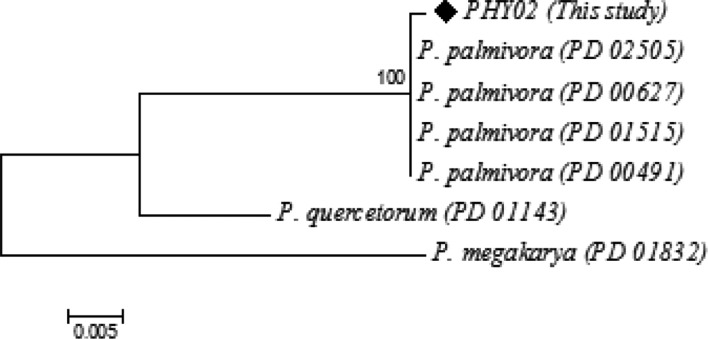
The sequence with 951 bases of the ITS rDNA PCR product of PHY02 was determined and used as a query to search the Genbank DNA database of the Phytophthora Database using the BLAST search. It was found that there was 99.75% (809/811), 99.87% (782/783) to identity with Phytophthora palmivora accession number PD_00627 and PD_02505, respectively. The phylogenetic tree, which illustrated relationships between PHY02 and related taxa was constructed as shown in Fig. 3. The isolate PHY02 was identified as Phytophthora palmivora (Butl.), based on its morphology and the molecular analysis.
Fig. 3. Phylogenetic relationship between Phytophthora palmivora PHY02 and related taxa inferred using a neighbor-joining method with internal transcribed spacer (ITS) rDNA sequences. Bootstrap value based on 1,000 replications is shown above the branch.
Bi-culture test
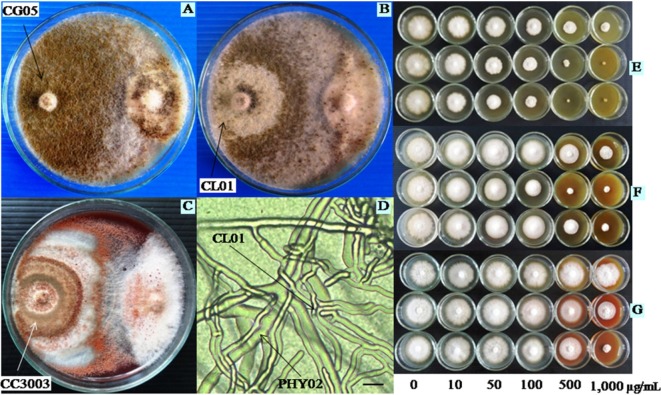
As shown in Table 2, the tested antagonists led to 50~61% growth inhibition and reduced 92~99% sporangial production of P. palmivora PHY02 in bi-culture plates at 30 days, when compared to the controls. Moreover, CG05 and CL01 were seen to grow rapidly over PHY02 colony after 10~15 days. In all bi-culture plates, both antagonists degraded mycelia of the pathogen, resulting in change of color from white to light yellow-brown and a part or entire colony death (Fig. 4). Differently, C. cupreum CC3003 was a slow growing fungus, as it grew over gradually and resulting in degradation of colony of PHY02 in some cases. Mycelia of all the tested antagonists were observed to penetrate mycelia of PHY02 in some cases. In both inhibitions of mycelial growth and sporangial production, CC3003 was significantly less efficient than CG05 and CL01.
Table 2. Inhibition of mycelial growth and sporagial production of Phytophthora palmivora PHY02 in bi-culture test with Chaetomium spp.
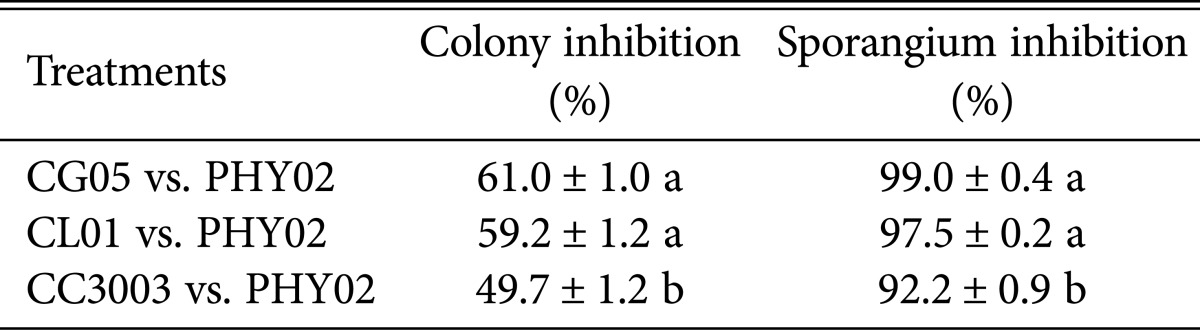
Average of four replications, the same letter represents no significant difference between treatments, based on the Duncan's multiple range test at p = 0.05.
Fig. 4. Growth of Phytophthora palmivora PHY02 in bi-culture tests (at 30 days) and in crude extracts (at 10 days) of Chaetomium spp. A~C, PHY02 and Chaetomium spp. in bi-culture plates; D, Interaction between mycelia of CL01 and PHY02 in bi-culture test (scale bar = 10 µm); E~G, Growth of PHY02 at different concentrations of crude extracts of CG05, CL01, and CC3003, respectively (upper row: hexane extracts; middle row: ethyl acetate extracts; lower row: methanol extracts).
In vitro tests of crude extracts from Chaetomium spp. to inhibit P. palmivora PHY02
Total nine crude extracts from CG05, CL01, and CC3003 were tested at different concentrations to evaluate their capacities to inhibit mycelial growth and sporangium formation of P. palmivora (PHY02). The effective doses (ED50) of each crude extract on mycelial growth and sporangial production were also examined to determine their fungicidal spectrum.
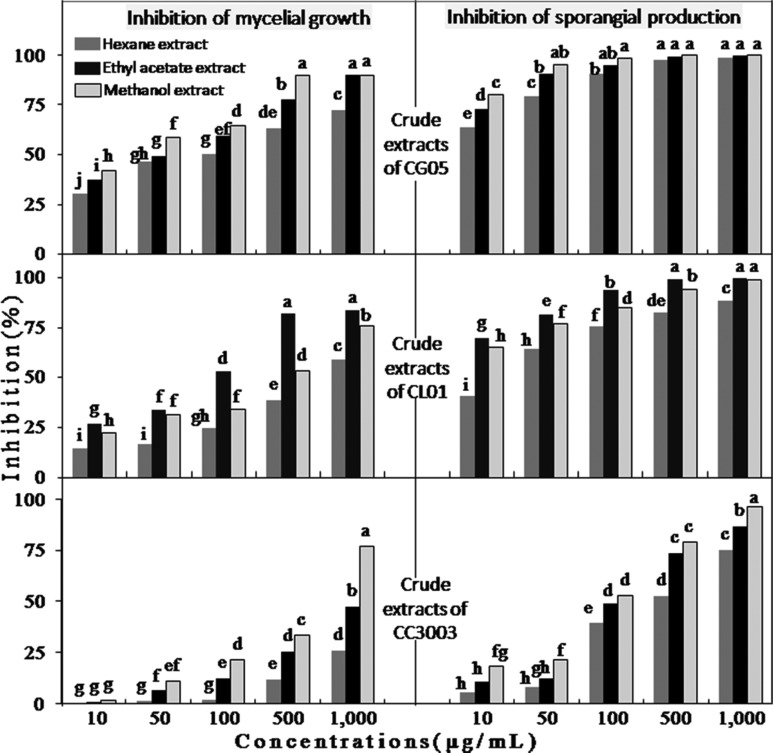
As shown in Table 3, the three crude extract of CG05 exhibited more antifungal activities against mycelial growth of PHY02 as compared to crude extracts of CL01 and CC3003. Particularly, the tested pathogen did not grow at all in the presence of higher 500 µg/mL methanol extract and 1,000 µg/mL ethyl acetate extract of CG05. At tested concentrations from 10~500 µg/mL, the methanol extract was more effective than the others (Figs. 4 and 5). Conversely, all crude extracts of CC3003 were less effective on mycelial growth of PHY02 with higher ED50 values (596.8~2,495 µg/mL). The methanol extract of CC3003 was more effective than the hexane and ethyl acetate at concentrations from 100~1,000 µg/mL. However, none of them showed significant inhibition of mycelial growth of PHY02 at 10 µg/mL. Whereas, mycelial growth of PHY02 was significantly inhibited by all crude extracts of CL01 at 10 µg/mL. Of which, the ethyl acetate extract was more significantly effective than the others at concentrations from 100~1,000 µg/mL, with ED50 value of 77.03 µg/mL and exhibited 83.5% growth inhibition of PHY02 at 1,000 µg/mL. Meanwhile, ED50 values of the methanol, hexane extract were 143.9 and 919.1 µg/mL, respectively. Unlike in bi-culture test, mycelia of PHY02 were not degraded by crude extracts of the antagonistic fungi.
Table 3. Effective dose values (ED50) of different crude extracts of Chaetomium spp. on mycelial growth and sporangial production of Phytophthora palmivora PHY02.
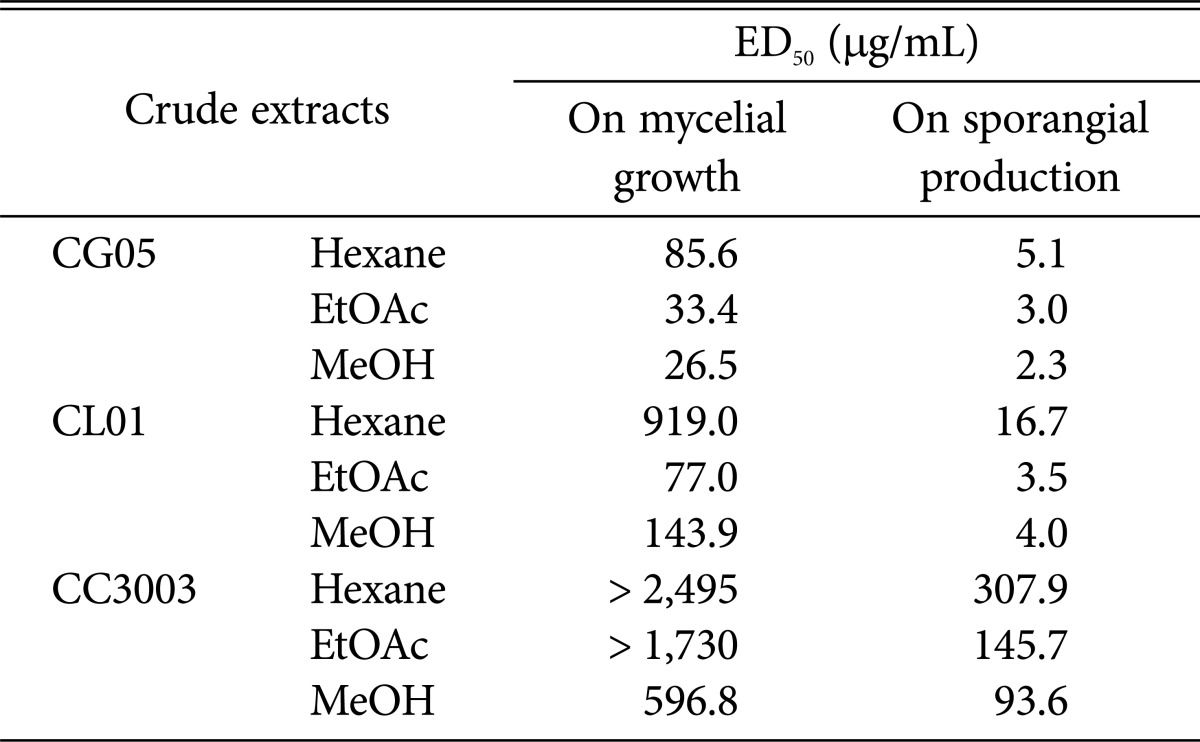
Hexane, hexane extract; EtOAc, ethyl acetate extract; MeOH, methanol extract.
Fig. 5. Inhibitory effects of crude extracts of Chaetomium spp. on mycelial growth (the charts on the left site) and sporangial production (the charts on the right site) of Phytophthora palmivora PHY02 at different concentrations. The same letter above columns in each chart represents no significant difference between treatments, based on the Duncan's multiple range test at p = 0.01.
All the tested crude extracts exhibited stronger inhibitory effects on sporangium formation than on mycelial growth of PHY02 with much lower ED50 values. The sporangium formation of PHY02 was most sensitive to crude extracts of CG05 with ED50 values of as 5.1, 3.0, and 2.3 µg/mL for the hexane, ethyl acetate and methanol extract, respectively.
Meanwhile, all three crude extracts of CC3003 were least effective on sporangial production of PHY02 with higher ED50 values. Of which, the methanol extract was more significantly effective than the others with ED50 value of 93.6 µg/mL, and gave an inhibitory rate of 96.5% at 1,000 µg/mL. The hexane and ethyl acetate extract gave the highest ED50 values among nine tested crudes, which were 307.9 and 145 µg/mL, respectively.
To reduce 50% sporangial production of PHY02, the hexane, ethyl acetate and methanol extract of CL01 required concentrations of 16.7, 3.5, and 4.0 µg/mL, respectively. However, none of them could reduce 90% sporangial production of PHY02 at 50 µg/mL. At concentrations from 10~500 µg/mL, the ethyl acetate extract of CL01 was more significantly effective than the others, showing an inhibitory rate of 94.0% at 100 µg/mL. While at concentration of 500 µg/mL, the hexane and methanol extract reduced 82.3% and 93.8% sporangial production of PHY02, respectively.
All the hexane extracts of tested antagonists were less effective than the ethyl acetate and methanol in both inhibitions of mycelia growth and sporangium formation of P. palmivora PHY02.
DISCUSSION
We have designated the species isolated from root rot symptoms of pomelo (Citrus maxima) in Thailand as Phytophthora palmivora (Butl.). The highly pathogenic of P. palmivora PHY02 to roots of pomelo here is supported by previous studies which demonstrated that this species is more aggressive and damages even larger root than P. parasitica on citrus [4, 5]. Serious root rot disease of citrus caused by P. palmivora has been recorded in India, America [3, 14].
The antagonistic activity of bio-control microorganisms is often demonstrated by the inhibition of growth, infection or reproduction of pathogen [15]. The tested strains of Chaetomium spp. as antagonists here not only inhibited colony growth and degraded mycelia but also reduced 92~99% sporangial production of P. palmivora PHY02. The degradation of mycelia of tested pathogen probably resulted from the lytic enzymes which are commonly secreted by Chaetomium species [8, 9]. On other hand, every tested antagonist in this study was demonstrated to produce antibiotics exhibiting antifungal activities against different plant pathogens [10, 13, 16]. It refers that the inhibitions of P. palmivora here were resulted from both antibiotics and lytic enzymes secreted by the antagonists. C. globosum and C. cupreum have ever been reported to control Phytophthora spp. in dual culture test [17, 18]. C. lucknowense was concluded to control Fusarium oxysporum causing tomato wilt [16]. However, to best our knowledge, this is the first report of C. lucknowense as an effective antagonist against a species of Phytophthora.
Chaetomium species are known as producers of many different bioactive metabolites which play important roles in their biological control activities [7, 9]. One Chaetomium species may produce many metabolites with different bioactivities and molecular weight, this lead to differences in antifungal activity of its crude extracts [16, 19]. That explains why there were variations in responses of P. palmivora PHY02 to different crude extracts in this study. The less efficiency of hexane extracts of Chaetomium spp. in comparison with the ethyl acetate and methanol against fungal pathogens were also confirmed by previous authors [16, 20].
C. globosum CG05 has been known to produce many bioactive compounds such chaetoglobosin (A, G, C, V, etc.) and chaetoviridins (A, B) [10]. Of which, the chaetoglobosin C is usually referred as antifungal principle of this strain and was reported to inhibit colony growth, sporangium formation of P. parasitisca and other fungal pathogens [10, 19]. However, the chaetoviridins A produced from C. globosum F0142 also has strong antifungal activities against P. infestans, P. capsici [21]. Thus, it suggests that chaetoglobosin C and also other bioactive compounds involved in crude extracts of C. globosum CG05 caused the high inhibitions of P. palmivora PHY02 in this study. C. cupreum CC3003 has been known to produce compound rubrorotiorin which inhibited growth of Candida albicans at low concentration (EC50 = 0.6 µg/mL) [13]. However, crude extracts of C. cupreum CC3003 here showed least inhibitory effects on P. palmivora with high ED50 values. Similarly, crude extracts of C. cupreum have been known to be less effective against Pythium aphanidermatum [20]. Both the crude extracts and chaetoglobosin C produced by C. lucknowense CL01 have been reported to inhibit colony growth and spore formation of Fusarium oxysporum causing tomato wilt [16]. However, this is the first time the inhibitory effects of metabolites from C. lucknowense on a species of Phytophthora are reported.
Sporangium formation is the most sensitive stage in life cycle of Phytophthora species [2]. This study demonstrated that sporangium formation of P. palmivora PHY02 was much more sensitive than mycelial growth to all tested crude extracts. The high inhibitory effects of crude extracts from Chaetomium spp. on spore formation of Fusarium oxysporum and P. parasitisca have been noted by previous authors [16, 19].
It is clear that only metabolites which were not decomposed through the extraction and autoclaving of the crude extracts resulted in the inhibitions of P. palmivora PHY02. All enzymes and many antibiotics will be decomposed because of heat and pressure when autoclaving [2]. That why mycelia of the pathogen were not degraded when growing in crude extracts. Therefore, the antifungal activities of crude extracts here probably did not account for all biocontrol activities of the tested antagonists against P. palmivora PHY02. Beside the known metabolites, degrading enzymes should be considered as effective factors involved in biocontrol activities of the tested antagonists against the pathogen. The roles and secretion of lytic enzymes of Chaetomium species under antagonism conditions with Phytophthora need to be investigated in the future. The effective crude extracts may possible develop to be microbial elicitors to induce immunity in citrus plants against P. palmivora.
ACKNOWLEDGEMENTS
This work was acknowledged under grant supports of the King Mongkut's Institute of Technology Ladkrabang (KMITL), Bangkok, Thailand.
References
- 1.Drenth A, Sendall B. Economic impact of Phytophthora diseases in Southeast Asia. In: Drenth A, Guest DI, editors. Diversity and management of Phytophthora in Southeast Asia. ACIAR Monograph No. 114. Canberra: Australian Centre for International Agriculture; 2004. pp. 10–28. [Google Scholar]
- 2.DC Erwin, Ribeiro OK. Phytophthora diseases worldwide. St. Paul: APS Press; 1996. [Google Scholar]
- 3.Naqvi SA. Diagnosis and management of certain important fungal diseases of citrus. In: Naqvi SA, editor. Diseases of fruits and vegetables. Dordrecht: Kluwer Academic Publishers; 2004. pp. 247–290. [Google Scholar]
- 4.Zitko SE, Timmer LW, Sandler HA. Isolation of Phytophthora palmivora pathogenic to citrus in Florida. Plant Dis. 1991;75:532–535. [Google Scholar]
- 5.Zitko SE, Timmer LW. Competitive parasitic abilities of Phytophthora parasitica and P. palmivora on fibrous roots of citrus. Phytopathology. 1994;84:1000–1004. [Google Scholar]
- 6.Bruin GC, Edgington LV. The chemical control of diseases caused by zoosporic fungi: a many-sided problem. In: Buczacki ST, editor. Zoosporic plant pathogens: a modern perspective. London: Academic Press; 1983. pp. 193–233. [Google Scholar]
- 7.Zhang Q, Li HQ, Zong SC, Gao JM, Zhang AL. Chemical and bioactive diversities of the genus Chaetomium secondary metabolites. Mini Rev Med Chem. 2012;12:127–148. doi: 10.2174/138955712798995066. [DOI] [PubMed] [Google Scholar]
- 8.Longoni P, Rodolfi M, Pantaleoni L, Doria E, Concia L, Picco AM, Cella R. Functional analysis of the degradation of cellulosic substrates by a Chaetomium globosum endophytic isolate. Appl Environ Microbiol. 2012;78:3693–3705. doi: 10.1128/AEM.00124-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun H, Yang J, Lin C, Huang X, Xing R, Zhang KQ. Purification and properties of a beta-1,3-glucanase from Chaetomium sp. that is involved in mycoparasitism. Biotechnol Lett. 2006;28:131–135. doi: 10.1007/s10529-005-5132-0. [DOI] [PubMed] [Google Scholar]
- 10.Soytong K, Kanokmedhakul S, Kukongviriyapa V, Isobe M. Application of Chaetomium species (Ketomium®) as a new broad spectrum biological fungicide for plant disease control: a review article. Fungal Divers. 2001;7:1–15. [Google Scholar]
- 11.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. New York: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 12.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanokmedhakul S, Kanokmedhakul K, Nasomjai P, Louangsysouphanh S, Soytong K, Isobe M, Kongsaeree P, Prabpai S, Suksamrarn A. Antifungal azaphilones from the fungus Chaetomium cupreum CC3003. J Nat Prod. 2006;69:891–895. doi: 10.1021/np060051v. [DOI] [PubMed] [Google Scholar]
- 14.Graham JH, Timmer LW. Phytophthora diseases of Citrus. In: Singh US, Mukhopadhay AN, Kumar J, Chaube HS, editors. Plant diseases of international importance: diseases of vegetables and oil seed crops. Englewood Cliffs: Prentice-Hall Inc.; 1992. pp. 250–269. [Google Scholar]
- 15.Alabouvette C, Olivain C, Steinberg C. Biological control of plant diseases: the European situation. Eur J Plant Pathol. 2006;114:329–341. [Google Scholar]
- 16.Sibounnavong P, Charoenporn C, Kanokmedhakul S, Soytong K. Antifungal metabolites from antagonistic fungi used to control tomato wilt fungus Fusarium oxysporum f. sp. lycopersici. Afr J Biotechnol. 2011;10:19714–19722. [Google Scholar]
- 17.Heller WE, Theiler-Hedtrich R. Antagonism of Chaetomium globosum, Gliocladium virens and Trichoderma viride to four soil-borne Phytophthora species. J Phytopathol. 1994;141:390–394. [Google Scholar]
- 18.Pecchprome S, Soytong K. Proceeding of First International Symposium on Biopesticides; 1996 Oct 27-31; Phitsanulok, Thailand. Bankok: Chulalongkorn University Press; 1997. Integrated biological control of Durian stem and root rot caused by Phytophthora palmivora; pp. 228–237. [Google Scholar]
- 19.Kanokmedhakul S, Kanokmedhakul K, Soytong K, Suksamrarn A. Proceeding of International Conference on Engineering, Applied Science and Technology; 2007 Nov 21-23; Bangkok, Thailand. Bangkok: King Mongkut's Institute of Technology Ladkrabang; 2007. Bioactive compounds from Chaetomium cupreum, Chaetomium globosum and Trichoderma harzianum for plant disease control and plant growth stimulant; pp. 626–629. [Google Scholar]
- 20.Pornsuriya C, Soytong K, Kanokmedhakul S, Lin FC. Efficacy of antifungal metabolites from some antagonistic fungi against Pythium aphanidermatum. J Agric Technol. 2010;6:299–308. [Google Scholar]
- 21.Park JH, Choi GJ, Jang KS, Lim HK, Kim HT, Cho KY, Kim JC. Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum. FEMS Microbiol Lett. 2005;252:309–313. doi: 10.1016/j.femsle.2005.09.013. [DOI] [PubMed] [Google Scholar]