Abstract
There is now considerable evidence to support the hypothesis that psychotic symptoms are the result of abnormal salience attribution, and that the attribution of salience is largely mediated through the prefrontal cortex, the striatum, and the hippocampus. Although these areas show differential activation under the influence of delta-9-tetrahydrocannabinol (delta-9-THC) and cannabidiol (CBD), the two major derivatives of cannabis sativa, little is known about the effects of these cannabinoids on the functional connectivity between these regions. We investigated this in healthy occasional cannabis users by employing event-related functional magnetic resonance imaging (fMRI) following oral administration of delta-9-THC, CBD, or a placebo capsule. Employing a seed cluster-based functional connectivity analysis that involved using the average time series from each seed cluster for a whole-brain correlational analysis, we investigated the effect of drug condition on functional connectivity between the seed clusters and the rest of the brain during an oddball salience processing task. Relative to the placebo condition, delta-9-THC and CBD had opposite effects on the functional connectivity between the dorsal striatum, the prefrontal cortex, and the hippocampus. Delta-9-THC reduced fronto-striatal connectivity, which was related to its effect on task performance, whereas this connection was enhanced by CBD. Conversely, mediotemporal-prefrontal connectivity was enhanced by delta-9-THC and reduced by CBD. Our results suggest that the functional integration of brain regions involved in salience processing is differentially modulated by single doses of delta-9-THC and CBD and that this relates to the processing of salient stimuli.
Introduction
Accumulating evidence suggest that regular cannabis use increases the risk of development of psychotic disorders (Kuepper et al, 2011; Moore et al, 2007). However, the extract of cannabis sativa has many different ingredients (Bhattacharyya et al, 2009a). Delta-9-tetrahydrocannabiol (delta-9-THC), the main psychoactive ingredient, is responsible for inducing transient psychotic and anxiety symptoms (Bhattacharyya et al, 2009a; D'Souza et al, 2004). In contrast, cannabidiol (CBD), another major ingredient may have anxiolytic (Crippa et al, 2009; Fusar-Poli et al, 2009) and antipsychotic-like effects (Zuardi et al, 2009) and may oppose the neural effects of delta-9-THC (Bhattacharyya et al, 2010) and block the psychotogenic (Bhattacharyya et al, 2010; Morgan and Curran, 2008) and cognitive (Morgan et al, 2010a, 2010b) effects of delta-9-THC. We have recently reported that the induction of psychotic symptoms by delta-9-THC may be related to its effects on the striatum and the prefrontal cortex, integral components of a network of brain areas involved in processing of salient information (Bhattacharyya et al, 2012c), consistent with emerging evidence regarding the role of aberrant salience attribution in psychosis (Jensen and Kapur, 2009; Palaniyappan and Liddle, 2012). Furthermore, we showed that CBD and delta-9-THC had opposite effects on the striatum, the hippocampus, and the inferior frontal gyrus, which are key components of a network of brain areas involved in the processing of salience (Rubia et al, 2007; Strange and Dolan, 2001; Zink et al, 2006). The striatum is engaged when salient stimuli are encountered in an unexpected environmental context, with the level of activation linked to how salient they are (Zink et al, 2006). The hippocampus detects novelty by comparing past and present experience (Kumaran and Maguire, 2006), and is normally engaged when novelty contributes to stimulus salience. The inferior prefrontal cortex has a central role in the control of both goal-directed and stimulus-related attention (Asplund et al, 2010), and its engagement during a salience detection task may be related to the attentional component of processing a salient stimulus. However, whether delta-9-THC and CBD modulate the integration of these key areas during the processing of salience has not been examined. To date, only one previous study has examined the effect of delta-9-THC and CBD on the functional integration of brain regions in the context of an emotional (fear) processing task (Fusar-Poli et al, 2010). The purpose of the present study was to specifically examine whether delta-9-THC and CBD had opposite effects on the functional integration (as indexed by functional connectivity) of areas involved in the processing of salient information, which we have previously shown to be modulated in different directions by these cannabinoids (Bhattacharyya et al, 2012c). Functional connectivity, defined as temporal correlation between spatially remote neurophysiological events, can be computed from correlations between functional magnetic resonance imaging (fMRI) time series within an activated functional system (Friston, 2011), which however cannot provide estimates of directional influences between brain regions.
We hypothesized that relative to placebo, ingestion of delta-9-THC or CBD would be associated with modulation of the functional connectivity of the striatum, inferior frontal cortex, and hippocampus during a visuo-spatial attention allocation task. These regions were selected as seed clusters for the connectivity analysis on the basis of our previous finding that delta-9-THC and CBD have opposite effects on the blood oxygen level-dependent (BOLD) response in these regions during the oddball salience processing task and that the induction of psychotic symptoms by delta-9-THC was related to its the effect on activation in the striatum (Bhattacharyya et al, 2012c). Furthermore, these regions have been implicated in psychotic disorders such as schizophrenia (Meyer-Lindenberg, 2010; Vita et al, 2006). In particular, psychotic symptoms in schizophrenia have been related to increased dopaminergic activity in the striatum (Guillin et al, 2007) and may be exacerbated by cannabis use (D'Souza et al, 2005). Specifically, in light of the already noted antagonism between these two cannabinoid molecules (Bhattacharyya et al, 2010) and their opposing effect upon psychotic symptoms (Zuardi et al, 2012) we predicted that delta-9-THC and CBD would have an opposite effect on the functional connectivity within these regions. Furthermore, we predicted that the effect of delta-9-THC, but not of CBD, on functional connectivity within these brain regions would be correlated with its effects on performance. As the striatum and the inferior frontal cortex respectively have critical roles in salience attribution and allocation of attentional resources toward salient stimuli (Rubia et al, 2007; Zink et al, 2006), we predicted that such a relationship would exist between the effects of delta-9-THC on task performance and its effects on fronto-striatal connectivity.
Materials and Methods
Data presented here are based on that acquired during a previous experimental study, the methods of which have already been reported in detail (Bhattacharyya et al, 2009b, 2012c). Further description of the methods including details about subjects and experimental procedure including imaging are included in Supplementary Material. In brief, 15 healthy, right-handed, occasional (<15 times/lifetime) cannabis user males with minimal other drug use and alcohol use less than 21 units/week were tested on three separate occasions at least 1 month apart. None of the subjects had any personal or family history of psychiatric illness and alcohol or other drug abuse or dependence. Nine subjects were current tobacco smokers. Seven subjects smoked 2.92 (SD, 4.9) (range, 0–15/day) cigarettes per day; 2 subjects smoked >10 cigarettes per day. All subjects were requested to abstain from use of recreational drugs for the duration of the study, alcohol intake for 24 h and caffeine intake for 12 h before the study and also to avoid smoking tobacco on the morning of the study. Following a light standardized breakfast, a urine sample was collected for screening for recent use of amphetamines, benzodiazepines, cocaine, methamphetamine, opiates, and THC using immunometric assay kits. None of the participants tested positive on any of the sessions. They were studied with fMRI on three separate occasions 1 h after they were given identical capsules of 10 mg delta-9-THC, 600 mg CBD or placebo (flour) orally, while there was a sustained level of the drug in their peripheral blood. There was an interval of at least 1 month between scans. Psychopathology ratings in a number of measures including psychotic symptoms (Positive and Negative Syndrome Scale, PANSS; Kay et al, 1987) were recorded and drug plasma levels were obtained immediately before drug administration (baseline) and again after 1, 2, and 3 h following drug administration. Inside the scanner, participants performed a simple visual oddball detection task (further details in Supplementary Material), which involved pressing a left/right button to indicate the direction of arrows presented on a screen for 600 ms in series pointing to the right or left with equal probability. Arrows were followed by a blank screen for an average of 1.2 s (jittered between 1 and 1.4 s, total mean inter-trial interval=1.8 s). There were 160 horizontal arrows (standard stimuli) and interspersed pseudo-randomly among them were 24 oddball stimuli, which were identical to the horizontal arrows but presented at a 23° angle. T2*-weighted images were acquired on a 1.5-T (GE) system with TE=40 ms, TR=1.8 s. Data from all subjects met criteria for image quality and movement (<3 mm displacement in any one direction) and were included for analysis.
Data Analysis
Measures of task performance and symptom ratings were normally distributed and were analyzed using repeated-measures ANOVA used to compare drug conditions. When significant differences were found, the Tukey test for pair-wise comparisons was applied. The effects of between-drug differences in symptom levels on activation were examined by correlating measures of activation with the change in the rating from baseline to the mean of those at 1 and 2 h.
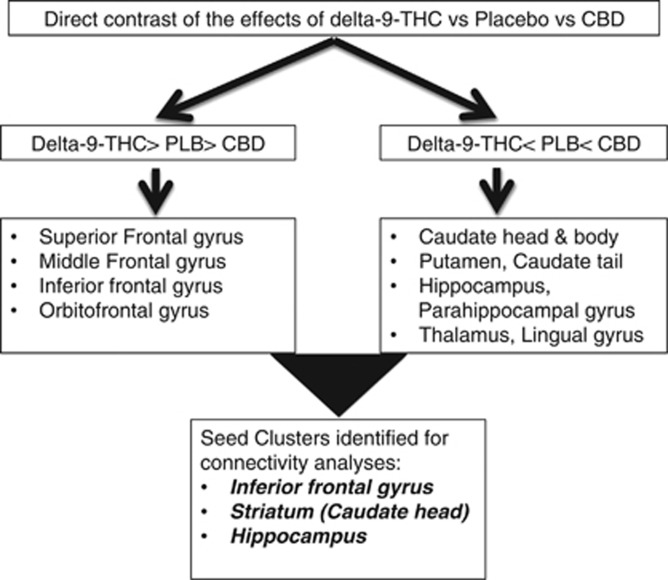
Imaging data obtained under each of the three drug conditions were analyzed using XBAMv3.4 (http://www.brainmap.co.uk/) software. Pre-processing and initial subject-level analysis of neuroimaging data has been already described before (Bhattacharyya et al, 2012c), but is reported in brief here for the sake of completeness. Following realignment and smoothing of the imaging data with an 8-mm FWHM Gaussian filter, we convolved the experimental design with two gamma variate functions to model the blood oxygen level-dependent (BOLD) hemodynamic response. This was followed by a least squares fitting of the convolved model to the time series at each voxel and estimation of the sum of squares ratio (SSQ ratio of model component to residual sum of squares) for the oddball vs standard contrast (ie, ‘oddball—standard' stimuli). ‘Oddball stimuli' were modelled against the ‘standard stimuli', which comprised the implicit baseline. The significance of the estimated SSQ values at each voxel was determined using permutation testing (Bullmore et al, 2001). We transformed the SSQ ratio maps for each individual into standard Talairach stereotactic space (Talairach and Tournoux, 1988) and then computed group activation maps for each drug by determining the median SSQ ratio at each voxel. We studied the effect of different conditions and contrasted them using non-parametric repeated-measures ANOVA (Brammer et al, 1997), with a voxel-wise threshold of p=0.05 and the cluster-wise threshold set such that the total number of false positive clusters per brain volume was <1; the p-value at which the later occurred is quoted. Cluster-level testing incorporates information from more than one voxel in the test statistic. Hence, the principal advantages of cluster-level testing are that it confers greater sensitivity and also mitigates the multiple comparisons problem, by substantially reducing the search volume or number of tests required for a whole-brain analysis. Effect of each drug was estimated by contrasting the oddball condition with the standard condition for each drug condition (delta-9-THC, CBD, and placebo). The effects of delta-9-THC and CBD in the whole brain were examined by comparing the activation maps for each drug condition separately with the activation map for the placebo condition. Finally, we identified areas within the whole brain where the effects of delta-9-THC and CBD relative to placebo were in opposite directions (Bhattacharyya et al, 2012c). This involved identifying brain areas where there was a linear relationship, either delta-9-THC>placebo>CBD or delta-9-THC<placebo<CBD. The seed clusters employed in the subsequent connectivity analyses were selected from the delta-9-THC>placebo>CBD group effect contrast (inferior frontal gyrus cluster) and the delta-9-THC<placebo<CBD contrast (dorsal striatal and posterior hippocampal cluster, see below).
Connectivity Analysis
To investigate the effect of delta-9-THC and CBD on functional connectivity, we selected seed clusters based on the group comparisons performed using the GLM-based whole-brain analysis described above. We used the inferior frontal (X=51, Y=26, Z=−13; cluster size=20 voxels), dorsal striatal (X=−22, Y=26, Z=9; cluster size=6 voxels) and posterior hippocampal foci (X=−29, Y=−41, Z=4; cluster size=10 voxels) as seed clusters. Cluster sizes were based on the size of the clusters in the three-way group contrasts (ie, delta-9-THC vs placebo vs CBD). Previous studies have implicated these regions in the processing of novel, deviant or rare stimuli (Rubia et al, 2007; Strange and Dolan, 2001; Zink et al, 2006) and as reported in Bhattacharyya et al (2012c) we have found the effect of delta-9-THC and CBD on the BOLD response in these regions to be in opposition to one another during the oddball salience processing task.
Using these seed clusters as regions of interest (ROIs), we then extracted the average time series over the whole ROI for each subject in each subject's space. The average time series for each subject was then used as a model for a whole-brain correlation analysis producing functional connectivity maps. Functional connectivity maps for each subject were then transformed to standard Talairach space where group connectivity maps were computed for each drug condition by determining the median correlation coefficient (across subjects) at each voxel.
Group comparisons were then carried out by employing a whole-brain analysis approach, using repeated-measures ANOVA with non-parametric (permutation-based) statistical inference. First, separate group-level analyses were performed to investigate the effect of each active drug (CBD or delta-9-THC) on functional connectivity with each of the seed clusters by contrasting each drug separately against the placebo condition, ie, CBD vs placebo and delta-9-THC vs placebo. We then investigated whether there was a linear relationship between the effects of the three drug conditions on functional connectivity with each of the seed clusters by contrasting delta-9-THC>placebo>CBD and delta-9-THC<placebo<CBD. We used a voxel-wise threshold of p=0.05 and a cluster-wise threshold set such that the total number of false positive clusters per whole brain was <1: the p-value at which the latter occurred is quoted in the text as appropriate. Effect of delta-9-THC on fronto-striatal connectivity was significant on a direct comparison of the effects of delta-9-THC and CBD relative to the placebo condition. Hence, this measure was employed in correlational analyses to test relationship between the effects of delta-9-THC on task performance and fronto-striatal connectivity.
Results
To make it easier for the reader to follow the presentation of results of the functional connectivity analysis that we have carried out here, we are briefly summarizing the most salient behavioural and functional neuroimaging results from our previous study (Bhattacharyya et al, 2012c), on which the results of the present study are built. They are also presented as Supplementary Material.
Summary of Previous Behavioural and fMRI Results
Administration of delta-9-THC, but not CBD, resulted in a significant (P=0.001) effect on psychotic symptom ratings relative to the placebo condition. Both delta-9-THC (P<0.001) and CBD (P=0.02) resulted in significantly reduced response latency across the oddball and standard conditions relative to placebo. However, there was a significant (P=0.01) effect of interaction between drug and stimulus condition on response latency, which was driven by a greater effect of delta-9-THC relative to CBD and placebo conditions during the standard compared with oddball stimuli. As reported in detail previously (Bhattacharyya et al, 2012c), direct contrasts revealed that delta-9-THC and CBD had opposite effects on activation in several regions, summarized in Figure 1 (details in Supplementary Table S2 and Supplementary Figure S1, Supplementary Material). Seed clusters were chosen based on these results. As reported previously, effect of delta-9-THC in the left caudate was inversely correlated with its effect on psychotic symptoms and task performance.
Figure 1.
Summary results of direct comparison of the effects of delta-9-THC, CBD, and placebo while processing oddball relative to standard stimuli.
Functional Connectivity Analyses
Main effect of delta-9-THC on whole-brain functional connectivity
Inferior frontal gyrus seed cluster. Relative to the placebo condition, delta-9-THC augmented the functional connectivity of the right inferior frontal gyrus with the right medial frontal gyrus and with the left claustrum and the right anterior lobe of the cerebellum, with the latter two clusters not surviving the <1 false positive cluster threshold (Supplementary Table S3, Supplementary Figure S2, Supplementary Material).
Dorsal striatum seed cluster. Relative to the placebo condition, delta-9-THC augmented the functional connectivity of the left dorsal striatum with the left anterior lobe of the cerebellum and the right superior frontal gyrus. In contrast, the connectivity of the dorsal striatal seed cluster with the left subcallosal gyrus and the left inferior frontal gyrus was attenuated by delta-9-THC relative to the placebo condition (uncorrected for <1 false positive cluster; Supplementary Table S3, Supplementary Material).
Hippocampal seed cluster. Relative to the placebo condition, delta-9-THC augmented the functional connectivity of the left posterior hippocampal cluster with the right medial frontal gyrus and attenuated the functional connectivity with the right postcentral gyrus and the left paracentral lobule (uncorrected for <1 false positive cluster; Supplementary Table S3, Supplementary Material).
Main effect of CBD on whole-brain functional connectivity
Inferior frontal gyrus seed cluster. Relative to the placebo condition, CBD attenuated the functional connectivity of the right inferior frontal gyrus with the right insula (uncorrected for <1 false positive cluster). A decrease in functional connectivity under the influence of CBD as compared with placebo was also found with a cluster of regions, with a peak in the left anterior lobe of the cerebellum, the left lingual gyrus, and the left thalamus (Supplementary Table S4, Supplementary Figure S3, Supplementary Material).
Dorsal striatum seed cluster. Relative to placebo, CBD augmented the functional connectivity of the left dorsal striatum with the body of the left caudate nucleus and the left inferior frontal gyrus and attenuated the functional connectivity with the left anterior cingulate and the left medial frontal gyrus (Supplementary Table S4, Supplementary Figure S4, Supplementary Material).
Hippocampal seed cluster. The functional connectivity of the left posterior hippocampal cluster with the left parahippocampal gyrus was augmented by CBD, whereas the functional connectivity with the right parahippocampal gyrus, the left posterior cingulate, and the tail of the left caudate was attenuated under the influence of CBD (Supplementary Table S4, Supplementary Figure S5, Supplementary Material).
Direct comparison of the effects of delta-9-THC and CBD on functional connectivity
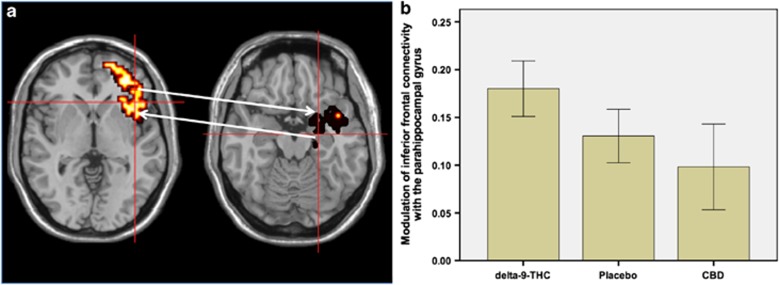
Inferior frontal gyrus seed cluster. Relative to the placebo condition, delta-9-THC augmented the functional connectivity of the right inferior frontal gyrus with the parahippocampal gyrus. On the other hand, CBD attenuated the functional connectivity between these two regions (Table 1, Figure 2a and b).
Table 1. Talairach Coordinates of Brain Regions Where Functional Connectivity to Seed Clusters Was Oppositely Modulated by Delta-9-THC and CBD Relative to Placebo Condition (All Results Corrected for<1 False Positive Cluster Unless Indicated Otherwise; Clusters⩾20 Voxels in Size and p<0.01 Underlined).
| Area |
Talairach coordinates |
Cluster size | p | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Opposite effects of delta-9-THC and CBD on functional connectivity with the inferior frontal (51,26,−13) cluster (delta-9-THC>Placebo>CBD) | |||||
| Parahippocampal gyrus | 22 | −15 | −18 | 56 | <0.03 |
| Opposite effects of delta-9-THC and CBD on functional connectivity with the striatal (−22,26,9) cluster (delta-9-THC<Placebo<CBD) | |||||
| Inferior frontal gyrus | −29 | 19 | −7 | 23 | <0.001 |
| −29 | 22 | −13 | |||
| Striatum | −22 | 7 | −7 | 49 | <0.001 |
| −22 | 19 | −2 | |||
| −18 | 22 | 4 | |||
| Opposite effects of delta-9-THC and CBD on functional connectivity with the hippocampal (−29,41,4) cluster (delta-9-THC<Placebo<CBD) | |||||
| Parahippocampal gyrus | −32 | −41 | −2 | 11 | 0.04 |
| −32 | −41 | −7 | |||
| Opposite effects of delta-9-THC and CBD on functional connectivity with the hippocampal (−29,41,4) cluster (delta-9-THC>Placebo>CBD) | |||||
| Superior frontal gyrus | 7 | 52 | 31 | 22 | 0.004 |
| Middle frontal gyrus | −43 | 26 | 20 | 24 | |
| −36 | −4 | 42 | |||
| Inferior frontal gyrus | −36 | 33 | −7 | 37 | |
| −36 | 33 | 9 | |||
| −51 | 22 | 9 | |||
| Anterior cingulate/medial prefrontal gyrus | 7 | 37 | 26 | 48 | |
| 4 | 48 | 37 | |||
| −4 | 52 | 20 | |||
| Precentral gyrus | −29 | −7 | 48 | 17 | |
Figure 2.
(a) Effects of delta-9-THC and CBD on inferior frontal connectivity (brain on the left side of panel; cross-hairs) with parahippocampal gyrus (brain on the right side of panel; cross-hairs) during visual oddball salience processing. The left side of the brain is shown on the left side of the images. (b) The bar graph (mean±SEM) shows that connectivity of the inferior frontal seed cluster with the parahippocampal gyrus (y axis; arbitrary units) in (a) was augmented by delta-9-THC but attenuated by CBD.
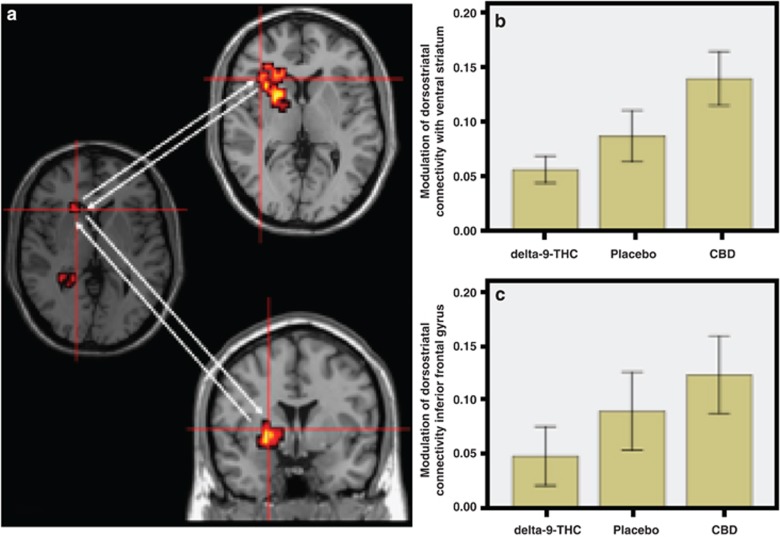
Dorsal striatum seed cluster. Relative to placebo, delta-9-THC reduced the functional connectivity of the left dorsal striatum with the ipsilateral ventral striatum and the inferior frontal gyrus. Conversely, CBD augmented the functional connectivity between the dorsal striatum and these two regions (Table 1, Figure 3a–c).
Figure 3.
(a) Effects of delta-9-THC and CBD on dorsostriatal connectivity (brain on the left side of panel; cross-hairs) with ventral striatum (brain on top half of middle panel; cross-hairs) and inferior frontal gyrus (brain on bottom half of middle panel; cross-hairs) during visual oddball salience processing. The left side of the brain is shown on the left side of the images. (b) The bar graph (mean±SEM) shows that connectivity of the dorsostriatal seed cluster with the ventral striatum (y axis; arbitrary units) in (a) was attenuated by delta-9-THC but augmented by CBD. (c) The bar graph (mean±SEM) shows that connectivity of the dorsostriatal seed cluster with the inferior frontal gyrus (y axis; arbitrary units) in (a) was attenuated by delta-9-THC but augmented by CBD.
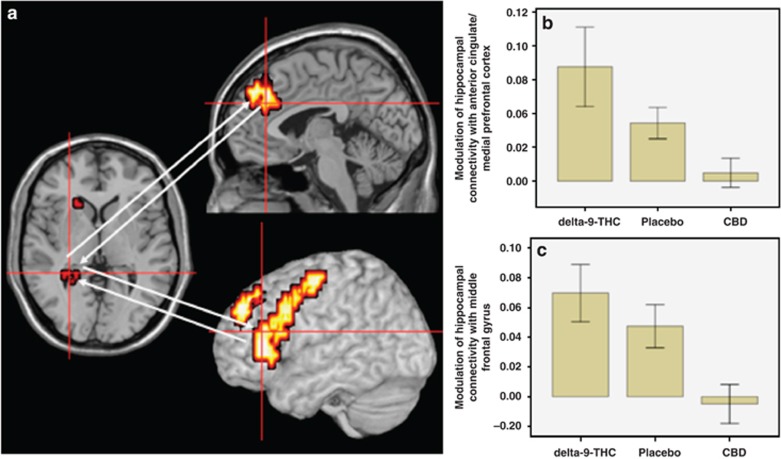
Hippocampal seed cluster. The functional connectivity of the left posterior hippocampal cluster with the left superior, middle and inferior frontal gyri, the anterior cingulate/medial prefrontal cortex, and the left precentral gyrus was augmented by delta-9-THC (Figure 4a). In contrast, CBD attenuated the connectivity of the posterior hippocampal cluster with these regions (Table 1, Figure 4a–c). Conversely, delta-9-THC attenuated the functional connectivity of the left posterior hippocampal cluster with the parahippocampal gyrus, whereas CBD augmented its connectivity with this region (Table 1, Figure 4d and e).
Figure 4.
(a) Effects of delta-9-THC and CBD on hippocampal connectivity (brain on the left side of panel; cross-hairs) with anterior cingulate/medial prefrontal cortex (brain on top half of middle panel; cross-hairs) and middle frontal gyrus (brain on bottom half of middle panel; cross-hairs) during visual oddball salience processing. The left side of the brain is shown on the left side of the images. (b) The bar graph (mean±SEM) shows that connectivity of the hippocampal seed cluster with the anterior cingulate/medial prefrontal cortex (y axis; arbitrary units) in (a) was augmented by delta-9-THC but attenuated by CBD. (c) The bar graph (mean±SEM) shows that connectivity of the hippocampal seed cluster with the middle frontal gyrus (y axis; arbitrary units) in (a) was augmented by delta-9-THC but attenuated by CBD. (d) Effects of delta-9-THC and CBD on hippocampal connectivity (brain on the left side of panel; cross-hairs) with parahippocampal cortex (brain on the right side of panel; cross-hairs) during visual oddball salience processing. The left side of the brain is shown on the left side of the images. (e) The bar graph (mean±SEM) shows that connectivity of the hippocampal seed cluster with the parahippocampal cortex (y axis; arbitrary units) in (d) was attenuated by delta-9-THC but augmented by CBD.
Relationship between delta-9-THC effects on functional connectivity and behaviour
The effect of delta-9-THC on the functional connectivity of the left striatal seed cluster with the inferior frontal gyrus (obtained in the three-way contrast between the effect of delta-9-THC and CBD relative to the placebo condition) was inversely correlated (r=−0.519, p=0.028) with its effect on performance during the visual oddball detection task. This was such that, the more delta-9-THC attenuated the connectivity between striatum and inferior frontal gyrus, the more prominent was its effect on response latency during the standard condition relative to the oddball condition. However, post hoc testing indicated that there was no such relationship between the effect of delta-9-THC on functional connectivity between the inferior frontal gyrus and medial temporal cortex and task performance.
Discussion
We employed a whole-brain analysis approach to investigate the effects of delta-9-THC and CBD, the principal ingredients of cannabis sativa, on the functional connectivity of the dorsal striatum, prefrontal cortex, and hippocampus, three brain regions that have a critical role in the processing of salient stimuli (Rubia et al, 2007; Strange and Dolan, 2001; Zink et al, 2006), which we have previously shown to be differentially modulated under their influence (Bhattacharyya et al, 2012c). In accordance with our first hypothesis, both cannabinoids modulated the functional connectivity of the striatum, inferior frontal cortex, and hippocampus. In the case of delta-9-THC, most of these effects did not survive correction for false positives. However, when delta-9-THC and CBD were directly contrasted relative to placebo in a three-way analysis to investigate whether the drugs had opposite effect on connectivity between the seed clusters and the rest of the brain, consistent with our second hypothesis, they had distinct, antagonistic effects on the connectivity of these regions. Furthermore, delta-9-THC-induced alterations in the connectivity between the striatal cluster and the inferior frontal gyrus correlated with response latency during the standard condition relative to the oddball condition in the visual oddball detection task. This is consistent with a similar relationship that we have observed between striatal effects of delta-9-THC and response latency to standard vs oddball stimuli in our previous study and perhaps suggests that altered fronto-striatal connectivity that we have observed may be functionally related to aberrant salience attribution in this sample that we have reported before (Bhattacharyya et al, 2012c) and summarized here (see Supplementary Material).
The antagonistic effects of delta-9-THC and CBD were evident on the connectivity of the inferior frontal gyrus, striatum, and hippocampus with other brain areas known to be involved in the processing of salience, such as the anterior cingulate/medial prefrontal cortex, parahippocampal gyrus, and ventral striatum (Palaniyappan and Liddle, 2012). This is consistent with differential modulation of both the processing of salient information in humans and its underlying neural substrates by delta-9-THC and CBD, which has been demonstrated in our previous study (Bhattacharyya et al, 2012c). Functional interaction of specific structures involved in salience processing, as identified in this study, is also in accordance with findings from human functional and structural imaging studies (Burianova et al, 2010) as well as mapping studies in primates (Selemon and Goldman-Rakic, 1985) that demonstrate their anatomical and functional relationship. Functional connectivity between the striatum and inferior frontal cortex was attenuated by delta-9-THC. One may interpret this as suggesting that the striatal regions were no longer subject to prefrontal control under the influence of delta-9-THC. Attenuation of striatal engagement while viewing the oddball stimuli relative to standard stimuli as observed under the influence of delta-9-THC, despite increased prefrontal engagement, may thus reflect this loss of cortical control under its influence, resulting in aberrant attribution of salience to standard stimuli. However, an important caveat to this interpretation is that it is not possible to infer directionality or causality regarding the influence that activity in a specified seed cluster may exert on other brain regions, based on the functional connectivity analyses reported here. Nevertheless, this interpretation is also consistent with the relationship between the effect of delta-9-THC on response latency while viewing standard stimuli and its effect on connectivity between the striatum and inferior frontal cortex. Conversely, functional connectivity of the inferior frontal cortex and striatum was enhanced under the influence of CBD. This may have facilitated the normal attribution of salience to oddball stimuli by augmenting the striatal response under the influence of CBD, rather than result in the aberrant attribution of salience to the standard stimuli that was observed under delta-9-THC (Bhattacharyya et al, 2012c). Alteration in fronto-striatal connectivity measured using fMRI (while performing a response inhibition task) has also been reported to correlate with the number of cannabis use occasions per day in regular cannabis users, though the direction of this relationship was opposite in dependent vs non-dependent users (Filbey and Yezhuvath, 2013). While it is not possible to directly compare the results of our study with this previous study because of obvious methodological differences, including the measurement of acute (using pharmacological challenge) vs non-acute effect of exposure, different cannabis usage patterns in study participants and different task parameters, it nevertheless identifies the fronto-striatal connection as a substrate for both the acute and non-acute effects of cannabinoids, perhaps reflecting the high distribution of cannabinoid CB1 receptors (CB1R) in these regions (Pertwee, 2008). Results presented here are also consistent with previous reports of effect of delta-9-THC on the coherence and synchrony of electrical signals in the frontal cortex (Morrison et al, 2011; Stone et al, 2012).
Although opposite effects of delta-9-THC and CBD on brain activation have been described in previous studies (Bhattacharyya et al, 2010, 2012c) their influence on functional connectivity has only been addressed in one study so far. However, unlike Fusar-Poli et al (2010) who employed dynamic causal modelling to investigate connectivity and reported that CBD but not delta-9-THC altered effective connectivity between the anterior cingulate cortex and the amygdala during emotional processing of fearful stimuli, we employed seed-based functional connectivity approach that allowed us to investigate drug-induced changes in connectivity throughout the brain. Opposite effects of delta-9-THC and CBD on connectivity between the prefrontal and medial temporal cortex and striatum are also consistent with previous studies that have reported an acute effect of these drugs on the engagement of these regions during other cognitive and emotional processing paradigms (Bhattacharyya et al, 2009a, 2009b, 2010, 2012a, 2012b; Borgwardt et al, 2008) as well as evidence from studies that have investigated the chronic effects of cannabis on brain structure and function (Batalla et al, 2013).
Results presented here are also consistent with the role of striatal dopamine in mediating salience processing (Floresco et al, 2003), evidence regarding the effect of cannabinoids on dopaminergic neurotransmission (see Kuepper et al, 2010 for a review) as well as evidence that variation in genes that regulate central dopamine neurotransmission may modulate the effect of delta-9-THC on striatal function that correlates with psychotic symptoms induced under its influence (Bhattacharyya et al, 2012a). Together with the results of our previous study, results presented here suggest that a potential mechanism for the psychotogenic effects of delta-9-THC and in particular its effects on salience processing might lie in its effect on the functional integration of components of a network processing salient information.
Consistent with these results, previous studies have reported the effect of other psychotogenic substances such as ketamine on the processing of salient information (Corlett et al, 2006). It has been suggested that the psychotropic effects of ketamine might also be related to alterations in the connectivity between specific brain regions, which has been demonstrated both in animal (Bolton et al, 2012) and in human studies (Salvadore et al, 2010).
Our results are also in line with studies demonstrating altered brain network connectivity under the influence of drugs that induce psychotic symptoms (Diaconescu et al, 2010; Esposito et al, 2013; Pujol et al, 2013) and studies demonstrating altered connectivity in different stages of schizophrenia (Pettersson-Yeo et al, 2011). This may suggest that altered functional connectivity under the influence of delta-9-THC might be a plausible mechanism through which regular cannabis consumption increases the risk of development of schizophrenia. However, it is worth noting that results presented here only refer to the acute effects of delta-9-THC on functional connectivity in those with minimal previous exposure to cannabis, which may be different from the long-term effects of regular cannabis use on functional connectivity.
At the cellular level, both delta-9-THC and CBD act on cannabinoid receptors and one of these, the CB1R, has been implicated in the modulation of salience in animals (Laviolette and Grace, 2006). It has also been proposed that delta-9-THC and CBD may have opposite mechanisms of action on the CB1R (Pertwee, 2008), and it is possible that the opposite effects of delta-9-THC and CBD on regions involved in processing salience may be a result of this, as CB1Rs have a high density in brain regions that form part of the salience network (Pertwee, 2008).
The opposite effects of delta-9-THC and CBD on functional connectivity provide further complementary evidence for the potential role of CBD as an antipsychotic (Bhattacharyya et al, 2010; Leweke et al, 2012; Morgan and Curran, 2008; Morgan et al, 2010b; Zuardi et al, 2012) and suggest that the antipsychotic effects of CBD might be related to its effects on brain activation in regions, which have been consistently found to be associated with psychosis, such as the striatum and the temporal cortex.
Limitations
One of the main limitations of this study is that functional connectivity analysis that depends on correlation between time series from different brain regions is only as good as the temporal and spatial resolution available with fMRI scanning. Whether temporal correlations observed between the time series from different brain regions truly reflect correlation between neural activity rather than correlation between drug-induced changes in cerebral blood flow remains difficult to be certain. However, it has been shown that the shape of the hemodynamic response that is used to estimate effects in fMRI studies is not altered by drugs known to have vascular effects (Gollub et al, 1998; Luo et al, 2003; Murphy et al, 2006). Furthermore, even if the effects of delta-9-THC and/or CBD on global cerebral blood flow were to have influenced the results of the connectivity analysis, such effects would not have been localized to specific brain regions as observed here. Another factor that may limit the generalizability of these results to the clinical setting is related to the fact that we have only reported the acute effects of the drugs on brain connectivity. Future studies investigating these effects over a longer time period are warranted for an understanding of the effects of delta-9-THC and CBD on brain connectivity to be relevant to an understanding of the role of these cannabinoids in psychosis and its treatment, respectively.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We thank Glynis Ivin for help with the blinding procedure, storage and dispensing of the drugs. This work was supported by a Joint MRC/Priory Clinical research training fellowship award (G0501775) from the Medical Research Council (MRC), UK to SB and a grant from the Psychiatry Research Trust, UK to PM. SB has received support from the National Institute of health Research (NIHR) (NIHR Clinician Scientist Award; NIHR CS-11-001) and the MRC (MR/J012149/1). IF was supported by the GA Lienert Foundation, Adolf-Schmidtmann-Foundation, FAZIT-Foundation and the German Academic Exchange Service. JAC is the recipient of a Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) Productivity fellowship. The authors acknowledge infrastructure support from the NIHR Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. SB had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nat Neurosci. 2010;13:507–512. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogué S, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS ONE. 2013;8:e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, Kambeitz J, Prata D, et al. Preliminary report of biological basis of sensitivity to the effects of cannabis on psychosis: AKT1 and DAT1 genotype modulates the effects of δ-9-tetrahydrocannabinol on midbrain and striatal function. Mol Psychiatry. 2012;17:1152–1155. doi: 10.1038/mp.2011.187. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, McGuire PK. Neural mechanisms for the cannabinoid modulation of cognition and affect in man: a critical review of neuroimaging studies. Curr Pharm Des. 2012;18:5045–5054. doi: 10.2174/138161212802884636. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Crippa JA, Allen P, Martin-Santos R, Borgwardt S, Fusar-Poli P, et al. Induction of psychosis by Δ9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry. 2012;69:27–36. doi: 10.1001/archgenpsychiatry.2011.161. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Crippa JA, Martin-Santos R, Winton-Brown T, Fusar-Poli P. Imaging the neural effects of cannabinoids: current status and future opportunities for psychopharmacology. Curr Pharm Des. 2009;15:2603–2614. doi: 10.2174/138161209788957465. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O'Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66:442–451. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton MM, Heaney CF, Sabbagh JJ, Murtishaw AS, Magcalas CM, Kinney JW. Deficits in emotional learning and memory in an animal model of schizophrenia. Behav Brain Res. 2012;233:35–44. doi: 10.1016/j.bbr.2012.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry. 2008;64:966–973. doi: 10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Brammer MJ, Bullmore ET, Simmons A, Williams SC, Grasby PM, Howard RJ, et al. Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. Magn Reson Imaging. 1997;15:763–770. doi: 10.1016/s0730-725x(97)00135-5. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, et al. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp. 2001;12:61–78. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage. 2010;49:865–874. doi: 10.1016/j.neuroimage.2009.08.066. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Honey GD, Aitken MRF, Dickinson A, Shanks DR, Absalom AR, et al. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: linking cognition, brain activity, and psychosis. Arch Gen Psychiatry. 2006;63:611–621. doi: 10.1001/archpsyc.63.6.611. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Martín-Santos R, Bhattacharyya S, Atakan Z, McGuire P, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515–523. doi: 10.1002/hup.1048. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu Y-T, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Diaconescu AO, Menon M, Jensen J, Kapur S, McIntosh AR. Dopamine-induced changes in neural network patterns supporting aversive conditioning. Brain Res. 2010;1313:143–161. doi: 10.1016/j.brainres.2009.11.064. [DOI] [PubMed] [Google Scholar]
- Esposito R, Cilli F, Pieramico V, Ferretti A, Macchia A, Tommasi M, et al. Acute effects of modafinil on brain resting state networks in young healthy subjects. PLoS ONE. 2013;8:e69224. doi: 10.1371/journal.pone.0069224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey F, Yezhuvath U. Functional connectivity in inhibitory control networks and severity of cannabis use disorder. Am J Drug Alcohol Abuse. 2013;39:382–391. doi: 10.3109/00952990.2013.841710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, et al. Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. 2010;13:421–432. doi: 10.1017/S1461145709990617. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Gollub RL, Breiter HC, Kantor H, Kennedy D, Gastfriend D, Mathew RT, et al. Cocaine decreases cortical cerebral blood flow but does not obscure regional activation in functional magnetic resonance imaging in human subjects. J Cereb Blood Flow Metab. 1998;18:724–734. doi: 10.1097/00004647-199807000-00003. [DOI] [PubMed] [Google Scholar]
- Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- Jensen J, Kapur S. Salience and psychosis: moving from theory to practise. Psychol Med. 2009;39:197–198. doi: 10.1017/S0033291708003899. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kuepper R, Morrison PD, van Os J, Murray RM, Kenis G, Henquet C. Does dopamine mediate the psychosis-inducing effects of cannabis? A review and integration of findings across disciplines. Schizophr Res. 2010;121:107–117. doi: 10.1016/j.schres.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Kuepper R, van Os J, Lieb R, Wittchen H-U, Höfler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol. 2006;4:e424. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci. 2006;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Wu G, Li Z, Li S-J. Characterization of effects of mean arterial blood pressure induced by cocaine and cocaine methiodide on BOLD signals in rat brain. Magn Reson Med. 2003;49:264–270. doi: 10.1002/mrm.10366. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468:194–202. doi: 10.1038/nature09569. [DOI] [PubMed] [Google Scholar]
- Moore THM, Zammit S, Lingford-Hughes A, Barnes TRE, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Morgan CJA, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192:306–307. doi: 10.1192/bjp.bp.107.046649. [DOI] [PubMed] [Google Scholar]
- Morgan CJA, Freeman TP, Schafer GL, Curran HV. Cannabidiol attenuates the appetitive effects of Delta 9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology. 2010;35:1879–1885. doi: 10.1038/npp.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJA, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected] Br J Psychiatry. 2010;197:285–290. doi: 10.1192/bjp.bp.110.077503. [DOI] [PubMed] [Google Scholar]
- Morrison PD, Nottage J, Stone JM, Bhattacharyya S, Tunstall N, Brenneisen R, et al. Disruption of frontal theta coherence by Δ9-tetrahydrocannabinol is associated with positive psychotic symptoms. Neuropsychopharmacology. 2011;36:827–836. doi: 10.1038/npp.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Dixon V, LaGrave K, Kaufman J, Risinger R, Bloom A, et al. A validation of event-related fMRI comparisons between users of cocaine, nicotine, or cannabis and control subjects. Am J Psychiatry. 2006;163:1245–1251. doi: 10.1176/ajp.2006.163.7.1245. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now. Neurosci Biobehav Rev. 2011;35:1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Pujol J, Blanco-Hinojo L, Batalla A, López-Solà M, Harrison BJ, Soriano-Mas C, et al. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. 2013;51:68–78. doi: 10.1016/j.jpsychires.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Temporal lobe dysfunction in medication-naïve boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry. 2007;62:999–1006. doi: 10.1016/j.biopsych.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Brugger S, Nottage J, Bhattacharyya S, Sumich A, et al. Communication breakdown: delta-9 tetrahydrocannabinol effects on pre-speech neural coherence. Mol Psychiatry. 2012;17:568–569. doi: 10.1038/mp.2011.141. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Adaptive anterior hippocampal responses to oddball stimuli. Hippocampus. 2001;11:690–698. doi: 10.1002/hipo.1084. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme Medical: New York; 1988. [Google Scholar]
- Vita A, De Peri L, Silenzi C, Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res. 2006;82:75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006;29:977–983. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JAS, Hallak JEC, Bhattacharyya S, Atakan Z, Martín-Santos R, et al. A critical review of the antipsychotic effects of Cannabidiol: 30 years of a translational investigation. Curr Pharm Des. 2012;13:5131–5140. doi: 10.2174/138161212802884681. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JAS, Hallak JEC, Pinto JP, Chagas MHN, Rodrigues GGR, et al. Cannabidiol for the treatment of psychosis in Parkinson's disease. J Psychopharmacol (Oxford) 2009;23:979–983. doi: 10.1177/0269881108096519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.