Abstract
Poor-quality maternal diet during pregnancy, and subsequent gestational growth disturbances in the offspring, have been implicated in the etiology of multiple neurodevelopmental disorders, including ADHD, schizophrenia, and autism. These disorders are characterized, in part, by abnormalities in responses to reward and errors of executive function. Here, we demonstrate dissociable deficits in reward processing and executive function in male and female mice, solely due to maternal malnutrition via high-fat or low-protein diets. Gestational exposure to a high-fat diet delayed acquisition of a fixed ratio response, and decreased motivation as assessed by progressive ratio. In contrast, offspring of a low-protein diet displayed no deficits in operant learning, but were more prone to assign salience to a cue that predicts reward (sign-tracking) in a Pavlovian-conditioned approach task. In the 5-choice serial reaction time task (5-CSRTT), gestational exposure to a high-fat diet promoted impulsivity, whereas exposure to a low-protein diet led to marked inattention. These dissociable executive function deficits are known to be mediated by the medial prefrontal cortex (PFC), which displays markers of epigenetic dysregulation in neurodevelopmental disorders. Following behavioral characterization, we assayed PFC gene expression using a targeted PCR array and found that both maternal diets increased overall transcription in PFC. Cluster analysis of the relationships between individual transcripts and behavioral outcomes revealed a cluster of primarily epigenetic modulators, whose overexpression was linked to executive function deficits. The overexpression of four genes, DNA methyltransferase 1 (DNMT1), δ-opioid receptor (OPRD1), cannabinoid receptor 1 (CNR1), and catechol-o-methyltransferase (COMT), was strongly associated with overall poor performance. All 5-CSRTT deficits were associated with DNMT1 upregulation, whereas impulsive behavior could be dissociated from inattention by overexpression of OPRD1 or COMT, respectively, as well as a distinct cluster of epigenetic regulators. These data provide molecular support for dissociable domains of executive function.
Introduction
The risk of adult mental disorder is known to be influenced by gestational exposure to adverse events, such as severe stress or infection, and recent data also implicates the rising importance of poor maternal nutrition and obesity (Grissom and Reyes, 2012; Ornoy, 2011; Vesco et al, 2011). Poor maternal diet and excessive gestational weight gain significantly increase the risk of children being born either small for gestational age (SGA) or large for gestational age (LGA), respectively, with each occurring in ∼10% of pregnancies in the United States (Grissom and Reyes, 2012; Ornoy, 2011). Both abnormal gestational growth trajectories are biomarkers of later metabolic (Ornoy, 2011) and neurodevelopmental pathology, including schizophrenia, attention deficit/hyperactivity disorder (ADHD), impulsivity associated with conduct disorders, and autism (Krakowiak et al, 2012; Van Lieshout and Boyle, 2011; Van Lieshout et al, 2011; Moore et al, 2012). These neurodevelopmental disorders involve deficits in medial prefrontal cortex (PFC)-mediated executive function, including attention, impulse control, and behavioral flexibility (Bari and Robbins, 2013; Duncan, 2013; Floresco and Jentsch, 2011).
Gestational growth in mice is sensitive to maternal diet, as it is in humans. SGA and LGA can be modeled using maternal diets deficient in protein or high in fat, respectively. We have found that this maternal malnutrition alters the expression of genes related to dopamine and opioid function in the PFC (Grissom and Reyes, 2012; Grissom et al, 2013; Vucetic et al, 2010a, 2010b). Vulnerability of the developing PFC also involves epigenetic dysregulation, specifically DNA methylation (Grayson and Guidotti, 2013). Both SGA and LGA mice demonstrate decreased genome-wide DNA methylation in PFC (Grissom and Reyes, 2012; Vucetic et al, 2010a). PFC from patients with schizophrenia and autism have substantial alterations in DNA methylation and overexpress the DNA methyltransferase DNMT1 (Grayson and Guidotti, 2013; Ladd-Acosta et al, 2013). Collectively, these findings suggest that epigenetic dysregulation and aberrant transcription in the PFC may be a crucial mechanism linking gestational adversity to neurodevelopmental disorders.
Behavioral deficits in executive function disorders can occur independently, for eg, impulsivity in the absence of inattention (Johnson et al, 2007; Kuntsi et al, 2013; Ben Shalom et al, 2013), and are linked with opposing patterns of prefrontal cortex activation (Pezze et al, 2014). In these experiments, we use two models of gestational malnutrition, resulting in SGA and LGA offspring, to demonstrate dissociable deficits in behaviors related to motivation, attention, and impulse control in both males and females, as assessed by the 5-CSRTT. We were able to dissociate two ADHD-relevant endophenotypes driven by gestational malnutrition, namely stimulus salience-related inattention in offspring of a maternal low-protein diet, vs motivation-related impulsivity in offspring of a maternal high-fat diet. Currently, neither specific etiologies nor molecular mechanisms have been linked to dissociable executive function domains. We assessed PFC gene expression in animals after behavioral testing was completed, and performed cluster analyses between transcript expression and behavioral performance. These analyses revealed that transcript overabundance, particularly in the cluster enriched for epigenetic function, were associated with poor performance in the 5-CSRTT. However, specific deficits in attention or impulse control were associated with the overexpression of different sets of epigenetic regulators. These data indicate that different executive function deficits (inattention vs impulsivity) may be associated with specific transcriptional profiles, triggered by divergent gestational nutrition and growth patterns in utero.
Materials and Methods
Animals and Behavioral Preparations
All animals were cared for according to the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee. The offspring used in behavioral testing were (C57BL/6 J × DBA/2 J) F1 mice. Experimental timeline schematic is seen in Figure 1. Maternal diets were given as previously published (see Grissom and Reyes, 2012; Vucetic et al, 2010a, 2010b; Whitaker et al, 2011 for information on the maternal and offspring phenotypes). All diets from Test Diet, Richmond, IN, USA; control: Test Diet 5755, 4.09 kcal/g with 18% of total energy calories from protein, 22% from fat, and 60% from carbohydrate; low protein: Test Diet 5769, 4.13 kcal/g with 8.5% of total energy calories from protein, 22% from fat, and 69.5% from carbohydrate; high fat: Test Diet 58G9, 5.21 kcal/g with 18% of total energy calories from protein, 60% from fat, and 22% from carbohydrate). Mean litter size (and SEM) did not differ between groups; HF=8.4 (0.65), LP=7.7 (0.29), and CTL=8.3 (0.42). Litters are culled if the number of pups exceeds 10, but this is rare and did not occur in these cohorts. Litter sex compositions were not different between groups, with HF=46% males: 53% females, LP=45% males: 55% females, and CTL=46% males: 53% females. On p21, animals were weaned onto ad libitum standard chow (Lab Diet 5001), and housed in groups of 4–5. At 12–16 weeks, animals were moved to a 9 a–9 p-reversed light cycle to permit testing during the dark period, and food restriction was begun (Young et al, 2009). Male and female experiments were conducted sequentially to avoid possible effects of opposite-sex odors in the chamber on operant performance, with males tested first. In some cases, planned comparisons (t-tests) were used to analyze female data (Figures 1d and 2d) to examine whether that pattern of responses observed in males were replicated in females. At the start of experimentation, mean body weights (and SEM) for male CTL=37.9 (0.51), male LP=31.6 (0.55), male HF=37.2 (1.24), female CTL=22.3 (0.35), female LP=18.7 (0.26), female HF=20.8 (0.23). At this adult baseline, male LP offspring were significantly lighter than CTL males (F(2,45)=17.5, p<0.0001), and both female HF and LP offspring were significantly lighter than CTL females (F(2,47)=37.3, p<0.0001). To maintain operant responding, all animals were food restricted to 80–95% of their free-feeding body weight for the duration of experiments, as is standard for operant testing protocols. The percent of body weight change from free-feeding weights was not different between the groups. Animals were tested in darkened nine-hole mouse operant chambers (Lafayette Instruments, Lafayette, IN, USA) with peristaltic pumps to deliver liquid reward (Yoohoo, Mott's, Plano, TX, USA). The five odd-numbered holes are depicted in Figures 1 and 2; even numbered holes were inactive throughout the experiment. The holes are in the rear of the chamber, and contain recessed lights and infrared beams, which register nose pokes, whereas the liquid-delivery magazine is at the front (Figure 1). There are also two infrared beams at the front (∼1 cm from the magazine) and back (∼1 cm from the holes), and a house light. Estrous cycling was continuously monitored via vaginal cytology. Cycle stage did not exert an effect on operant performance on 5-CSRTT. A two-way ANOVA performed on % correct performance on the most difficult 5-CSRTT schedule (three maternal diet conditions (CTL, LP, HF) × 3 cycle stages (estrous, meta-/diestrous, proestrous) revealed no significant effect of the estrous stage on % correct performance (F(2,83)=1.59, p=0.21), and no significant interaction between maternal diet and the estrous stage (F(4,83)=0.84, p=0.51). A one-way ANOVA collapsing across maternal diet groups also found no significant effect of the estrous stage on percent correct performance (F(2,88)=1.52, p=0.12). This is consistent with reports indicating that the estrous cycle and female steroid hormones do not impact operant behavior on simpler tasks (Gray et al, 1977; Hest and Haaren, 1989; Kritzer et al, 2007; Roberts et al, 1998).
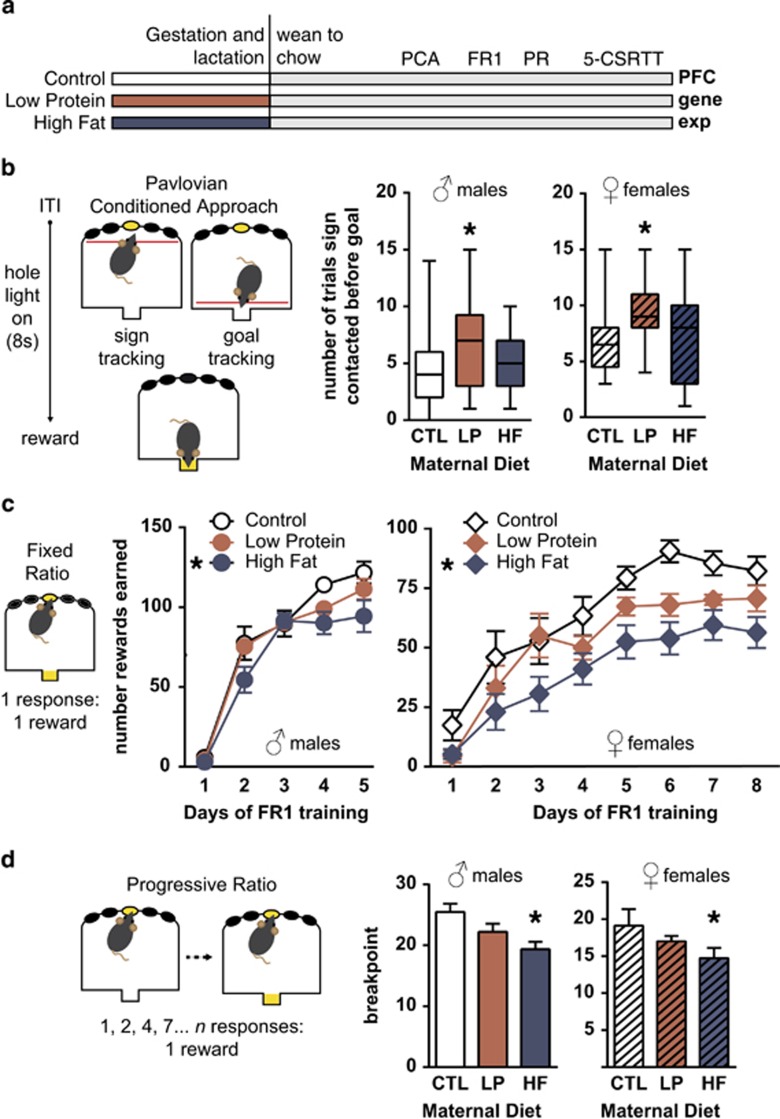
Figure 1.
Altered reward-related behavior profiles are dissociably induced by different forms of gestational malnutrition. (a) Mouse dams were provided unbalanced diets (low protein, LP; high fat, HF) or a standard breeder diet (control, CTL) throughout gestation and lactation, at which point offspring were weaned onto colony chow. Animals were then run on a series of operant tasks described below, and finally killed to examine gene expression in the prefrontal cortex (PFC). (b) LP show increased approach to cues that predict reward in the Pavlovian-conditioned approach (PCA). The task was modified for mouse nine-hole operant boxes. After a variable intertrial interval (ITI), the center hole was lit for 8 s, providing a cue or ‘sign' predicting food delivery. Following this, reinforcer was delivered at the magazine or ‘goal' at the front of the chamber (yellow rectangle). Approach to the sign or goal during the cue was assessed by infrared beam breaks at the front and rear of the chamber. Male and female LP were more likely to approach the sign before approaching the goal (male: Mann–Whitney U=65, p=0.04; female: U=50.5, p=0.01), suggesting the sign held a greater incentive value and draws more attention in these animals. (c) Offspring of a maternal high-fat diet have deficits in acquiring motivated behavior. Animals now had to nosepoke at the center hole once to elicit a reinforcer (fixed ratio 1, FR1), as illustrated. Male and female HF made fewer responses over FR1 training (main effect of maternal diet; male: F(2,45)=4.2, p=0.02; female: F(2,43)=8.9, p=0.0006). (d) HF offspring are less motivated to work for reinforcers on a progressive ratio (PR) schedule. Once FR1 criteria were achieved, animals were required to perform progressively increasing numbers of nose pokes to the center hole to earn a reinforcer, assessing motivation. HF males terminated responding in PR testing after fewer trials than controls (male: F(2,45)=5.7, p=0.006). When these groups were directly compared in the females, similar deficits in motivation were seen: HF vs CTL (t(27)=0.04). LP animals showed a nonsignificant tendency toward reduced motivation. *p⩽0.05 vs the control group. n: male: CTL=15, LP=14, HF=19, female: CTL=14, LP=15, HF=17 in all figures. (b) Box-and-whisker plots of the full response range, c and d depict mean±SEM. 5-CSRTT, 5-choice serial reaction time test.
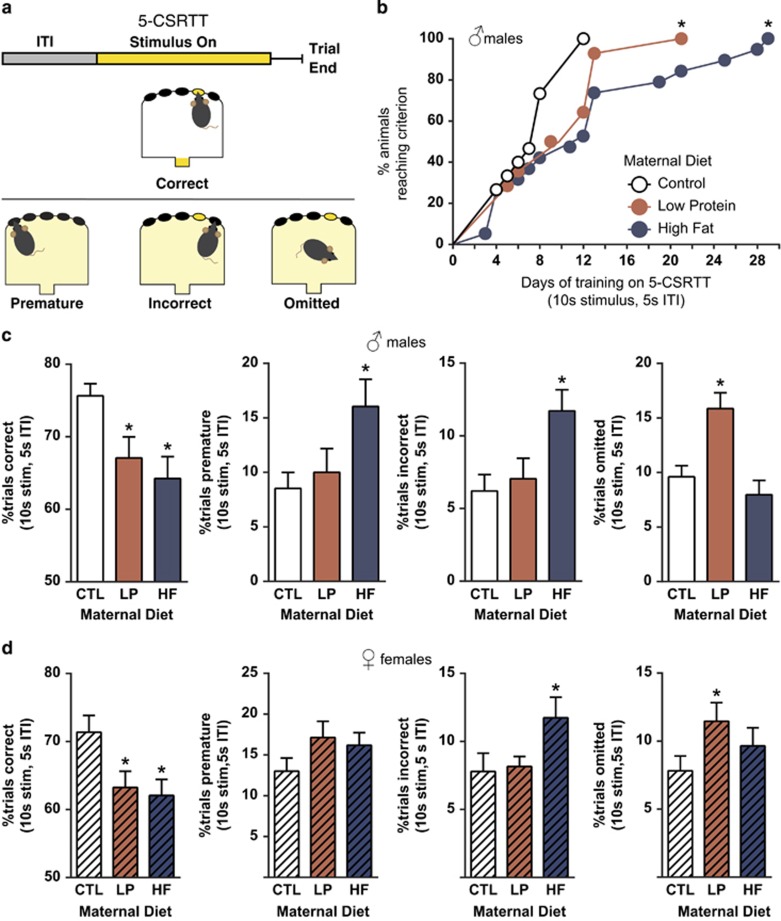
Figure 2.
Inattention and impulsiveness are dissociably induced in adult offspring by a gestational diet. (a) Trial schematic for the 5-choice serial reaction time test (5-CSRTT). After trial initiation, animals were required to wait a short intertrial interval (ITI) prior to the lighting of one of five possible stimulus holes. Correct responses at the lit hole earned reinforcers. Premature responses prior to hole lighting, and incorrect responses to a hole other than one that was lit indicated impulsivity and were punished by 5-s time out with house light illumination. Lack of response by trial end was logged as an omitted trial and punished. (b) Task acquisition was impaired in both male low-protein (LP) and high-fat (HF) offspring (LP χ2=4.6, p=0.03, HF χ2=5.5, p=0.02). Animals were trained on the first stage of 5-CSRTT (10 s stimulus, 5 s ITI) until each individual met advancement criterion (see Materials and Methods). (c and d) Different forms of maternal malnutrition program dissociable executive function deficits. Performance data were collected for each animal from the day he or she met advancement criterion. (c) Both maternal LP and HF diets lead male adult offspring to make fewer correct responses (F(2,45)=4.9, p=0.01), due to different causes. HF offspring made significantly more premature (F(2,45)=3.6, p=0.03) and incorrect (F(2,45)=5.1, p=0.01) responses, indicating impulsivity. In contrast, LP offspring omitted a higher percentage of trials (F(2,45)=9.9, p=0.0003) indicative of inattention. (d) Female offspring of maternal malnutrition display similar deficits to males (correct responses F(2,43)=4.3, p=0.02). When the female groups were compared with test for the same deficits found in the male groups (planned comparisons), female HF offspring were also found to commit more incorrect responses (t(29)=1.95, p=0.03), and female LP offspring were found to make more omission errors (t(27)=2.1, p=0.04), indicating inattention. *p⩽0.05 vs the control group. (b) A survival curve, c and d depict mean±SEM. CTL, control.
Pavlovian-Conditioned Approach (PCA)
PCA training ran 25 trials/day on days 1–14, the duration needed for mice to demonstrate approach (Tomie et al, 2011). Each trial was preceded by a variable intertrial interval (ITI) averaging 60 s. Following ITI, the center hole would light for 8 s. Following this, the cue would terminate and Yoohoo would be delivered at the magazine (Figure 1b). Beam breaks to the front and back of the chamber were tracked automatically, and animal behavior was videoed. For each of the 25 trials in the PCA, two human coders assessed the number of approaches to the sign and the goal (which was averaged across the 25 trials), the latency to first contact the sign and the goal (averaged), and the number of seconds spent in contact with the sign and the goal (averaged). We also assessed these measures as beam breaks in the chamber, averaged across trials as with the human data. Thus, each measure was not binary (sign or goal) but instead a numeric, continuous variable. The value for each one of these measures, from each person and from the computer, for every mouse was analyzed on an omnibus correlation matrix, using Spearman correlations (nonparametric, corrected for the number of comparisons). Two human coders naive to condition were highly correlated with each other on all measures of sign tracking (correlation: 0.713–0.851, all p<0.0001) and with automated beam break data (correlation: 0.575–0.710, all p<0.0001). The beam break measurements are presented, as they provided greater precision. PCA data were analyzed using ANOVA and t-tests, and nonparametric tests when data did not follow a normal distribution. Statistics were performed with GraphPad Prism 6.0.
Fixed Ratio (FR) and Progressive Ratio (PR) Responding
After completion of PCA, animals were transitioned to FR1 (Figure 1c). The center hole was lit and the reinforcer could be earned by a single nose poke. Responses per day and days to criterion were logged for each animal, and analyzed using repeated measures ANOVA. FR1 training continued until all animals reached a criterion of 70 responses/30 min (for males) or 50 responses/30 min (for females). A baseline sex difference was observed with females showing an overall lower level of performance (fewer overall responses), which also resulted in an increased number of FR1 training sessions in females. The PR schedule was as previously described (Young et al, 2011) and was administered after all animals acquired FR1, and analyzed with ANOVA. Following PR, animals were transitioned to an FR1 where a response at any hole was rewarded in preparation for 5-choice testing.
5-Choice Serial Reaction Time Test (5-CSRTT)
5-CSRTT was administered as previously described (Young et al, 2009). Schematic of the task can be seen in Figure 2a. Errors were punished by time out (house light on for 5 s, no reinforcer, operanda inactive). Magazine entry was necessary to initiate the next trial after correct and error trials, thus 5-CSRTT was self-paced. After magazine entry, an ITI passed, followed by one of the five odd-numbered holes illuminating for a brief period of time. If an animal made a response during the ITI, this was logged as a premature trial, the trial was terminated, and the error was punished. If an animal responded at the incorrect hole during the stimulus or the 2-s-limited hold following the stimulus, this was logged as an incorrect trial, the trial was terminated, and was punished. If an animal failed to respond during the stimulus, this was logged as an omitted trial and punished. Only a response at the correct hole during the stimulus or limited hold was reinforced. Performance measures (correct, premature, and incorrect responses, and omissions) were reported as a percentage of the total number of trials (the sum of correct, premature, incorrect, and omitted trials) (McTighe et al, 2013; Scott and Taylor, 2014).
Animals were maintained on each schedule until criteria were met for that schedule (>20 responses, with >50% correct, for two consecutive days). These criteria were always exceeded in animals that acquired the behavior, and the largest differences in group performance were seen at early testing (Supplementary Figure 3), with incremental performance improvements over training as is also seen in humans performing continuous performance tasks under reinforcement conditions (Bubnik et al, 2014). Time to acquire 5-CSRTT was analyzed using χ2-tests. Performance data were taken from the day when an individual met criteria for the given schedule and analyzed using ANOVA. The task began with a fixed 5-s ITI, followed by lighting one of the five stimulus holes for 10 s. As animals achieved criteria with these settings, the stimulus duration was progressively shortened and the ITI was made variable (Supplementary Figures 4 and 5) in the following pattern: 10 and 5 s ITI; 8 s stimulus and 5 s ITI; 4 s stimulus and 5 s ITI; 4 s stimulus and 3–7 s ITI; 2 s stimulus and 3–7 s ITI.
Gene expression in Prefrontal Cortex
After completion of all operant training, male animals were killed the following day between 10 a and 12 p. Females were not analyzed, as these animals were needed for separate experiments. Brains were placed in RNA later and stored at −20 °C. The PFC (consisting of anterior cingulate, prelimbic, and infralimbic) was dissected from a 2-mm coronal slice from bregma +2.3 to +0.3, and DNA and RNA was extracted using Qiagen AllPrep DNA/RNA Mini kit. Hundred μg/μl cDNA was synthesized using Applied Biosystems High Capacity Reverse Transcriptase kit. Gene expression was assayed using Taqman primers on custom OpenArray real-time PCR plates assaying housekeeping genes (GAPDH, ACTB) and 18 genes of interest (Figure 3). Expression of housekeeping genes did not differ between groups. Expression of targets was normalized to the geometric mean of housekeeping genes and expressed as 2−ΔCt (Schmittgen and Livak, 2008), then converted to z-scores, which removes differences in expression levels between genes to highlight differences in expression levels between animals (Figure 3a and b), and were analyzed by ANOVA. Pairwise Pearson correlations were calculated between each animal's performance at criterion or on the last testing day for all behaviors, and the expression of each gene as 2−ΔCt (Schmittgen and Livak, 2008) resulting in an omnibus correlation matrix comparing behavioral performance to gene expression (Figure 3a), including corrected p-values. Full correlation matricies, both omnibus and separated by maternal diet, can be seen in Supplementary Figures 7 and 8. Unsupervised hierarchical clustering of average linkage between genes based on their pattern of correlation to behavior was computed using Cluster 3.0 and JavaTreeview (Eisen et al, 1998).
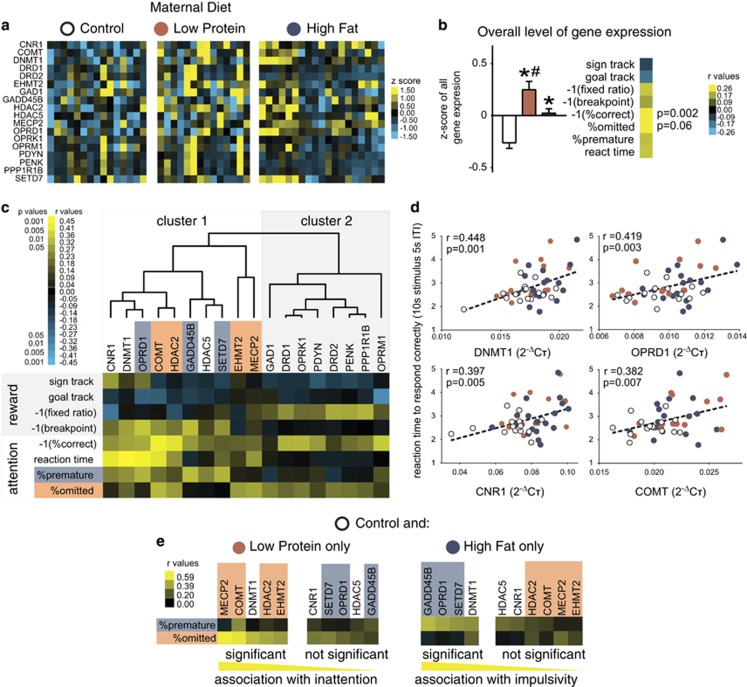
Figure 3.
Gestational malnutrition increases transcription in medial prefrontal cortex, and overexpression of different epigenetic modifiers associate with dissociable executive function deficits. (a) Levels of expression of 18 target genes expressed as a z-score normalized 2−ΔCT values in the medial prefrontal cortex (PFC) of individual males (each column) after behavioral completion. Lowest levels of expression (blue) are most frequently found in control offspring, whereas highest levels of expression (yellow) are found in low protein (LP), high fat (HF), or both LP and HF, although all groups show substantial variability. (b) Gene expression values depicted in a are significantly higher in HF than control (CTL), and significantly higher in LP than either CTL or HF (F(2,846)=17.7, p<0.0001). An animal's overall level of gene expression in the PFC is strongly correlated with lower correct performance (inverse of correct percentage and average gene expression z-score r=0.421, p=0.003) and tends to be associated with omitted, inattentive errors (omitted percentage and average gene expression z-score r=0.269, p=0.06). (c) Cluster analysis of the correlations between expression of 18 target genes in PFC with individual performance of behavioral tests indicate that the genes most strongly associated with executive function deficits are either directly related to epigenetic function or cluster with epigenetic regulators. Certain behavioral indices were converted to their inverse (% correct, fixed ratio 1 (FR1), breakpoint) if higher scores were associated with better performance, to better illustrate the direct relationship between poor performance and elevated transcription. Two major gene clusters were defined. Cluster 1 containing all tested genes involved in epigenetic regulation, as well as cannabinoid receptor 1 (CNR1), δ-opioid receptor (OPRD1), and catechol-o-methyltransferase (COMT). Cluster 2 contained dopamine receptors, other opioid receptor subtypes and endogenous opioid precursors, the GABA precursor GAD1, and dopamine and cAMP-regulated neuronal phosphoprotein (DARPP-32/PPP1R1B). Although few associations were found between members of cluster 2 and 5-choice serial reaction time test (5-CSRTT) performance, strong associations with most or all indices of attention were found with cluster 1 (full correlation matrix in SI5). In addition, particular epigenetic cluster genes associated strongly with only premature/impulsive errors (shaded blue) or omitted/inattentive errors (shaded orange) in 5-CSRTT. Three genes in cluster 2, dopamine receptor 1 (DRD1), δ-opioid receptor (OPRK1), and μ-opioid receptor (OPRM1), were associated with correct responses, but with no other indices of 5-CSRTT performance. (d) Scatterplots and trendlines for the four strongest correlations in c, which were all related to reaction times in 5-CSRTT, related to overexpression of DNA methyltransferase 1 (DNMT1), OPRD1, CNR1, and COMT. Notably, animals who had low expression of these genes demonstrated quicker reaction times regardless of maternal diet, but maternal malnutrition drove overexpression and poor performance. (e) Correlations were calculated as in c, individually comparing LP or HF with the control. Displayed are relationships between error type and epigenetic cluster expression, sorted by decreasing correlation. When comparing LP and CTL offspring, only DNMT1 and the four genes previously found to be associated with inattention in c were significantly associated with omitted trials. When comparing HF and CTL offspring, only DNMT1 and the three genes associated with impulsivity were significantly associated with premature trials. *p⩽0.05 vs the control group, #p⩽0.05 vs HF. (b) Mean±SEM. (d) Scatterplot and linear trendline. (a–c and e) Heat map data, with degree of expression or correlation indicated by a colorbar of z- or r-values located next to each figure. ITI, intertrial interval.
Results
Offspring of a Maternal Low-Protein Diet Show Increased Attention to Reward-Predicting Cues, Whereas Offspring of Maternal High-Fat Diet Have Decreased Motivation to Earn Reward
Offspring of maternal low-protein (LP), high-fat (HF), or control (CTL) diets were weaned to standard chow at postnatal day 21 and began operant testing in adulthood (12–16 weeks old) (Figure 1a). PCA assesses the sensitivity of animals to a stimulus that predicts imminent reward. Increased approach to this stimulus or ‘sign-tracking' discriminates animal populations prone to a constellation of negative behaviors, including addiction, hyperactivity, and inattention (Saunders and Robinson, 2013; Tomie et al, 2011). LP males and females more frequently approached the lit hole, which predicted reward, than controls (Figure 1b; Supplementary Figures 1 and 2), and there were no differences in approach behavior across groups when the stimulus was absent. Following PCA, animals began training to respond at the center hole of the nine-hole operant array at a FR1 schedule to prepare them for future operant testing. Both male and female HF offspring responded at lower levels and were delayed at meeting criterion (Figure 1c), whereas LP offspring were not significantly different than controls. Once the nose-poke response was learned, PR performance was used to assess motivation to work for reward by increasing the number of responses needed to elicit a reinforcer (Young et al, 2011). Both male and female HF offspring were less motivated, seen as reduced breakpoints (Figure 1d). Male and female LP offspring showed an intermediate phenotype towards reduced motivation, although this was not significant.
Maternal Low Protein Drives Offspring Inattention, Whereas Maternal High Fat Drives Offspring Impulsivity
The rodent 5-CSRTT task is a translationally relevant rodent behavioral task, similar to the continuous performance test, which is useful in discriminating attention and impulsivity-related endophenotypes in humans (Bari and Robbins, 2013; Young et al, 2009). The task is able to discriminate impulsivity (via incorrect trials and premature trials) from inattention (via omitted trials) (Figure 2a). Both LP and HF males were delayed at acquiring 5-CSRTT, with the slowest LP and HF needing 2–3 × the training compared with the slowest CTL (Figure 2b), whereas female LP were faster at acquiring the task than controls (Supplementary Figure 4). Therefore, performance was assessed for each animal based on data from the day that animal met advancement criteria. 5-CSRTT has traditionally been employed in otherwise normal, adult animals that can be fully trained prior to acute adult pharmacological manipulations. However, this approach, which calls for performance assessments after months of 5-CSRTT training on the part of the animal, may be confounded by neurodevelopmental insults such as those employed here, or by genetic manipulations (Pennanen et al, 2013), which will impact the ability of animals to perform the task early in training, but which may be recoverable by overtraining. Indeed, repeated exposure to the continuous performance test with reinforcement in humans leads to an amelioration of performance deficits in patients with ADHD and a loss of the ability to distinguish the vulnerable population (Bubnik et al, 2014). Therefore, we focused our analyses on early performance deficits measured at a time when animals have acquired the task, but have not had time to incrementally improve (Supplementary Figure 3).
Both male and female LP and HF animals were impaired at the 5-CSRTT when the task was relatively new, getting fewer correct responses (Figure 2c and d; Supplementary Figure 3) and making correct responses more slowly (Supplementary Figures 4 and 5). However, the source of errors was dissociably programmed in both sexes by maternal diet. Male and female LP made high numbers of inattentive errors (increased omissions). In contrast, HF males made high numbers of incorrect and impulsive premature responses, and HF females made high levels of incorrect response errors. There were no differences in premature response rates between groups in the females; however, the rate of premature responding in CTL females was comparable to HF males, suggesting that premature response rates in females may have been at a ceiling at this time point. The double dissociation in the attention phenotype was apparent in the earliest 5-CSRTT schedule (Figure 2c and d; Supplementary Figure 3). However, as training continued, these deficits became less apparent (Supplementary Figures 4 and 5), which is typically observed over the course of the 5-CSRTT training (Bari and Robbins, 2013). The exception was in performance on trials with a 1-s stimulus duration, which was tested acutely on animals stably performing at a 2-s stimulus duration, to prevent adaptation to the shortened cue. Acute testing with a 1-s stimulus duration reproduced the inattention deficits seen in low-protein male offspring during early training (Supplementary Figure 6), and led to impulsive premature responses in high-fat female offspring.
DNMT1, CNR1, COMT, OPRD1, and Transcriptional Regulators in PFC are Linked to Executive Function Errors and Dissociate Impulsivity from Inattention
After behavioral testing was completed, males were killed at the time of the day when they would normally be tested, without behavioral testing. Only male brains were examined for two reasons: first, behavioral phenotypes were either similar between males and females, or more severe in males, and second, female animals were needed for separate experiments. Normalized expression of 18 genes of interest, including components of DA, opioid, and cannabinoid signaling, as well as epigenetic regulators, was assessed in the PFC of each animal (Figure 3). Genes of interest were selected on the basis of previous differential expression in HF or LP offspring (ie, dopamine- and opioid-related signaling molecules, DNMT1, and MeCP2) (Grissom and Reyes, 2012; Grissom et al, 2013; Vucetic et al, 2010a, 2010b), or a close mechanistic relationship with these differentially expressed transcripts (ie, CNR1 to dopamine and opioid signaling; histone-modifying enzymes to DNA methylation machinery). Expression was converted to z-scores to observe whether gene expression varied by maternal diet. Correlations between expression of each gene and key behavioral end points were calculated, and the correlation matrix underwent hierarchical cluster analysis of genes to determine functional groupings.
Levels of gene expression were clearly altered by maternal diet. Compared with controls, exposure to LP or HF diet led to net overexpression of all target genes (Figure 3a), with LP also expressing more transcript on average than HF (Figure 3b). Surprisingly, overall increases in gene expression were associated with fewer correct responses and tended to be linked with omission errors, indicating inattention, an effect primarily driven by LP animals (Figure 3b). However, considerable variability in overall levels of gene expression was noted within groups (Figure 3a). Although it is not clear whether gene expression was impacted by behavioral training, we have previously observed upregulation in many of these gene targets, including DNMT1, in naive offspring of these maternal diet conditions in a pattern similar to that observed here (Grissom and Reyes, 2012; Grissom et al, 2013; Vucetic et al, 2010a, 2010b), and tissue was collected 24 h following the last behavioral testing, in an effort to minimize the effect of acute activity-dependent expression.
We then correlated individual expression levels with behavioral measures, and performed hierarchical clustering to understand the relationship between networks of transcripts associated with executive function. We expected genes to form two clusters generally dividing epigenetic modifiers from genes involved in neurotransmission. Genes did form two main clusters (Figure 3c). The first was comprised primarily of epigenetic modifiers, but also cannabinoid receptor 1 (CNR1), catechol-o-methyltransferase (COMT), and δ-opioid receptor (OPRD1) (cluster 1), whereas the other cluster was comprised of dopamine-, opioid-, and GABA-related transcripts, but no epigenetic regulators (cluster 2). The inclusion of CNR1, COMT, and OPRD1 in the epigenetic cluster reflected similar patterns of correlation between these genes and epigenetic regulators in relationship to behavior. Indeed, PFC overexpression of cluster-1 members was strongly associated with multiple parameters of executive function. In contrast, genes in cluster 2 were surprisingly not strongly related to most indices of attention. In all cases, poor performance was associated with overexpression of a target transcript and never a decrease in expression. The full correlation matrix can be seen in Supplementary Figure 7.
The strongest associations were seen between reaction time and four members of cluster 1, DNA methyltransferase 1 (DNMT1), OPRD1, CNR1, and COMT. Reaction time variability in the continuous performance test, the human analog of 5-CSRTT, is the most frequently observed deficit in neurodevelopmental disorders (Epstein et al, 2003; Johnson et al, 2007; Kuntsi et al, 2013; Tuch et al, 2005). Overexpression of each of these genes was associated with elevated reaction time, as well as with decreased correct responses (Figure 3c). Plotting reaction time against the expression of these transcripts revealed a linear relationship between expression and performance in all groups, with particularly elevated expression in certain LP and HF offspring corresponding with particularly poor performance (Figure 3d).
Individual genes also defined specific types of executive function errors (Figure 3c). Premature errors (highlighted in dark blue) were specifically associated with overexpression of OPRD1 and a sub-cluster of transcriptional activators, including the DNA-damage regulator and putative DNA demethylator GADD45B, and the histone methyltransferase Set9 (SETD7). Increased inattention (highlighted in orange) was associated with COMT overexpression, as well as overexpression of the DNA methyl-binding protein MeCP2, and the histone deacetylase HDAC2 (at p=0.06). To further analyze the dissociation of these two constructs, we conducted separate correlations of genes and behavior in LP and control or HF and control separately (Figure 3e; Supplementary Figure 8). DNMT1 was the only transcript that associated with both risk of inattention and impulsivity. In LP offspring, the associations between inattentive errors and COMT, MeCP2, and HDAC2 expression remained, whereas no significant relationships between premature responses and gene expression were seen. Further, in the HF analysis, the associations between premature responses and OPRD1, GADD45B, and SETD7 remained significant, whereas no relationship was found between gene expression and omitted trials. Overall, deficits in executive function, which can be seen at the behavioral level, appear to be associated with dissociable changes at the molecular level.
Discussion
PFC dysfunction is implicated in a number of mental disorders, and is reflected in deficits in one or more aspects of executive function. However, executive function is a nonspecific term for a number of tasks involved in directing behavior, such as sustained attention and impulse control, and different psychiatric diagnoses can be associated with dissociable executive function deficits (Johnson et al, 2007; Kuntsi et al, 2013; Ben Shalom et al, 2013). Consequently, it has been difficult to tease out the regulation of specific deficits at the molecular level. The understanding of epigenetic contributions to mental disorders is in its infancy, but it is clear that PFC epigenetic function is compromised in major psychiatric disorders (Grayson and Guidotti, 2013; Ladd-Acosta et al, 2013). However, the effect of this epigenetic dysregulation is largely unexplored. Here, dissociable behavioral deficits were driven by exposing animals to one of two unbalanced gestational diets, coupled with specific patterns of abnormal gene expression in the PFC. Developmental disorders involving PFC function have been repeatedly linked with gestational adversity, and here we have demonstrated that specific forms of suboptimal maternal nutrition can lead to specific kinds of PFC dysfunction. We then examined transcriptional regulation in the PFC of individual animals about which we had extensive behavioral information. We found that the overexpression of specific genes, as well as higher levels of mRNA generally, dissociate different forms of executive dysfunction.
Behavioral deficits were apparent in early reward-related behavior and later attention problems. Low-protein males and females engaged in more sign tracking in a conditioned approach paradigm, indicating increased attention to cues predicting reward (Saunders and Robinson, 2013), as might be expected in animals with elevated dopamine transmission (Saunders and Robinson, 2012), which we have previously observed in LP offspring (Vucetic et al, 2010b). This is the first demonstration that the susceptibility to sign-tracking behavior can be programmed by in utero or an early-life environment. When tested in the 5-CSRTT, these animals were then found to be more inattentive. Outbred rats prone to sign tracking exhibit similar deficits in sustained attention (Paolone et al, 2013), which have been related to both dopamine- and cholinergic-signaling alterations. Collectively, these data suggest that increased salience of cues (sign tracking) can increase inattention and ‘distractability' (increased attention to irrelevant cues). Gestational programming of the Pavlovian approach behavior may indicate mechanisms for later pathology that involve inappropriate attention to environmental cues, including obesity and addiction (Saunders and Robinson, 2013), which are elevated in SGA offspring (Grissom and Reyes, 2012). HF offspring displayed reduced motivation early in operant training. These animals have increased sucrose preference (Vucetic et al, 2010a), indicating a dissociation between preference-related ‘liking' vs effort-related ‘wanting' (Berridge et al, 2010). Later, the 5-CSRTT revealed impulsive behavior in these animals. Patients with ADHD display impulsivity specifically under conditions of low reward value (Slusarek et al, 2001), suggesting that HF mice may be a useful model of impulsive behavior due to reduced motivation to direct effort toward inhibiting responses. An important caveat to note is that these maternal diets can affect peripheral metabolism and food intake (Whitaker et al, 2011), which could affect how the mice responded to the food-restriction protocol. However, to the extent possible, this confound was mitigated by keeping the extent of the food restriction equivalent across all groups.
Gene expression in the PFC was linked with these dissociable behavioral outcomes. First, high gene expression across all target genes was related to poor executive function performance. One reason for elevated transcription may be related to disrupted epigenetic mechanisms in HF and LP. The current data indicate that this overexpression is indicative of substantial behavioral deficits, and further suggest that epigenetic complexes involved in transcriptional repression (EHMT2, MECP2, and HDAC2) vs activation (SETD7 and GADD45B) may be differentially involved in inattention and impulsivity, respectively. However, overexpression DNMT1, a key participant in DNA methylation, was linked with both inattentive and impulsive deficits. Overexpression of DNMT1 in cortex is a hallmark finding in psychosis (Grayson and Guidotti, 2013), and we have previously observed this overexpression in the brain in these mouse models of poor-quality maternal diet (Grissom and Reyes, 2012). Interestingly, HF and LP offspring show reductions in global DNA methylation in the PFC (Grissom and Reyes, 2012; Vucetic et al, 2010a), indicating that overexpressed DNMT1 may not necessarily be fully able to participate in DNA methylation, leading to a hypomethylated phenotype. In other words, overexpressed DNMT1 may represent a compensatory mechanism in response to diminished activity of DNMT1. It is not clear from the current data how transcript overexpression and behavioral dysregulation are linked, but these observations with DNMT1 suggest that disruptions in epigenetic machinery, which regulate both behavior and gene expression, may be at fault rather than a simple causal relationship between specific transcript overproduction and errors of executive function.
Three nonepigenetic genes were linked with executive function: CNR1, OPRD1, and COMT. All are implicated in cognitive function, and our results indicate that they participate in dissociable executive function domains. OPRD1, although understudied relative to other opioid receptors, has a role in forming action–outcome associations, emotional responses, and impulsivity (Klenowski et al, 2014; Olmstead et al, 2009). Polymorphisms in COMT have repeatedly been linked with prefrontal cortical dysfunction (Roffman et al, 2008). Cannabis use is thought to interact with COMT in the development of schizophrenia (Costas et al, 2011), and cannabinoid function in the PFC is essential for emotional regulation and sustained attention (Arguello and Jentsch, 2004; McLaughlin et al, 2014). This evidence strongly indicates these three genes in mental disorders associated with PFC dysfunction, and the current data indicate that COMT and OPRD1, in particular, may have opposing roles in mediating executive function. However, it remains unknown why these genes would associate so much more strongly with epigenetic modifiers than with the remaining dopamine and opioid neurotransmission-related transcripts. One possible reason why COMT clustered with epigenetic genes may have to do with its catalytic activity as a methyltransferase. Polymorphisms in COMT and MTHFR, which is involved in one-carbon metabolism regulating the availability of methyl groups to transfer to monoamines, proteins, and DNA, interact to alter executive function in schizophrenia (Roffman et al, 2008). It is noteworthy that the gene most ubiquitously linked with deficits was DNMT1, a DNA methyltransferase. Alterations in neuronal DNMT1 function have been observed not only in schizophrenia, but also in dementia (Desplats et al, 2011; Mastroeni et al, 2013), indicating that DNA methylation is of central importance in regulating cognition. In addition, the expression of two histone methyltransferases (EHMT2 and SETD7) dissociated inattention from impulsivity. Histone methylation changes in the PFC and altered transcription have previously been found in rats that showed abnormal attention after adolescent cocaine exposure (Black et al, 2006). It is possible that methylation reactions reliant on one-carbon metabolism may represent a common mechanism of dysregulation of transcription and catecholamine metabolism in PFC, impacting executive function. Examining epigenetic or transcriptional networks may elucidate mechanisms of mental disorder that have escaped target gene approaches.
FUNDING AND DISCLOSURE
This work was supported by MH087978 and MH091372 to TMR. The authors declare no conflict of interest.
Acknowledgments
We acknowledge the technical support of Ariel Miller, Sarah McKee, Robert George, and Emily Shuldiner, and the helpful comments of Dr Lucia Peixoto, Dr Benjamin Saunders, and Dr Jared Young in preparation of this manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Arguello PA, Jentsch JD. Cannabinoid CB1 receptor-mediated impairment of visuospatial attention in the rat. Psychopharmacology (Berl) 2004;177:141–150. doi: 10.1007/s00213-004-1953-0. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Ben Shalom D, Ronel Z, Faran Y, Meiri G, Gabis L, Kerns KA.2013A double dissociation between inattentive and impulsive traits, on tasks of visual processing and emotion regulation J Atten Disorde-pub ahead of print 10 December 2013doi: 10.1177/1087054713510351 [DOI] [PubMed]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black YD, Maclaren FR, Naydenov AV, Carlezon Wa, Baxter MG, Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J Neurosci. 2006;26:9656–9665. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubnik MG, Hawk LW, Pelham WE, Waxmonsky JG, Rosch KS.2014Reinforcement enhances vigilance among children with ADHD: comparisons to typically developing children and to the effects of methylphenidate J Abnorm Child Psychole-pub ahead of print 17 June 2014doi: 10.1007/s10802-014-9891-8 [DOI] [PMC free article] [PubMed]
- Costas J, Sanjuán J, Ramos-Ríos R, Paz E, Agra S, Tolosa A, et al. Interaction between COMT haplotypes and cannabis in schizophrenia: a case-only study in two samples from Spain. Schizophr Res. 2011;127:22–27. doi: 10.1016/j.schres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Desplats P, Spencer B, Coffee E, Patel P, Michael S, Patrick C, et al. Alpha-synuclein sequesters Dnmt1 from the nucleus: a novel mechanism for epigenetic alterations in Lewy body diseases. J Biol Chem. 2011;286:9031–9037. doi: 10.1074/jbc.C110.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron. 2013;80:35–50. doi: 10.1016/j.neuron.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations between continuous performance test performance measures and ADHD behaviors. J Abnorm Child Psychol. 2003;31:543–554. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Jentsch JD. Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology. 2011;36:227–250. doi: 10.1038/npp.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Rickwood L, Drewett RF, Dunne E. Gonadal hormones and effects of partial reinforcement on appetitive behaviour in the rat. Physiol Behav. 1977;19:41–45. doi: 10.1016/0031-9384(77)90156-1. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Guidotti A. The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology. 2013;38:138–166. doi: 10.1038/npp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom NM, Lyde R, Christ L, Sasson IE, Carlin J, Vitins AP, et al. Obesity at conception programs the opioid system in the offspring brain. Neuropsychopharmacology. 2013;39:801–810. doi: 10.1038/npp.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom NM, Reyes TM. Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics. Int J Dev Neurosci. 2012;31:406–414. doi: 10.1016/j.ijdevneu.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hest AVan, Haaren FVan. The effects of gonadectomy and chronic testosterone suppletion on the autoshaped response of male and female Wistar rats. Bull Psychon Soc. 1989;27:9–12. [Google Scholar]
- Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Dáibhis A, et al. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia. 2007;45:2234–2245. doi: 10.1016/j.neuropsychologia.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenowski P, Morgan M, Bartlett SE.2014The role of delta opioid receptors in learning and memory underlying the development of addiction Br J Pharmacole-pub ahead of print 12 February 2014doi: 10.1111/bph.12618 [DOI] [PMC free article] [PubMed]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121–e1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Pinto R, Price TS, van der Meere JJ, Frazier-Wood AC, Asherson P. The separation of ADHD inattention and hyperactivity-impulsivity symptoms: pathways from genetic effects to cognitive impairments and symptoms. J Abnorm Child Psychol. 2013;42:127–136. doi: 10.1007/s10802-013-9771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd-Acosta C, Hansen KD, Briem E, Fallin MD, Kaufmann WE, Feinberg AP. Common DNA methylation alterations in multiple brain regions in autism. Mol Psychiatry. 2013;19:862–871. doi: 10.1038/mp.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroeni D, Chouliaras L, Grover A, Liang WS, Hauns K, Rogers J, et al. Reduced RAN expression and disrupted transport between cytoplasm and nucleus; a key event in Alzheimer's disease pathophysiology. PLoS One. 2013;8:e53349. doi: 10.1371/journal.pone.0053349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev. 2014;42C:116–131. doi: 10.1016/j.neubiorev.2014.02.006. [DOI] [PubMed] [Google Scholar]
- McTighe SM, Neal SJ, Lin Q, Hughes ZA, Smith DG. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLoS One. 2013;8:e62189. doi: 10.1371/journal.pone.0062189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing G. Autism risk in small- and large-for-gestational-age infants. Am J Obstet Gynecol. 2012;206:314.e1–9. doi: 10.1016/j.ajog.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Ouagazzal A-M, Kieffer BL. Mu and delta opioid receptors oppositely regulate motor impulsivity in the signaled nose poke task. PLoS One. 2009;4:e4410. doi: 10.1371/journal.pone.0004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol. 2011;32:205–212. doi: 10.1016/j.reprotox.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33:8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennanen L, van der Hart M, Yu L, Tecott LH. Impact of serotonin (5-HT)2C receptors on executive control processes. Neuropsychopharmacology. 2013;38:957–967. doi: 10.1038/npp.2012.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze M, McGarrity S, Mason R, Fone KC, Bast T. Too little and too much: hypoactivation and disinhibition of medial prefrontal cortex cause attentional deficits. J Neurosci. 2014;34:7931–7946. doi: 10.1523/JNEUROSCI.3450-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res. 1998;22:1564–1569. [PubMed] [Google Scholar]
- Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Wong DH, et al. Interactive effects of COMT Val108/158Met and MTHFR C677T on executive function in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:990–995. doi: 10.1002/ajmg.b.30684. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: implications for addiction. Neurosci Biobehav Rev. 2013;37:1955–1975. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Scott D, Taylor JR. Chronic nicotine attenuates phencyclidine-induced impulsivity in a mouse serial reaction time task. Behav Brain Res. 2014;259:164–173. doi: 10.1016/j.bbr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarek M, Velling S, Bunk D, Eggers C. Motivational effects on inhibitory control in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40:355–363. doi: 10.1097/00004583-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Tomie A, Lincks M, Nadarajah SD, Pohorecky La, Yu L. Pairings of lever and food induce Pavlovian conditioned approach of sign-tracking and goal-tracking in C57BL/6 mice. Behav Brain Res. 2011;226:1–8. doi: 10.1016/j.bbr.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci USA. 2005;102:12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lieshout R, Boyle M. Canadian youth born large or small for gestational age and externalizing and internalizing problems. Can J Psychiatry. 2011;56:227–234. doi: 10.1177/070674371105600406. [DOI] [PubMed] [Google Scholar]
- Van Lieshout RJ, Taylor VH, Boyle MH. Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obes Rev. 2011;12:e548–e559. doi: 10.1111/j.1467-789X.2010.00850.x. [DOI] [PubMed] [Google Scholar]
- Vesco KK, Sharma AJ, Dietz PM, Rizzo JH, Callaghan WM, England L, et al. Newborn size among obese women with weight gain outside the 2009 Institute of Medicine recommendation. Obstet Gynecol. 2011;117:812–818. doi: 10.1097/AOG.0b013e3182113ae4. [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:1–9. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, et al. Early life protein restriction alters dopamine circuitry. Neuroscience. 2010;168:359–370. doi: 10.1016/j.neuroscience.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker KW, Totoki K, Reyes TM. Metabolic adaptations to early life protein restriction differ by offspring sex and post-weaning diet in the mouse. Nutr Metab Cardiovasc Dis. 2011;22:1067–1074. doi: 10.1016/j.numecd.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Meves JM, Tarantino IS, Caldwell S, Geyer MA. Delayed procedural learning in α7-nicotinic acetylcholine receptor knockout mice. Genes Brain Behav. 2011;10:720–733. doi: 10.1111/j.1601-183X.2011.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.