Abstract
Serotonin critically affects the neural processing of emotionally salient stimuli, including indices of threat; however, how alterations in serotonin signaling contribute to changes in brain function is not well understood. Recently, we showed in a placebo-controlled study of 32 healthy males that brain serotonin 4 receptor (5-HT4) binding, assessed with [11C]SB207145 PET, was sensitive to a 3-week intervention with the selective serotonin reuptake inhibitor fluoxetine, supporting it as an in vivo model for fluctuations in central serotonin levels. Participants also underwent functional magnetic resonance imaging while performing a gender discrimination task of fearful, angry, and neutral faces. This offered a unique opportunity to evaluate whether individual fluctuations in central serotonin levels, indexed by change in [11C]SB207145 binding, predicted changes in threat-related reactivity (ie, fear and angry vs neutral faces) within a corticolimbic circuit including the amygdala and medial prefrontal and anterior cingulate cortex. We observed a significant association such that decreased brain-wide [11C]SB207145 binding (ie, increased brain serotonin levels) was associated with lower threat-related amygdala reactivity, whereas intervention group status did not predict change in corticolimbic reactivity. This suggests that in the healthy brain, interindividual responses to pharmacologically induced and spontaneously occurring fluctuations in [11C]SB207145 binding, a putative marker of brain serotonin levels, affect amygdala reactivity to threat. Our finding also supports that change in brain [11C]SB207145 binding may be a relevant marker for evaluating neurobiological mechanisms underlying sensitivity to threat and serotonin signaling.
INTRODUCTION
Serotonin is a neuromodulator with significant effects on emotional behavior and it is implicated in the pathophysiology of mood and anxiety disorders (Holmes, 2008; Albert et al, 2012). Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed to treat these disorders. The primary pharmacological action of SSRIs is to block the serotonin transporter (5-HTT), the mechanism for clearing extracellular serotonin, putatively increasing central serotonin levels (Nutt et al, 1999). The clinical response to SSRI treatment typically emerges 2–3 weeks after treatment onset, at which point response is significantly predictive of long-term treatment response (Baldwin et al, 2009). This time frame coincides with hypothesized molecular mediators of antidepressant response including serotonin 1A autoreceptor desensitization and downregulation of 5-HTT (Benmansour et al, 2002; Blier and de Montigny, 1999; Richardson-Jones et al, 2010). Linking fluctuations in serotonin signaling within this clinically relevant 2–3 week time frame with changes in specific neural pathways would implicate neurobiological mechanisms mediating SSRI effects on emotional behavior and treatment responsiveness.
A corticolimbic circuit comprising the amygdala and medial prefrontal cortex (mPFC), including the anterior cingulate cortex, critically modulates behavioral and physiological responses to salient environmental stimuli (Sergerie et al, 2008; Quirk and Mueller 2008; Phelps et al, 2004). Human neuroimaging studies in healthy and clinical cohorts implicate this circuit in processing threat-related stimuli and the pathophysiology of mood and anxiety disorders (Phillips et al, 2003; Stuhrmann et al, 2011; Fusar-Poli et al, 2009). Converging evidence from human functional magnetic resonance imaging (fMRI) studies implicates serotonin signaling in modulating threat-related corticolimbic circuit function (Grady et al, 2013; Hornboll et al, 2013; Rhodes et al, 2007; Hariri et al, 2005; Fisher et al, 2011). Despite this, our understanding of how changes in central serotonin signaling modulate this circuit is limited.
The effect of a single SSRI dose on amygdala reactivity remains equivocal with reports of heightened (Bigos et al, 2008), lowered (Murphy et al, 2009), and no difference in (Norbury et al, 2009) amygdala reactivity. Behaviorally, the recognition of fearful faces was heightened following a single SSRI dose in healthy individuals (Browning et al, 2007). Conversely, healthy individuals taking citalopram for 7 days showed lower amygdala reactivity to fearful faces compared with placebo (Harmer et al, 2006). Only one study has evaluated SSRI effects in healthy individuals over a more clinically relevant time period of 3 weeks, reporting an inverse association between threat-related reactivity and escitalopram urine concentrations (Arce et al, 2008). Clinically, SSRI effects on treatment response and threat-related amygdala reactivity show substantial individual variability (Ruhe et al, 2012). Thus, long-term SSRI intervention appears to decrease threat-related amygdala reactivity; however, there is an outstanding need for additional studies, which may benefit from modeling individual variation in change in brain function with a more proximal measure of central serotonin levels.
Recently, we showed that serotonin type 4 receptor (5-HT4) brain-binding, assessed with [11C]SB207145 positron emission tomography (PET), may be a useful proxy for changes in brain serotonin levels. Three-week fluoxetine intervention (40 mg) was associated with a 5% decrease in 5-HT4 binding (Haahr et al, 2014). This same cohort completed a gender-matching threat-related face fMRI paradigm, offering a unique opportunity to determine the association between fluctuations in central serotonin levels, assessed with PET, and threat-related corticolimbic reactivity assessed with fMRI. On the basis of previous long-term SSRI intervention studies in healthy individuals, we hypothesized that the change in [11C]SB207145, a putative marker for change in central serotonin levels from baseline to re-scan, would be negatively associated with the change in threat-related amygdala reactivity. We compared these effects against those estimated considering intervention group status to determine the importance of this more proximal measure of the central serotonin system.
MATERIALS AND METHODS
Participants
Healthy males were recruited by advertisement for a research protocol approved by the regional Danish Ethics Committee (H-KF-2006-20, Amendment 23830). All participants were generally healthy (see Supplementary Materials and Methods for more details) as described previously (Haahr et al, 2014). Of the 35 participants who were recruited for the study, four did not complete the entire protocol (two because of PET scanner failure, one elected to dropout, and one did not complete the fMRI paradigm). Thus, our sample consisted of 31 individuals. fMRI data included here have not been previously described, whereas PET data included here have been reported previously (Fisher et al, 2012; Haahr et al, 2014; Haahr et al, 2013).
Intervention Protocol
Using a randomized, double-blind design participants received either fluoxetine or placebo. Intervention group assignment was block-randomized on the basis of age, education, and 5-HTTLPR status by a member of our research group uninvolved in contact with the participants or the data analysis. Participants received oral and verbal instructions for taking medication. Participants were instructed to take 20 mg (one capsule) each of the first 3 days of the intervention period. From the fourth day until the final re-scan, the participants took 40 mg, an effective clinical dose (Charlier et al, 2000). We instructed the participants to take the capsules at ∼22 hours or before going to bed. Side effects were monitored during the intervention period and blood samples were taken to verify treatment compliance. The participants completed the second scan sessions ∼3 weeks after beginning the intervention (Table 1). See Supplementary Materials and Methods for more details.
Table 1. Demographic, Behavioral, and Intervention Data.
| Active | Placebo | p-value | |
|---|---|---|---|
| N | 16 | 15 | — |
| 5-HTTLPR genotype status (LA/LA vs non-LA/LA) | 4/12 | 5/10 | 0.70 |
| Intervention days (mean±s.d. (median, range)) | 19.3±3.5 (20.5, 12–24) | 21.1±2.9 (22, 13–25) | 0.14 |
| Age (years) | 25.7±5.2 | 25.9±3.9 | 0.91 |
| Body mass index (kg/m2) | 23.1±1.9 | 23.4±3.1 | 0.76 |
| Education (years) | 15.7±1.9 | 15.8±1.6 | 0.66 |
| Neuroticism | 65.9±15.8 | 74.1±20.1 | 0.23 |
| Harm avoidance | 10.0±6.9 | 9.9±6.5 | 0.96 |
| Major depressive inventory | 7.6±5.7 | 4.6±3.6 | 0.09 |
| Cohen's perceived stress | 7.8±4.9 | 8.6±4.8 | 0.61 |
| Serum fluoxetine, between scans (μg/l) | 120.6±51.1 (44.5–200.2) | <15 | — |
| Serum norfluoxetine, between scans (μg/l) | 92.8±30.7 (38.1–154.4) | <15 | — |
| Serum fluoxetine, at second scan (μg/l) | 168.4±49 (69.7–241.1) | <15 | — |
| Serum norfluoxetine, at second scan (μg/l) | 147.8±37.4 (94.2–212.6) | <15 | — |
| Reaction time, faces task, baseline (ms) | 764.4±116.5 | 748.2±95.9 | 0.68 |
| Percent correct, faces task, baseline (% correct) | 97.3±1.6 | 97.6±1.2 | 0.56 |
| Reaction time, faces task, re-scan (ms) | 772.0±111.7 | 773.6±98.9 | 0.97 |
| Percent correct, faces task, re-scan (% correct) | 97.3±1.5 | 97.1±1.7 | 0.82 |
Values are expressed as mean±SD with the median and range in parentheses. All p-values reflect a two-sample t-test (active vs placebo) except for 5-HTTLPR status, which reflects a χ2 test where χ2=0.2 and degrees of freedom=1. Neuroticism scores are derived from the NEO-PI-R questionnaire and harm avoidance scores from the TCI questionnaire.
Genotyping
Genotype status for the 5-HTTLPR polymorphism (LA, LG, and S allele) was determined as described previously (Fisher et al, 2013). All the participants self-identified as Caucasian except for one who self-identified as mixed (African/Caucasian).
Magnetic Resonance Imaging Data Acquisition and Paradigm
Blood-oxygen level-dependent (BOLD) fMRI scans were acquired using a T2*-weighted gradient echo planar imaging sequence and high-resolution T1-weighted structural MR scans were acquired as described previously (Grady et al, 2013) and in Supplementary Materials and Methods.
The participants completed a gender discrimination task on individual threat-related (ie, fearful or angry) and neutral faces presented in the middle of the screen as described previously (Grady et al, 2013). In total, 32 blocks of neutral faces were interleaved between 16 blocks of fearful and 16 blocks of angry faces presented across two runs with a brief break between runs. See Supplementary Materials and Methods for more details.
fMRI Data Analysis
We pre-processed fMRI data sets using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). Functional images were realigned to a subject-specific mean functional image to which the T1-weighted structural image was co-registered. Functional images were normalized into Montreal Neurological Institute space on the basis of normalization of the T1-weighted image within VBM5 (http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5/) and then smoothed with an 8-mm Gaussian kernel. Smoothed images were high-pass-filtered (frequency 128 s, 0.008 Hz) to remove low-frequency drift and entered into a general-linear model employing a canonical hemodynamic response function to estimate condition-specific and task-specific BOLD activation (ie, beta and contrast images, respectively) including estimated motion parameters and respiratory measures as covariates. Single-subject contrast images (ie, weighted sum of task-related beta images) were entered in second-level random effects models to determine task-related reactivity.
To address the issue of multiple comparisons for voxel-level comparisons, 3dClustSim, a software program within AFNI (http://afni.nimh.nih.gov/afni) was used to determine cluster extent thresholds for specific ROIs unlikely to have occurred by chance (α<0.05) at a voxel-level statistical threshold of p<0.01, uncorrected. Amygdala and mPFC ROIs were defined using WFU Pickatlas v3.0.3 (Maldjian et al, 2003; Lancaster et al, 1997). Our mPFC ROI was defined as Brodmann Area's 24, 25, and 32 (3D dilation=1) so as to include mPFC regions that share anatomical connectivity with the amygdala (Barbas, 1995; Ongur and Price, 2000). The cluster extent, k, required for our amygdala and mPFC search volume was >14 and 196 voxels, respectively.
PET Data Acquisition
[11C]SB207145 was synthesized using an automated radio-synthesis system as previously described (Marner et al, 2010). Immediately following intravenous bolus injection of [11C]SB207145, a 120-minute dynamic 3D emission scan was acquired within 38 time frames using a Siemens ECAT HRRT scanner with an approximate in-plane resolution of 1.5 mm (Olesen et al, 2009). Scans were reconstructed using the iterative point-spread function reconstruction with attenuation map improvements (Sureau et al, 2008). All doses were below the recommended limits, ensuring tracer conditions (Madsen et al, 2011).
Quantification of [11C]SB207145 Binding and β Values
Quantification of PET data was completed as described previously (Haahr et al, 2014). On the basis of this study, we interpret the percent change in binding, as determined by an occupancy plot for each participant, to reflect fluctuations in global central serotonin levels. In these plots, the baseline binding potential (BPND) for each brain region was plotted on the x axis against re-scan BPND for the same regions on the y axis. The slope of the fitted line, β, represents an estimate of the change in specific [11C]SB207145 binding, indexing the change in serotonin levels. A β<1 indicates an increase in serotonin levels (ie, a decrease in the receptor availability), whereas a β>1 indicates a decrease in serotonin levels (ie, an increase in receptor availability). Thus, a single β value was estimated for each participant. β values used here were identical to those reported previously (Haahr et al, 2014).
Personality Measures
Danish versions of state measures of personality, depressive symptoms, and stress were collected to determine effects of intervention. Participants completed the Major Depression Inventory (Forsell, 2005) and Cohen's Perceived Stress (Cohen et al, 1983) at each PET scan. Trait measures were collected to determine whether participants differed in relevant personality measures at baseline. Participants completed the NEO PI-R (Hansen et al, 2004), providing a trait measure of neuroticism, and the Temperament and Character Inventory (Cloninger et al, 1994), providing a trait measure of harm avoidance.
Regression Analysis
All statistical analyses outside of SPM were carried out in R v3.0.2 (http://www.r-project.org/). First, we evaluated the main effects of task-related reactivity (ie, fear and angry vs neutral faces contrast) at baseline in SPM. To ensure that consistent and task-responsive brain areas were evaluated, the mean contrast estimates from clusters within ROIs showing significant task-related reactivity were extracted from SPM and modeled in correlation analyses including either fluctuation in serotonin levels or intervention group status.
We determined the association between change in task-related reactivity and intervention measures using a linear regression model that can be expressed generally as: BOLDR=β0+β1 × BOLDB+β2 × Intervention+ɛ. ‘BOLDR' and ‘BOLDB' correspond to the BOLD contrast fear and angry—neutral faces for one of the three regions (left amygdala, right amygdala, and mPFC) at re-scan and baseline, respectively. ‘Intervention' corresponds to one of two intervention measures tested (fluctuation in serotonin levels or intervention group status). Each intervention measure was evaluated in a separate model for each regional BOLD contrast. In effect, these models predict the change in task reactivity. This model is advantageous for test-retest designs relative to computing the ‘delta' (ie, BOLDR−BOLDB) for a particular measure (Vickers and Altman, 2001).
To consider effects of pharmacologically induced and spontaneous changes in [11C]SB207145 binding, we included all participants in each model evaluating the association between BOLD measures and the β value. Support for combining the fluoxetine and placebo groups included a nonsignificant interaction between the β value and the group status on any reactivity estimate (p>0.47), suggesting that this association did not differ between groups. All statistics reported in the Results do not include the interaction term. A threshold of p<0.05 (two-tailed) was considered statistically significant.
RESULTS
Threat-Related Corticolimbic Reactivity at Baseline
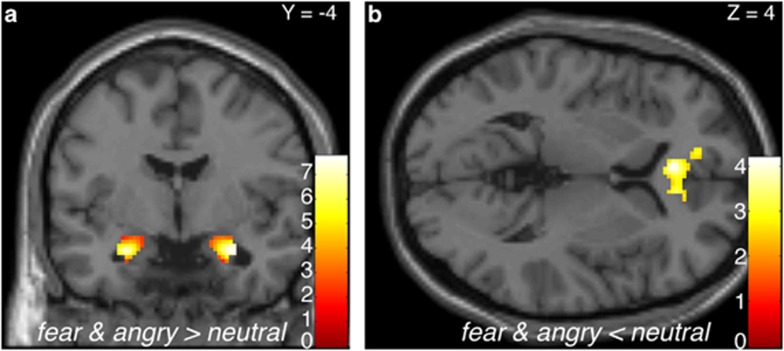
Across all participants at baseline, we observed significant task-related corticolimbic reactivity including bilateral threat-related amygdala reactivity (ie, fear and angry>neutral) and an mPFC cluster that was significantly more responsive to neutral compared with threat-related faces (Figure 1).
Figure 1.
Threat-related corticolimbic reactivity across all participants at baseline. Statistical parametric map highlighting (a) bilateral threat-related amygdala reactivity as well as the (b) medial prefrontal cortex (mPFC) region that was significantly more responsive to neutral faces compared with threat-related faces, at baseline. Right amygdala: (30, −4, −20), z=5.69, k=132 voxels, p<0.05, corrected; left amygdala: (−26, −4, −20), z=5.48, k=120 voxels, p<0.05, corrected; mPFC: (−4, 34, 4), z=−3.68, k=419 voxels, p<0.05, corrected). Color bars indicate t-scores. Right is right in both images.
Effects of Change in [11C]SB207145 Binding on Corticolimbic Reactivity
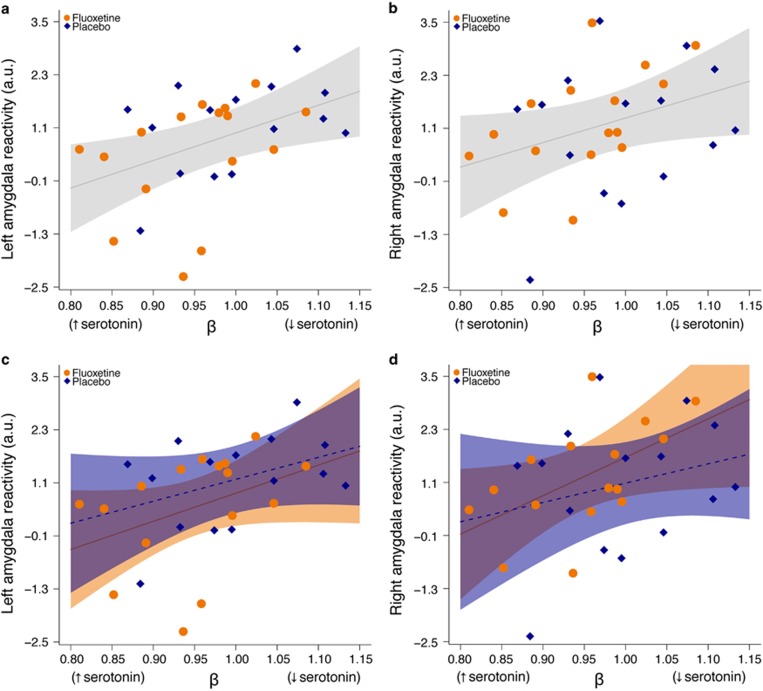
In light of our recent finding that [11C]SB207145 PET may be sensitive to fluctuations in endogenous serotonin levels (Haahr et al, 2014), we evaluated the association between change in brain-wide [11C]SB207145 binding, β, and change in corticolimbic reactivity, thereby relating putative changes in central serotonin levels and corticolimbic circuit function. Change in threat-related left amygdala reactivity was significantly associated with change in [11C]SB207145 binding (slope (95% C.I.): 6.11 (1.21, 11.30), p=0.017), and the right amygdala showed a similar, although borderline, significant effect (slope: 5.54 (−0.35, 11.40), p=0.064; Figure 2a and b). Put another way, as specific [11C]SB207145 binding decreased (ie, putatively increased central serotonin levels) there was a linear decrease in amygdala reactivity from baseline to re-scan. We observed similar effects at lower statistical significance when evaluating the fluoxetine and placebo groups separately (fluoxetine (N=16): left amygdala: 6.13 (−3.30, 15.60), p=0.18; right amygdala: 8.47 (0.33, 16.6), p=0.04; placebo (N=15): left amygdala: 5.03 (−1.94, 12.00), p=0.14, right amygdala: 4.14, (−7.11, 15.4), p=0.44; Figure 2c and d). Changes in mPFC reactivity estimates were not associated with changes in [11C]SB207145 binding (slope: 0.07 (−0.42, 0.57), p=0.77).
Figure 2.
Change in [11C]SB207145 binding associated with amygdala reactivity. (a, b) Plots showing the association between change in [11C]SB207145 binding, β, and change in left and right threat-related amygdala reactivity. A β<1 reflects decreased specific binding from baseline to rescan (ie, putatively increased serotonin levels), whereas a β>1 reflects increased specific binding (ie, putatively decreased serotonin levels). The mean left and right threat-related (ie, fear and angry—neutral contrast) amygdala reactivity values at re-scan, adjusted for baseline, are plotted for all 31 participants (orange points: fluoxetine group, blue points: placebo group). The gray line and shading represent the best fit line and 95% confidence interval, respectively, across the entire group. (c, d) Plot of same data but independent best fit lines and 95% confidence intervals are shown for fluoxetine (solid line) and placebo (hatched line) groups.
Effect of Intervention Group on Corticolimbic Reactivity
In contrast to our measure of change in [11C]SB207145 binding, intervention group (active vs placebo) was not significantly predictive of change in the amygdala or mPFC reactivity (intervention effect: left amygdala (95% CI): −0.61 (−1.51, 0.29), p=0.17; right amygdala: (95% CI): 0.16 (−0.92, 1.23), p=0.77; mPFC: 0.03 (−0.95, 1.01), p=0.95).
Personality Measures, Task Response, and Intervention Compliance
There were no significant group differences at baseline in demographics, state, or trait measures of personality, depressive symptoms, or perceived stress (Table 1; Haahr et al, 2014). Nor did we observe evidence for an effect of intervention on our state measures of depressive symptoms and stress (p>0.70). Participants performed the fMRI face paradigm with a high-degree of accuracy and we did not observe evidence for an effect of intervention on reaction time or accuracy (Table 1). Fluoxetine significantly reduced body mass index (intervention group effect (95% CI): −0.53 (−0.74; −0.33), p=1.1 × 10−5).
Three individuals receiving fluoxetine indicated that they missed one treatment day. We observed little difference in the reported side effects between groups. Eight and nine participants in the fluoxetine and placebo groups, respectively, reported no side effects. Seven and five participants in the fluoxetine and placebo groups, respectively, reported a few nonspecific symptoms (UKU rating <5). One individual from the fluoxetine and one from the placebo group reported sexual dysfunction and concentration problems (UKU score >5).
DISCUSSION
In this study we used a multimodal pharmaconeuroimaging strategy to evaluate the association between a recently reported effect of a 3-week fluoxetine intervention on change in [11C]SB207145 binding, putatively reflecting change in central serotonin levels and response of a threat-related corticolimbic circuit, assessed with fMRI. We observed a significant association such that decreased brain-wide [11C]SB207145 binding was associated with lower threat-related amygdala reactivity. Remarkably, this association included participants who received fluoxetine and placebo, suggesting that pharmacologically induced and naturally occurring fluctuations in [11C]SB207145 binding correlate with change in the amygdala response to threat among healthy individuals. These findings provide more direct evidence linking in vivo changes in the central serotonin neurotransmission and amygdala response to threat. Notably, the intervention group status (ie, active vs placebo) did not significantly predict the change in corticolimbic reactivity, whereas interindividual differences in central serotonin signaling significantly predicted change in left amygdala reactivity. These findings support a specific association between serotonin signaling and threat-related amygdala reactivity, and underscore the benefit of a more direct measure of the central serotonin system.
Although previous studies have evaluated effects of SSRI intervention on threat-related corticolimbic brain function, the ability to relate these effects to a more direct measure of alterations in central serotonin levels has been hindered by the availability of such a measure with PET or other methods (Paterson et al, 2010). We report that estimated change in [11C]SB207145, which we interpret to reflect change in central serotonin levels, is significantly associated with change in threat-related amygdala reactivity. This effect is directionally consistent with previous studies evaluating SSRI effects in healthy individuals (Harmer et al, 2006; Arce et al, 2008). Whereas previous neuroimaging studies support an association between serotonergic signaling mechanisms and sensitivity to threat (Rhodes et al, 2007), the present findings provide intriguing evidence linking dynamic changes in central serotonin levels and threat-related amygdala reactivity. Furthermore, this study provides a methodological framework for evaluating neurobiological mechanisms responsive to changes in serotonin signaling, even among healthy individuals, and may serve as a benchmark for future studies evaluating SSRI effects in clinical cohorts.
Here we modeled spontaneous fluctuations in [11C]SB207145 binding using the placebo cohort of our study. Although there is reason to be cautious about this interpretation because the extent to which serotonin levels fluctuate spontaneously in humans is not clear, primarily because there is not a validated way to measure it, we believe that our findings provide support for its consideration. A study in a group of non-human primates reported cerebrospinal fluid 5-HIAA levels more than double baseline levels 8 weeks after the introduction of a novel female (Higley et al, 1996). This finding alludes to the dynamic range of the central serotonin system, which may underlie the changes in [11C]SB207145 binding in the placebo group. As with any test–retest study, there is noise in the estimate of change in [11C]SB207145 binding. However, the similar direction and magnitude of associations between change in [11C]SB207145 binding and threat-related amygdala reactivity between the placebo and fluoxetine groups suggests that a limitation of our current study may be a small sample size and that we are simply under-powered to observe a significant effect within either group, individually. Thus, we suggest that our observed change in [11C]SB207145 binding, including the placebo group, captures a change in serotonin neurotransmission, which affects the threat-related neural processing.
Within our current study, we did not observe a significant effect of intervention group on estimates of reactivity, which we speculate is because of a combination of related factors. The magnitude of change in central serotonin levels (∼5%) was smaller than the 16–47% change observed in a study in rodents (Licht et al, 2009) but similar to another molecular neuroimaging study in healthy humans (Nord et al, 2013). This suggests that the effect of SSRIs on central serotonin levels may be smaller than expected, at least in healthy individuals. In addition, we observed that [11C]SB207145 binding increased in some individuals and decreased in others following fluoxetine intervention. This is consistent with the large variability in responsiveness to SSRI treatment and evidence that treatment decreases threat-related amygdala reactivity in clinical responders (Ruhe et al, 2012). This individual variability in responsiveness to SSRI intervention diminishes the sensitivity of a simple intervention group comparison and reinforces the value of modeling interindividual differences in change in central serotonin levels. Although the extent to which our observed effects in a healthy population generalize to clinical cohorts with mood and/or anxiety disorders is unclear, our findings and the notion that serotonin dysfunction represents a neurobiological feature of these disorders suggests that SSRIs would more strongly affect central serotonin levels and related brain function in treatment responders.
The specificity of our observed effect in the amygdala may reflect that its response to this task is more tightly linked to serotonin signaling than to the prefrontal cortex, which may be relatively more strongly regulated by other neurotransmitter systems. Previous studies have reported effects of serotonin signaling on response to threat in other brain regions including the insula, fusiform gyrus, temporal gyri, and striatum (Grady et al, 2013; Arce et al, 2008). We considered these regions in post hoc analyses and did not observe evidence for an association with our measure of endogenous serotonin levels or intervention group status (p>0.13).
A previous study reported an inverse association between urinary concentrations of escitalopram and threat-related brain function, but acknowledged the uncertainty about its relation to blood or brain levels of drug (Arce et al, 2008). As described previously, we observed that drug levels in the blood and change in central serotonin levels were unrelated (Haahr et al, 2014). Furthermore, post hoc analyses did not suggest an association between change in threat-related brain function and drug levels in the blood at the time of re-scan (p>0.29). Taken together, these findings are consistent with the idea that [11C]SB207145 PET more closely relates to change in central serotonin levels and resulting effects on brain function. It remains clear that identifying a low- to noninvasive biomarker predictive of SSRI responsiveness would be beneficial, but our findings suggest that this neuroimaging measure of change in serotonin levels may be a useful proxy for studies delineating neural pathways responsive to SSRIs. We used a commonly employed fMRI task to probe threat-related brain function but it is important to note that this task does not comprehensively measure the neural processes underlying the response to threat. Future studies evaluating fMRI paradigms targeting complementary features of threat-related brain function or additional neural pathways would benefit our understanding of how changes in serotonin levels may affect other relevant aspects of brain function.
Although our study has the strengths of a rigorous placebo-controlled, double-blind design, it is not without limitations. In order to avoid potential confounds of hormonal fluctuations, we included only males. However, there is compelling evidence for an interaction between serotonin signaling and gender or sex hormones (Moses-Kolko et al, 2011); thus, future studies should evaluate these effects in women. Owing to logistical constraints, PET and fMRI scans were not always acquired on the same day. Although we cannot rule this out as a confound, we do not think that it substantially affected our results because the time between re-scans was generally small (median 1 day between fMRI and PET re-scan) and 5-HT4 binding is insensitive to acute changes in serotonin levels (Marner et al, 2010). Furthermore, days between re-scan did not differ between intervention groups (p=0.71, Wilcoxon test). Although acute SSRI intervention does not alter [11C]SB207145 binding (Marner et al, 2010), one may consider whether a 3-week SSRI intervention, if associated with a global decrease in blood flow, could lead to an underestimation of [11C]SB207145 BPND. However, it has been shown previously that even profound changes in blood flow introduce only negligible bias (Marner et al, 2009). To the best of our knowledge, there are no data to support that the 3-week SSRI intervention in healthy volunteers is associated with substantial, global alterations in blood flow.
In summary, we report evidence that fluctuations in central serotonin levels correlate with amygdala response to threat but not to the intervention group status. Remarkably, this association included pharmacologically induced and spontaneously occurring changes in serotonin signaling, underscoring it as a key molecular mechanism affecting amygdala reactivity, a central brain structure for identifying and responding to salient environmental stimuli, including indices of threat. Our current results provide novel support for the value of modeling individual differences in change in serotonin levels, which may prove an informative biomarker for understanding individual variability in treatment responsiveness in clinical cohorts. These findings provide additional insight into the neurobiological mechanisms responsive to changes in central serotonin levels and provide a framework for future studies evaluating these effects in individuals at high-risk for or with mood and anxiety disorders.
FUNDING AND DISCLOSURE
This study was funded in part by an unrestricted grant from GlaxoSmithKline and by a center grant to the Center for Integrated Molecular Brain Imaging from the Lundbeck Foundation. PMF is funded by a grant from the Danish Council for Independent Research (DFF). HRS has received honoraria as speaker from Lundbeck A/S, Valby, Denmark, Biogen Idec, Denmark A/S, and Genzyme, Denmark, honoraria as the editor of Neuroimage from Elsevier Publishers, Amsterdam, The Netherlands, and Springer Publishing, Stuttgart, Germany, and travel support from MagVenture, Denmark. GMK is a consultant of H. Lundbeck, Valby, Denmark and received an honorarium as a field editor for The International Journal of Neuropsychopharmacology. All other authors declare no conflict of interest.
Acknowledgments
We thank the John and Birthe Meyer Foundation for the donation of the Cyclotron and PET-scanner, the Simon Spies Foundation for the donation of the Siemens Trio MRI scanner; Sussi Larsen, Julian Macoveanu, and Pernille Iversen from the Danish Research Centre for Magnetic Resonance for assistance in MR data collection and management; Lone Freyr and Dorthe Givard for their coordinated effort in maintaining the tablets and blinding
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Albert PR, Benkelfat C, Descarries L. The neurobiology of depression—revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos Trans R Soc B. 2012;367:2378–2381. doi: 10.1098/rstb.2012.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berl) 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin DS, Stein DJ, Dolberg OT, Bandelow B. How long should a trial of escitalopram treatment be in patients with major depressive disorder, generalised anxiety disorder or social anxiety disorder? An exploration of the randomised controlled trial database. Hum Psychopharmacol. 2009;24:269–275. doi: 10.1002/hup.1019. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766–6772. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacology. 1999;21:91S–98S. doi: 10.1016/S0893-133X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ. A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacol. 2007;21:684–690. doi: 10.1177/0269881106074062. [DOI] [PubMed] [Google Scholar]
- Charlier C, Pinto E, Ansseau M, Plomteux G. Relationship between clinical effects, serum drug concentration, and concurrent drug interactions in depressed patients treated with citalopram, fluoxetine, clomipramine, paroxetine or venlafaxine. Hum Psychopharmacol. 2000;15:453–459. doi: 10.1002/1099-1077(200008)15:6<453::AID-HUP228>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The Temperament and Character Inventory (TCI): Guide to its Development and Use. Center for Psychobiology Personality, Washington University in St. Louis: St. Louis, MO; 1994. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Fisher PM, Holst KK, McMahon B, Haahr ME, Madsen K, Gillings N, et al. 5-HTTLPR status predictive of neocortical 5-HT4 binding assessed with [(11)C]SB207145 PET in humans. Neuroimage. 2012;62:130–136. doi: 10.1016/j.neuroimage.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Madsen MK, McMahon B, Holst KK, Andersen SB, Laursen HR, et al. Three-week bright-light intervention has dose-related effects on threat-related corticolimbic reactivity and functional coupling. Biol Psychiatry. 2013;76:332–339. doi: 10.1016/j.biopsych.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Price JC, Meltzer CC, Moses-Kolko EL, Becker C, Berga SL, et al. Medial prefrontal cortex serotonin 1 A and 2 A receptor binding interacts to predict threat-related amygdala reactivity. Biol Mood Anxiety Disord. 2011;1:2. doi: 10.1186/2045-5380-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsell Y. The major depression inventory versus schedules for clinical assessment in neuropsychiatry in a population sample. Soc Psychiatry Psychiatr Epidemiol. 2005;40:209–213. doi: 10.1007/s00127-005-0876-3. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Siebner HR, Hornboll B, Macoveanu J, Paulson OB, Knudsen GM. Acute pharmacologically induced shifts in serotonin availability abolish emotion-selective responses to negative face emotions in distinct brain networks. Eur Neuropsychopharmacol. 2013;23:368–378. doi: 10.1016/j.euroneuro.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Haahr ME, Fisher P, Holst K, Madsen K, Jensen CG, Marner L, et al. The 5-HT4 receptor levels in hippocampus correlates inversely with memory test performance in humans. Hum Brain Mapp. 2013;34:3066–3074. doi: 10.1002/hbm.22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahr ME, Fisher PM, Jensen CG, Frokjaer VG, Mahon BM, Madsen K, et al. Central 5-HT4 receptor binding as biomarker of serotonergic tonus in humans: a [(11)C]SB207145 PET study. Mol Psychiatry. 2014;19:427–432. doi: 10.1038/mp.2013.147. [DOI] [PubMed] [Google Scholar]
- Hansen HS, Mortensen EL, Schiøtz HK.2004NEO-PI-R, manualIn: Hansen HS, Mortensen EL, (eds). Dokumentation for Den Danske Udgave af NEO PI-R og NEO PI-R Kort Version Dansk Psykologisk Forlag: Copenhagen, Denmark [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Higley JD, King ST, Jr, Hasert MF, Champoux M, Suomi SJ, Linnoila M. Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology. 1996;14:67–76. doi: 10.1016/S0893-133X(96)80060-1. [DOI] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornboll B, Macoveanu J, Rowe J, Elliott R, Paulson OB, Siebner HR, et al. Acute serotonin 2 A receptor blocking alters the processing of fearful faces in the orbitofrontal cortex and amygdala. J Psychopharmacol. 2013;27:903–914. doi: 10.1177/0269881113494106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Summerln JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon, a database server for Talairach Atlas Labels. Neuroimage. 1997;5:S633. [Google Scholar]
- Licht CL, Marcussen AB, Wegener G, Overstreet DH, Aznar S, Knudsen GM. The brain 5-HT4 receptor binding is down-regulated in the Flinders Sensitive Line depression model and in response to paroxetine administration. J Neurochem. 2009;109:1363–1374. doi: 10.1111/j.1471-4159.2009.06050.x. [DOI] [PubMed] [Google Scholar]
- Madsen K, Marner L, Haahr M, Gillings N, Knudsen GM. Mass dose effects and in vivo affinity in brain PET receptor studies—a study of cerebral 5-HT4 receptor binding with [11C]SB207145. Nucl Med Biol. 2011;38:1085–1091. doi: 10.1016/j.nucmedbio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marner L, Gillings N, Comley RA, Baare WF, Rabiner EA, Wilson AA, et al. Kinetic modeling of 11C-SB207145 binding to 5-HT4 receptors in the human brain in vivo. J Nucl Med. 2009;50:900–908. doi: 10.2967/jnumed.108.058552. [DOI] [PubMed] [Google Scholar]
- Marner L, Gillings N, Madsen K, Erritzoe D, Baaré WFC, Svarer C, et al. Brain imaging of serotonin 4 receptors in humans with [11C]SB207145-PET. Neuroimage. 2010;50:855–861. doi: 10.1016/j.neuroimage.2010.01.054. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Price JC, Shah N, Berga S, Sereika SM, Fisher PM, et al. Age, sex, and reproductive hormone effects on brain serotonin-1 A and serotonin-2 A receptor binding in a healthy population. Neuropsychopharmacology. 2011;36:2729–2740. doi: 10.1038/npp.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, O'Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry. 2009;194:535–540. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury R, Taylor MJ, Selvaraj S, Murphy SE, Harmer CJ, Cowen PJ. Short-term antidepressant treatment modulates amygdala response to happy faces. Psychopharmacology (Berl) 2009;206:197–204. doi: 10.1007/s00213-009-1597-1. [DOI] [PubMed] [Google Scholar]
- Nord M, Finnema SJ, Halldin C, Farde L. Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int J Neuropsychopharmacol. 2013;16:1577–1586. doi: 10.1017/S1461145712001617. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Forshall S, Bell C, Rich A, Sandford J, Nash J, et al. Mechanisms of action of selective serotonin reuptake inhibitors in the treatment of psychiatric disorders. Eur Neuropsychopharmacol. 1999;9 (Suppl 3:S81–S86. doi: 10.1016/s0924-977x(99)00030-9. [DOI] [PubMed] [Google Scholar]
- Olesen OV, Sibomana M, Keller SH, Andersen F, Jensen J, Holm S, et al. Spatial resolution of the HRRT PET scanner using 3D-OSEM PSF reconstruction. In. Nuclear Science Symposium Conference Record (NSS/MIC), 2009 IEEE. pp 3789-3790 2009.
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paterson LM, Tyacke RJ, Nutt DJ, Knudsen GM. Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J Cereb Blood Flow Metab. 2010;30:1682–1706. doi: 10.1038/jcbfm.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction Learning in Humans: Role of the Amygdala and vmPFC. Neuron. 2004;43:897. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes RA, Murthy NV, Dresner MA, Selvaraj S, Stavrakakis N, Babar S, et al. Human 5-HT transporter availability predicts amygdala reactivity in vivo. J Neurosci. 2007;27:9233–9237. doi: 10.1523/JNEUROSCI.1175-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe HG, Booij J, Veltman DJ, Michel MC, Schene AH. Successful pharmacologic treatment of major depressive disorder attenuates amygdala activation to negative facial expressions: a functional magnetic resonance imaging study. J Clin Psychiatry. 2012;73:451–459. doi: 10.4088/JCP.10m06584. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1:10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau FC, Reader AJ, Comtat C, Leroy C, Ribeiro MJ, Buvat I, et al. Impact of image-space resolution modeling for studies with the high-resolution research tomograph. J Nucl Med. 2008;49:1000–1008. doi: 10.2967/jnumed.107.045351. [DOI] [PubMed] [Google Scholar]
- Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. Br Med J. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.