Abstract
Purpose
The aims of this study were to compare the expression of sarcosine metabolism-related proteins between invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC) and to determine the implications of these results.
Materials and Methods
Tissue microarrays were constructed, containing 30 samples from normal breast tissue, 114 samples from patients with ILC, and 692 samples from patients with IDC. Immunohistochemical staining was performed to examine the expression of sarcosine metabolism-related proteins [glycine N-methyltransferase, sarcosine dehydrogenase, and l-pipecolic acid oxidase (PIPOX)].
Results
The sarcosine metabolic phenotype differed between ILC and IDC (p<0.001). In IDC, sarcosine metabolic phenotype was distributed as null type (61.7%)>low sarcosine type (30.4%)>high sarcosine type (5.0%)>intermediate type (2.9%). However, in ILC, the sarcosine metabolic phenotype was distributed as low sarcosine type (61.4%)>null type (32.5%)>intermediate type (5.3%)>high sarcosine type (0.9%). PIPOX showed higher expression in ILC than in IDC (p<0.001) and correlated with androgen receptor (AR) positivity (p=0.001) in ILC.
Conclusion
Expression of sarcosine metabolism-related proteins differed between ILC and IDC. Low sarcosine type was the majority sarcosine metabolic phenotype of ILC. PIPOX expression was predominant in ILC and correlated with AR positivity.
Keywords: Breast cancer, lobular cancer, sarcosine
INTRODUCTION
Breast cancer is one of most common cancers in women and comprises about 23% of all female carcinomas.1 With regard to histologic subtypes, invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC) are the major two subtypes of invasive carcinoma of the breast.1 Among the various subtypes, ILC accounts for about 5-15% of all invasive carcinomas,2,3 and the incidence of ILC has increased more rapidly than that of IDC in the past decade due to widely-prescribed hormone replacement therapy and increasing alcohol consumption.4,5 ILC is distinguished from IDC by several clinical and histologic features. For example, ILC usually shows multifocal manifestation and tends to occur in both breasts simultaneously.6,7 Histologically, ILC is characterized by discohesive cancer cells lacking E-cadherin expression.8 Metastatic sites in ILC are often unusual regions including the bones, gastrointestinal tract, uterus, meninges, and ovaries, which are rarely involved in cases of IDC.7,9,10
Sarcosine (N-methylglycine) is a non-proteinogenic amino acid that is made during the metabolism and catabolism of glycine. Several major enzymes involved in the sarcosine metabolism pathway include glycine N-methyltransferase (GNMT), sarcosine dehydrogenase (SARDH), and l-pipecolic acid oxidase (PIPOX). GNMT participates in the production of sarcosine by transmitting the methyl group from S-adenosylmethionine to glycine. SARDH and PIPOX, both sarcosine-metabolizing enzymes, produce glycine from sarcosine by oxidative demethylation.11 Sarcosine has been reported as a potential oncometabolite more than non-proteinogenic amino acid. In prostate cancer, sarcosine was studied as a sensitive tumor biomarker and was also found to have a relationship with tumor progression and the metastatic process.12,13 Like prostate cancer, breast cancer is hormone-dependent cancer, although in this case, it is predominantly related to estrogen receptor (ER) and progesterone receptor (PR). However, androgen receptor (AR) is also expressed in breast cancer, especially in ILC.14 In contrast, the expression of sarcosine metabolism-related proteins in breast cancer remains to be elucidated. We aimed to investigate the expression of sarcosine metabolism-related proteins in ILC and IDC, to compare the expression rates in ILC and IDC, and to determine the implications of the results.
MATERIALS AND METHODS
Patient selection and clinicopathologic evaluation
Between January 2000 and December 2012, formalin-fixed, paraffin-embedded (FFPE) tissue samples were collected at Severance Hospital. Tissue samples were obtained from patients who had undergone breast surgery due to ILC. For the control group, tissue samples were prepared from patients diagnosed with IDC, not otherwise specified from 2006 to 2010. This study was approved by the Institutional Review Board of Severance Hospital. Patients who had received preoperative chemotherapy were excluded from this study. Histologic examination was performed by hematoxylin and eosin (H&E) staining, and all slides were reviewed retrospectively by a trained breast pathologist (Koo JS). The histologic grade was assessed using the Nottingham grading system.15 Tumor staging was based on the seventh American Joint Committee on Cancer criteria. Disease-free survival was calculated from the date of the first curative surgery to the date of the first loco-regional or systemic relapse or to death without any type of relapse. Overall survival was estimated from the date of the first curative operation to the date of the last follow-up or death from any cause. Clinicopathologic parameters evaluated in each type of breast cancer included patient age at initial diagnosis, lymph node metastasis, tumor recurrence, distant metastasis, and patient survival.
Tissue microarray
Through retrospective review of the H&E-stained slides, the most appropriate FFPE tumor tissue samples were retrieved, and representative tumor areas were circled on the FFPE blocks. Two 3.0-mm tissue cores were taken from the representative region of each paraffin block and inserted into 6×5 recipient blocks.
Immunohistochemistry
The antibodies used for immunohistochemistry in this study are shown in Table 1. Three-micrometer paraffin sections were deparaffinized and rehydrated by xylene and alcohol solution. Immunohistochemistry was performed using a Ventana Discovery XT automated staining system (Ventana Medical System, Tucson, AZ, USA). Antigen retrieval was performed using CC1 buffer [Cell Conditioning 1; citrate buffer (pH 6.0), Ventana Medical System]. Appropriate positive and negative controls for immunohistochemical staining were also included.
Table 1. Source, Clone, and Dilution of Antibodies Used in This Study.
| Antibody | Company | Clone | Dilution |
|---|---|---|---|
| Sarcosine metabolism-related | |||
| GNMT | Abcam, Cambridge, UK | Polyclonal | 1:100 |
| SARDH | Abcam, Cambridge, UK | Polyclonal | 1:100 |
| PIPOX | Abcam, Cambridge, UK | Polyclonal | 1:100 |
| Molecular subtype-related | |||
| ER | Thermo Scientific, San Siego, CA, USA | SP1 | 1:100 |
| PR | DAKO, Glostrup, Denmark | PgR | 1:50 |
| HER-2 | DAKO, Glostrup, Denmark | Polyclonal | 1:1500 |
| Ki-67 | Abcam, Cambridge, UK | MIB | 1:1000 |
| Androgen-related | |||
| AR | DAKO, Glostrup, Denmark | AR441 | 1:150 |
GNMT, glycine N-methyltransferase; SARDH, sarcosine dehydrogenase; PIPOX, l-pipecolic acid oxidase; ER, estrogen receptor; PR, progesterone receptor; AR, androgen receptor; HER-2, human epidermal growth factor receptor-2.
Interpretation of immunohistochemical results
A cut-off value of 1% or more positively-stained nuclei was used to define ER and AR positivity.16 Human epidermal growth factor receptor-2 (HER-2) staining was analyzed according to the American Society of Clinical Oncology/College of American Pathologists guidelines using the following categories: 0=no immunostaining; 1+=weak incomplete membranous staining in less than 10% of tumor cells; 2+=complete membranous staining either uniform or weak in at least 10% of tumor cells; and 3+=uniform intense membranous staining in at least 30% of tumor cells.17 HER-2 immunostaining was considered positive when strong (3+) membranous staining was observed whereas cases with 0 to 1+ staining were regarded as negative. The cases showing 2+ HER-2 expression were evaluated for HER-2 amplification by fluorescent in situ hybridization (FISH).
Immunohistochemical markers for GNMT, SARDH, and PIPOX were accessed by light microscopy. Interpretation of immunohistochemical staining for autophagy and redox-related proteins was determined by multiplying the proportion of stained cells (0%=0; 1-29%=1; 30-100%=2) by the immunostaining intensity (negative=0; weak=1; moderate=2; strong=3). Final scores of 0-1 were interpreted as negative, and scores of 2-6 were considered positive.18 The Ki-67 labeling index (LI) was scored by percentage of nuclear stained cells in total tumor cells (0-100%).
Tumor phenotype classification
In this study, we classified breast cancer phenotypes according to the immunohistochemistry results for ER, PR, HER-2, and Ki-67 LI. FISH results for HER-2 were classified as follows:19 luminal A type: ER and/or PR positive, HER-2 negative, and Ki-67 LI<14%; luminal B type: (HER-2 negative) ER and/or PR positive, HER-2 negative, and Ki-67 LI≥14% and (HER-2 positive) ER and/or PR positive and HER-2 overexpressed or amplified; HER-2 type: ER and PR negative and HER-2 overexpressed or amplified; triple-negative breast cancer (TNBC) type: ER, PR, and HER-2 negative.
Sarcosine metabolism phenotypes
According to immunohistochemistry results for GNMT, SARDH, and PIPOX, sarcosine metabolism phenotypes were categorized as follows: high sarcosine type: GNMT (+), SARDH and PIPOX (-); low sarcosine type: GNMT (-), SARDH and/or PIPOX (+); intermediate sarcosine type: GNMT (+), SARDH or PIPOX (+); and null type: GNMT (-), SARDH and PIPOX (-).
Statistical analysis
Data were statistically processed using SPSS for Windows version 12.0 (SPSS Inc., Chicago, IL, USA). Student's t-test and Fisher's exact test were used for continuous and categorical variables, respectively. Statistical significance was assumed when p<0.05. Kaplan-Meier survival curves and log-rank statistics were employed to evaluate time to survival. Multivariate regression analysis was performed using the Cox proportional hazards model.
RESULTS
Basal characteristics of invasive lobular carcinoma
The clinicopathologic characteristics of patients with ILC are summarized in Table 2. There were 114 patients with ILC, subdivided into 102 classic-type and 12 pleomorphic-type patients. The pleomorphic type showed significant association with older age (p=0.033), a higher nuclear grade (p<0.001), a higher histologic grade (p<0.001), a higher T stage (p=0.026), PR negativity (p=0.005), HER-2 positivity (p=0.002), a higher Ki-67 LI (p<0.001), and the non-luminal A subtype (p<0.001) compared with the classic type.
Table 2. Clinicopathologic Characteristics of Invasive Lobular Carcinoma.
| Parameters | Total, n=114 (%) | Classic type, n=102 (%) | Pleomorphic type, n=12 (%) | p value |
|---|---|---|---|---|
| Age (yrs) | 0.033 | |||
| <50 | 63 (55.3) | 60 (58.8) | 3 (25.0) | |
| ≥50 | 51 (44.7) | 42 (41.2) | 9 (75.0) | |
| Nuclear grade | <0.001 | |||
| 1/2 | 102 (89.5) | 102 (100.0) | 0 (0.0) | |
| 3 | 12 (10.5) | 0 (0.0) | 12 (100.0) | |
| Histologic grade | <0.001 | |||
| I/II | 109 (95.6) | 102 (100.0) | 7 (58.3) | |
| III | 5 (4.4) | 0 (0.0) | 5 (41.7) | |
| T stage | 0.026 | |||
| T1 | 67 (58.8) | 64 (62.7) | 3 (25.0) | |
| T2/T3 | 47 (41.2) | 38 (37.3) | 9 (75.0) | |
| Lymph node metastasis | 0.749 | |||
| Absent | 80 (70.2) | 72 (70.6) | 8 (66.7) | |
| Present | 34 (29.8) | 30 (29.4) | 4 (33.3) | |
| ER | 0.158 | |||
| Negative | 7 (6.1) | 5 (4.9) | 2 (16.7) | |
| Positive | 107 (93.9) | 97 (95.1) | 10 (83.3) | |
| PR | 0.005 | |||
| Negative | 19 (16.7) | 13 (12.7) | 6 (50.0) | |
| Positive | 95 (83.3) | 89 (87.3) | 6 (50.0) | |
| HER-2 | 0.002 | |||
| Negative | 107 (93.9) | 99 (97.1) | 8 (66.7) | |
| Positive | 7 (6.1) | 3 (2.9) | 4 (33.3) | |
| AR | 0.515 | |||
| Negative | 48 (42.1) | 44 (43.1) | 4 (33.3) | |
| Positive | 66 (57.9) | 58 (56.9) | 8 (66.7) | |
| Ki-67 LI | <0.001 | |||
| ≤14 | 92 (80.7) | 88 (86.3) | 4 (33.3) | |
| >14 | 22 (19.3) | 14 (13.7) | 8 (66.7) | |
| Molecular type | <0.001 | |||
| Luminal A | 86 (75.4) | 83 (81.4) | 3 (25.0) | |
| Luminal B | 22 (19.3) | 15 (14.7) | 7 (58.3) | |
| HER-2 | 1 (0.9) | 0 (0.0) | 1 (8.3) | |
| TNBC | 5 (4.4) | 4 (3.9) | 1 (8.3) |
ER, estrogen receptor; PR, progesterone receptor; AR, androgen receptor; LI, labeling index; HER-2, human epidermal growth factor receptor-2; TNBC, triple-negative breast cancer.
When comparing clinicopathologic parameters of ILC with those of IDC, ILC was associated with a lower histologic grade (p<0.001), no lymph node metastasis (p=0.026), ER positivity (p<0.001), PR positivity (p<0.001), HER-2 negativity (p<0.001), a lower Ki-67 LI (p<0.001), and a higher incidence of luminal A type (p<0.001) (Table 3).
Table 3. Comparison of Clinicopathologic Characteristics between Invasive Lobular Carcinoma and Invasive Ductal Carcinoma.
| Parameters | Total, n=835 (%) | IDC, n=721 (%) | ILC, n=114 (%) | p value |
|---|---|---|---|---|
| Age (yrs) | 0.832 | |||
| <50 | 491 (58.8) | 428 (59.4) | 63 (55.3) | |
| ≥50 | 344 (41.2) | 293 (40.6) | 51 (44.7) | |
| Histologic grade | <0.001 | |||
| I/II | 594 (71.1) | 485 (67.3) | 109 (95.6) | |
| III | 241 (28.9) | 236 (32.7) | 5 (4.4) | |
| T stage | 0.058 | |||
| T1 | 422 (50.5) | 355 (49.2) | 67 (58.8) | |
| T2/T3 | 413 (49.5) | 366 (50.8) | 47 (41.2) | |
| Lymph node metastasis | 0.026 | |||
| Absent | 507 (60.7) | 427 (59.2) | 80 (70.2) | |
| Present | 328 (39.3) | 294 (40.8) | 34 (29.8) | |
| ER | <0.001 | |||
| Negative | 266 (31.9) | 259 (35.9) | 7 (6.1) | |
| Positive | 569 (68.1) | 462 (64.1) | 107 (93.9) | |
| PR | <0.001 | |||
| Negative | 366 (43.8) | 347 (48.1) | 19 (16.7) | |
| Positive | 469 (56.2) | 374 (51.9) | 95 (83.3) | |
| HER-2 | <0.001 | |||
| Negative | 672 (80.5) | 565 (78.4) | 107 (93.9) | |
| Positive | 163 (19.5) | 156 (21.6) | 7 (6.1) | |
| Ki-67 LI | <0.001 | |||
| ≤14 | 501 (60.0) | 409 (56.7) | 92 (80.7) | |
| >14 | 334 (40.0) | 312 (43.3) | 22 (19.3) | |
| Molecular type | <0.001 | |||
| Luminal A | 389 (46.6) | 303 (42.0) | 86 (75.4) | |
| Luminal B | 191 (22.9) | 169 (23.4) | 22 (19.3) | |
| HER-2 | 72 (8.6) | 71 (9.8) | 1 (0.9) | |
| TNBC | 183 (21.9) | 178 (24.7) | 5 (4.4) |
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; ER, estrogen receptor; PR, progesterone receptor; LI, labeling index; HER-2, human epidermal growth factor receptor-2; TNBC, triple-negative breast cancer.
Expression of sarcosine metabolism-related proteins in normal tissue, ILC, and IDC
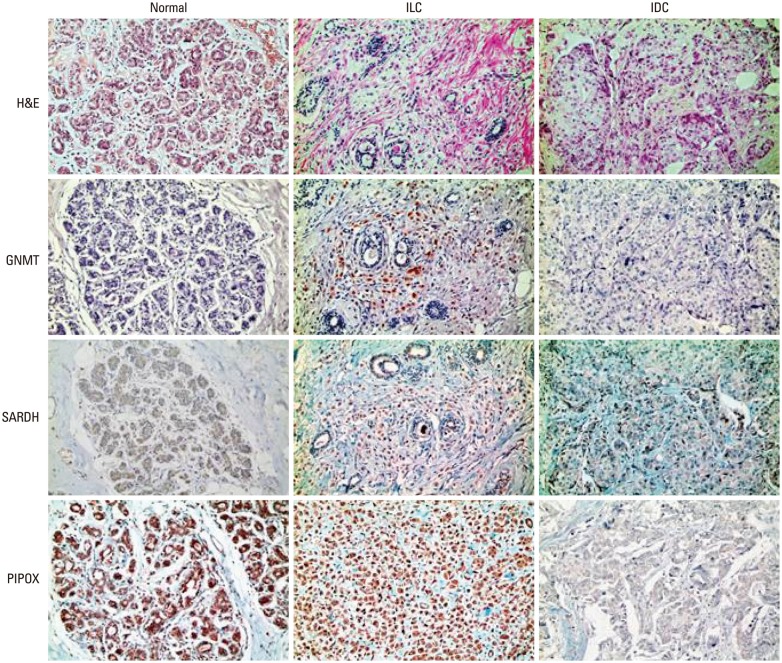
No difference was observed in the expression of sarcosine metabolism-related proteins between the subtypes of ILC, although expressions of SARDH and PIPOX were higher in ILC compared to normal tissue (SARDH: p=0.014; PIPOX: p<0.001) (Table 4). When comparing ILC with IDC, ILC showed a higher expression of PIPOX (p<0.001) (Table 5, Fig. 1). This difference of expression was also observed between ILC and IDC luminal types (p<0.001) (Table 6).
Table 4. Expressions of Sarcosine Metabolism-Related Proteins in Normal Breast Tissue and ILC.
| Parameters | Total, n=114 (%) | Normal tissue, n=30 (%) | ILC | p value† | ||
|---|---|---|---|---|---|---|
| Classic type, n=102 (%) | Pleomorphic type, n=12 (%) | p value* | ||||
| GNMT | 0.349 | 0.345 | ||||
| Negative | 107 (93.9) | 30 (100.0) | 95 (93.1) | 12 (100.0) | ||
| Positive | 7 (6.1) | 0 (0.0) | 7 (6.9) | 0 (0.0) | ||
| SARDH | 0.473 | 0.014 | ||||
| Negative | 94 (82.5) | 30 (100.0) | 85 (83.3) | 9 (75.0) | ||
| Positive | 20 (17.5) | 0 (0.0) | 17 (16.7) | 3 (25.0) | ||
| PIPOX | 0.714 | <0.001 | ||||
| Negative | 42 (36.8) | 28 (93.3) | 37 (36.3) | 5 (41.7) | ||
| Positive | 72 (63.2) | 2 (6.7) | 65 (63.7) | 7 (58.3) | ||
| Sarcosine metabolic type | 0.829 | <0.001 | ||||
| High sarcosine type | 1 (0.9) | 0 (0.0) | 1 (1.0) | 0 (0.0) | ||
| Intermediate sarcosine type | 6 (5.3) | 0 (0.0) | 6 (5.9) | 0 (0.0) | ||
| Low sarcosine type | 70 (61.4) | 2 (6.7) | 62 (60.8) | 8 (66.7) | ||
| Null type | 37 (32.5) | 28 (93.3) | 33 (32.4) | 4 (33.3) | ||
ILC, invasive lobular carcinoma; GNMT, glycine N-methyltransferase; SARDH, sarcosine dehydrogenase; PIPOX, l-pipecolic acid oxidase.
*Compared between classic type and pleomorphic type.
†Compared between normal tissue and ILC.
Table 5. Expressions of Sarcosine Metabolism-Related Proteins in IDC and ILC.
| Parameters | Total, n=835 (%) | Normal tissue, n=30 (%) | IDC, n=721 (%) | ILC, n=114 (%) | p value |
|---|---|---|---|---|---|
| GNMT | 0.231 | ||||
| Negative | 771 (92.3) | 30 (100.0) | 664 (92.1) | 107 (93.9) | |
| Positive | 64 (7.7) | 0 (0.0) | 57 (7.9) | 7 (6.1) | |
| SARDH | 0.045 | ||||
| Negative | 691 (82.8) | 30 (100.0) | 597 (82.8) | 94 (82.5) | |
| Positive | 144 (17.2) | 0 (0.0) | 124 (17.2) | 20 (17.5) | |
| PIPOX | <0.001 | ||||
| Negative | 612 (73.3) | 28 (93.3) | 570 (79.1) | 42 (36.8) | |
| Positive | 223 (26.7) | 2 (6.7) | 151 (20.9) | 72 (63.2) |
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; GNMT, glycine N-methyltransferase; SARDH, sarcosine dehydrogenase; PIPOX, l-pipecolic acid oxidase.
Fig. 1. Comparison of sarcosine metabolism-related proteins among normal breast tissue, invasive ductal carcinoma (IDC), and invasive lobular carcinoma (ILC). ILC was found to have a higher expression rate of PIPOX than IDC. H&E, hematoxylin and eosin; GNMT, glycine N-methyltransferase; SARDH, sarcosine dehydrogenase; PIPOX, l-pipecolic acid oxidase.
Table 6. Expressions of Sarcosine Metabolism-Related Proteins in ILC and Luminal-Type IDC.
| Parameters | Total, n=586 (%) | IDC, luminal type, n=472 (%) | ILC, n=114 (%) | p value |
|---|---|---|---|---|
| GNMT | 0.228 | |||
| Negative | 533 (91.0) | 426 (90.3) | 107 (93.9) | |
| Positive | 53 (9.0) | 46 (9.7) | 7 (6.1) | |
| SARDH | 0.923 | |||
| Negative | 485 (82.8) | 391 (82.8) | 94 (82.5) | |
| Positive | 101 (17.2) | 81 (17.2) | 20 (17.5) | |
| PIPOX | <0.001 | |||
| Negative | 410 (70.0) | 368 (78.0) | 42 (36.8) | |
| Positive | 176 (30.0) | 104 (22.0) | 72 (63.2) |
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; GNMT, glycine N-methyltransferase; SARDH, sarcosine dehydrogenase; PIPOX, l-pipecolic acid oxidase.
Comparison of sarcosine metabolism phenotypes among normal tissue, IDC, and ILC
Sarcosine metabolic phenotypes based on the expression of sarcosine metabolism-related proteins showed different distributions among normal breast tissue, IDC, and ILC (Table 7). Sarcosine metabolic phenotypes of normal breast tissue were null type (93.3%) and low sarcosine type (6.7%). In IDC, sarcosine metabolic phenotypes were composed of null type (61.7%)>low sarcosine type (30.4%)>high sarcosine type (5.0%)>intermediate type (2.9%); however, in ILC, the proportion of sarcosine metabolic phenotypes was low sarcosine type (61.4%)>null type (32.5%)>intermediate type (5.3%)>high sarcosine type (0.9%) (p<0.001). These different distributions of sarcosine metabolic phenotypes between IDC and ILC were sustained when compared with the sarcosine metabolic phenotypes in luminal-type IDC (p<0.001) (Table 8).
Table 7. Comparison of Sarcosine Metabolic Phenotypes between IDC and ILC.
| Metabolic phenotypes | Total, n=835 (%) | Normal tissue, n=30 (%) | IDC, n=721 (%) | ILC, n=114 (%) | p value |
|---|---|---|---|---|---|
| Sarcosine metabolic type | <0.001 | ||||
| High sarcosine type | 37 (4.4) | 0 (0.0) | 36 (5.0) | 1 (0.9) | |
| Intermediate type | 27 (3.2) | 0 (0.0) | 21 (2.9) | 6 (5.3) | |
| Low sarcosine type | 289 (34.6) | 2 (6.7) | 219 (30.4) | 70 (61.4) | |
| Null type | 482 (57.7) | 28 (93.3) | 445 (61.7) | 37 (32.5) |
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
Table 8. Comparison of Sarcosine Metabolic Phenotypes between Luminal-Type IDC and ILC.
| Metabolic phenotypes | Total, n=586 (%) | IDC, luminal type, n=472 (%) | ILC, n=114 (%) | p value |
|---|---|---|---|---|
| Sarcosine metabolic type | <0.001 | |||
| High sarcosine type | 29 (4.9) | 28 (5.9) | 1 (0.9) | |
| Intermediate sarcosine type | 24 (4.1) | 18 (3.8) | 6 (5.3) | |
| Low sarcosine type | 217 (37.0) | 147 (31.1) | 70 (61.4) | |
| Null type | 316 (53.9) | 279 (59.1) | 37 (32.5) |
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
Correlation between sarcosine metabolism-related proteins and clinicopathologic factors in ILC
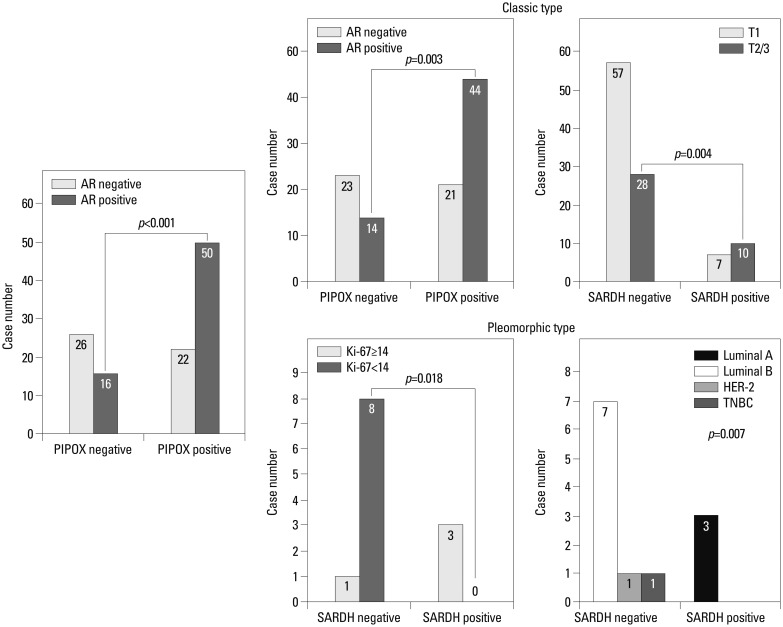
In ILC, PIPOX expression was correlated with AR positivity (p=0.001) (Fig. 2). In the classical type, PIPOX expression was also correlated with AR positivity (p=0.003), and SARDH expression was associated with a higher T stage (p=0.004). In the pleomorphic type, SARDH expression was correlated with a higher Ki-67 LI (p=0.018) and associated with the luminal A molecular type. All SARDH-positive cases were luminal A molecular type, and all SARDH-negative cases were non-luminal A molecular type (p=0.007).
Fig. 2. Correlation between clinicopathologic factors and sarcosine metabolism-related proteins in invasive lobular carcinoma. AR, androgen receptor; PIPOX, l-pipecolic acid oxidase; SARDH, sarcosine dehydrogenase; HER-2, human epidermal growth factor receptor-2; TNBC, triple-negative breast cancer.
Impact of expression status for sarcosine metabolism-related proteins on prognosis in ILC
In ILC, no significant association was found between metabolism-related proteins and prognosis with univariate analysis (Table 9).
Table 9. Univariate Analysis by Log-Rank Test of the Impact of Sarcosine Metabolism-Related Protein Expression in Invasive Lobular Carcinoma on Disease-Free Survival and Overall Survival Times.
| Parameters | Disease-free survival | Overall survival | ||
|---|---|---|---|---|
| 95% CI | p value | 95% CI | p value | |
| GNMT | N/A | N/A | ||
| Negative | N/A | N/A | ||
| Positive | N/A | N/A | ||
| SARDH | N/A | 0.538 | ||
| Negative | N/A | 163 (157-169) | ||
| Positive | N/A | 91 (83-98) | ||
| PIPOX | 0.941 | N/A | ||
| Negative | 164 (156-171) | N/A | ||
| Positive | 93 (89-96) | N/A | ||
GNMT, glycine N-methyltransferase; SARDH, sarcosine dehydrogenase; PIPOX, l-pipecolic acid oxidase; CI, confidence interval; N/A, not applicable.
DISCUSSION
The purpose of the present study was to investigate and compare the expressions of sarcosine metabolism-related proteins in ILC and IDC. Compared to IDC, PIPOX had a higher expression rate in ILC (p<0.001). Sarcosine metabolic phenotypes, based on expressions of sarcosine metabolism-related proteins, were found to have different proportional distributions between ILC and IDC. Low sarcosine type was most common in ILC, whereas null type was most common in IDC. In the present study, sarcosine metabolism-related proteins and sarcosine metabolic phenotypes were different in ILC and IDC, and there were also clinical and histologic distinctions found between ILC and IDC. Although expressions of sarcosine metabolism-related proteins are not yet well-studied in breast cancer, an increased sarcosine level has been associated with cancer progression in prostate cancer. The addition of exogenous sarcosine was reported to induce invasive phenotypes in benign prostate lesions,11 and elevated sarcosine levels in prostate cancer have also been found.20 Also, sarcosine elevation is known to be associated with prostate cancer growth and progression in vitro and in vivo.12 Different sarcosine metabolism-related proteins and sarcosine metabolic phenotypes in ILC and IDC could result in different tumor biology and behavior between ILC and IDC, as sarcosine has been reported to affect to tumor biology in prostate cancer, although further investigation is required in breast cancer.
In the present study, sarcosine metabolism-related proteins (GNMT, SARDH, and PIPOX) were evaluated instead of sarcosine level measurements in breast cancer tissue. In a prior study, sarcosine levels in prostate cancer tissue were well-correlated with elevated GNMT (a sarcosine-generating enzyme) and reduced SARDH and PIPOX, both of which are sarcosine-metabolizing enzymes.12 Considering that ILC in the present study had a higher PIPOX expression rate and more of the low sarcosine phenotype than other types, we expect that a reduced sarcosine level may be found in ILC. However, further validation is needed.
Meanwhile, most of ILC is of the luminal type in molecular classification, whereas IDC is composed of HER-2 and basal-like types, addition to luminal type. In contrast to luminal-type IDC, ILC persistently had a higher PIPOX expression rate and a larger proportion of the low sarcosine phenotype. Thus, in spite of differences of composition of molecular subtypes between ILC and IDC, sarcosine metabolism in ILC and IDC may be unrelated to molecular subtype. In the present study, expression of PIPOX in ILC was correlated with AR positivity (p=0.001), which is incompatible with the previous study on prostate cancer that showed correlation with GNMT expression and AR positivity.21 This discordance may originate from organ-specific differences; therefore, further study on AR and sarcosine metabolism in ILC is required.
In conclusion, ILC and IDC exhibited different expressions of sarcosine metabolism-related proteins. The low sarcosine type comprised the majority of ILC, and PIPOX expression was dominant in ILC.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2012R1A1A1002886).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Tavassoli FA, Devilee P International Agency for Research on Cancer, World Health Organization. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IAPS Press; 2003. [Google Scholar]
- 2.Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421–1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 3.Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046–1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li CI, Chlebowski RT, Freiberg M, Johnson KC, Kuller L, Lane D, et al. Alcohol consumption and risk of postmenopausal breast cancer by subtype: the women's health initiative observational study. J Natl Cancer Inst. 2010;102:1422–1431. doi: 10.1093/jnci/djq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeves GK, Beral V, Green J, Gathani T, Bull D Million Women Study Collaborators. Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol. 2006;7:910–918. doi: 10.1016/S1470-2045(06)70911-1. [DOI] [PubMed] [Google Scholar]
- 6.Lesser ML, Rosen PP, Kinne DW. Multicentricity and bilaterality in invasive breast carcinoma. Surgery. 1982;91:234–240. [PubMed] [Google Scholar]
- 7.Silverstein MJ, Lewinsky BS, Waisman JR, Gierson ED, Colburn WJ, Senofsky GM, et al. Infiltrating lobular carcinoma. Is it different from infiltrating duct carcinoma? Cancer. 1994;73:1673–1677. doi: 10.1002/1097-0142(19940315)73:6<1673::aid-cncr2820730620>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.De Leeuw WJ, Berx G, Vos CB, Peterse JL, Van de Vijver MJ, Litvinov S, et al. Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol. 1997;183:404–411. doi: 10.1002/(SICI)1096-9896(199712)183:4<404::AID-PATH1148>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, et al. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996;77:113–120. doi: 10.1002/(SICI)1097-0142(19960101)77:1<113::AID-CNCR19>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Lamovec J, Bracko M. Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol. 1991;48:28–33. doi: 10.1002/jso.2930480106. [DOI] [PubMed] [Google Scholar]
- 11.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 12.Khan AP, Rajendiran TM, Ateeq B, Asangani IA, Athanikar JN, Yocum AK, et al. The role of sarcosine metabolism in prostate cancer progression. Neoplasia. 2013;15:491–501. doi: 10.1593/neo.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baum CE, Price DK, Figg WD. Sarcosine as a potential prostate cancer biomarker and therapeutic target. Cancer Biol Ther. 2010;9:341–342. doi: 10.4161/cbt.9.5.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riva C, Dainese E, Caprara G, Rocca PC, Massarelli G, Tot T, et al. Immunohistochemical study of androgen receptors in breast carcinoma. Evidence of their frequent expression in lobular carcinoma. Virchows Arch. 2005;447:695–700. doi: 10.1007/s00428-005-0003-6. [DOI] [PubMed] [Google Scholar]
- 15.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 16.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 18.Won KY, Kim GY, Kim YW, Song JY, Lim SJ. Clinicopathologic correlation of beclin-1 and bcl-2 expression in human breast cancer. Hum Pathol. 2010;41:107–112. doi: 10.1016/j.humpath.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jentzmik F, Stephan C, Lein M, Miller K, Kamlage B, Bethan B, et al. Sarcosine in prostate cancer tissue is not a differential metabolite for prostate cancer aggressiveness and biochemical progression. J Urol. 2011;185:706–711. doi: 10.1016/j.juro.2010.09.077. [DOI] [PubMed] [Google Scholar]
- 21.Lee CM, Yen CH, Tzeng TY, Huang YZ, Chou KH, Chang TJ, et al. Androgen response element of the glycine N-methyltransferase gene is located in the coding region of its first exon. Biosci Rep. 2013;33:pii: e00070. doi: 10.1042/BSR20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]