Abstract
Purpose
Continuous renal replacement therapy (CRRT) has been established for critically ill acute kidney injury (AKI) patients. In addition, some centers consist of a specialized CRRT team (SCT) with physicians and nurses. To our best knowledge, however, ona a few studies have yet been carried out on the superiority of SCT management.
Materials and Methods
A total of 551 patients, who received CRRT between January 2008 and March 2009, were divided into two groups based on the controller of CRRT. The impact of the CRRT management on 28-day mortality was compared between two groups by Kaplan-Meier curve and Cox analysis.
Results
During the study period, the number of filters used, down-time per day, and intensive care unit length of day were significantly higher in non-SCT group than in SCT group (6.2 hrs vs. 5.0 hrs, p=0.042; 5.0 hrs vs. 3.8 hrs, p<0.001; 27.5 days vs. 21.1 days, p=0.027, respectively), while net ultrafiltration rate was significantly lower in non-SCT group than SCT group (28.0 mL/kg/hr vs. 29.5 mL/kg/hr, p=0.043, respectively). In addition, 28-day mortality rate was significantly lower in SCT group than with non-SCT group (p=0.031). Moreover, Cox regression analysis showed that 28-day mortality rate was significantly lower in SCT control group, even after adjusting for age, gender, severity scores, biomarkers, risk, injury, failure, loss, and end-stage renal disease, and contributing factors (hazard ratio 0.91, p=0.046).
Conclusion
A well-trained CRRT team could be beneficial for mortality improvement of AKI patients requiring CRRT.
Keywords: SCT management, acute kidney injury, continuous renal replacement therapy, 28-day mortality
INTRODUCTION
Severe acute kidney injury (AKI) is a well-known complication in critically ill patients and has a significant impact on morbidity, mortality, and health resource utilization in these patients.1,2,3,4,5 Although general treatment such as fluid and hemodynamic optimization was provided only for these patients in the past,6 continuous renal replacement therapy (CRRT) has been recently an essential part of critical care, and is considered an established treatment modality for AKI patients.7 However, the mortality rate in these patients still remains extremely high,8,9,10 even though the technical devices for CRRT management have been advanced recently. Given the complexity of AKI progression and handling of extracorporeal system, high qualified CRRT managements, which include proper exchanges of extracorporeal circuits, frequent monitoring for dose of CRRT and optimal anticoagulation, and replacement of electrolytes, are regarded as one of potential candidates for improving patient's outcomes.9,11
Some centers operate a specialized CRRT team (SCT) with nurses and physicians from their disciplines,12 and they surmise that clinical outcomes of the patients treated by SCT should be superior to those from non-SCT. However, to our best knowledge, only a few studies have been carried out on the comparison between before and after SCT approach.12 Therefore, we investigated the benefit of SCT management for 28-day all-cause mortality of AKI patients treated with CRRT. In addition, we initiated the SCT approach for the management of CRRT in August 2008, therefore, we compared the outcomes and quality of CRRT management based on the SCT management.
MATERIALS AND METHODS
Patients
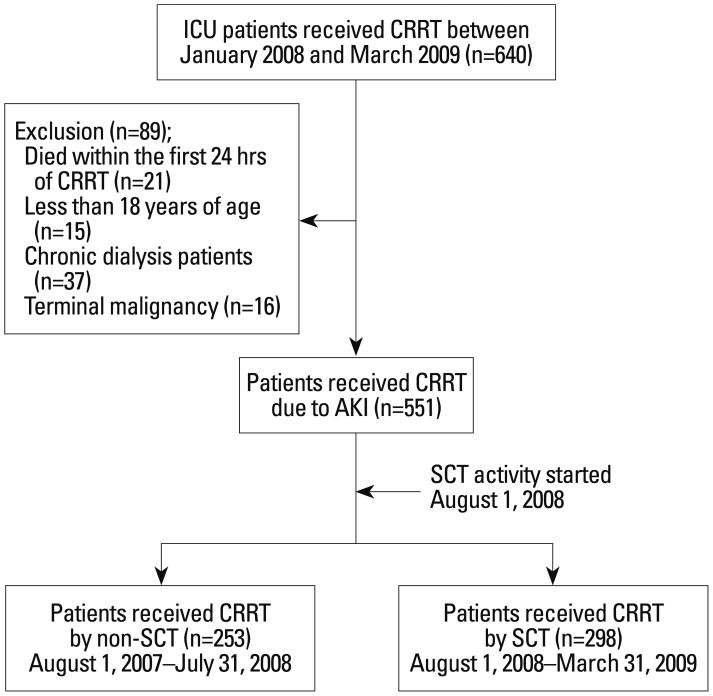
A total of 640 intensive care unit (ICU) patients who received CRRT for severe AKI between January 2008 and March 2009 were initially analyzed. We excluded 89 patients who died within the first 24 hours of CRRT, were less than 18 years of age, were on chronic dialysis, or were diagnosed with terminal malignancy and less than 3 month-life expectancy. In the final analysis, 551 patients were enrolled and investigated. The SCT was set up in August 2008, therefore, we divided patients into two groups, based on that point (Fig. 1).
Fig. 1. Flow diagram of patient selection and outcomes. A total of 640 ICU patients who received CRRT for severe AKI between August 2007 and September 2009 were initially analyzed. We excluded 89 patients because they died within the first 24 hours of CRRT, were less than 18 years of age, were on chronic dialysis, or were diagnosed with terminal malignancy which was considering less than 3 month-life expectancy. In the final analysis, 551 patients were enrolled and investigated. ICU, intensive care; CRRT, continuous renal replacement therapy; AKI, acute kidney injury; non-SCT, conventional team approach; SCT, specialized CRRT team.
The study protocol was approved by the Institutional Review Board (IRB) of Yonsei University Health System (YUHS) Clinical Trial Center. Since this study was a retrospective medical record-based study and the study subjects were de-identified, the IRB waived the need for a written consent from the patients.
Data collection
Patients' data were retrieved from the CRRT Database of YUHS, Seoul, Korea. Demographic, clinical, and biochemical data at the time of CRRT initiation were recorded. For the assessment of disease severity, Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation (APACHE) II score, and Charlson Comorbidity Index (CCI) were also calculated at the start of CRRT. Moreover, we counted the transfused number of packed red blood cell (RBC) during the period of CRRT treatment except for transfusions conducted due to active bleeding. Such active bleeding is considered to be a situation when the patients need transfusion of more than 10 units of packed RBCs within 24 hrs or are bleeding more than 1- to 1.5-fold of the body's entire blood volume as previously described.13 Down-time (hours/day) was defined as the period of time when CRRT was not applied between the initiation and end of CRRT, as defined by Uchino, et al.14
Definitions and SCT roles
SCT is defined as a team including physicians and nurses especially trained and educated in performing CRRT. The members of SCT are composed of 2 specialized nephrologists, 2 nephrologic fellows, an ICU specialist, 3 ICU residents, and 5 CRRT specialized nurses. They create and share the educational programs and management protocol on CRRT. Additionally, a monthly quality control to correct management protocols and problems about CRRT such as electrolytes, coagulation, and hemodynamic status is performed. SCT team has complementary roles to each other. ICU specialist and residents have a primary responsibility for patient general care and perform overall decision makings about medical problems of ICU patients. Especially, nephrologists are authorized to initiate, maintain and stop CRRT and switch to intermittent hemodialysis or peritoneal dialysis. They decide to start CRRT based on patients' hemodynamic status, declining urine output, electrolyte and acid-base imbalances. They also settled mode and dose of CRRT, and net ultrafiltration rate during CRRT. In nursing part, CRRT-nurses work on three shifts daily. He/she goes around ICU at regular interval to monitor the patients treated with CRRT, and checks hemodynamic stability, removal rate of ultrafiltration, and CRRT kit status etc. Their primary role is CRRT management, apart from ICU general care performed by bed-side nurses. In addition, they are also in charge to educate bed-side nurses on basic CRRT-handling methods every three months. Based on daily rounds with specialized team, they discussed and tried to solve the potential problems on the management of CRRT (Table 1 and 2).12
Table 1. The Modules of Educational Program for SCT Nurses.
| Module | Lessons | In-practice training | |
|---|---|---|---|
| I | General intensive care course | 72 hrs | 80 hrs on general ICU |
| II | CRRT specialist training course | 16 hrs | 32 hrs on general ICU |
CRRT, continuous renal replacement therapy; SCT, specialized CRRT team; ICU, intensive care unit; AKI, acute kidney injury.
Module I is corresponded to all ICU nurses. Module II is relevant to SCT nurses for CRRT. Module I includes several topics about the general management of AKI patients including nursing care for CRRT patients and practical installation and monitoring of the CRRT machine. CRRT specialist training course; basic principles and optimal prescription for CRRT, overview and practical operation of CRRT, trouble shooting in CRRT, assessment and management of failed dialysis catheter.
Table 2. The Protocol for Replacement of Electrolytes and Anticoagulation during CRRT.
| Electrolytes | |
| K+ (mEq/L) | |
| >4.5 | No KCl mix in the 5 L hemozol® |
| 3.6-4.5 | 20 mEq KCl mix in the 5 L hemozol® |
| <3.6 | 40 mEq KCl mix in the 5 L hemozol® |
| P (mEq/L) | |
| ≥2.0 | No phosten® mix in the 5 L hemozol® |
| <2.0 | 20 mL phosten® mix in the 5 L hemozol® |
| Anticoagulation | |
| Initial | |
| High risk | No anticoagulation/saline flushing |
| Low risk | Systemic heparinization |
| Maintenance | |
| High risk-1 | No anticoagulation/saline flushing |
| High risk-2 | Regional anticoagulation (citrate or nafamostat) |
| Low risk | Systemic heparinization |
CRRT, continuous renal replacement therapy.
Potassium and phosphate level check 2 times per day. Phosten®; potassium phosphate. Hemosol®; hemozol B0. Definitions; 1) High risk; active bleeding, post-operative within 48 hours, low platelet count <50000/mm3, prolonged PT/PTT ≥2.0 INR/60 sec. 2) Low risk; all patients except for high risk patients. 3) High risk-1 and high risk-2 were divided according to assessment of clotting in extracorporeal system during CRRT.
ICU setting
The investigation site was a self-contained, 112-bed medical and surgical ICU in a 2089-bed teaching hospital in Seoul, Korea that was equipped with 15 CRRT machines. The decisions related CRRT treatment make no difference between before and after SCT group, because they are determined by same nephrologist. One bed-side nurse cared 2 ICU patients, but they also had to perform monitoring and maintenance of the CRRT system before SCT set-up. However, after SCT approach, additional nursing cares were available for CRRT care.
CRRT protocol
Vascular access for CRRT was approached via femoral, internal jugular, or subclavian vein route. In most patients, continuous veno-venous hemodiafiltration was performed using the PRISMA (Gambro, Hechingen, Germany) platform. CRRT was initiated at a blood flow rate of 100 mL/min, which was gradually increased to 150 mL/min. The ultrafiltration dose was prescribed as 35 mL/kg/hr, and Hemosol (Gambro) was replaced by predilution method. We also measured delivered drainage amount daily and recorded it to calculate delivered CRRT dose. CRRT circuits were exchanged, if the blood pump stopped. However, circuit-exchanges were conducted regularly after 48 hrs of use, even if the blood pumps were not stopped.
Statistical analysis
Statistical analysis was performed using SPSS for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean±standard deviation and categorical variables as numbers and percentages, if they were statistically normally distributed, and they were compared using Student's t-test for continuous variables and the χ2 test for categorical variables. However, medians and interquartile ranges are presented unless variables are clearly normally distributed, and they were compared using Mann-Whitney test. In the present study, we evaluated 28-day all-cause mortality as an endpoint.
Based on the SCT-processing, we divided these enrolled patients into two groups, a SCT group and a non-SCT group. Survival curves were designed using the Kaplan-Meier method, and comparisons were made using the log-rank test. The impact of SCT control on 28-day mortality in AKI patients treated with CRRT was determined using the Cox proportional hazards model, and the results were presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Especially, variables less than 0.1 of p-value in univariate analysis, including age and gender, were used for multivariate analysis. All tests were two-sided, and p<0.05 was considered significant.
RESULTS
Baseline characteristics
The baseline characteristics of these patients are shown in Table 3. The mean age was 62.1 years, and 351 patients (63.7%) were male. The most common comorbid disease was malignancy (52.1%), followed by hypertension (37.2%) and diabetes mellitus (29.8%). In 206 patients (37.4%), CRRT was started at the 'risk' stage of the risk, injury, failure, loss and end kidney disease (RIFLE) classification, and 287 patients (52.1%) were treated with CRRT due to sepsis.
Table 3. Baseline Characteristics at the Time of CRRT Start in AKI Patients Treated with CRRT.
| Variables | Total (n=551) | Non-SCT group (n=253) | SCT group (n=298) | p value |
|---|---|---|---|---|
| Demographic data | ||||
| Age (yrs) | 62.1±14.6 | 63.4±13.6 | 61.6±15.1 | 0.19 |
| Male (%) | 351 (63.7) | 150 (59.3) | 200 (67.1) | 0.11 |
| MAP (mm Hg) | 79.5±16.6 | 80.5±17.4 | 78.1±16.1 | 0.13 |
| APACHE II score | 27.3±7.7 | 28.6±8.4 | 27.1±7.0 | 0.06 |
| SOFA score | 12.3±3.1 | 12.4±3.0 | 12.3±3.2 | 0.91 |
| RIFLE, n (%) | 0.27 | |||
| Risk | 206 (37.4) | 86 (34.0) | 120 (40.3) | |
| Injury | 205 (37.2) | 104 (41.1) | 101 (33.9) | |
| Failure | 140 (25.4) | 63 (24.9) | 77 (25.8) | |
| Contributing factor, n (%) | 0.89 | |||
| Sepsis | 287 (52.1) | 129 (51.0) | 158 (53.0) | |
| Hemodynamic instability without sepsis | 177 (32.1) | 84 (33.2) | 93 (31.2) | |
| Major surgery | 87 (15.8) | 40 (15.8) | 47 (15.8) | |
| Use of anticoagulation (%) | 428 (77.7) | 203 (80.2) | 225 (75.5) | 0.21 |
| Biochemical data | ||||
| Hb (g/dL) | 9.2±1.8 | 9.2±1.8 | 9.2±1.8 | 0.98 |
| WBC (103/mm3) | 14.6±12.1 | 15.6±12.2 | 14.1±10.6 | 0.17 |
| Platelet (×103/mm3) | 141.0±115.5 | 135.1±113.9 | 144.5±116.5 | 0.40 |
| BUN (mg/dL) | 54.7±28.1 | 57.9±27.3 | 53.9±29.5 | 0.13 |
| Cr (mg/dL) | 3.5±2.1 | 3.4±1.9 | 3.5±2.3 | 0.68 |
| T. cholesterol (mg/dL) | 93.4±44.2 | 95.2±45.5 | 92.0±43.4 | 0.46 |
| Albumin (g/dL) | 2.7±0.6 | 2.6±0.6 | 2.7±0.6 | 0.11 |
| CRP (mg/dL) | 13.1±12.5 | 14.3±12.0 | 12.3±12.7 | 0.10 |
| Arterial pH | 7.3±0.3 | 7.4±0.1 | 7.3±0.4 | 0.18 |
| T. bilirubin (mg/dL) | 3.8±6.7 | 4.0±7.2 | 3.7±6.4 | 0.62 |
| HCO3- (mEq/L) | 19.5±5.3 | 19.9±4.9 | 19.2±5.5 | 0.15 |
| BMI | 23.0±5.3 | |||
| Age-adjust CCI | 5.8±2.3 |
CRRT, continuous renal replacement therapy; AKI, acute kidney injury; BMI, body mass index; RIFLE, risk, injury, failure, loss, and end kidney disease; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; CCI, Charlson Comorbidity Index; MAP, mean arterial pressure; Hb, hemoglobin; WBC, white blood cell; BUN, blood urea nitrogen; Cr, creatinine; CRP, C-reactive protein; SCT, specialized continuous renal replacement therapy team.
Data are n (%), mean±SD.
At the time of CRRT application, the mean APACHE II, SOFA score, and age-adjusted CCI were 27.3, 12.3, and 5.8, respectively. The mean hemoglobin (Hb) concentration was 9.2 g/dL, white blood cell count 14.6×103/mm3, platelet count 141.0×103/mm3, blood urea nitrogen level 54.7 mg/dL, and serum creatinine concentration 3.5 mg/dL. The mean total cholesterol level was 93.4 mg/dL, serum albumin concentration 2.7 g/dL, and C-reactive protein (CRP) level was 13.1 mg/dL, indicating that most patients were in inflammatory and undernourished conditions. In addition, arterial pH was 7.3, serum total bilirubin 3.8 mg/dL, serum sodium 137.8, potassium 4.3, and bicarbonate (HCO3-) was 19.5 mEq/L.
Comparisons of clinical outcomes between the two groups during follow-up duration
The total CRRT time, down-time per day, RBC-transfused numbers, and ICU length of days during CRRT treatment were significantly lower under SCT management compared to the group under non-SCT, while net ultrafiltration rate in SCT group was significantly higher than that in the non-SCT group. Moreover, the numbers of dialyzers used during 48 hrs in two groups for evaluating circuit failure was also significantly lower in SCT group than those in the non-SCT group (Table 4).
Table 4. Comparisons of CRRT Outcomes between Two Groups at the 28-Day Follow-Up.
| Non-SCT group (n=253) | SCT group (n=298) | p value | |
|---|---|---|---|
| Total CRRT time (days) | 7 (1-48) | 4 (1-33) | 0.033 |
| Down-time per day (hrs) | 5.1 (3.7-15.4) | 3.2 (2.8-5.9) | 0.002 |
| Ultrafiltration rate (mL/kg/hr) | 23.5 (20.5-28.0) | 27.9 (25.3-30.2) | 0.037 |
| Number of TF during CRRT | 9 (1-22) | 6 (1-13) | 0.011 |
| ICU length of day (days) | 21 (8-37) | 18 (8-26) | 0.037 |
| CRRT mortality (%) | 176 (69.5) | 165 (55.3) | 0.031 |
| Number of dialyzers during 48 hrs | 1.28 (1-2.4) | 1.87 (1-4) | 0.042 |
CRRT, continuous renal replacement therapy; non-SCT, conventional team approach; SCT, specialized CRRT team; TF, transfusion; ICU, intensive care unit.
Data are n (%), median (interquartile ranges).
SCT is independently associated with 28-day patients' survival
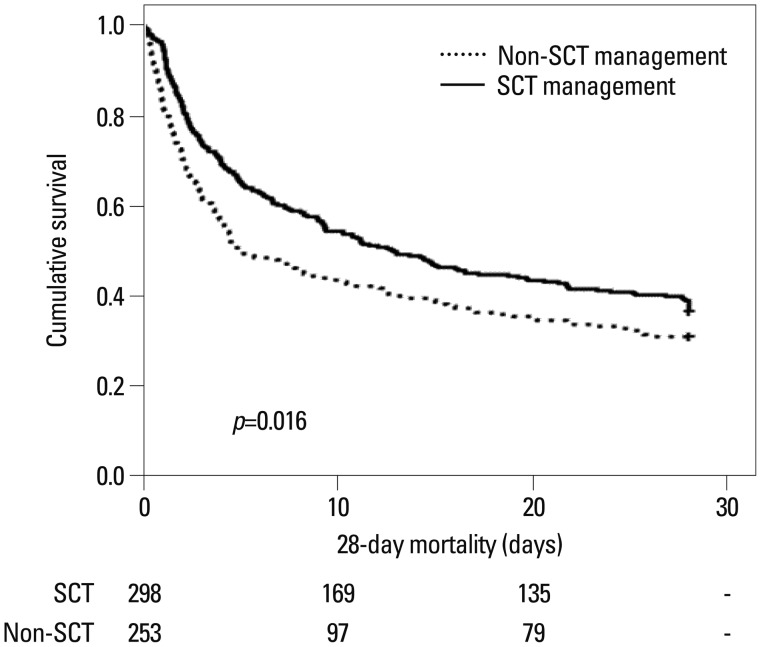
During the follow-up duration, 28-day all-cause mortality was significantly higher in the non-SCT group than in the SCT group (69.5% vs. 55.3%, p=0.031) (Table 4). In addition, Kaplan-Meier plots also showed that the 28-day all-cause mortality was significantly higher in the non-SCT group (p=0.016) (Fig. 2). Cox regression analysis revealed that the crude 28-day mortality rate was significantly lower in the SCT group than in the non-SCT group (HR: 0.77; 95% CI: 0.63-0.95; p=0.016) (Model 1). Moreover, the lower mortality rate in the SCT group remained significant even after adjusting for age, gender, mean arterial pressure, CRP level, and APACHE II, and SOFA scores (HR: 0.76; 95% CI: 0.58-0.98; p=0.039) (Model 2). Furthermore, additional adjustments for Hb, serum albumin, total cholesterol, and total bilirubin levels still revealed that SCT was significantly associated with reducing 28-day all-cause mortality (HR: 0.87; 95% CI: 0.66-0.92; p=0.041) (Model 3). Finally, we carried out further adjustments for RIFLE and contributing factors, however, they did not change the benefit for 28-day mortality of the SCT approach (HR: 0.91; 95% CI: 0.63-0.94; p=0.047) (Model 4) (Table 5).
Fig. 2. Kaplan-Meier plots for cumulative 28-day mortality. 28-day all-cause mortality rates after the SCT approach were significantly reduced (log rank p=0.016). non-SCT, conventional team approach; SCT, specialized continuous renal replacement therapy team.
Table 5. Cox Proportional Hazards Analysis for 28-Day Mortality Based on SCT.
| Compared with non-SCT management | |||
|---|---|---|---|
| HR | 95% CI | p value | |
| Model 1 | 0.77 | 0.63-0.95 | 0.016 |
| Model 2 | 0.76 | 0.58-0.98 | 0.039 |
| Model 3 | 0.87 | 0.66-0.92 | 0.041 |
| Model 4 | 0.91 | 0.69-0.94 | 0.046 |
SCT, specialized CRRT team; CRRT, continuous renal replacement therapy; non-SCT, conventional team approach; HR, hazard ratio; CI, confidence interval; MAP, mean arterial pressure; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; RIFLE, risk, injury, failure, loss, and end kidney disease.
Model 1: unadjusted relative risk. Model 2: adjusted for age, gender, MAP, APACHE II score and SOFA score, CRP. Model 3: adjusted for Model 2 plus, hemoglobin, albumin, eGFR, total cholesterol, total bilirubin. Model 4: adjusted for Model 3 plus RIFLE and contributing factors.
DISCUSSION
In this study, we found that the SCT approach for managing CRRT increased survival at 28-day intervals in AKI patients. Furthermore, all-cause mortality was decreased by 9.0% in the SCT group, in comparison with the non-SCT group (p=0.046). These findings suggest that well-trained CRRT team approaches may have beneficial effects on clinical outcomes in AKI patients treated with CRRT in the ICU. The down time per day and RBC-transfused number during CRRT treatment were significantly reduced after SCT management compared with non-SCT management, and these factors are considered to play important roles in improving clinical outcomes in AKI patients when treated with CRRT. The SCT activities contributing to improving these factors were increased specialty and rapidity in solving problems by well-trained members who could focus on CRRT related tasks seperated from ICU general care.
We considered that the most significant change after SCT approach was to add 5 CRRT nurses to CRRT management. CRRT nurses have to go through more educational programs for CRRT management (ex; CRRT kit changes, anticoagulation use, and ultrafiltration dose etc.) in addition to general ICU care (Table 1). Gilbert, et al.12 reported that CRRT program definitely enhanced the level of care of the patient by allowing the "bedside-nurse" to concentrate on general patient care (ex; turning, suctioning, medication administration, overall management and documentation) free from CRRT-related tasks. At the same time, the trained CRRT-nurse could entirely focus on the CRRT treatment, thereby providing an additional care and minimizing potential mistakes. Moreover, regular interval rounding by CRRT nurses made it possible to continuously access to patients' hemodynamic status and CRRT operating status, which could be beneficial for detection and solving problems promptly. Furthermore, they are also in charge for education of bed-side nurse for basic CRRT management. Besides addition of CRRT nurses, the SCT monthly meeting for quality control could make it possible to regularly check the problems related to CRRT management and discuss with each other. Moreover, such regular meeting and discussion could make CRRT-nurses more professional and skillful. Therefore, we infer that professional and well-organized training and regular feedback system could get better quality of CRRT management, and consequentially, might have an effect on favorable clinical outcomes.
CRRT has been preferred by patients with hemodynamic instability to control uremia, electrolyte, acid-base and volume balance in many ICUs.9 However, prolonged blood pump halting and prolonged manipulation time for the replacement of circuit can result in inadequate treatment doses and blood loss in patients.15,16 In this study, down time and numbers of blood transfusion were significantly lower than before SCT management. Because CRRT machines give a warning sign before complete circuit-stopping, CRRT circuit management is controlled properly, and therefore, circuit exchange originated from circuit failure is not necessarily required. Moreover, the CRRT machines are highly complicated and require operating skill. Consequently, there should be specialized approach for quick and accurate problem solving. Because well trained CRRT team with specialty and many experiences could more quickly and properly solve the problems such as circuit halt, vascular access related problems and mechanical errors, down time, blood loss and the number of circuit exchanges would be lower in SCT group than non-SCT group. In this study, circuit-exchanges were conducted regularly after 48 hrs of use, even if the blood pumps were not stopped. We investigated the numbers of dialyzers used during 48 hrs for evaluating circuit failure, and the result showed that the numbers of dialyzers used in SCT group was significantly lower than those in non-SCT groups [in SCT group: 1.56 (1-2.4), in non-SCT group: 1.98 (1-4); p=0.042]. Uchino, et al.14 pointed out that the term "continuous" in CRRT is somewhat inaccurate due to down-time developed through interruptions of CRRT treatment, and suggested 3.0 hrs of median down-time (1.0-8.3), and was 15.0 hrs of median filter life-time (8.9-26.1) per day. They also concluded that down-time adversely affects azotemia control, therefore, physicians who prescribe CRRT should be aware of the consequences of such down-time on the quality and quantity of renal replacement therapy delivered.14 Clinical experience indicated that circuit down-time is the main factor for whether proper cumulative ultrafiltration reaches the goal or not.14 In this study, the down-time per day was 3.2 hrs in the SCT group compared to 5.1 hrs in non-SCT group, with significant differences (p=0.002, respectively). As for the association with ultrafiltration doses and survival rate in patients treated with CRRT, some studies showed that intensive renal support of critically ill patients with AKI did not decrease the mortality,17,18,19 while others showed that increasing the ultrafiltration dose, especially for low molecular weight solutes removal, resulted in a better survival in severely ill patients with AKI.20 In randomized controlled trial of continuous venovenous hemodialysis (CVVH), Ronco, et al.21 found that patients who received at least 85% of the prescribed dose of hemofiltration showed mean measured CRRT dose significantly higher in SCT group than with the dose in non-SCT group (40200 mL/d vs. 33800 mL/d, p=0.032) and delivered ultrafiltration rate was 27.9 mL/kg/hr (79.7% of prescribed dose) in the SCT group, while 23.5 mL/kg/hr (67.1% of prescribed dose) in the non-SCT group with significant differences (p=0.037). Although there are still controversies about the optimal dose of CRRT in AKI patients, the minimal required dose is necessary for adequate solute control and the correction of electrolyte and acid base imbalance. We think that the difference of CRRT dose between two groups could be due to management of the circuit failure and exchange, and that SCT approach helped maintain delivered CRRT dose above the minimal dose and achieved the daily goal more effectively.
Our study has several potential limitations that should be noted. First, despite the fact that our results are based on a large number of samples, this study was a retrospective cohort study without a pre-specified hypothesis. So, the characteristics of these patients are quite heterogeneous and risk factors for mortality of patients requiring CRRT are frequently inter-related. Second, these two groups (non-SCT group vs. SCT group) were not conducted at the same time. However, the gap of the periods was less than 6 month, the same ICU policy was applied to patients at a single center, and the patients in this study experienced no differences in decision-making process because of same nephrologist and ICU specialists. Moreover, there were no remarkable differences in indications for CRRT, filter types, anticoagulation types and management between the two groups. Furthermore, as shown in Table 3, there were no significant differences in the baseline characteristics between the two groups, except for estimated glomerular filtration rate level. Therefore, the bias caused by the different time may be negligible. However, in regard with ICU clinical care practices, there would be differences between two groups, because of adding CRRT nurses and separating general care from CRRT related tasks. This was important change in SCT management which could have influences on clinical outcomes. Third, because there are diversities in physician factors, critical care programs, patient to nurse ratios and ICU systems, depending on centers, we are not certain whether identical result would be found in all other centers. Nevertheless, a major strength of this study is that it is based on a relatively large number of patients requiring CRRT.
In conclusion, a well-trained CRRT team may be beneficial for the management of AKI patients requiring CRRT treatment. However, additional multicenter studies are required to confirm these findings.
ACKNOWLEDGEMENTS
This work was supported by the Brain Korea 21 Project for Medical Science, Yonsei University, by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011-0030711), and by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (H10C2020).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Ahlström A, Tallgren M, Peltonen S, Räsänen P, Pettilä V. Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med. 2005;31:1222–1228. doi: 10.1007/s00134-005-2681-6. [DOI] [PubMed] [Google Scholar]
- 4.Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgera S, Kraft AK, Siebert G, Luft FC, Neumayer HH. Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis. 2002;40:275–279. doi: 10.1053/ajkd.2002.34505. [DOI] [PubMed] [Google Scholar]
- 6.Ostermann M, Chang RW. Correlation between parameters at initiation of renal replacement therapy and outcome in patients with acute kidney injury. Crit Care. 2009;13:R175. doi: 10.1186/cc8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lins RL, Elseviers MM, Van der Niepen P, Hoste E, Malbrain ML, Damas P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant. 2009;24:512–518. doi: 10.1093/ndt/gfn560. [DOI] [PubMed] [Google Scholar]
- 8.Allegretti AS, Steele DJ, David-Kasdan JA, Bajwa E, Niles JL, Bhan I. Continuous renal replacement therapy outcomes in acute kidney injury and end-stage renal disease: a cohort study. Crit Care. 2013;17:R109. doi: 10.1186/cc12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, et al. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 2007;33:1563–1570. doi: 10.1007/s00134-007-0754-4. [DOI] [PubMed] [Google Scholar]
- 10.Oh HJ, Park JT, Kim JK, Yoo DE, Kim SJ, Han SH, et al. Red blood cell distribution width is an independent predictor of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Nephrol Dial Transplant. 2012;27:589–594. doi: 10.1093/ndt/gfr307. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Ni H, Lu B. Variables associated with circuit life span in critically ill patients undergoing continuous renal replacement therapy: a prospective observational study. ASAIO J. 2012;58:46–50. doi: 10.1097/MAT.0b013e31823fdf20. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert RW, Caruso DM, Foster KN, Canulla MV, Nelson ML, Gilbert EA. Development of a continuous renal replacement program in critically ill patients. Am J Surg. 2002;184:526–532. doi: 10.1016/s0002-9610(02)01056-5. [DOI] [PubMed] [Google Scholar]
- 13.Meißner A, Schlenke P. Massive Bleeding and Massive Transfusion. Transfus Med Hemother. 2012;39:73–84. doi: 10.1159/000337250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchino S, Fealy N, Baldwin I, Morimatsu H, Bellomo R. Continuous is not continuous: the incidence and impact of circuit "down-time" on uraemic control during continuous veno-venous haemofiltration. Intensive Care Med. 2003;29:575–578. doi: 10.1007/s00134-003-1672-8. [DOI] [PubMed] [Google Scholar]
- 15.Webb AR, Mythen MG, Jacobson D, Mackie IJ. Maintaining blood flow in the extracorporeal circuit: haemostasis and anticoagulation. Intensive Care Med. 1995;21:84–93. doi: 10.1007/BF02425162. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin IC, Elderkin TD. Continuous hemofiltration: nursing perspectives in critical care. New Horiz. 1995;3:738–747. [PubMed] [Google Scholar]
- 17.Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol. 2008;19:1233–1238. doi: 10.1681/ASN.2007111173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VA/NIH Acute Renal Failure Trial Network. Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RENAL Replacement Therapy Study Investigators. Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 20.Saudan P, Niederberger M, De Seigneux S, Romand J, Pugin J, Perneger T, et al. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006;70:1312–1317. doi: 10.1038/sj.ki.5001705. [DOI] [PubMed] [Google Scholar]
- 21.Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]