Abstract
Purpose
The aim of this study was to evaluate the effects and safety of a sleep aid for postoperative analgesia in patients undergoing arthroscopic rotator cuff repair.
Materials and Methods
Seventy-eight patients were prospectively assigned to either the zolpidem group (multimodal analgesia+zolpidem; 39 patients) or the control group (multimodal analgesia; 39 patients). Self-rated pain levels were assessed twice a day using a visual analog scale (VAS). The need for additional rescue analgesic, duration of functional recovery, and adverse effects were assessed for the first 5 days after surgery.
Results
The mean number of times that additional rescue analgesic was required during 5 days after surgery was 2.1±2.0 in the zolpidem group and 3.3±2.8 in the control group, a significant difference. There were no significant differences between the two groups in mean VAS pain scores during the first 5 days after surgery, although the zolpidem group had lower VAS pain scores than the control group. Additionally, there were no significant differences in duration of functional recovery and adverse effects between the two groups.
Conclusion
The use of zolpidem for analgesia after arthroscopic rotator cuff repair provided a significant reduction in the need for rescue analgesic without increasing adverse effects. Nevertheless, mean VAS pain scores during the first 5 days after surgery did not differ between the zolpidem group and the control group.
Keywords: Rotator cuff, arthroscopic repair, postoperative analgesia, sleep aid, zolpidem
INTRODUCTION
Although arthroscopic rotator cuff repair is now moving from an inpatient setting to an outpatient setting,1 it is still associated with severe postoperative pain that can compromise effective rehabilitation and prolong hospital stay.2,3,4 Thus, the development of effective analgesia for early postoperative pain after rotator cuff repair is a critical and challenging issue for both surgeon and anesthesiologist.
Various methods, including injection or infusion of local analgesic, regional nerve block, intravenous patient-controlled analgesia, and multimodal analgesia have been proposed to effectively reduce early postoperative pain after arthroscopic rotator cuff repair.1,2,5,6,7,8,9,10,11 However, patients who undergo rotator cuff repair may report sleep disturbance, as well as moderate to severe pain, in the early postoperative period. Postoperative pain, especially at night, disturbs sleep, which can lead to a vicious cycle of acute pain, anxiety, and additional sleep deprivation.1,12 Rosenberg, et al.13 wrote that postoperative sleep disturbance represents an important research field because inadequate pain control and sleep disturbance in the early postoperative period have a negative impact on health-related quality of life and can adversely affect the outcomes of surgery.
Zolpidem is a short-acting nonbenzodiazepine hypnotic drug that has demonstrated efficacy in the treatment of insomnia in elderly patients, as well as in the general population.14,15,16 Its main action is sedation, although weak anticonvulsive, anxiolytic, and myorelaxant effects have been also demonstrated in human and animal studies.16,17,18 Recently, studies of its effects on pain control and sleep disturbance in the early postoperative period have demonstrated that zolpidem can reduce postoperative pain and the amount of narcotics required after knee surgery.17,18 To our knowledge, there has been no study of the use of a sleep aid as part of a postoperative pain-control regimen in patients undergoing rotator cuff repair. Therefore, the aim of the study was to evaluate the effects and safety of a sleep aid for analgesia after rotator cuff repair. Our hypothesis was that use of zolpidem would reduce requirements for rescue analgesia without significant adverse effects in patients who underwent arthroscopic rotator cuff repair.
MATERIALS AND METHODS
Our study was approved by our Institutional Review Board and written informed consent was obtained from all participants. Seventy-eight consecutive patients scheduled for arthroscopic rotator cuff repair were eligible for the study. The inclusion criteria were 1) undergoing arthroscopic rotator cuff repair without any additional procedures, such as biceps tenodesis, repair of a superior labrum anterior-to-posterior tear, or distal clavicle resection; 2) an American Society of Anesthesiologists (ASA) physical status of 1 or 2 (normal or patient with mild systemic disease); and 3) the ability to understand and cooperate with the study protocol. Exclusion criteria were 1) the presence of a massive rotator cuff tear (>5 cm), 2) an ASA physical status ≥3 (patient with severe systemic disease), 3) a history of drug addiction, 4) a severe neurological lesion, and 5) an allergy to any medication or local anesthetics used in the study.
Patients were sequentially assigned to receive either multimodal analgesia with zolpidem (zolpidem group; 39 patients) or multimodal analgesia only (control group; 39 patients) for postoperative pain control after rotator cuff repair. All patients received general anesthesia combined with an interscalene block according to our standard protocol, and all operations were performed by a single surgeon who used an arthroscopic repair technique. For postoperative pain control in both groups, we followed a multimodal pain control protocol that was developed at our institution. The protocol included preemptive oral medication (controlled-release oxycodone HCl, acetaminophen), regional nerve block (interscalene block), intra-operative injection of local analgesics (morphine HCl, 0.75% ropivacaine), and postoperative oral medication (postoperative day 0 to 2: immediate-release oxycodone HCl, acetaminophen, and cyclooxygenase-2 inhibitor; postoperative day 3 to 5: cyclooxtygenase-2 inhibitor plus a tablet containing a combination of 37.5 mg of tramadol and 325 mg of acetaminophen). If a patient required additional pain control beyond that provided by our protocol, 2 mL of intramuscular diclofenac was administered as a rescue analgesic.
The zolpidem group received a prescription for 10 mg of zolpidem (for those younger than 60 years) or 5 mg of zolpidem (for those aged 60 years or older) that was given orally immediately before bedtime for the first 5 days after surgery. Study participants rated their pain themselves before surgery, immediately after surgery (at 3 and 10 hours), and on days 1 through 5 after surgery (6 AM and 6 PM) using a visual analog scale (VAS) that ranged from 0 (no pain) to 10 points (unbearable pain). The duration of functional recovery was defined as the number of days until patients could achieve 120° of flexion and 30° of external rotation. The number of times that rescue analgesic was administered and adverse effects, such as nausea, vomiting, urinary retention, constipation, diarrhea, dizziness, urticaria, headache, respiratory depression, and drowsiness, were recorded.
The primary outcome variable was the number of times that additional rescue analgesic was administered during the 5 days immediately after surgery. The secondary outcome variables were twice-daily VAS pain scores and adverse effects throughout the first 5 days after surgery.
Statistical analysis
Sample size was calculated using the number of times additional rescue analgesic was administered as the primary outcome variable. The number of patients required to achieve 90% power (α error of 0.05 and β error of 0.10) and to detect an effect size of 0.7 was a minimum of 36 patients for each group.
Statistical analysis was performed using SPSS software (version 20.0E; IBM, Armonk, NY, USA). Baseline and preoperative characteristics were analyzed using the independent t-test and the chi-square test. The rescue analgesic requirements, VAS pain scores, and adverse effects were compared using the independent t-test, the chi-square test, Fisher's exact test, and repeated-measures analysis of variance to assess the significance of difference between the two groups. All p-values<0.05 were considered statistically significant.
RESULTS
There were no significant differences between the two groups in baseline and preoperative characteristics, such as age, sex, body mass index, ASA physical status, involved side, tear size, duration of symptoms, preoperative stiffness, and mean preoperative VAS pain score (Table 1).
Table 1. Baseline and Preoperative Characteristics of the Patients.
| Parameter | Zolpidem group | Control group | p value |
|---|---|---|---|
| Age (yrs) | 59.6±7.4 | 59.0±8.7 | 0.323 |
| Gender (male:female) | 13:26 | 10:29 | 0.456 |
| Body mass index (kg/m2) | 24.2±3.3 | 23.5±2.9 | 0.329 |
| ASA physical status (1:2) | 20:19 | 27:12 | 0.105 |
| Involved side (dominant:non-dominant) | 31:8 | 26:13 | 0.202 |
| Tear size (no. of cases) | 0.951 | ||
| Partial | 8 | 10 | |
| Small | 9 | 9 | |
| Medium | 16 | 15 | |
| Large | 6 | 5 | |
| Duration of symptoms | 18.3±26.4 | 28.2±35.7 | 0.171 |
| Preoperative stiffness (no. of cases) | 7 | 6 | 0.761 |
| Preoperative VAS pain score | 6.7±2.1 | 6.4±2.3 | 0.507 |
ASA, American Society of Anesthesiologists; VAS, visual analog scale.
The mean number of times that rescue analgesic was administered during the first 5 days after surgery was a mean of 2.1±2.0 times in the zolpidem group and 3.3±2.8 times in the control group (Table 2), a significant difference (p=0.033).
Table 2. Rescue Analgesic Requirement and Comparison of Functional Recovery between the Two Groups.
| Parameter | Zolpidem group | Control group | p value |
|---|---|---|---|
| Rescue analgesic (no. of times) | 2.1±2.0 | 3.3±2.8 | 0.033 |
| Functional recovery (days) | 3.8±1.8 | 3.8±1.4 | 0.890 |
Statistical significance (p<0.05).
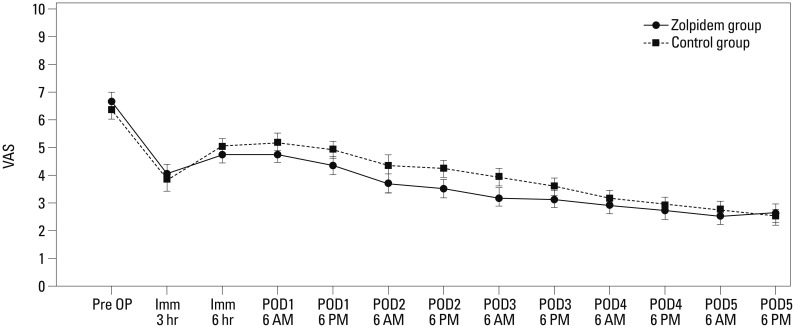
Mean VAS pain scores at 3 and 10 hours after surgery were, respectively, 4.1±2.7 and 4.8±2.4 in the zolpidem group and 3.9±2.3 and 5.0±2.0 in the control group. Mean VAS pain scores on days 1 through 5 after surgery, assessed twice a day at 6 AM and 6 PM, respectively, were 4.8±2.4 and 4.4±2.0 (day 1), 3.7±2.1 and 3.5±1.8 (day 2), 3.2±1.8 and 3.1±1.8 (day 3), 2.9±1.6 and 2.7±1.5 (day 4), and 2.5±1.4 and 2.6±1.5 (day 5) in the zolpidem group and were 5.2±1.8 and 4.9±1.8 (day 1), 4.3±1.5 and 4.2±1.7 (day 2), 3.9±1.6 and 3.6±1.5 (day 3), 3.2±1.2 and 2.9±1.3 (day 4), and 2.7±1.3 and 2.5±1.2 (day 5) in the control group, representing no significant difference between the two groups (Fig. 1).
Fig. 1. Mean visual analog scale (VAS) pain scores measured before surgery, immediately after surgery (at 3 and 10 hours), and on days 1 to 5 after surgery (at 6 AM and 6 PM). There were no significant differences between the two groups in VAS pain scores for the first 5 days after surgery, although the zolpidem group had lower VAS pain scores than the control group. POD, postoperative day.
The average duration of functional recovery was 3.8±1.8 days in the zolpidem group and 3.8±1.4 days in the control group, not a significant difference. In addition, there were no significant differences between the two groups in regards to medication-related adverse effects, including nausea, vomiting, urinary retention, constipation, diarrhea, dizziness, urticaria, headache, respiratory depression, or drowsiness (Table 3).
Table 3. Side Effects Related to Medication.
| Adverse effect | n (%) | p value | |
|---|---|---|---|
| Zolpidem group | MPC group | ||
| Nausea | 15 (38.5) | 14 (35.9) | 0.815 |
| Vomiting | 6 (15.4) | 4 (10.3) | 0.737 |
| Urinary retention | 3 (7.7) | 2 (6.7) | 1.000 |
| Constipation | 11 (28.2) | 17 (43.6) | 0.157 |
| Diarrhea | 2 (6.7) | 0 (0.0) | 0.494 |
| Dizziness | 5 (12.8) | 4 (10.3) | 1.000 |
| Urticaria | 1 (2.6) | 2 (6.7) | 1.000 |
| Headache | 1 (2.6) | 2 (6.7) | 1.000 |
| Respiratory depression | 0 (0.0) | 0 (0.0) | 1.000 |
| Drowsiness | 0 (0.0) | 0 (0.0) | 1.000 |
DISCUSSION
The current study revealed that the use of zolpidem for analgesia after arthroscopic rotator cuff repair provides a significant reduction in the need for rescue analgesic without increasing adverse effects. However, VAS pain scores during the first 5 days after surgery did not reach significant difference between the zolpidem group and the control group.
Rotator cuff repair is well recognized as having the potential to cause severe postoperative pain.2,4 Although arthroscopic rotator cuff repair is minimally invasive, severe pain during the first several days after surgery is common.2,6 Various methods, including injection or infusion of local analgesic, regional nerve block, and intravenous patient-controlled analgesia, have been proposed to effectively reduce postoperative pain.1,2,5,9,11 However, all of these techniques involve their own particular risks, limitations, and adverse effects.1,2,9,10 Oh, et al.11 reported that aggressive pain management in the early postoperative period is a major issue for patients who pursue "well-being" and satisfaction with the treatment of their disorders. The evidence presented in many studies clearly indicate the necessity of developing more effective analgesic strategies for patients with rotator cuff repair, especially in ambulatory surgery.
Multimodal analgesia is an increasingly popular approach to preventing postoperative pain.8 It involves administering a combination of opioid and nonopioid analgesics before, during, and after surgery that act at different sites within the central and peripheral nervous systems in an effort to improve pain control while eliminating opioid-related adverse effects.3,7,8 Cho, et al.6 demonstrated that multimodal pain control offers more effective postoperative pain control with fewer adverse effects than intravenous patient-controlled analgesia. However, they also noted that achieving adequate pain control within the first 48 hours after surgery remains challenging and that development of more effective and safer multimodal pain control protocols is required.
It is well known that patients may experience problems with pain management and sleep at night in the early postoperative period.17,19,20,21,22 Also, it is not uncommon for patients to take more pain medication at night to get to sleep.17 Patients who undergo rotator cuff repair are especially likely to complain of sleep disturbance, as well as moderate to severe pain, in the early postoperative period. Postoperative pain, especially at night, disturbs sleep, which can lead to a vicious cycle of acute pain, anxiety, and additional sleep deprivation.1,12 Furthermore, sleep deprivation has been shown to be associated with increased sensitivity to pain.12 Reportedly, inappropriate pain control and sleep disturbance in the early postoperative period have a negative impact on health-related quality of life and can adversely affect postoperative outcomes.22,23
Kain and Caldwell-Andrews20 reported that a significant number of patients who undergo outpatient elective surgery experience postoperative sleep disturbance and that sleep problems cannot be solely attributed to the influence of postoperative pain. It may be that other factors, such as anxiety or opioid medications, play a major role in the etiology of postoperative sleep problems.20,21,23 We believe this suggests the appropriateness of using a sleep aid to act as a sedative, anxiolytic, and myorelaxant for both postoperative pain control and improved sleep after surgery. Accordingly, our study demonstrated the effectiveness and safety of using a sleep aid for postoperative analgesia in patients undergoing arthroscopic rotator cuff repair.
Zolpidem is a short-acting nonbenzodiazepine hypnotic drug and has been widely used as a sleep aid in the elderly, as well as in the general population.3,6,14 Zolpidem is known to have sedative, anticonvulsant, anxiolytic, and some myorelaxant properties.16,17 There are no known analgesic properties of the medication.17 Recently, its effects on pain control after orthopedic surgery have been studied.17,18 Tompkins, et al.18 studied the effect of zolpidem in a multimodal approach to pain control after anterior cruciate ligament reconstruction and demonstrated that zolpidem helps to lower the amount of narcotic pain medication required for adequate analgesia, although there were no significant differences in postoperative pain and fatigue levels between their zolpidem and placebo groups. Our findings are consistent with those of Tompkins, et al.18 In our patients, the use of zolpidem for analgesia after arthroscopic rotator cuff repair significantly reduced the need for rescue analgesics. However, VAS pain scores in the first 5 days after surgery did not differ between our two groups. It is our opinion that the results that we saw from zolpidem were due not to any analgesic action but instead to the drug's sedative, anti-anxiolytic, or myorelaxant actions.
The most common adverse effects reported for the short-term use of zolpidem are nausea, dizziness, drowsiness, and diarrhea.16,18 Other effects, including an increased risk of hip fracture, dependence, tolerance, and seizure, have also been reported, although rarely.24,25,26 The current study discovered no significant differences in adverse effects between the zolpidem and control groups, although our results may not be generalizable because our sample size was relatively small for comparing adverse effects between groups. Thus, clinicians must consider the potential adverse effects if they prescribe zolpidem in this setting.
Our study has several limitations. First, there was no sample randomization because study participants of each group were enrolled consecutively. Second, this was not a blinded study using a control group with placebo. Third, the number of study participants was too small to determine the incidence of medication-related adverse effects. Fourth, there was no assessment of sleep status using a measurement tool in the early postoperative period. Fifth, use of different dosages between patients of ages under or over 60 years in the zolpidem group was a bias. Additional randomized controlled trials with large sample sizes are necessary to clarify the characteristics and relationships of postoperative sleep disturbance and to determine the effects and safety of sleep aids in patients undergoing rotator cuff repair. Further efforts should be made to develop effective strategies for prevention and treatment of pain and sleep disturbance after rotator cuff repair.
Sleep aids may be considered a useful part of a multimodal pain control regimen after orthopedic surgery, especially outpatient arthroscopic rotator cuff repair. Herein, the use of zolpidem for analgesia after arthroscopic rotator cuff repair provided a significant reduction in the need for rescue analgesic without increasing adverse effects. Nevertheless, mean VAS pain scores during the first 5 days after surgery did not differ between the zolpidem group and the control group.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Ruiz-Suarez M, Barber FA. Postoperative pain control after shoulder arthroscopy. Orthopedics. 2008;31:1130. doi: 10.3928/01477447-20081101-25. [DOI] [PubMed] [Google Scholar]
- 2.Boss AP, Maurer T, Seiler S, Aeschbach A, Hintermann B, Strebel S. Continuous subacromial bupivacaine infusion for postoperative analgesia after open acromioplasty and rotator cuff repair: preliminary results. J Shoulder Elbow Surg. 2004;13:630–634. doi: 10.1016/j.jse.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Chauvin M. State of the art of pain treatment following ambulatory surgery. Eur J Anaesthesiol Suppl. 2003;28:3–6. [PubMed] [Google Scholar]
- 4.Chung F, Ritchie E, Su J. Postoperative pain in ambulatory surgery. Anesth Analg. 1997;85:808–816. doi: 10.1097/00000539-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Bishop JY, Sprague M, Gelber J, Krol M, Rosenblatt MA, Gladstone J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87:974–979. doi: 10.2106/JBJS.D.02003. [DOI] [PubMed] [Google Scholar]
- 6.Cho CH, Song KS, Min BW, Lee KJ, Ha E, Lee YC, et al. Multimodal approach to postoperative pain control in patients undergoing rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2011;19:1744–1748. doi: 10.1007/s00167-010-1294-y. [DOI] [PubMed] [Google Scholar]
- 7.Dorr LD, Raya J, Long WT, Boutary M, Sirianni LE. Multimodal analgesia without parenteral narcotics for total knee arthroplasty. J Arthroplasty. 2008;23:502–508. doi: 10.1016/j.arth.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Elvir-Lazo OL, White PF. Postoperative pain management after ambulatory surgery: role of multimodal analgesia. Anesthesiol Clin. 2010;28:217–224. doi: 10.1016/j.anclin.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Filos KS, Lehmann KA. Current concepts and practice in postoperative pain management: need for a change? Eur Surg Res. 1999;31:97–107. doi: 10.1159/000008627. [DOI] [PubMed] [Google Scholar]
- 10.Fredrickson MJ, Krishnan S, Chen CY. Postoperative analgesia for shoulder surgery: a critical appraisal and review of current techniques. Anaesthesia. 2010;65:608–624. doi: 10.1111/j.1365-2044.2009.06231.x. [DOI] [PubMed] [Google Scholar]
- 11.Oh JH, Rhee KY, Kim SH, Lee PB, Lee JW, Lee SJ. Comparison of analgesic efficacy between single interscalene block combined with a continuous intra-bursal infusion of ropivacaine and continuous interscalene block after arthroscopic rotator cuff repair. Clin Orthop Surg. 2009;1:48–53. doi: 10.4055/cios.2009.1.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakki Onen S, Alloui A, Jourdan D, Eschalier A, Dubray C. Effects of rapid eye movement (REM) sleep deprivation on pain sensitivity in the rat. Brain Res. 2001;900:261–267. doi: 10.1016/s0006-8993(01)02320-4. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg J, Rosenberg-Adamsen S, Kehlet H. Post-operative sleep disturbance: causes, factors and effects on outcome. Eur J Anaesthesiol Suppl. 1995;10:28–30. [PubMed] [Google Scholar]
- 14.Cotroneo A, Gareri P, Nicoletti N, Lacava R, Grassone D, Maina E, et al. Effectiveness and safety of hypnotic drugs in the treatment of insomnia in over 70-year old people. Arch Gerontol Geriatr. 2007;44(Suppl 1):121–124. doi: 10.1016/j.archger.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Hindmarch I, Legangneux E, Stanley N, Emegbo S, Dawson J. A double-blind, placebo-controlled investigation of the residual psychomotor and cognitive effects of zolpidem-MR in healthy elderly volunteers. Br J Clin Pharmacol. 2006;62:538–545. doi: 10.1111/j.1365-2125.2006.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs. 2000;59:865–889. doi: 10.2165/00003495-200059040-00014. [DOI] [PubMed] [Google Scholar]
- 17.Tashjian RZ, Banerjee R, Bradley MP, Alford W, Fadale PD. Zolpidem reduces postoperative pain, fatigue, and narcotic consumption following knee arthroscopy: a prospective randomized placebo-controlled double-blinded study. J Knee Surg. 2006;19:105–111. doi: 10.1055/s-0030-1248088. [DOI] [PubMed] [Google Scholar]
- 18.Tompkins M, Plante M, Monchik K, Fleming B, Fadale P. The use of a non-benzodiazepine hypnotic sleep-aid (Zolpidem) in patients undergoing ACL reconstruction: a randomized controlled clinical trial. Knee Surg Sports Traumatol Arthrosc. 2011;19:787–791. doi: 10.1007/s00167-010-1368-x. [DOI] [PubMed] [Google Scholar]
- 19.Büyükyilmaz FE, Şendir M, Acaroğlu R. Evaluation of night-time pain characteristics and quality of sleep in postoperative Turkish orthopedic patients. Clin Nurs Res. 2011;20:326–342. doi: 10.1177/1054773811406110. [DOI] [PubMed] [Google Scholar]
- 20.Kain ZN, Caldwell-Andrews AA. Sleeping characteristics of adults undergoing outpatient elective surgery: a cohort study. J Clin Anesth. 2003;15:505–509. doi: 10.1016/j.jclinane.2003.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Krenk L, Jennum P, Kehlet H. Sleep disturbances after fast-track hip and knee arthroplasty. Br J Anaesth. 2012;109:769–775. doi: 10.1093/bja/aes252. [DOI] [PubMed] [Google Scholar]
- 22.Wylde V, Rooker J, Halliday L, Blom A. Acute postoperative pain at rest after hip and knee arthroplasty: severity, sensory qualities and impact on sleep. Orthop Traumatol Surg Res. 2011;97:139–144. doi: 10.1016/j.otsr.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Onen SH, Onen F, Courpron P, Dubray C. How pain and analgesics disturb sleep. Clin J Pain. 2005;21:422–431. doi: 10.1097/01.ajp.0000129757.31856.f7. [DOI] [PubMed] [Google Scholar]
- 24.Cubała WJ, Landowski J. Seizure following sudden zolpidem withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:539–540. doi: 10.1016/j.pnpbp.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Huang MC, Lin HY, Chen CH. Dependence on zolpidem. Psychiatry Clin Neurosci. 2007;61:207–208. doi: 10.1111/j.1440-1819.2007.01644.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Zolpidem use and hip fractures in older people. J Am Geriatr Soc. 2001;49:1685–1690. doi: 10.1111/j.1532-5415.2001.49280.x. [DOI] [PubMed] [Google Scholar]