Abstract
Purpose
Decision to perform concurrent ipsilateral thyroidectomy on patients with hypopharyngeal cancer is important, and unnecessary thyroidectomy should be avoided if oncologically feasible. We hypothesized that concurrent ipsilateral thyroidectomy is not routinely required to prevent occult metastasis. This study aimed to determine the prevalence of histological thyroid invasion in patients with hypopharyngeal cancer, and to refine the indications for prophylactic ipsilateral thyroidectomy in patients with hypopharyngeal cancer.
Materials and Methods
A retrospective review of the medical records from the Department of Otolaryngology at Yonsei University College of Medicine was conducted from January 1994 to December 2009. A total of 49 patients underwent laryngopharyngectomy with thyroidectomy as a primary treatment of hypopharyngeal cancer.
Results
The incidence of thyroid gland involvement was 10.2%. The most common route of invasion was direct extension through the thyroid cartilage. Thyroid cartilage invasion (p=0.034) was the most significant factor associated with thyroid invasion. Disease-specific survival at 5 years was lower in patients with than without thyroid gland invasion (26.7% vs. 55.2%, respectively; p=0.032). Disease-free survival at 5 years was also lower in patients with than without thyroid gland invasion (20.0% vs. 52.1%, respectively; p=0.024).
Conclusion
Ipsilateral thyroidectomy in combination with total laryngopharyngectomy is indicated when invasion of the thyroid cartilage is suspected in patients with hypopharyngeal cancer.
Keywords: Hypopharyngeal cancer, total laryngopharyngectomy, thyroidectomy, thyroid cartilage invasion
INTRODUCTION
Extralaryngeal spread of laryngopharyngeal cancer to the thyroid gland can theoretically occur by three pathways: direct extension, lymphatic spread, or hematogenous spread. Of these three mechanisms, direct extension is the main mechanism because of the close anatomical relationship of the thyroid gland to the laryngopharyngeal region.1,2,3 Ipsilateral hemithyroidectomy and isthmectomy are commonly performed as part of the total laryngectomy or laryngopharyngectomy procedure to facilitate resection of clinically occult metastases of laryngopharyngeal cancer.4 There is no controversy regarding the performance of thyroidectomy during laryngectomy when thyroid gland invasion has been confirmed during the preoperative evaluation.5,6,7 However, the questions of whether to perform thyroidectomy and the optimal extent of thyroidectomy (total or hemithyroidectomy) in patients with advanced laryngopharyngeal carcinoma without definite thyroid gland invasion remain controversial.4,5
Many studies have evaluated the role of thyroidectomy in advanced laryngeal and pharyngolaryngeal carcinoma.3,4,8 However, most of these studies focused mainly on laryngeal cancer.4,5 Although laryngeal and hypopharyngeal carcinomas have many similarities, including their etiology, clinical presentation, and surgical treatment, hypopharyngeal cancer differs from laryngeal cancer with respect to the comparative lack of anatomic boundaries of neighboring structures that are as limiting as those around the larynx.5 Thus, functional disturbances are not seen until the disease is advanced; moreover, the disease is frequently detected in its advanced stages because of the relative paucity of initial symptoms.9 Because this area also contains richer lymphatic drainage channels than does the larynx, regional metastasis (such as metastasis to the neck lymph nodes, thyroid cartilage, and thyroid gland) is not an uncommon finding, especially in advanced stages of the disease.7,10 Furthermore, hypopharyngeal cancer exhibits a high association with heavy alcohol use and other systemic disorders; thus, morbidity in the postoperative period is more frequent in patients with hypopharyngeal cancer than in patients with laryngeal cancer.6 Therefore, it is more critical for the surgeon to decide whether to resect the thyroid during laryngopharyngectomy.9,11 However, to the best of our knowledge, few studies have examined the effect of concurrent thyroidectomy during total laryngopharyngectomy in patients with hypopharyngeal cancer.4,5
The aims of this study were to determine the incidence of thyroid gland invasion in a cohort of patients with hypopharyngeal cancer, and to evaluate the preoperative factors predicting thyroid gland involvement.
MATERIALS AND METHODS
We reviewed 49 patients who were diagnosed with hypopharyngeal squamous cell carcinoma and treated by thyroidectomy with total laryngopharyngectomy at the Department of Otolaryngology at Yonsei University College of Medicine from January 1994 to December 2009. Patients were excluded from the study if they had a pathological diagnosis other than primary hypopharyngeal cancer or thyroid cancer, were previously treated with chemoradiation or salvage surgery, had distant metastatic disease, or had incomplete medical records.
The study involved 43 men and 6 women with a mean age of 61.5 years (range, 37-83 years). The minimum follow-up period was 2 years. All patients underwent total laryngopharyngectomy by one experienced head and neck surgeon; 13 patients also underwent upper esophagectomy. Of the 49 patients, 2 with suspected multifocal thyroid gland invasion (1 was false-positive) underwent total thyroidectomy, and 47 underwent hemithyroidectomy with isthmectomy. The indications for thyroidectomy as a part of total laryngopharyngectomy included advanced-stage disease, subglottic extension, thyroid cartilage erosion, and thyroid gland invasion on preoperative evaluation. Before surgery, all patients underwent upper aerodigestive endoscopy with biopsy by one experienced otolaryngologist and preoperative contrast-enhanced head and neck CT and chest CT by one experienced radiologist. The clinical stage was determined according to the 7th edition of the American Joint Committee on Cancer Staging (AJCC). One patient was staged as I, 4 as II, 10 as III, and 34 as IV.
The preoperative clinical endoscopic findings (including the anatomic lesion of the primary cancer, vocal cord mobility, and laryngeal invasion) and preoperative imaging findings (including invasion of the thyroid cartilage, cricoid cartilage, thyroid gland, esophagus, and larynx) are included in the analysis. In the pathologic study, approximately 40 coronal-plane sections at 1-mm intervals were analyzed with hematoxylin-eosin staining by an attending pathologist. We examined the correlation between thyroid involvement and the endoscopic and radiologic findings. In addition, we examined the patterns of the spread of thyroid invasion (direct, lymphatic, or hematogenous). Finally, we evaluated the prognosis of thyroid invasion in terms of the 2- and 5-year disease-specific survival (DSS) and disease-free survival (DFS) rates.
Statistical analysis was performed by the chi-squared test or binary logistic regression test. A p value of <0.05 indicated statistical significance. Kaplan-Meier analysis was performed to evaluate the survival rate according to thyroid invasion using SPSS v.18.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Five (10.2%) of 49 patients exhibited histological signs of thyroid gland invasion, and all patients were determined to have stage IV disease. The clinical characteristics of five patients are listed in Table 1. Of these five patients, the primary site of the mass was the pyriform sinus (PS) in four and the post-cricoid region in one.
Table 1. Clinical Characteristics of the Patients with Thyroid Gland Invasion.
| No. | Sex/age | Primary site | Stage | Operation | Treatment modality | Recur | Recur gap (M) | Outcome | F/U (M) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/64 | PS (l) | T4aN2b | T/L+P/P+ND+hemiT | S+R | - | DIOD | 20 | |
| 2 | M/58 | PS (m) | T4aN2c | T/L+P/P+P/E+ND+hemiT | S+R | - | NED | 193 | |
| 3 | M/63 | PS (m) | T4aN2c | T/L+P/P+ND+hemiT | S+R | Lung | 11 | DOD | 38 |
| 4 | M/56 | PS (m) | T4aN2c | T/L+P/P+ND+hemiT | S+R | Lung | 19 | DOD | 49 |
| 5 | M/56 | Post-cricoid | T2N2b | T/L+P/P+ND+totalT | S+R | Neck, lung | 13 | AWD | 32 |
ND, neck dissection; T/L, total laryngectomy; P/P, partial pharyngectomy; P/E, partial esophagectomy; hemiT, hemi-thyroidectomy; totalT, total-thyroidectomy; S, surgery; R, radiotherapy; AWD, alive with disease; DOD, dead of disease; DIOD, dead irrelevant of disease; NED, no evidence of disease; F/U, follow up.
PS (m), Pyriform sinus medial wall; PS (l), Pyriform sinus lateral wall.
We divided the possible predictive factors into two groups: endoscopic signs (Table 2) and CT/MRI findings (Table 3). Laryngeal invasion, extension to the PS apex, and ipsilateral vocal cord palsy were included. Tumors invading the thyroid gland also invaded the PS apex (80%), thyroid cartilage (80%), larynx (80%), and ipsilateral true vocal cord (60%). No patients exhibited crossing of the midline or extension to the esophagus. We next evaluated clinical factors such as the clinical T and N stages (p=0.511 and 0.15), thyroid invasion on CT/MRI findings (p=0.005), thyroid cartilage invasion (p=0.034), and midline crossing (p=0.17). Our data indicated that histological thyroid gland invasion was statistically significantly correlated with CT/MRI signs of thyroid cartilage invasion (p=0.034) and thyroid gland invasion (p=0.005) in univariate analysis. Multivariate analysis revealed significant associations (p<0.05) between histologic thyroid gland involvement and thyroid cartilage or thyroid gland invasion on CT/MRI.
Table 2. Independent Endoscopic Correlates of Thyroid Gland Invasion.
| Preoperative endoscopy | Postoperative histology | p value | Se (%) | Sp (%) | |
|---|---|---|---|---|---|
| Thyroid gland invasion | No thyroid gland invasion | ||||
| Larynx invasion | |||||
| + | 4 | 29 | 0.82 | ||
| Supraglottic | 3 | 22 | |||
| Transglottic | 1 | 7 | |||
| - | 1 | 15 | |||
| Invasion of PS apex | |||||
| + | 4 | 16 | 0.06 | 80.0 | 60.0 |
| - | 1 | 28 | |||
| Ipsilateral VC fixation | |||||
| + | 3 | 23 | 0.56 | ||
| - | 2 | 21 | |||
Se, sensitivity; Sp, specificity; PS, pyriform sinus; VC, vocal cord.
Table 3. Independent Preoperative CT/MRI Correlates of Thyroid Gland Invasion.
| Preoperative CT/MRI | Postoperative histology | p value | Se (%) | Sp (%) | |
|---|---|---|---|---|---|
| Thyroid gland invasion | No thyroid gland invasion | ||||
| cT | |||||
| T1/T2 | 1 | 14 | 0.511 | ||
| T3/T4 | 4 | 30 | |||
| cN | |||||
| N0/N1 | 0 | 15 | 0.15 | ||
| N2/N3 | 5 | 29 | |||
| Thyroid gland invasion | |||||
| + | 3 | 2 | 0.005 | 60.0 | 95.5 |
| - | 2 | 42 | |||
| Thyroid cartilage invasion | |||||
| + | 4 | 12 | 0.034 | 80.0 | 72.7 |
| - | 1 | 32 | |||
| Midline Cross | |||||
| + | 0 | 14 | 0.17 | ||
| - | 5 | 30 | |||
| Esophagus | |||||
| + | 0 | 4 | 0.64 | ||
| - | 5 | 40 | |||
Se, sensitivity; Sp, specificity.
The most common mechanism of invasion to the thyroid gland was direct extension through the thyroid cartilage and the anterior commissure (four of five patients) (Fig. 1). One of five patients with thyroid gland invasion had lymphatic and multifocal spread with the presence of tumor emboli within the perithyroidal lymphatics, situated in areas that were not approximate to the site of the primary cancer (Fig. 2, Table 4).
Fig. 1. Direct thyroid gland invasion of hypopharyngeal cancer. (A) Thyroid cartilage (ThyC) destruction is noted on contrast-enhanced neck CT (thick arrow). Thyroid gland invasion by tumor extension (thin arrows) through the area of thyroid cartilage destruction is noted on the axial and coronal views of neck CT. (B) Histological section showing direct thyroid gland invasion through the thyroid cartilage (left, H&E ×12; right, H&E ×30): squamous cell carcinoma (thick arrows), thyroid gland (thin arrows). The scale bar denotes 1 mm.
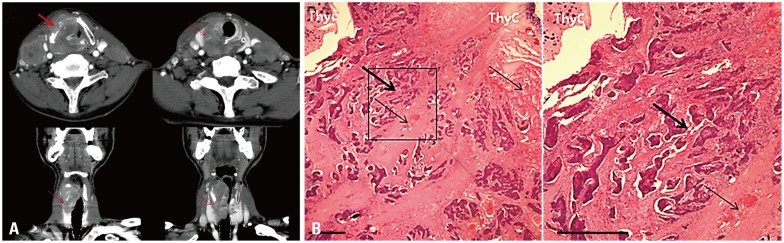
Fig. 2. Multifocal thyroid gland invasion by ipsilateral metastasis of hypopharyngeal cancer without direct extension through the thyroid cartilage. (A) An approximately 3.5-mm primary mass lesion extending from the post-cricoid region to the pyriform sinus is noted without definite thyroid cartilage involvement (upper left). Multiple foci of metastatic thyroid gland invasion (thin arrows) are noted without direct connections to the primary lesion (thick arrow) on the coronal view of contrast-enhanced neck CT (upper right). Thyroid invasion of hypopharyngeal cancer at the mid-pole (lower left) and upper pole (lower right) of the thyroid gland are revealed without destruction of the surrounding soft tissue, including the cartilage. (B and C) Histological section showing no direct thyroid gland invasion. (B) Squamous cell carcinoma fully invaded the hypopharynx (thick arrow), but the fibroadipose tissue and thyroid cartilage are well preserved without cancer invasion (H&E, ×12). (C) There is no associated lesion between the metastasis of the upper pole of the thyroid (thin arrow) and primary tumor (H&E, ×12). The scale bar denotes 1 mm. (D) Histological section showing tumor emboli in the lymphatic channel (H&E, ×40). The scale bar denotes 0.25 mm. Thick arrow: tumor emboli. ThyC, thyroid cartilage; V, vessel; L, lymphatic channel.
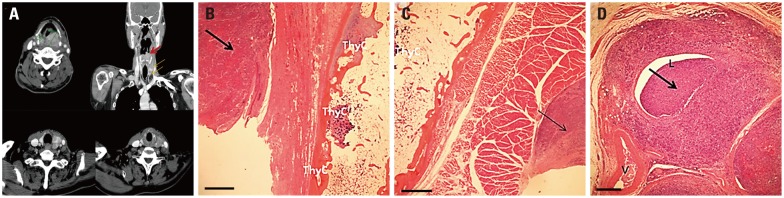
Table 4. Patterns of Spread of Thyroid Invasion.
| Patient | Primary site | Direct invasion | Lymphatic spread | Vascular channel spread | Multifocal invasion |
|---|---|---|---|---|---|
| 1 | PS lateral wall | + | - | - | - |
| 2 | PS medial wall | + | - | - | - |
| 3 | PS medial wall | + | - | - | - |
| 4 | PS medial wall | + | - | - | - |
| 5 | Post cricoid | - | + | - | + |
PS, pyriform sinus.
No patients with thyroid gland invasion exhibited primary recurrence. Regional or distant metastasis occurred in three patients. All distant metastases occurred in the lungs, and one patient had simultaneous lung metastasis and nodal recurrence at neck level V.
Examination of thyroid function in the patients who had undergone total laryngopharyngectomy with ipsilateral hemithyroidectomy demonstrated a 68.4% incidence of hypothyroidism (26 of 38 patients) in those who underwent radiotherapy (2 were excluded because total thyroidectomy was performed) and a 33.3% incidence (3 of 9 patients) in those who did not undergo radiotherapy. All patients were well managed with proper medical treatment provided by the endocrine department of our institute.
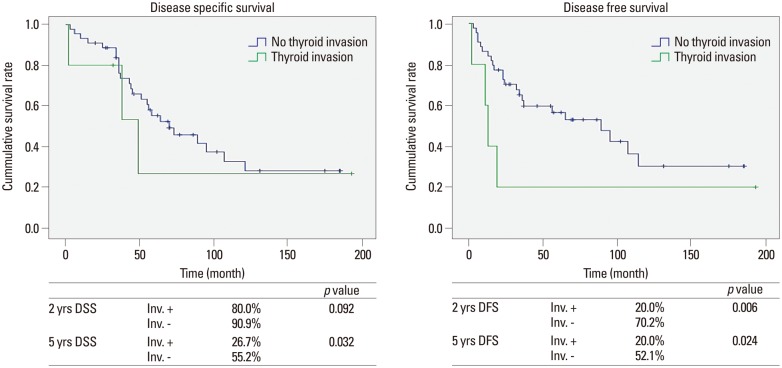
The 5-year DSS among patients with thyroid gland invasion was 26.7%, while that among patients without thyroid gland invasion was 55.2% (p=0.032). The 5-year DFS among patients with thyroid gland invasion was 20.0%, while that among patients without thyroid gland invasion was 52.1% (p=0.024) (Fig. 3). Almost all deaths of patients with thyroid gland invasion occurred from 2 to 5 years postoperatively, and all recurrences in these patients were revealed within 2 years.
Fig. 3. Prognosis according to thyroid gland invasion. The prognosis of patients with hypopharyngeal cancer with thyroid gland invasion was worse than that of patients with noninvasive tumors. DSS, disease-specific survival; DFS, disease-free survival.
DISCUSSION
Thyroid gland involvement in patients with laryngopharyngeal cancer is associated with the anatomical proximity of the thyroid gland to the laryngopharynx.12,13,14 Concomitant thyroidectomy has been performed to remove any known or occult tumor extension or metastasis, thereby ensuring local control, because thyroid gland invasion by squamous cell carcinoma is associated with a worse prognosis.1
The reported incidence of thyroid gland involvement in patients with laryngopharyngeal cancer varies from 0.0% to 23.0%, which is comparable with the incidence of 10.2% observed in the present study.3,8,15,16 Thyroidectomy with simultaneous total laryngectomy can, therefore, be considered to be unnecessarily performed in about 90% of cases, an unacceptable figure in view of the potential endocrine complications associated with this procedure, as preservation of both thyroid lobes is associated with a lower long-term prevalence of hypothyroidism.2
Ozgursoy and Jacobs17 suggested that hemithyroidectomy may not be necessary in the treatment of PS cancer unless there is evidence of thyroid gland invasion. However, their study had several limitations: it was confined to only PS cancer, the cohort was small (n=27), and the authors used only preoperative CT scans to assess thyroid gland invasion. Joshi, et al.18 recently reported that post-cricoid area invasion, subglottic extension, extralaryngeal spread, or prior tracheotomy were associated with a higher risk of thyroid gland involvement. However, their study was confined to only patients with T3 and T4 stage disease.
Laryngopharyngeal cancer can involve the thyroid gland by direct extension or indirect extension via lymphatic or vascular structures.3,5,8,16,19 Direct extension is the most common course of invasion in patients with hypopharyngeal cancer.16 In our cohort, 80% of patients (four of five) with thyroid gland invasion had direct invasion through the thyroid cartilage. Lymphatic drainage from the PS passes through the thyroid membrane primarily to the jugulodigastric lymph node and midjugular and spinal accessory chains.20 Lymphatic drainage from the inferior portion of the hypopharynx (PS apex) and from the post-cricoid region also passes to the paratracheal and paraesophageal nodes, which are located near the thyroid gland. In our study, one patient exhibited lymphatic metastasis without direct invasion (Fig. 2); his primary site of cancer was the post-cricoid region. This skipped metastasis to the thyroid gland was highly related to the primary anatomic site and lymphatic drainage in this case.
Our present data showed that thyroid cartilage invasion on preoperative CT imaging is the only statistically significant predictive factor for histologic thyroid gland invasion when thyroid gland invasion is not definitively confirmed during the preoperative evaluation (p=0.034). Histological thyroid gland invasion was observed in only 3% of cases (one case of metastasis) in which the thyroid cartilage appeared to be intact on preoperative CT. Therefore, thyroidectomy may not be indicated when the thyroid cartilage appears to be intact during the preoperative evaluation (negative predictive value=97.0%). Although unnecessary thyroidectomy was still performed (75.0%, 12 of 16 patients), the incidence was substantially reduced from 95.7% (44 of 46 patients) when considering a merely predictive value such as thyroid cartilage involvement. This incidence is especially low when we consider the intraoperative appearance of the thyroid gland.8 Although no statistically significant correlation was observed in this study, PS apex invasion (p=0.06) may be shown to be a predictive factor for thyroid gland invasion if more cases are accumulated. Identification of more predictive factors would decrease unnecessary thyroidectomy.
Moreover, no histologic invasion of hypopharyngeal cancer to the contralateral thyroid lobe was observed in our series. Although we could not demonstrate statistical significance because of the low number of patients, we can assume that ipsilateral hemithyroidectomy appears to be adequate when preoperative CT imaging clearly demonstrates thyroid gland invasion and/or thyroid cartilage destruction.
Hypothyroidism is a well-recognized complication of radiotherapy and surgery in the treatment of laryngopharyngeal cancer and is associated with various morbidities such as delayed wound healing, pharyngeal fistula, mood depression, and cardiac morbidity.21,22 Approximately 65% of patients who undergo hemithyroidectomy develop hypothyroidism after laryngopharyngectomy.6 Additionally, when hemithyroidectomy is combined with radiotherapy, the incidence of hypothyroidism is as high as 70% to 90%. However, the incidence of hypothyroidism decreases substantially when the whole thyroid gland is preserved.6,7 Our data showed a relatively higher rate of hypothyroidism among patients with laryngeal and hypopharyngeal cancer than that shown by other study groups, even when postoperative irradiation was not performed. One possible reason for this finding is that the patients in our study were older, relatively speaking, at the time of diagnosis because of the differences in the characteristics of their disease. Palmer, et al.23 reported that the incidence of hypothyroidism was 44% among 37 patients who underwent radiotherapy, total laryngectomy, and thyroid lobectomy. Alexander, et al.21 reported that hypothyroidism was observed in 55% of patients who underwent hemithyroidectomy and irradiation and in 22% of patients who underwent hemithyroidectomy alone. Their study also demonstrated that these patients with hypothyroidism had significant difficulties with postoperative wound healing and mental depression associated with hypothyroidism. Thus, patients should be evaluated postoperatively and carefully monitored with serial thyroid function tests.
This study has limitations in that it was retrospective and involved a relatively small cohort. Moreover, central lymph node treatment was not considered. Because the histological results of central lymph node dissection were poorly documented, we were unable to analyze the incidence of central lymph node metastasis despite its intimate anatomical relationship.
In conclusion, 10.2% of patients with hypopharyngeal cancer exhibited thyroid gland involvement in this study, and thyroid cartilage invasion was associated with a high risk of thyroid gland invasion. Although, our results require confirmation by additional large-scale prospective studies, they are expected to help select more accurately which patients with hypopharyngeal cancer would benefit most from thyroidectomy.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Brennan JA, Meyers AD, Jafek BW. The intraoperative management of the thyroid gland during laryngectomy. Laryngoscope. 1991;101:929–934. doi: 10.1288/00005537-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Biel MA, Maisel RH. Indications for performing hemithyroidectomy for tumors requiring total laryngectomy. Am J Surg. 1985;150:435–439. doi: 10.1016/0002-9610(85)90149-7. [DOI] [PubMed] [Google Scholar]
- 3.Sparano A, Chernock R, Laccourreye O, Weinstein G, Feldman M. Predictors of thyroid gland invasion in glottic squamous cell carcinoma. Laryngoscope. 2005;115:1247–1250. doi: 10.1097/01.MLG.0000165454.75480.EA. [DOI] [PubMed] [Google Scholar]
- 4.Mendelson AA, Al-Khatib TA, Julien M, Payne RJ, Black MJ, Hier MP. Thyroid gland management in total laryngectomy: meta-analysis and surgical recommendations. Otolaryngol Head Neck Surg. 2009;140:298–305. doi: 10.1016/j.otohns.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Elliott MS, Odell EW, Tysome JR, Connor SE, Siddiqui A, Jeannon JP, et al. Role of thyroidectomy in advanced laryngeal and pharyngolaryngeal carcinoma. Otolaryngol Head Neck Surg. 2010;142:851–855. doi: 10.1016/j.otohns.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Kim JW, Han GS, Byun SS, Lee DY, Cho BH, Kim YM. Management of thyroid gland invasion in laryngopharyngeal cancer. Auris Nasus Larynx. 2008;35:209–212. doi: 10.1016/j.anl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Turgut OK, Erişen L, Coşkun H, Basut O, Onart S, Hizalan I. Hypothyroidism after primary surgical treatment for laryngeal and hypopharyngeal cancer. Kulak Burun Bogaz Ihtis Derg. 2008;18:125–130. [PubMed] [Google Scholar]
- 8.Dadas B, Uslu B, Cakir B, Ozdoğan HC, Caliş AB, Turgut S. Intraoperative management of the thyroid gland in laryngeal cancer surgery. J Otolaryngol. 2001;30:179–183. doi: 10.2310/7070.2001.20211. [DOI] [PubMed] [Google Scholar]
- 9.Montero EH, Trufero JM, Romeo JA, Terré FC. Comorbidity and prognosis in advanced hypopharyngeal-laryngeal cancer under combined therapy. Tumori. 2008;94:24–29. doi: 10.1177/030089160809400106. [DOI] [PubMed] [Google Scholar]
- 10.Wagner MM, Curé JK, Caudell JJ, Spencer SA, Nabell LM, Carroll WR, et al. Prognostic significance of thyroid or cricoid cartilage invasion in laryngeal or hypopharyngeal cancer treated with organ preserving strategies. Radiat Oncol. 2012;7:219. doi: 10.1186/1748-717X-7-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckel HE, Schröder U, Jungehülsing M, Guntinas-Lichius O, Markitz M, Raunik W. [Surgical treatment options in laryngeal and hypopharyngeal cancer] Wien Med Wochenschr. 2008;158:255–263. doi: 10.1007/s10354-008-0530-2. [DOI] [PubMed] [Google Scholar]
- 12.Ceylan A, Köybaşioğlu A, Yilmaz M, Uslu S, Asal K, Inal E. Thyroid gland invasion in advanced laryngeal and hypopharyngeal carcinoma. Kulak Burun Bogaz Ihtis Derg. 2004;13:9–14. [PubMed] [Google Scholar]
- 13.Lefebvre JL, Lartigau E. Preservation of form and function during management of cancer of the larynx and hypopharynx. World J Surg. 2003;27:811–816. doi: 10.1007/s00268-003-7106-5. [DOI] [PubMed] [Google Scholar]
- 14.Aimoni C, Scanelli G, D'agostino L, Pastore A. Thyroid function studies in patients with cancer of the larynx: preliminary evaluation. Otolaryngol Head Neck Surg. 2003;129:733–738. doi: 10.1016/S0194-59980301588-2. [DOI] [PubMed] [Google Scholar]
- 15.Fagan JJ, Kaye PV. Management of the thyroid gland with laryngectomy for cT3 glottic carcinomas. Clin Otolaryngol Allied Sci. 1997;22:7–12. doi: 10.1046/j.1365-2273.1997.00835.x. [DOI] [PubMed] [Google Scholar]
- 16.Herranz J, Sarandeses A, Fernández MF, Barro CV, Vidal JM, Gavilán J. Complications after total laryngectomy in nonradiated laryngeal and hypopharyngeal carcinomas. Otolaryngol Head Neck Surg. 2000;122:892–898. doi: 10.1016/S0194-59980070020-9. [DOI] [PubMed] [Google Scholar]
- 17.Ozgursoy OB, Jacobs JR. Necessity of routine ipsilateral hemithyroidectomy during laryngopharyngectomy for pyriform sinus cancer. Am J Otolaryngol. 2012;33:562–564. doi: 10.1016/j.amjoto.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Joshi P, Nair S, Chaturvedi P, Nair D, Shivakumar T, D'Cruz AK. Thyroid gland involvement in carcinoma of the hypopharynx. J Laryngol Otol. 2014;128:64–67. doi: 10.1017/S0022215113003277. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert J, Forastiere AA. Organ preservation trials for laryngeal cancer. Otolaryngol Clin North Am. 2002;35:1035–1054. doi: 10.1016/s0030-6665(02)00035-x. [DOI] [PubMed] [Google Scholar]
- 20.Sessions DG. Surgical pathology of cancer of the larynx and hypopharynx. Laryngoscope. 1976;86:814–839. doi: 10.1288/00005537-197606000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Alexander MV, Zajtchuk JT, Henderson RL. Hypothyroidism and wound healing: occurrence after head and neck radiation and surgery. Arch Otolaryngol. 1982;108:289–291. doi: 10.1001/archotol.1982.00790530025007. [DOI] [PubMed] [Google Scholar]
- 22.Chang HJ, Kim KW, Choi SH, Lim S, Park KU, Park do J, et al. Endothelial function is not changed during short-term withdrawal of thyroxine in patients with differentiated thyroid cancer and low cardiovascular risk. Yonsei Med J. 2010;51:492–498. doi: 10.3349/ymj.2010.51.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer BV, Gaggar N, Shaw HJ. Thyroid function after radiotherapy and laryngectomy for carcinoma of the larynx. Head Neck Surg. 1981;4:13–15. doi: 10.1002/hed.2890040105. [DOI] [PubMed] [Google Scholar]