Abstract
B7-H1 is a recently identified B7 family member that along with one of its receptors, PD-1 has been involved in multiple immunopathological scenarios. However, the nature of B7-H1 and PD-1 in human hepatocellular carcinoma (HCC) remains poorly defined. We investigated the expression and functional relevance of this pathway in patients with HCC. We showed that B7-H1 expression on Kupffer cells (KCs) was increased in tumor tissues compared to surrounding non-tumor liver tissues in patients with HCC and this correlated with poorer survival. Co-culture of HCC cells with monocytes demonstrated that tumor associated IL-10 contributed to the induction of B7-H1 in the HCC environment. We further observed that the levels of PD-1+CD8+ T cells were higher in tumor tissues than non-tumor tissues. B7-H1+ KCs and PD-1+ T cells were co-localized in the HCC stroma. PD-1+CD8+ T cells had decreased proliferative ability and effector function as shown by reduced granule and cytokine expression compared to PD-1- T cells. Importantly, blocking KC B7-H1 interaction with PD-1+CD8+ cells using neutralizing antibodies recovered effector T cell function. Our data indicates that the B7-H1/PD-1 axis contributes to immune suppression in human HCC with blockade of this pathway carrying important therapeutic implications.
Keywords: B7-H1 (PD-L1), PD-1, Kupffer Cell, effector T cells, tumor immunity and Hepatocellular Carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy and is the second most common cause of cancer-related deaths in the world. While resection and transplantation are possible therapeutic options, 5-year recurrence rates following resection can exceed 50% (1). Several variables affect the risk of recurrence following resection, including tumor size, tumor number, and vascular invasion. However the role of tumor immunity is increasingly being recognized as being important to controlling tumor progression or recurrence in cases of breast, ovarian, and pancreatic cancers along with many others (2). Recently, HCC recurrence and stage has been shown to be independently predicted by the degree of CD8+ tumor infiltrating T cells (3). CD8+ cytotoxic T-lymphocytes are the major effectors against tumor cells (4). However, the progression of HCC and other tumors despite the presence of infiltrating CD8+ T cells stresses the pathological significance of the regulatory mechanisms within the tumor microenvironment. Recent evidence suggests that CD8+ T cells may become dysfunctional or functionally exhausted within the tumor microenvironment (5).
PD-1 (programmed death-1; CD279) may have an important role in the exhaustion of CD8+ T cells in infectious diseases (6, 7). Functionally exhausted CD8+ T cells express high levels of PD-1. PD-1 has two ligands with distinct expression patterns: B7-H1 (PD-L1; programmed death-ligand 1; CD274) and B7-DC (PD-L2; programmed death-ligand 2; CD273). B7-H1 is expressed on resting T cells, B cells, dendritic cells, and macrophages and is further up-regulated upon activation (8). B7-H1, which acts as an anti-apoptotic receptor, is also expressed on some tumor cells (9). In contrast, B7-DC is expressed only on DC and macrophages following induction (10). Ligation of PD-1 by B7-H1 transduces a negative signal when transmitted simultaneously with TCR signaling. This negative regulatory function is accomplished through the cytoplasmic domain of the molecule, which recruits intracellular phosphatases SHP-1 and SHP-2 (6). The recruitment of these phosphatases to the membrane leads to the dephosphorylation of several key signaling intermediates and thus the attenuation of TCR-mediated signals and T cell activation. It has been shown that B7-H1/PD-1 interactions contribute to the suppression of anti-viral immunity in liver or anti-tumor immunity (11-16). However, little is known about the functional relevance and mechanism of B7-H1 and PD-1 in human HCC.
The liver is an immunologically privileged site as studied in oral tolerance and transplantation models (17). Toleragenic or suppressive antigen presenting cells (APCs), numerous in liver tissue, are thought to control toleragenic phenotypes. Recently, B7-H1 has been shown to be expressed on hepatocytes and liver residential APCs contributing to deletion of intrahepatic T cells, preventing hepatic autoimmunity and suppressing anti-viral immune responses in animal models (18, 19). Given the relative paucity of data on the HCC microenvironment, we studied tumor tissues and surrounding non-tumor liver tissues in HCC patients undergoing surgical resection, and investigated the expression and functional relevance of B7-H1 and PD-1 in the human HCC microenvironment. Evidence is provided that Kupffer cells (KCs) primarily express B7-H1 in human HCC and mediate decreased effector function via interaction with PD-1 on effector T cells. Furthermore, this suppression appears to be reversible following disruption of these interactions, carrying potential immunotherapeutic implications.
Material and Methods
Human tissues
For functional analysis, fresh HCC tissues and surrounding non-tumor liver tissues were obtained from HCC patients undergoing surgical resection. The surrounding liver tissues from benign liver tumors served as normal liver controls. For survival analysis, 71 patients with HCC following surgical resection at the the Sun Yat-Sen University Cancer Center (Guang Zhou, China) from July 2000 to December 2002 were studied. These tissues were analyzed for tumor tissue B7-H1 expression by immunohistochemistry (IHC). The clinical characteristics of all patients are summarized in Table 1. All patients gave written, informed consent. The study was approved by the local institutional review boards.
Table 1. Clinical characteristics of HCC patients.
| Variables | Results |
|---|---|
| Number of patients | 71 |
| Age, years (median, range) | 48, 23-75 |
| Gender (male / female) | 65 / 6 (92/8) |
| HBsAg (negative / positive) | 8 / 63 (11/89) |
| Cirrhosis (absent / present) | 16/ 55(23/77) |
| ALT, U/L (median, range) | 40, 11-179 |
| AFP, ng/ml (≤25 / >25) | 27 / 44 (38/62) |
| Tumor size, cm (≤5 / >5) | 36 / 35 (51/49) |
| Tumor multiplicity (solitary / multiple) | 63 / 8 (89/11) |
| Vascular invasion (absent / present) | 69 / 2 (91/9) |
| Intrahepatic metastasis (no / yes) | 68 / 3 (96/4) |
| TNM stage (I+II / III+IV) | 57 / 14 (80/20) |
| Tumor differentiation (I+II / III+IV) | 58 / 13 (82/18) |
| Fibrous capsule (absent / present) | 13 / 58 (18/82) |
| B7-H1+ cells (median, range) | 94 ,16-227 |
Data in parentheses are percentages. AFP, alpha-fetoprotein.
Cell isolation
Cells were obtained from fresh HCC or surrounding liver tissues. The tissues were minced and digested with 50 mL of HBSS (Life Technologies, Herdon, VA) containing 40 mg of collagenase, 4 mg of DNase I, and 100 units of hyaluronidase (Sigma, St. Louis, MO) for 2 hours at room temperature. Tumor infiltrating leukocytes (TILs) or non-tumor infiltrating leukocytes (NILs) were isolated by Ficoll gradient centrifugation and analyzed by LSR II (BD Biosciences). CD8+ T cells and CD14+ tumor associated KCs were isolated and purified with paramagnetic beads (StemCell Technology, Vancouver, British Columbia, CA). Lin-CD45-CD14‐HCC tumor cells were sorted with FACSaria using DiVa software (Becton Dickinson Immunocytometry Systems).
Cell culture
CD8+ T cells (5× 105/ml) were added to 96-well round-bottom cluster plates and stimulated with anti-human CD3 (2.5 ug/mL, clone: UCHT1, BD Biosciences) and anti-human CD28 (1.2 ug/mL, clone: CD28.2, BD Biosciences) in the presence of HCC associated KCs (1× 105/ml) from the same tissue or CD14+ cells from the blood of healthy donors for 5 days. Neutralizing monoclonal antibody against human PD-1 (clone: M3, 5ug/ml) and B7-H1 (clone: 5H1, 5ug/ml) (17) or isotype controls were used in co-culture as indicated. After co-culture, cells were subjected to FACS analysis or proliferation assay.
Flow cytometry
Anti-human CD3, CD4, CD8, CD11c, CD14, CD45, CD123, HLA-DR, B7-H1, PD-1, Foxp3, granzyme B (Gran B), perforin, IFNγ and TNFα antibodies (BD Biosciences) were used in FACS and compared to isotype antibody controls as appropriate. Anti-human Ki67 (BD Biosciences) staining was used to identify proliferating cells. For intracellular cytokine staining, cells were exposed for 4 hours with PMA (phorbol 12-myristate 13-acetate; 50ng/ml; Sigma), ionomycin (500ng/ml; Sigma), GolgiStop (1ul/1.5ml; BD Bioscience) and Golgiplug (1ul/ml; BD Bioscience) as previously described (20), followed by surface and intracellular staining of various cytokines according to the manufacturer's instructions (BD Bioscience).
Immunohistochemistry
Fluorescence immunohistochemistry (IHC) was performed on 8 um cytosections of tissues. Briefly, tissues were stained as indicated with primary antibodies including mouse anti-human B7-H1 (clone: MIH1, IgG1, 1:100, BD Biosciences), mouse anti-human CD68 (clone: Y1/82A, IgG2b,1:100; BD Biosciences), mouse anti-human PD-1 (clone: NAT, IgG1, 1:200, Abcam), or goat anti-human PD-1(catalog number: AF1086, IgG, 1:100, R&D), and rat anti-human CD8 (clone: YTC182.20, IgG2b, 1:200, Abcam). Secondary antibodies were Alexa Fluor 568 conjugated goat anti-mouse IgG1, Alexa Fluor 488 conjugated goat anti-mouse IgG2b, Alexa Fluor 488 conjugated donkey anti-goat IgG, Alexa Fluor 568 conjugated goat anti-mouse IgG1 or Alexa Fluor 488 conjugated goat anti-rat IgG (all 2ug/mL, Molecular Probes). DAPI (4′, 6-diamidino-2-phenylindole) (Invitrogen, Carlsbad, CA) was used for nuclear staining. Fluorescent expression was evaluated with the Leica DM 5000B fluorescence microscope and compared to antibody isotype controls. Positive cells were quantified by ImagePro Plus software (Media Cybernetics, Bethesda, MD) and expressed as the mean number of positive cells ± SEM in 10 high-powered fields. For survival analysis, experiments were performed by two independent observers who were blinded to the clinical outcome. At low power field (×100), the tissue sections were screened using a Leica DM IRB inverted research microscope (Leica Microsystems, Wetzlar, Germany), and the five most representative fields were selected. Thereafter, to evaluate the density of B7-H1+ cells, the respective areas of tumor tissue were measured at × 400 magnification, and the nucleated cells positive for B7-H1 in each area were counted and expressed as number of cells per field.
Transwell experiments
Hep G2 cells were obtained from American Type Culture Collection (Manassas, VA). The cells were grown in Dulbecco's modified complete Eagle's medium (DMEM). Six-well transwell chambers with a 0.4 um porous membrane (Corning-Costar) were used. Briefly, primary HCC tumor cells or Hep G2 cells (2×106/ml) where indicated were added in the upper chamber and CD14+ cells (0.5×106/ml) from blood of healthy donors) were plated to the lower chambers. Recombinant human IL-10 (10ng/ml, R&D Systems, Inc., Minneapolis, MN) or monoclonal antibody against human IL-10 (500ng/ml, R&D) were added to select wells in the upper chamber. After 48 hours, CD14+ cells were harvested and B7-H1 expression was determined by FACS. Where indicated, supernatants from transwell experiments and cultured cells were checked by human IL-10 ELISA kit (R&D systems, Minneapolis, MN).
Western blot analysis and Real-Time RT-PCR
Single cell suspensions from HCC tumor tissues and surrounding liver tissues were used for Western blot analysis and real-time RT-PCR. Briefly, for Western blot analysis whole-cell lysates were prepared by incubating cells in ice-cold lysis buffer (20 mM Tris [pH 7.8], 2 mM EDTA, 50 mM NaF, 1% Triton X-100, 5 μg of leupeptin per ml, 5 μg of pepstatin per ml, and 0.5 mM PMSF). The lysates were then centrifuged at 14,000 × g for 15 min at 4°C and assayed for protein with the Bio-Rad protein assay reagent (Hercules, CA). Equal amounts of protein were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The following antibodies for Western blots were used: mouse anti-human IL-10 (clone: 25209, IgG2b, 1:1,000, R&D) and mouse anti-human β-actin (clone: AC-15, IgG1, 1:2,000, Sigma). Images were visualized with an enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, Pittsburgh, PA) (21). For RT-PCR, total RNA from tumor tissues and liver tissue as indicated were isolated using RNeasy Kits (QIAGEN, Valencia, CA). cDNA synthesis was performed using SuperScript One-Cycle cDNA Kit (Invitrogen). The cDNA served as a template in Real-Time PCR using Fast SYBR Green Master Kit (Applied Biosystems, Foster City, CA). The following primers for RT-PCR were used: Human IL-10 primers: 5′-CATTCTTCACCTGCTCCACG-3′ (sense) and 5′- AACCTGCCTAACATGCTTCG-3′ (antisense) (22); Human GAPDH primers: 5′-CTGCCCCCTCTGCTGATG-3′ (sense) and 5′- TCCACGATACCAAAGTTGTCATG-3′ (antisense) (23). All reactions were performed in triplicate. IL-10 mRNA expression of different group specimens was normalized to GAPDH. Relative IL-10 mRNA levels are presented as unit values of 2−ΔCt = 2−(Ct (GAPDH) − Ct (IL-10)), where Ct is the threshold cycle value defined as the fractional cycle number at which the target fluorescent signal passes a fixed threshold above baseline.
Proliferation assay
After 5 days of co-culture, T cells were pulsed with [3H] thymidine for the final 16 hours of incubation. Incorporation of [3H] thymidine was measured with a microbeta liquid scintillation counter (PerkinElmer, Waltham, MA).
IFNγ ELISPOT assay
MultiScreen filtration plates (96-wells per plate; Millipore) were coated with 100 μL per well of 4 μg/mL purified anti-human IFNγ antibody (clone: NIB42; BD PharMingen) overnight at 4°C and then incubated for 90 min at room temperature with 150 μl per well 1% bovine serum albumin (Sigma) in PBS. CD8+ cells (1×105/100ul per well) and CD14+ cells (5×104/100ul per well) from tumor tissues were placed into 96-well round-bottom cluster plates in the presence of anti-human CD28 (1.2 ug/mL, clone: CD28.2, BD Biosciences) for 5 days at 37°C, 5% CO2 in presence or absence of anti-human B7-H1 (clone: 5H1, 5ug/ml) or anti-human PD-1(clone: M3, 5ug/ml) antibody. Then all the cells were transferred to MultiScreen filtration plates and incubated for 48 h in the presence of HCC tumor cells lysate (from 1 × 104 per well) at 37°C, 5% CO2. PMA (1ng/ml) and ionomycin (0.5μg/ml) treated CD8+ effector cells served as a positive control (about 100 spots per well, data not shown). Plates were washed and incubated overnight at 4°C with 100 μL per well of 4 μg/mL of biotinylated rat anti-human IFNγ antibody (clone: 4S.B3; BD PharMingen) followed by a 90-min incubation with 100 μL per well anti-biotin alkaline phosphatase (Vector Laboratories, Inc.) diluted 1:1,000. Spots were visualized with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium alkaline phosphatase substrate and counted using the ImmunoSpot analyzer (Cellular Technology, Ltd.).
Statistical analysis
Data are presented as the mean plus or minus the standard error of the mean (SEM). Significant differences between groups were analyzed using Wilcoxon Matched-Pairs Ranks test for non-parametric data or one-way ANOVA test, where appropriate. P-values of less than 0.05 were considered statistically significant. For survival analysis, survival curves were compared by the Kaplan-Meier method and the log-rank test, and survival was measured in months from resection to the last review. All analyses were done using SPSS v12.0 software.
Results
B7-H1 expression on antigen presenting cell (APC) subsets in human HCC
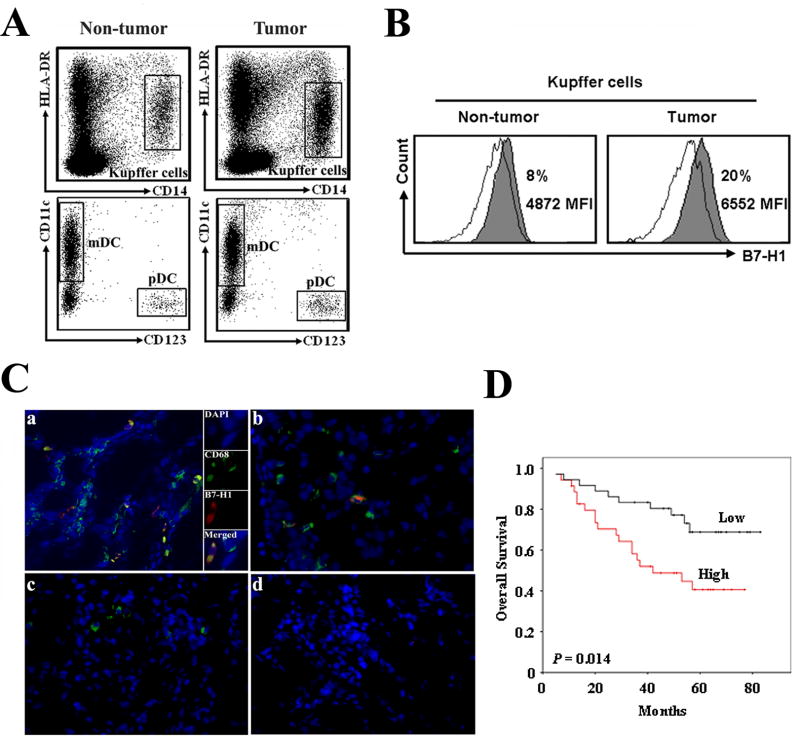
We analyzed the prevalence and phenotype of APC subsets within paired tumor tissues and surrounding liver tissues in patients with HCC (n = 20). The major human APC subsets, including HLA-DR+CD14+ Kupffer cells (KCs), lin-HLA-DR+CD4+CD11c+ myeloid dendritic cells (mDCs) and lin-HLA-DR+CD4+CD11c-CD123+ plasmacytoid dendritic cells (pDCs), were found in both tumor tissues and surrounding non-tumor liver tissues. The proportion of pDCs (1 ± 0.5%), mDCs (5 ± 2%), and KCs (20 ± 5%) in CD45+HLA-DR+ leukocytes was comparable between tumor tissues and non-tumor tissues (Fig. 1A). We next examined the expression of B7-H1 on these APC subsets. mDCs and pDCs expressed negligible levels of B7-H1 (Fig. S1). Similar to our previous observation on ovarian cancer associated macrophages (12), KCs were the predominant B7-H1+ population in HCC microenvironment. The percentage of B7-H1+ KCs was higher in tumor tissues than surrounding liver tissues (17.6 ± 4.1% versus 7 ± 2.7%, n = 15, P < 0.05, both by percentage of cells and mean fluorescence intensity) when compared to antibody isotype controls (Fig. 1B and S1). Further, the levels of B7-H1 expression on tumor associated KCs were significantly higher than that on other APC subsets in different compartments (Fig. S1). Using immune fluorescence staining, we confirmed that CD68+ KCs expressed more B7-H1 in HCC tumor tissues (Fig. 1C,panel a) than in surrounding non-tumor (Fig. 1C,panel b) and normal liver tissues (Fig. 1C,panel c). Cells were negative for staining with isotype control antibodies (Fig. 1C,panel d).
Figure 1.
KCs express increased B7-H1 in human HCC. APC subsets in human HCC were analyzed by FACS. A, CD45+ leukocytes were analyzed and gated on Lin-HLA-DR+ cells. Representative dot plots of KCs (HLA-DR+CD14+), pDCs (lin-HLA-DR+CD4+CD11c-CD123+), and mDCs (lin-HLA-DR+ CD4+CD11c+CD123-) from both tumor tissues or non-tumor tissues. B, Representative FACS histogram of B7-H1+ KCs. Filled histogram, B7-H1 expression; open histogram, antibody isotype control. MFI, Mean fluorescence intensity. C, B7-H1+KCs in HCC tumor tissue (a), surrounding non-tumor tissue (b) and normal liver tissue (c). The immunohistochemistry showed B7-H1 (red) staining was found on CD68+ KCs (green) in HCC tumor tissue. Nuclear was stained by DAPI (blue). Mouse IgG1 and IgG2b were used as isotype controls (d) for B7-H1 and CD68 staining, respectively. Representative sections are shown from 8 patients. ×400 magnification. D, Kaplan-Meier survival curves of HCC patients as determined by B7-H1 expression on IHC of tumor tissue black line, low expression (n = 36); red line, high expression (n = 35). p = 0.014.
To evaluate whether B7-H1 expression is a prognostic factor for survival after HCC tumor resection, 71 patients were divided into a low B7-H1 expressing and a high expressing group by using the median value of B7-H1+ cell density in tumor tissues on IHC. Overall, the high B7-H1 expressing group experienced worse survival when compared to the lower B7-H1 expressing group following log-rank analysis (Fig. 1D). The data indicate that high levels of B7-H1 are expressed in the HCC microenvironment which correlated with worse prognosis and that B7-H1 is primarily expressed on KCs in HCC tumor tissues.
Tumor associated IL-10 induced B7-H1 expression on KCs
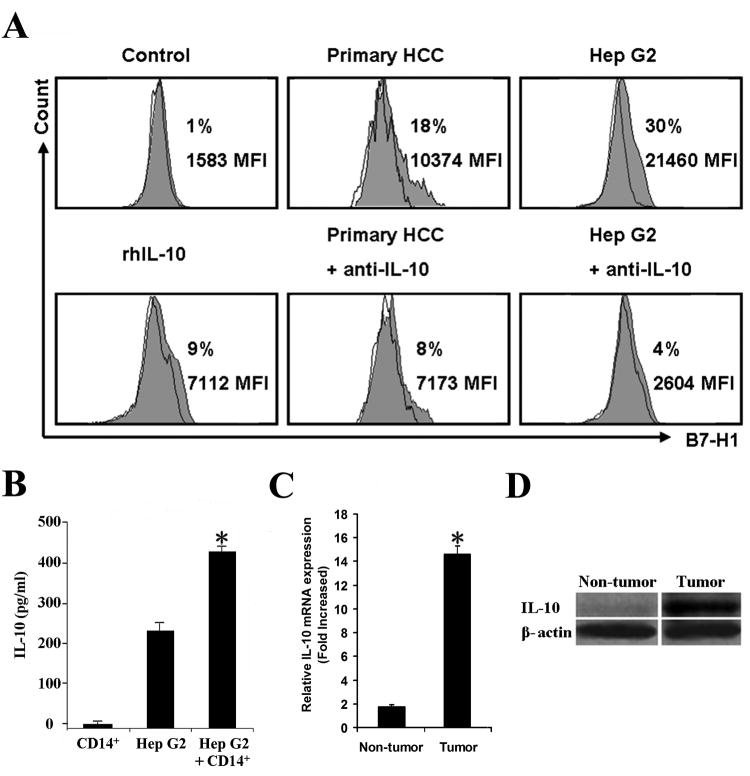
Our previous work in human ovarian carcinoma indicated that tumor associated IL-10 can up-regulate B7-H1 expression (15). Therefore we examined the potential factors in the HCC microenvironment contributing to KC B7-H1 up-regulation. We co-cultured blood CD14+ monocytes from healthy donors with primary HCC tumor cells or Hep G2 cells. Monocyte B7-H1 expression was significantly increased in the co-culture with HCC tumor cells or Hep G2 cells (Fig. 2A). Increased levels of IL-10 were also detected in the co-cultures (Fig. 2B). Additionally, both mRNA and protein expression of IL-10 were increased in primary HCC tumor tissues compared to surrounding liver tissues (Fig. 2C and D). The addition of recombinant IL-10 moderately induced B7-H1 expression on monocytes (Fig. 2A) while neutralizing antibody against IL-10 significantly blocked monocyte B7-H1 expression induced in co-culture. These results suggest that HCC associated IL-10 production may contribute to increased KC B7-H1 expression in the HCC microenvironment.
Figure 2.
HCC microenviromental factors induce monocyte B7-H1 expression. CD14+ monocytes from healthy donor blood were sorted and co-cultured with primary HCC tumor cells or Hep G2 cells in a transwell system for 48 h. B7-H1 expression on monocytes was determined by FACS. A, CD14+ monocytes co-cultured with primary HCC tumor cells or Hep G2 cells expressed more B7-H1 than CD14+ cells alone (control). B7-H1 expression increased moderately on monocytes alone with recombinant human IL-10 (10ng/ml). Co-culture treatment with anti-IL-10 neutralizing antibody inhibited B7-H1 expression. Data are representative of 5 independent experiments for Hep G2 and triplicate cultures for primary HCC. Filled histogram, B7-H1 expression; open histogram, antibody isotype control. MFI, Mean fluorescence intensity. B, Supernatants were collected from transwells and IL-10 was detected by ELISA. Results were shown as the mean ± SEM. *, P <0.05 vs. CD14+ monocytes alone or Hep G2 cells alone. n=5 experiments. C, representative Western blot for IL-10 protein in HCC vs. non-tumor liver tissue, n = 8 patients D, real-time RT-PCR for IL-10 expression *, P < 0.05. n= 8 patients.
PD-1 expression on T cell subsets in human HCC
We further analyzed the prevalence and phenotype of T cell subsets within paired tumor tissues and surrounding liver tissues in patients with HCC (n = 20). We observed significant and comparable CD4+ and CD8+ T cell infiltration in tumor tissues and surrounding liver tissues (Fig. 3A, upper panel). Since PD-1 is the co-inhibitory signal receptor for B7-H1 and CD8+ cytotoxic T cells are the main effector in anti-tumor immune responses (4), we examined the phenotype of PD-1+CD8+ T cells. Both in HCC tumor tissues and surrounding liver tissue, less than 5% of CD8+ T cells are CD45RA+ or CD62L+ (Fig. 3A, lower panel). The percentage of PD-1+CD8+ T cells was higher (63.0 ± 20.3%) in tumor tissues than that in surrounding tissues (11.5 ± 6.0%, P<0.05) (Fig. 3B and C). The expression of PD-1 on CD8+ T cells in HCC tumor tissue (Fig. 3D, panel a) was confirmed by IHC and was much higher than in surrounding non-tumor (Fig. 3D, panel b) and normal liver tissues (Fig. 3D, panel c). Cells were negative for staining with isotype control antibodies (Fig. 3D, panel d). PD-1+ cells were also found to be localized primarily to the HCC stroma (Fig. S2). CD80, another reported receptor for B7-H1 (24), was not detected on CD8+ cells nor was B7-DC, another ligand for PD-1 (10), detected on KC or DC (data not shown). These results indicate that most of CD8+ T cells within human HCC are effector phenotype with a greater percentage of these expressing PD-1 in the tumor microenviroment.
Figure 3.
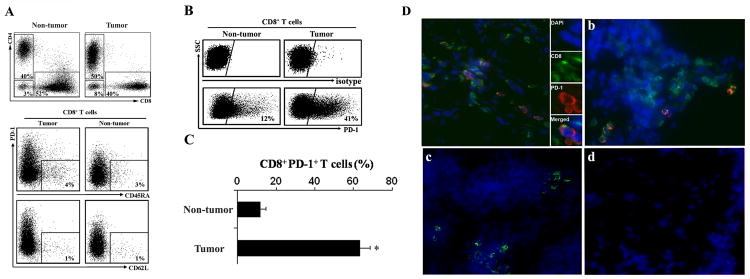
HCC tumor associated CD8+ T cells highly express PD-1. Human HCC T cell subsets were analyzed by FACS. A, Top, representative dot plots of CD4+ and CD8+ T cells from 20 HCC patients gated on CD3+ cells. The proportions of CD4+ and CD8+ T cells in both tumor tissue and non-tumor tissue. Bottom, representative phenotype dot plots of CD8+ T cells showed both in HCC tumor and non-tumor tissues less than 5% CD8+ T cells are CD45RA+ or CD62L+. n= 8 patients. B, Representative dot plots of PD-1+CD8+ T cells from 20 HCC patients in tumor and non-tumor tissue. Top, isotype control. Bottom, PD-1 expression on CD8+ cells. C, The mean percentage of PD-1+CD8+ T cells in tumor tissues and non-tumor tissues. *, P < 0.05. n= 20 patients. D, PD-1+CD8+ T cells in HCC tumor tissue (a), surrounding non-tumor tissue (b) and normal liver tissue (c). PD-1 (red) staining was largely limited to CD8 cells (green). Mouse IgG1 and rat IgG were used as antibody isotype controls (d) for PD-1 and CD8 staining, respectively. DAPI nuclear (blue), ×400 magnification, n = 8 patients.
Functional characteristics of tumor associated PD-1+CD8+ T cells in human HCC
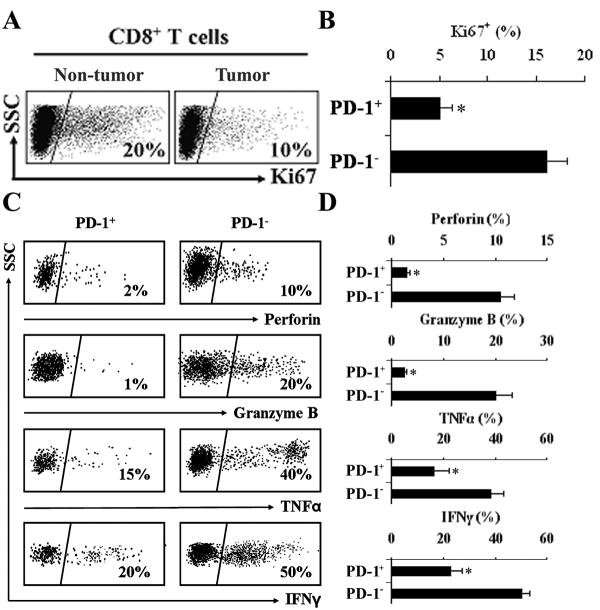
Given the high levels of PD-1 expression on CD8+ T cells in HCC, effector T cells might be functionally exhausted. We examined the functional characteristics of tumor associated PD-1+CD8+ T cells in human HCC. We initially compared the in vivo proliferation status of CD8+ T cells in tumor tissues versus surrounding liver tissues, and of PD-1+CD8+ T cells versus PD-1-CD8+ T cells in human HCC using Ki67, a proliferative marker for cells in mitosis. The levels of Ki67+CD8+ T cells were lower in tumor tissues than surrounding tissues (Fig. 4A). Furthermore, PD-1+CD8+ T cells were less proliferative than PD-1-CD8+ T cells in patients with HCC (Fig. 4B). PD-1 has previously been shown in animal models to negatively regulate CD8+ T cell effector function (19, 20). We further examined the functional markers of effector T cells including cytokine profile and cytotoxic enzymes. PD-1+CD8+ T cells in tumors exhibited markedly diminished expression of perforin and granzyme B (Fig. 4C and D). Furthermore, The percentage of TNFα+ and IFNγ+ T cells was significantly reduced in PD-1+CD8+ T cells than PD-1-CD8+ T cells (Fig. 4C and D). These results indicate that PD-1+CD8+ T cells in the HCC microenviroment have impaired effector function when compared to PD-1-CD8+ T cells.
Figure 4.
Decreased effector function of PD-1+CD8+ T cells in human HCC. A, Representative dot plots of Ki67 expression in CD8+ T cells from tumor tissues vs. non-tumor tissue are shown and B, Ki67 expressed as mean percentage cells in PD-1+CD8+ or PD-1-CD8+ T cells in human HCC tissues. *, P < 0.05. n = 20 patients. C, PD-1+CD8+ T cells in HCC tumor tissues demonstrated decreased effector function compared to PD1- T cells. Representative dot plots showing the percentage of perforin, granzyme B, TNFα and IFNγ expressing cells in PD-1+ vs. PD-1- CD8+ T cells and D, the mean percentages of perforin, granzyme B, TNFα, and IFNγ in PD-1+CD8+ T cells in HCC tumor tissues vs. PD1- T cells. *, P < 0.05. n = 20 patients.
B7-H1/PD-1 interactions mediate impaired effector T cell function in human HCC
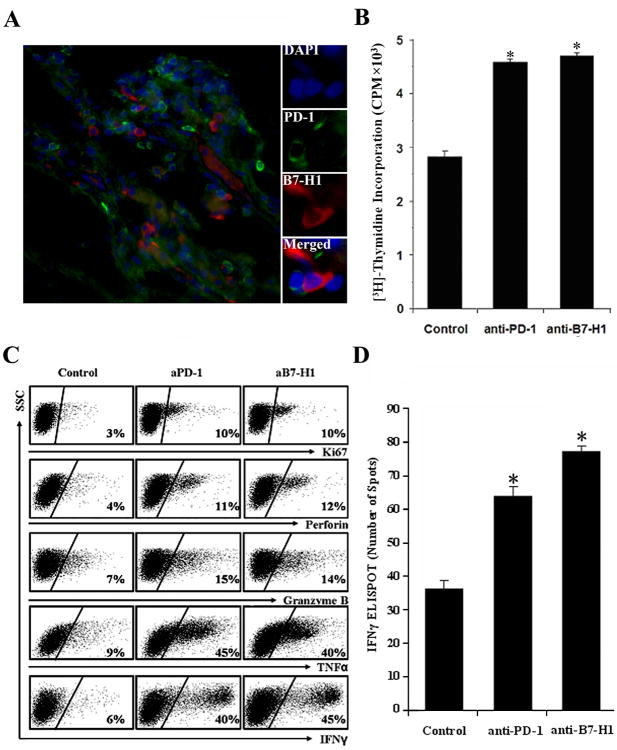
To identify whether B7-H1+ KCs interact with PD-1+CD8+ T cells in HCC, human HCC tumor specimens were further analyzed by IHC to determine their physical localization. A significant infiltration of B7-H1+ and PD-1+ cells was detected in the peri-tumor stroma when compared to the tumor nests (Fig. S2). In addition, B7-H1+ and PD-1+ cells appeared to be in close contact with each other, providing the possibility for direct interactions between these cells (Fig. 5A). To evaluate whether disruption of the B7-H1/PD-1 interaction would restore effector function to CD8+ T cells, CD8+ T cells and KCs were sorted from HCC tumor tissues and placed into co-culture. Tumor-derived KC caused no increase in CD8+ proliferation or cytokine proliferation (data not shown). However, blockade of B7-H1 or PD-1 with specific monoclonal antibodies resulted in enhanced T cell proliferation as shown by [3H]-thymidine incorporation (Fig. 5B) and Ki67 expression (Fig. 5C), T cell effector cytokine expression, granzyme B, and perforin expression compared to no treatment isotype control group (Fig. 5C). To evaluate whether B7-H1/PD-1 interactions affect tumor antigen-specific responses, we further performed HCC-specific IFNγ ELISPOT analysis using HCC-derived KCs loaded with HCC lysates to stimulate autologous HCC CD8+ T cells. Blocking B7-H1 or PD-1 using monoclonal antibodies increased the antigen-specific IFNγ-secreting spots (Fig. 5D), suggesting that B7-H1+ KCs interact with PD-1+CD8+ T cells and contribute to dysfunction of effector T cells in HCC. CD14+ cells from blood of healthy donors were used as control of tumor associated KCs and showed similar results as the CD8+ T cells alone cultures (data not shown) and were also similar to the mouse anti-human IgG1 isotype control (Fig. 5D).
Figure 5.
B7-H1+ and PD-1+ cells were co-localized in HCC and mediated T cell immune suppression. A, PD-1+ cells (green) were co-localized with B7-H1+ cells (red), DAPI nuclear (blue), and ×400 magnification. Representative data for 8 patients. B, C, Blocking B7-H1 or PD-1 increased T cell effector function. CD14+ and CD8+ cells were sorted from human HCC and co-cultured for 5 days with anti-CD3 and anti-CD28 antibodies. Where indicated, anti-PD-1 or anti-B7-H1 monoclonal antibodies were added and compared to control (mouse anti-human IgG1 isotype control for both anti-PD-1 and anti-B7-H1 antibodies). B, CD8+ T cells proliferation was determined by 3[H]-thymidine incorporation and expressed as the mean CPM ± SEM and are representative of n = 5 experiments. *, p<0.05 vs. isotype control. C, Expression of Ki67, perforin, granzyme B and cytokines was determined by FACS analysis with representative dot plots shown from 5 experiments. D. HCC-specific CD8+ T cell response. CD8+ T cells were stimulated with KCs loaded with autologous HCC lysates over 5 days and HCC-specific IFNγ was determined by ELISPOT in the presence or absence of anti-B7-H1 or anti-PD-1 antibody. Results were shown as the mean number of IFNγ spots per 105 CD8+ T cells ± SEM. *, p<0.05 vs. isotype control. n = 3 experiments.
Discussion
An immune suppressive environment has been previously described in patients with HCC comprising decreased numbers of CD8+ T cells and increased regulatory T cells (3, 25). However, the underlying mechanisms controlling effector function remain poorly defined within human HCC. Furthermore, the nature and significance of B7-H1 and PD-1 in human HCC are likewise incompletely defined. In the current investigation we have demonstrated that HCC environmental factors stimulate B7-H1 expression on KCs, and that the B7-H1/PD-1 pathway contributes to impaired effector T cell function in the HCC environment.
B7-H1 has been reported to be expressed on multiple APC subsets (7, 8). It has been shown that liver resident pDCs or mDC express B7-H1 after transplantation or virus infection (26, 27). We found that in the HCC environment, B7-H1 expression is confined to KCs but is minimally expressed on mDCs, pDCs, or HCC tumor cells. A significant number of B7-H1+ KCs in HCC tissue were noted compared with surrounding non-tumor tissue. Tumor associated IL-10 contributed to B7-H1 up-regulation in the co-culture system and increased IL-10 expression was observed in human HCC. IL-10 expression was elevated in supernatants of monocytes and tumor cells when co-cultured. Therefore both tumor cells and macrophages in the HCC microenvironment could be the source of IL-10 following their interaction with each other. It is also possible that B7-H1 expression could be induced by other tumor-associated cytokines, such as IFNγ and VEGF (7, 15, 28). Therefore IL-10 may not the only factor inducing B7-H1 expression in the HCC microenviroment and is consistent with our previous findings in human ovarian carcinoma (15). However, tumor conditioned KCs, are the likely mediator of B7-H1/PD-1 interactions in human HCC.
CD8+ effector T cells mediate anti-tumor effects directly by expression of either anti-tumor cytokines (TNFα and IFNγ) or cytotoxic granules (granzyme B and perforin). However, there was no significant difference in the prevalence of CD8+ T cell distribution and effector phenotype between HCC tissue and surrouding non-tumor tissue. Overexpression of PD-1 is a hallmark of exhausted T cells in chronic hepatitis (12, 29). We showed in HCC tissue that higher percentages CD8+ T cell express PD-1. These PD-1+CD8+ T cells were mainly localized in the HCC stroma as opposed to the tumor nests. Several studies reported the importance of PD-1 expression in dampening antiviral CD8+ T cell responses in human viral hepatitis (8, 9, 26). Our results in HCC are consistent with these previous findings and are important as chronic hepatitis or cirrhosis is a major risk factor for human HCC (1). We have shown that PD-1+ CD8+ T cells in HCC also display impaired effector phenotype indicating that the quality and function are altered in HCC.
Inhibitory B7-H1/PD-1 interaction can restrict immune responses against self-antigens. This pathway has emerged as a potential therapeutic target for treating cancer (13-16). In this study we demonstrate that CD8+ T cells and KCs in human HCC tumor tissues expressed high levels of PD-1 and B7-H1, respectively, while other molecules, namely CD80 and B7-DC, were not detected. Exhausted CD8+ cells were able to be rescued following B7-H1 or PD-1 blockade using monoclonal antibodies resulting in improved effector responses following TCR stimulation and stimulation with tumor specific antigens. We have previously demonstrated a role for B7-H1 to inhibit tumor immune responses in human ovarian carcinoma (15). Other investigators have observed that blocking B7-H1/PD-1 enhances the function of antigen-specific T cells in infectious diseases (12, 28, 30, 31).
To our knowledge, this is the first evidence that B7-H1+ APCs and PD-1+ T cells are physically localized in the same site of liver or within HCC and that T cell effector function could be rescued by blocking B7-H1/PD-1 interactions, providing important therapeutic implications. By overall survival analysis, we observed that elevated B7-H1 expression in HCC is indeed associated with poorer prognosis in HCC patients as has been recently reported by other investigators (32). Altogether, our data suggests that B7-H1/PD-1 interactions mediate immune suppression in human HCC and B7-H1 expression in tumor was associated with clinical outcome. The suppressive interaction between KCs and T cells using B7-H1/PD-1 interactions in HCC patients suggests that targeted therapies against this pathway represent a promising approach to enhance T cell immunity to human HCC.
Supplementary Material
Acknowledgments
These investigations were supported in part by the Tissue Procurement Core of the University of Michigan Comprehensive Cancer Center, Grant #CA46952. The investigators would also like to acknowledge the assistance of Dr. Li Dong Wang (University of Michigan Medical Center) for the Western blot analysis.
References
- 1.El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–63. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 2.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 3.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–93. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 4.Ossendorp F, Mengedé E, Camps M, et al. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187(5):693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 6.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169(10):5538–45. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 9.Azuma T, Yao S, Zhu G, et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–43. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Bajorath J, Flies DB, et al. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083–91. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans A, Riva A, Cooksley H, et al. Programmed death 1 expression during antiviral treatment of chronic hepatitis B: Impact of hepatitis B e-antigen seroconversion. Hepatology. 2008;48:759–69. doi: 10.1002/hep.22419. [DOI] [PubMed] [Google Scholar]
- 12.Nakamoto N, Kaplan DE, Coleclough J, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–37. 1937.e1–2. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 14.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 15.Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–5. [PubMed] [Google Scholar]
- 16.Yamamoto R, Nishikori M, Kitawaki T, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111:3220–4. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 17.Crispe IN, Giannandrea M, Klein I, et al. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–18. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 18.Dong H, Zhu G, Tamada K, et al. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity. 2004;20:327–36. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 19.Iwai Y, Terawaki S, Ikegawa M, et al. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198(1):39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kryczek I, Liu R, Wang G, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69(9):3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Heidt DG, Lee CJ, et al. Oncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilization. Cancer Cell. 2009;15(3):207–19. doi: 10.1016/j.ccr.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miura Y, Nishimura Y, Katsuyama H, et al. Involvement of IL-10 and Bcl-2 in resistance against an asbestos-induced apoptosis of T cells. Apoptosis. 2006;11(10):1825–35. doi: 10.1007/s10495-006-9235-4. [DOI] [PubMed] [Google Scholar]
- 23.Sindić A, Hirsch JR, Velic A, et al. Guanylin and uroguanylin regulate electrolyte transport in isolated human cortical collecting ducts. Kidney Int. 2005;67(4):1420–7. doi: 10.1111/j.1523-1755.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 24.Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–39. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 26.Tokita D, Mazariegos GV, Zahorchak AF, et al. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation. 2008;85(3):369–77. doi: 10.1097/TP.0b013e3181612ded. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Zhang Z, Chen W, et al. B7-H1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. J Immunol. 2007;178(10):6634–41. doi: 10.4049/jimmunol.178.10.6634. [DOI] [PubMed] [Google Scholar]
- 28.Lee SJ, Jang BC, Lee SW, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580(3):755–62. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Zhang JY, Wherry EJ, et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134:1938–49. 1949.e1–3. doi: 10.1053/j.gastro.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 30.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 31.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–10. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–9. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.