Abstract
Introduction:
The aims of this study were to investigate the effect of varicocelectomy on DNA fragmentation index and semen parameters in infertile patients before and after surgical repair of varicocele.
Materials and Methods:
In this prospective study, 72 men with at least 1-year history of infertility, varicocele and oligospermia were examined. Varicocele sperm samples were classified as normal or pathological according to the 2010 World Health Organization guidelines. The acridine orange test was used to assess the DNA fragmentation index (DFI) preoperatively and postoperatively.
Results:
DFI decreased significantly after varicocelectomy from 34.5% to 28.2% (P = 0.024). In addition all sperm parameters such as mean sperm count, sperm concentration, progressive motility and sperm morphology significantly increased from 19.5 × 106 to 30.7 × 106, 5.4 × 106/ml to 14.3 × 106/ml, and 19.9% to 31.2% (P < 0.001) and 2.6% to 3.1% (P = 0.017). The study was limited by the loss to follow-up of some patients and unrecorded pregnancy outcome due to short follow-up.
Conclusion:
Varicocele causes DNA-damage in spermatozoa. We suggest that varicocelectomy improves sperm parameters and decreases DFI.
Keywords: Acridine orange test, DNA damage, male infertility, varicocele
INTRODUCTION
Varicocele is one of the major causes of infertility in men, present in between 15 and 20% of the general male population.[1] Patients with varicocele may have altered spermatogenesis, which has been attributed to several factors, including reflux of renal/adrenal toxic metabolites, testicular hypoxia due to venous stasis, hormonal dysfunction, hypertension in the internal spermatic veins, and increase in testicular temperature.[2] The semen of these patients can have high levels of oxidative stress, as evidenced by increased levels of reactive oxygen species (ROS) and reduced total antioxidant capacity.[3,4] In addition, sperm DNA damage is correlated with high levels of ROS in men with varicocele.[5,6] Several groups reported that varicocele is associated with increased sperm DNA damage.[7,8] Since varicocelectomy significantly improves sperm concentration and motility in infertile men with varicocele and abnormal preoperative semen parameters, we hypothesized that surgical varicocelectomy may improve spermatozoal function caused by increased levels of sperm DNA damage.[9,10] Therefore in this study we evaluated DNA fragmentation in spermatozoa and semen parameters of infertile men clinically diagnosed with varicoceles before and after surgical varicocelectomy.
MATERIALS AND METHODS
Study group
This prospective study included 72 men with at least 1-year history of infertility, a palpable varicocele and oligospermia from November 2012 to January 2014 at the Clinic of Urology of Ankara Training and Research Hospital. Varicocele was diagnosed by scrotal palpation and testicular volume was assessed with a Prader orchidometer. All patients with urinary tract infections, fever occurring ~90 days before semen analysis, leukocytospermia, systemic diseases such as cancer and endrocrinopathies that would lead to testicular alterations and a history of smoking and excessive alcohol or drug use were excluded from study. All subjects provided informed consent, and the study was approved by research and ethics committee. Every patient provided a fresh ejaculated semen sample by masturbation after 3 days of ejaculatory abstinence and were analyzed within 1 h of collection. After liquefaction, seminal analysis was performed according to WHO criteria.[11] All varicoceles were treated with inguinal spermatic vein ligation. Postoperative semen was analyzed at 3 months after surgery.
Measurement of sperm DNA fragmentation
Acridine Orange Test (AOT) was performed as described by Evenson and co-workers.[12] The maturity of sperm nuclei can be determined by the use of AOT. The acridine orange molecules are intercalated into double-stranded DNA and green fluorescence is emitted from the sperm nuclei. Sperm DNA with immature nuclei is easily denatured into single strands and after acridine orange molecules aggregate in the nuclei, the color of fluorescence changes into orange-red.[13]
For assessment of sperm DNA integrity, the smears were air-dried for 1 h and then fixed overnight in freshly made Carnoy's solution. The slides were rinsed two times with distilled water and dipped in McIlvaine phosphate-citrate buffer (pH = 4) for 5 min. Each sample was then stained with freshly prepared acridine orange (0.19 mg/mL; Sigma Chemical Co, St Louis, USA) in MciIvaine phosphate- citrate buffer for 10 min in the darkness. The cell suspension was pipetted onto a glass slide and observed under a confocal fluorescent microscope (Zeiss LSM 510) with a 480–490 nm filter. The percentages of green (normal DNA integrity) and orange-red (abnormal DNA integrity) spermatozoa (per 100 spermatozoa in each sample) were calculated by a same single person [Figure 1]. An abnormal integrity of sperm nuclear DNA was considered as more than 30% denaturation (orange-red spermatozoa on acridine orange staining). The DNA fragmentation index (DFI), which is the ratio of the orange-red to the total (orange-red + green) fluorescence intensities of spermatozoa, was also calculated for the samples[14] and all the DFI calculations were performed by the same person since bias can be introduced if different personnel were involved. The observer performing DFI calculations was blinded as to whether the samples were pre or postoperative. A minimum of 500 spermatozoa for sample was scored under the × 100 objective of microscope.
Figure 1.
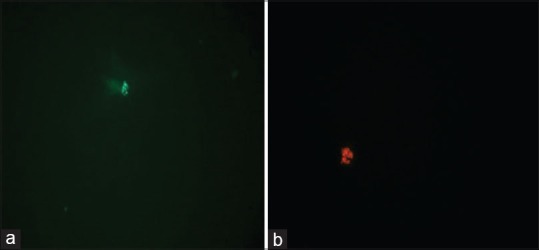
Acridine orange molecules aggregation in the sperm nuclei under fluorescent microscope (a) green (normal DNA integrity) (b) orange-red (abnormal DNA integrity)
Statistical analysis
Continuous variables are presented as mean ± standard deviation, while categorical variables are given as percentages. The Shapiro–Wilk test was used to verify the normality of the distribution of continuous variables. Statistical analysis of clinical data between two groups consisted of Mann Whitney U test for unpaired nonparametric data and Wilcoxon Sign Rank Test for paired nonparametric data. Correlations were assessed with the Spearman correlation coefficient. Analyses were performed with PASW 18 (SPSS/IBM, Chicago, IL, USA) software and two-tailed P value less than 0.05 was considered statistically significant.
RESULTS
The mean age of the 72 men was 29.3 ± 5.7 years. Left sided grade I, grade II and grade III varicocele were found in 19, 26 and 21 patients, respectively, and 6 had bilateral varicoceles. Of the 72 men who underwent varicocelectomy, 66 underwent left and 6 bilateral varicocelectomy. The mean follow-up after varicocelectomy was 6.2 ± 2.4 (median 6; 4–12) months. The mean sperm count, concentration, progressive motility and normal morphology were significantly higher after varicocelectomy and DFI was significantly lower after surgery. The mean DFI was 34.5 ± 3.3% and 28.2 ± 3.5% before and after varicocelectomy (P = 0.024). The mean values of semen parameters and DFI are presented in Table 1. We observed a negative significant correlation between the change in DFI and the change in sperm motility after varicocelectomy [Figure 2].
Table 1.
Semen parameters and DFI before and after varicocelectomy
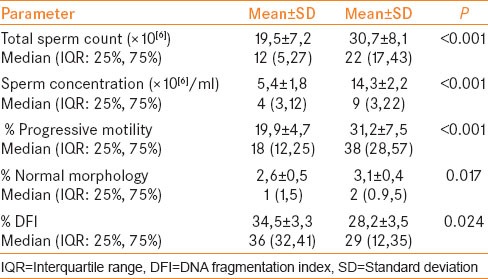
Figure 2.
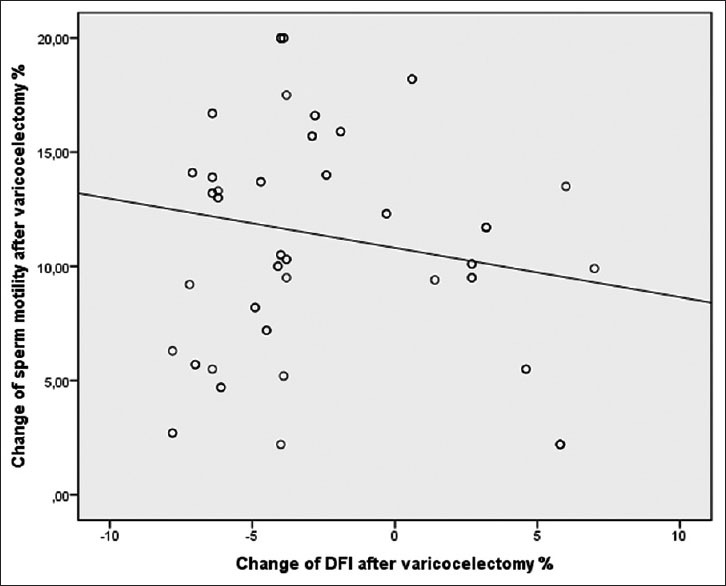
DFI change after varicocelectomy and subsequent sperm motility change (Spearman correlation r −0.267, P=0.043)
DISCUSSION
While varicocele is one of the most andrological pathology and affects sperm functions, the true effect of adult varicocelectomy on male fertility remains controversial.[15] In a previous study, Saleh et al.[7] reported the association between a clinical varicocele and high levels of sperm DNA damage. These investigators have implicated both elevated temperature and the elaboration of reactive oxygen species as potential mechanism responsible for varicocele-mediated sperm dysfunction and DNA damage. Although the benefit of varicocelectomy on male fertility remains controversial, in the present study we found a significant increase in postoperative sperm count, concentration, progressive motility and morphology after varicocele repair and a significant decrease in DFI.[15,16]
Sperm DNA fragmentation provides additional information about sperm quality and the ability of a couple to conceive.[17] There are several assays available to assess sperm nuclear integrity and, in clinical setting, AOT is one of the standard assays which is quick and easy to perform. This can be used for testing sperm maturity and predicting the fertilization capacity of ejaculated spermatozoa.[18] Evaluation of sperm nuclear integrity with AOT and fluorescent microscopy, as used in our study, is a routine procedure in andrology units; however, usage of flowcytometry is more efficient as described in a recent study.[19] Flowcytometry is an expensive technique and also time consuming, thus, less practical for daily evaluation of sperm DNA in assisted reproductive clinics.[14]
Studies show that varicocele samples contain a higher proportion of spermatozoa with abnormal and immature chromatin than those from fertile men as well as infertile men without varicocele.[20] A cause of this phenomenon may be the increased production of ROS in varicocele patients that is significantly higher in patients diagnosed with grade II and III varicocele.[21] Oxidative stress and testicular apoptosis are well-documented causes of increased sperm DNA fragmentation. Varicocele is associated with increased ROS production in spermatozoa and decreased antioxidant capacity.[3] In addition nitric oxide can increase sperm DNA fragmentation that is associated with infertility in men with varicocele.[22] Similar to our study, Smit et al. reported that varicocelectomy improves sperm parameters significantly and sperm DNA fragmentation was significantly decreased. Low DNA fragmentation index values are associated with a higher pregnancy rate.[23]
Our study has some limitations. First is the relatively small number of patients and short follow-up, which are the major limitations. Second, pregnancy outcome was not recorded due to the short follow-up. Future studies are needed to confirm the exact role of varicocelectomy and sperm DFI that may affect natural pregnancy.
CONCLUSION
In infertile men with varicocele, varicocelectomy can reduce sperm DNA fragmentation and improve sperm parameters. We suggest that varicocelectomy improves spermatogenesis and sperm function and provides an additional mechanism for the reported improvement in pregnancy rates after varicocele repair.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chan P. Management options of varicoceles. Indian J Urol. 2011;27:65–73. doi: 10.4103/0970-1591.78431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenberg ML, Lipshultz LI. Varicocele-induced infertility: Newer insights into its pathophysiology. Indian J Urol. 2011;27:58–64. doi: 10.4103/0970-1591.78428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheehan MM, Ramasamy R, Lamb DJ. Molecular mechanisms involved in varicocele-associated infertility. J Assist Reprod Genet. 2014;31:521–6. doi: 10.1007/s10815-014-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Sekhon LH. Oxidative stress and antioxidants for idiopathic oligoasthenoteratospermia: Is it justified? Indian J Urol. 2011;27:74–85. doi: 10.4103/0970-1591.78437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien J, Zini A. Sperm DNA integrity and male infertility: A review. Urology. 2005;65:16–22. doi: 10.1016/j.urology.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Fisher MA, Willis J, Zini A. Human sperm DNA integrity: Correlation with sperm cytoplasmic droplets. Urology. 2003;61:207–11. doi: 10.1016/s0090-4295(02)02098-8. [DOI] [PubMed] [Google Scholar]
- 7.Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ., Jr Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003;80:1431–6. doi: 10.1016/s0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]
- 8.Blumer CG, Fariello RM, Restelli AE, Spaine DM, Bertolla RP, Cedenho AP. Sperm nuclear DNA fragmentation and mitochondrial activity in men with varicocele. Fertil Steril. 2008;90:1716–22. doi: 10.1016/j.fertnstert.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short RA, Sabanegh E, et al. Efficacy of varicocelectomy in improving semen parameters: New meta-analytical approach. Urology. 2007;70:532–8. doi: 10.1016/j.urology.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Marmar JL, Agarwal A, Prabakaran S, Agarwal R, Short RA, Benoff S, et al. Re-assessing the value of varicocelectomy as a treatment for male subfertility with a new meta- analysis. Fertil Steril. 2007;88:639–48. doi: 10.1016/j.fertnstert.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 12.Shiraishi K, Matsuyama H, Takihara H. Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. Int J Urol. 2012;19:538–50. doi: 10.1111/j.1442-2042.2012.02982.x. [DOI] [PubMed] [Google Scholar]
- 13.Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: A systematic review and meta-analysis. Hum Reprod. 2012;27:2908–17. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 14.Khalili MA, Aghaie-Maybodi F, Anvari M, Talebi AR. Sperm nuclear DNA in ejaculates of fertile and infertile men: Correlation with semen parameters. Urol J. 2006;3:154–9. [PubMed] [Google Scholar]
- 15.Evers JL, Collins JA. Assessment of efficacy of varicocele repair for male subfertility: A systematic review. Lancet. 2003;361:1849–52. doi: 10.1016/S0140-6736(03)13503-9. [DOI] [PubMed] [Google Scholar]
- 16.Kroese AC, de Lange NM, Collins JA, Evers JL. Varicocele surgery, new evidence. Hum Reprod Update. 2013;19:317. doi: 10.1093/humupd/dmt004. [DOI] [PubMed] [Google Scholar]
- 17.Spanò M, Bonde JP, Hjøllund HI, Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human fertility. The Danish first pregnancy planner study team. Fertil Steril. 2000;73:43–50. doi: 10.1016/s0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 18.Hoshi K, Katayose H, Yanagida K, Kimura Y, Sato A. The relationship between acridine orange fluorescence of sperm nuclei and the fertilizing ability of human sperm. Fertil Steril. 1996;66:634–9. doi: 10.1016/s0015-0282(16)58581-1. [DOI] [PubMed] [Google Scholar]
- 19.Erenpreiss J, Jepson K, Giwercman A, Tsarev I, Erenpreisa J, Spano M. Toluidine blue cytometry test for sperm DNA conformation: Comparison with the flow cytometric sperm chromatin structure and TUNEL assays. Hum Reprod. 2004;19:2277–82. doi: 10.1093/humrep/deh417. [DOI] [PubMed] [Google Scholar]
- 20.González-Marín C, Gosálvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012;13:14026–52. doi: 10.3390/ijms131114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: An update. Am J Reprod Immunol. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 22.Abbasi M, Alizadeh R, Abolhassani F, Amidi F, Ragerdi KI, Fazelipour S, et al. Effect of aminoguanidine in sperm DNA fragmentation in varicocelized rats: Role of nitric oxide. Reprod Sci. 2011;18:545–50. doi: 10.1177/1933719110393028. [DOI] [PubMed] [Google Scholar]
- 23.Smit M, Romijn JC, Wildhagen MF, Veldhoven JL, Weber RF, Dohle GR. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2013;189:146–50. doi: 10.1016/j.juro.2012.11.024. [DOI] [PubMed] [Google Scholar]


