Abstract
Adrenal myelolipomas (AMLs) are rare, benign neoplasms of the adrenal gland with varied clinical presentations. The rarity of these tumors precludes any case-controlled or randomized study into their management. The available literature is limited to case reports and short series from referral centers. This review is an effort to put the available literature into perspective such that clinical decision making can be done with some clarity. The PubMed and Cochrane databases were searched with key words Adrenal Myelolipoma, Adrenal Incidentaloma (AI) and Adrenal Collision Tumor (ACT). From over 1300 search results, 547 relevant publications dating from 1954 to 2014 were reviewed. Details of about 1231 AMLs in the indexed literature were analyzed. Increasing usage of imaging studies has significantly increased the discovery of AMLs. Although AMLs are benign tumors, those measuring larger than 6 cm are prone to rupture and hemorrhage. Thorough endocrine work-up may benefit a selected group of patients, especially those who are hypertensive, diabetic/pre-diabetic, young patients (<50 years) and those with bilateral AML. Regular observation is needed for AML patients who are being treated non-operatively, as many of them may require surgery during follow-up. Although the AACE/AAES guidelines for AI (2009) exclude AML from mandatory metabolic work-up for a newly discovered AI, we feel that a significant number of patients with AML would benefit from metabolic work-up. In the literature, endocrine dysfunction in AML is 7% as compared with 11% in AI. Endocrine dysfunction in AML is probably underdiagnosed.
Keywords: Adrenal collision tumors, adrenal incidentaloma, adrenal myelolipoma, adrenalectomy
INTRODUCTION
History and background
Adrenal myelolipoma (AML) was first described by Arnold as “adrenal lipoma” in 1866, but it was Gierke in 1905 who described the myeloid component.[1] The term “myelolipoma” was coined by Oberling in 1929. Most of the early literature describes it as an incidental autopsy finding or as occasional case reports. In the last two decades, widespread use of imaging studies has resulted in a dramatic increase in the incidentally discovered adrenal masses. As a result, endocrine associations across the world are issuing successive guidelines for the management of adrenal incidentalomas (AIs).
Pathology
AML is a rare, benign tumor of the adrenal gland of unknown etiology. The tumor usually shows varying degrees of lipomatous as well as myeloid tissue. Typically, there is lipid tissue containing an active bone marrow component, showing normal trilineage hematopoiesis but with markedly increased number of megakaryocytes [Figure 1]. It is categorized as Type I: Predominantly lipomatous tissue with minimal hematopoietic elements and Type II: Hematopoietic elements predominate. Myelolipomas originate in the adrenal cortex and their surfaces consist of a pseudocapsule of compressed zona glomerulosa and fasciculate tissue.
Figure 1.
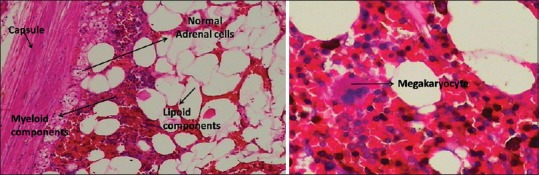
Adrenal myelolipoma — normal adrenal tissue in the periphery, tumor shows intermixed areas of lipomatous and myelomatous elements with a megakaryocyte
Theories of origin and genetics
The presence of hematopoietic cells and endocrine activity in these tumors has initiated many theories of origin of these cells, like ectopic myeloid tissue, embolism of bone marrow element, hamartosis, metaplasia and proliferation of hematopoietic cells by substances from tumor necrosis. Feng et al.[2] hypothesized that the fat component is derived from the mesenchymal stem cells of stromal fat of the adrenal cortex. Then, circulating hematopoietic cells are recruited possibly due to the release of granulocyte colony stimulating factor by adrenal cortical tissue. A widely accepted theory is metaplasia of the reticulo-endothelial cells of the blood capillaries. The majority of AMLs also have non-random X-chromosome inactivation, suggesting a clonal origin of these tumors. Stromal and vascular patterns are different from that of the bone marrow pattern.[3] Its association with different adrenal disorders like adrenal carcinoma, Cushing's syndrome, congenital 21-hydroxylase deficiency and pheochromocytoma is well documented. Although MEN-1 association has been suggested in some of the reported cases, complete DNA sequencing yielded no hint that defects of the MEN-1 gene is responsible for the formation of AML.[4] Low levels of p53 proteins in AML suggest the role of tumor suppressor gene in the tumorogenesis of AML.[5]
Chronic stimulation by ACTH has been implicated in the origin of AML. Increasing incidence with advancing age and stress has been cited as evidence for the role of hormonal overstimulation of the adrenals. Higher incidence of AML in patients with untreated congenital adrenal hyperplasia (CAH) and occurrence of bilateral AML in patients with CAH probably points toward the role of chronic ACTH overstimulation of the adrenals. An increasing incidence of large AMLs has been reported in patients with hemolytic anemia, and such an association suggests that tumor growth is under the extrinsic stimulation by erythropoietin. Thus, extramedullary adrenal hematopoiesis may contribute to the pathogenesis of AML.
Imaging features
Increasing usage of cross-sectional imaging has resulted in increased detection of AI. Most AMLs are small, benign and hormonally inactive, and can be managed conservatively. Ultrasound is usually inadequate for the diagnosis. On computed tomography (CT), AML appears as relatively well-circumscribed fat containing adrenal mass. The attenuation values are low (-20 to -30 Hounsfield units [HU]; Figure 2]; Evidence level 2 [EL 2]] reflecting, the mixture of adipose and myeloid elements. The amount of the fatty component is variable.
Figure 2.
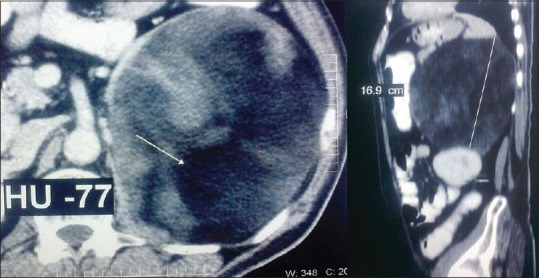
Computed tomography scan of a giant adrenal myelolipoma showing low attenuation (-70 HU)
AML may show atypical radiological features in the presence of a high bone marrow: fat ratio, hemorrhage or calcification. On CT, AML needs to be differentiated from adrenal adenoma, adrenal carcinoma and liposarcoma. Adenomas are usually small, homogeneous round masses with smooth margins with relatively low density and mild enhancement after intravenous (IV) contrast with up to 50% washout on delayed study (EL 2). They have typical attenuation values of -10 to -30 HU. (EL 3). Adrenal carcinoma usually has an irregular margin and non-homogeneous density with marked enhancement after IV contrast. For AI, a size of 4 cm is considered as a reasonable cut-off, above which the incidence of malignancy increases (EL 3). Liposarcomas have inhomogeneous attenuation with evidence of significant amount of soft tissue within the fatty mass. They show poor definition of adjacent structures and evidence of infiltration of surrounding structures. High-grade sarcomas are devoid of macroscopic fat.
Magnetic resonance imaging (MRI) also accurately depicts both microscopic and macroscopic fat in AML using chemical shift imaging and the explicit fat saturation technique, respectively. On T1-weighted sequence, AMLs appear hyperintense due to fat content. They typically show fat suppression on T1-(FS). On T2-weighted sequences, they are usually intermediate to hyperintense masses, but can vary depending on the content, especially the myeloid component. The T1-weighted sequence with contrast (Gadolinium) shows striking enhancement. In masses with mixed components, out-of-phase imaging may show signal loss because macroscopic fat cells usually have very little intracellular water.
Malignancies have low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. After Gadolinium, adenomas show rapid contrast enhancement and rapid wash-out, whereas malignant lesions show strong enhancement and slow washout. Presence of necrosis, hemorrhage and calcification is suspicious of malignancy, although they can occur in large benign lesions like AML.
Endocrine dysfunction
Hormonal dysfunction in AML is a subject that has not been highlighted adequately. AMLs are generally considered as non-secreting AIs. The National Institute of Health Consensus Panel on AI (2002) concluded that AML can be regarded as an exception to the mandatory metabolic work-up of a newly discovered adrenal mass.[6] Similar recommendations have been made by the American Association of Clinical Endocrinologists/American Association of Endocrine Surgeons (AACE/AAES) Guideline 2009[7] and the Italian Guidelines (2011) on AI.[8]
The rarity of this tumor limits the extent to which it can be studied. Producing Level 1 or 2 evidence is relatively difficult in such tumors. No randomized controlled trial or metaanalysis has been reported so far. Larger reported series from referral centers usually do not exceed 10–20 cases over a decade. We had recently managed a case of catecholamine-secreting giant AML at our institution. In view of the uncertainties faced while managing this patient, we have reviewed the available literature, particularly highlighting the endocrine dysfunction in AML.
MATERIALS AND METHODS
The PubMed (Medline) and Cochrane Library databases were searched with the keywords - Adrenal Myelolipoma, Adrenal Incidentaloma and Adrenal Collision Tumor. A total of 547 relevant publications from 1954 to 2014 were reviewed. One hundred and forty-two publications were studied in detail. There were about 584 cases of AML reported in 367 published case reports and case series of AML. In addition, there are 381 cases of AML reported in other published series of laparoscopic and open adrenalectomy performed for adrenal masses. Further, we could gather about 266 AMLs reported in the radiological series and series of AI. Thus, we could find over 1231 AMLs reported in the indexed literature.
RESULTS
Is it a rare neoplasm?
Widespread use of imaging modality has resulted in a 20-fold increase in the detection of AI. Less than 300 cases of AML were reported prior to the year 2000.[9] In the last decade, there has been a dramatic increase in the number of reported cases. We could collect 1231 reported cases of AML in the literature. The incidence is likely to rise further as the use of imaging modalities increases.
In the reported studies, the incidence of AI in cross-sectional imaging studies varies from 2% to 5%. Bovio et al.[10] reported the prevalence of AI in imaging studies to be 4.4%. Song et al.[11] reported the prevalence of AI at 5% in 1049 patients. The rate of detection of AI on the higher resolution of imaging study is up to 8.7% (EL 3).
Song et al. reported the incidence of AML in AI at 6%[11]; thus, AMLs are the second most common AIs after adrenal adenoma (75%). Bin et al. reported a 16% incidence of AML in AI in the Chinese population.[12] Mantero et al., in an Italian study, reported that 8% of AIs are AMLs (EL 3).[13]
In the literature, we found 173 AMLs among 2811 AIs, which were reported in 27 published series; thus, AML accounts for approximately 6% of the AIs. Also, in 36 large series of adrenalectomies, we found 171 cases of AML in over 4216 adrenalectomies for adrenal masses, i.e. AMLs account for 4% of adrenal masses requiring adrenalectomy (EL 4).
The incidence of AML increases with age. Most cases are reported in the fifth to seventh decades of life. The youngest patient reported is a 17-month-old child with Beckwith–Wideman syndrome[14] and the oldest patient operated for AML was 82 years old. Although AMLs occur in both the sexes, there are some studies suggesting a higher prevalence in men. Some publications report that AML is three times more common on the right side than on the left.[15] About 12% cases are bilateral. We found about 26 reported cases of bilateral AML.
Indications for surgery
Accepted indications for surgical excision are symptomatic tumor, size greater than 4 cm, metabolically active tumor and suspicion of malignancy on imaging study (Grade C recommendation, EL 3).
While smaller AMLs can be managed conservatively, size of more than 4 cm is the most common indication for surgery in AML. The AACE/AAES guideline 2009[7] recommends adrenalectomy for all AIs measuring more than 4 cm. AMLs can remain asymptomatic till they grow to a great size [Figure 3]. Those measuring more than 10 cm in diameter have been termed giant AMLs. We could gather 51 cases of giant AMLs in the literature. The biggest reported AML weighed 6 kgs. Larger AMLs tend to become symptomatic due to pressure effects and due to intralesional hemorrhage and infarctions.
Figure 3.
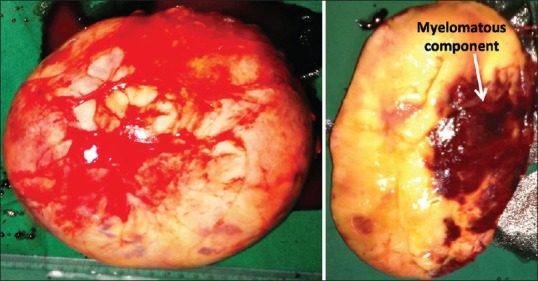
Resected specimen of a giant adrenal myelolipoma. The tumor was secreting catecholamines. The patient became normotensive after excision of the tumor
AMLs can present as acute abdomen due to retroperitoneal, intraperitoneal or intralesional bleeding. Larger AMLs are prone to spontaneous rupture or rupture due to trivial trauma. Such tumors are often mistaken for retroperitoneal liposarcoma or renal angiolipoma and unnecessary radical surgeries like nephrectomy have been reported.[16] There are about 24 reported cases of spontaneous rupture of AMLs in the literature. Rupture has been reported in tumors as small as 7 cm. This probably justifies excision of all AMLs larger than 4 cm, even if they are benign and asymptomatic (EL 4).
Non-operative treatment for retroperitoneal hemorrhage due to AML has also been reported.[17] In two of the reported cases, embolization was performed to control retroperitoneal hemorrhage and then excision of the AML was carried out as an elective procedure.[18,19] Acute abdomen due to vasoocclusive crisis in AML has been reported in a case of sickle cell disease.[20] Malignant degeneration has not been reported in the literature.
Imaging and image-guided biopsy
Imaging studies are accurate in diagnosing AML in up to 90% of the cases. In those where malignancy cannot be ruled out, fine needle aspiration cytology is indicated prior to surgery. On CT, macroscopic fat has an attenuation of -30 to -100 HU (EL 2]. On MRI, macroscopic fat shows high T1 and T2 signal intensities with signal loss following fat suppression.[21] MRI is unlikely to be of additional benefit in patients with equivocal findings on CT.[22] Particularly, AML with infarction or hemorrhage can show gradual persistent enhancement and highest attenuation during delayed contrast-enhanced CT or strongest enhancement during delayed contrast-enhanced MRI.[23] Twenty-seven percent of the AMLs may show calcification due to previous hemorrhage and necrosis.[24]
Multimodality imaging is required in patients with secreting AML, AML with adrenal collision tumor (ACT) and AML with a suspicion of malignancy. 18-Fluro-Deoxy Glucose (FDG)-PET shows increased uptake in the malignant component and guides the percutaneous biopsy.[25] Lipid-rich AI with FDG uptake more than that of the liver are likely to be secreting ones.[26] AML with extensive adenomatous and hematopoietic elements may mimic malignancy on FDG-PET.[27,28]
Image-guided biopsy may be needed in up to 7% of AI. Overall sensitivity and negative predictive value of adrenal biopsy was 73% and 60%, respectively.[29] Before biopsy, it is essential to rule out catecholamine secretion as pheochromocytomas are likely to have catecholamine surge during biopsy. Although some of the AML are found to be secreting catecholamine, we did not find any report of MIBG scan being of value in differentiating between AML and pheochromocytoma.
In the literature, the following entities have been mistaken for AML on imaging — marginal lymphoma, hybernomas, angiomyolipoma, teratoma, liposarcoma, pheochromocytoma, adenoma, ganglioneuroma, carcinoma, hemorrhagic cyst and metastatic lesions.
Imaging modalities are also useful in the long-term surveillance of the patients with AI/AML.
Is minimal access surgery feasible?
Laparoscopic adrenalectomy is being increasingly used in referral centers in the surgical management of AML, and we found over 21 published reports. Even larger AML with diameters up to 14–15 cm have been excised through transperitoneal laparoscopy.[30,31] Retroperitoneoscopy and hand-assisted laparoscopic excision have also been reported.[32,33] Operative time, blood loss, complication rates, etc., are comparable to open surgical excision. Because AMLs are well-encapsulated and less-vascular tumors, the conversion rate is also low. As AML patients tend to have very high BMI (36+/- 8 kg/sq.mt),[34] laparoscopy is of particular benefit.
Surgical complications are usually rare with small AML. However, complications may be expected with giant AML and functioning AML. Giant AMLs are known to cause pressure on the inferior vena cava on the right side. Significant displacement of the kidney may require nephropexy after resection of giant AML. Manipulation and retraction of giant AMLs is difficult during surgery, with a risk of avulsion of the adrenal vein. Shen et al. described retro-peritoneoscopic liposuction for large AMLs (8–14 cm diameter).[35] Tumor resection could easily be performed after deflation and shrinkage of tumor by liposuction. Blood loss was minimal and there was no recurrence after follow-up of 77 months. This may be an acceptable approach if carcinoma is unequivocally ruled out. We have found four reported cases of adrenal carcinoma coexisting with AML. Therefore, it is debatable whether liposuction is an acceptable approach.
In AML with endocrine dysfunction, pre-operative correction of metabolic derangement and hormonal dysfunction is optimal. In AML patients with undiagnosed CAH, perioperative complications can be expected if steroid replacement and correction of electrolyte imbalances is not performed before surgery. In our patient with catecholamine-secreting giant AML, in spite of pre-operative alfa and beta blockers to control hypertension, intraoperative manipulation of the AML resulted in a brief surge of blood pressure.
Is metabolic work-up not necessary?
AMLs are generally considered as non-secreting AIs. The AACE/AAES Guideline on Adrenal Incidentaloma (2009)[7] (and its earlier version, National Institutes of Health Consensus conference, 2002) states “The detection of an adrenal lesion should prompt biochemical evaluation unless it is an obvious myelolipoma. Adrenal myelolipomas are of low CT attenuation and also contain fat (-10 to -20 Hounsfield units [HU]); therefore, the diagnosis is generally clear.”
Our review of the literature showed that significant numbers of AML are associated with endocrine dysfunction. Reported cases in the literature show three distinct types of endocrinal dysfunctions: hormone secreting AMLs, AML occurring in patients with CAH, and AML occurring in association with a secreting ACT.
We could gather 85 reported cases of AML with endocrine dysfunction. Of them, 28 cases were secreting AMLs, 43 cases were AMLs occurring in association with CAH and 14 cases were of ACTs with hormonal hypersecretion. Among 28 cases of secreting AMLs, majority are reported with Cushing's syndrome or with hyperaldosteronism. There are five cases of catecholamine secretion and two cases of androgen-secreting AMLs. In addition to the 85 reported cases of endocrine abnormality, we found seven case reports of AMLs in hypertensive patients who became normotensive after resection of AML. There is a reported case of hypertensive central chorioretinopathy that completely resolved after the excision of AML.[36] There is also a reported case of dissecting aortic aneurysm in a woman with AML who underwent repair of aortic aneurysm followed by excision of AML, showing remission of hypertension after the excision of AML.[37] Camarero et al.[38] reported a case of AML where hyperaldosteronism was undiagnosed and the patient was observed for 5 years since the AML was a proven to be a benign tumor on fine needle aspiration biposy. During this period, the patient had severe hypertension and required renal transplantation due to malignant nephroangiosclerosis. In this patient, 2 years after renal transplantation, AML was implicated as a cause for the hypertension and renal failure. Removal of AML resulted in marked reduction in hypertension. Ahsan et al. reported a case of AML with Type-2 diabetes mellitus, whose diabetes was cured following excision of AML.[39] Joy et al. reported a case of giant AML presenting with cardiac failure due to uncontrolled hypertension and atrial fibrillation, in which excision of AML resulted in significant improvement of cardiac failure, atrial fibrillation and hypertension. Endocrine work-up was incomplete in this case as well.[40] We feel that endocrine abnormality in AML is underdiagnosed because of inadequate pre-operative metabolic work-up.
AML with CAH accounts for nearly half of the reported cases of AML with endocrine dysfunction. Etiology is hypothesized to be secondary to chronic stimulation of the adrenals by ACTH due to undiagnosed or inadequately treated CAH. Of the reported 43 cases, 10 patients had bilateral tumors, and most of the patients are younger than the average age of presentation of AML, the youngest patient reported being 10 years old. AML has been reported in CAH due to 21-Hydroxylase, 17-Hydroxylase and 11β Hydroxylase deficiencies.[41] In many of the cases, AML occurred in previously undiagnosed and uncontrolled cases of CAH, and the diagnosis of CAH is made during the metabolic work-up for AML.[42] Nermoen et al. reported a prevalence of 11% of AML in Norwegian patients with CAH who were on treatment (EL 2b).[43]
AMLs have been reported in association with other tumors arising from the adrenals. Such tumors have been named as ACT (probably, adrenal co-lesion tumors would have been a better terminology). We collected about 33 reports of ACTs. Non-functioning adrenal adenomas are the most common co-lesions occurring with AML. There are about eight cases of secreting adrenal adenomas presenting with Cushing's syndrome. Oncocytic adenoma with virilization, hybernoma, ganglioneuroma, pheochromocytoma, corticomedullary mixed tumors and Hodgkin's lymphoma are other reported co-lesion tumors with endocrinal dysfunction. There are four reported cases of adrenal carcinoma reported in association with AML. Pre-operative diagnosis of ACT is difficult and may require an image-guided biopsy. Most of the reported cases of ACTs are diagnosed post-operatively.
AIs are implicated in subclinical Cushing's syndrome and metabolic syndrome.[44] The overall reported incidence of hormonal hyperactivity in AI is about 11%.[7] Our review shows that 7% of AMLs (85 of 1231 cases) are associated with endocrine dysfunction (EL 4]. We feel that endocrine dysfunction is probably underreported in patients with AML because endocrine work-up is often not performed. Among patients with AML, those who have hypertension, diabetes/pre-diabetes, young patients with AML and patients with bilateral AML are the ones who have a high incidence of associated endocrine dysfunction [Figure 4]. Therefore, we feel that this subset of patients should undergo endocrine workup (Grade D recommendation, EL 4) for the pituitary–adrenal axis, rennin–angiotensin–aldosteron system, catecholamine metabolism and undiagnosed CAH. On the other hand, there can be reporting bias for the AMLs with hormonal dysfunction, i.e. AMLs with hormonal dysfunction are more likely to be reported and accepted for publication; thus, the figure of 7% may not be truly reflective of the incidence of hormonal dysfunction in AML.
Figure 4.
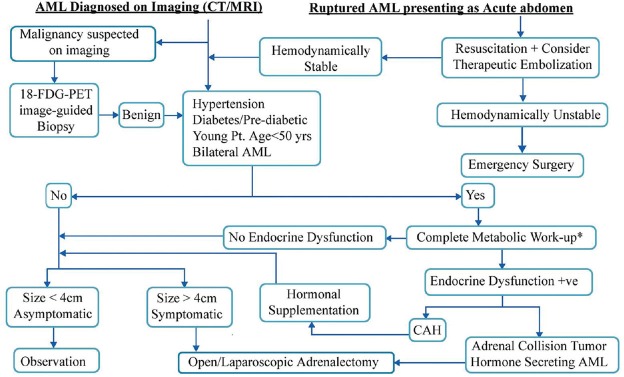
Flowchart for the management of adrenal myelolipoma. *For details of metabolic work-up, refer to the AACE/AAES Guideline 2009.[7] In addition, CAH has to be ruled out by serum level estimation of 17-Hydroxyprogesteron and 11-deoxycortisol
Is conservative treatment justified?
For asymptomatic AMLs that measure less than 4 cm, if malignancy is ruled out, the recommended line of management is observation. The AACE/AAES Guideline (2009) recommends that AIs that are observed without surgical excision should undergo radiological evaluation at 3 and 6 months and then annually for 1–2 years. Hormonal evaluations should be performed annually for 5 years (Grade C recommendation, EL 3). Melck et al.[45] concluded that the cost of surveillance of AI for more than 9 years exceeded the cost of surgery for AI. There are few reports of long-term follow-up in patients with AML. Imamura et al. reported tumor size doubling time of 16–31 months in a patient of bilateral AML.[46] Han et al. reported that nearly half of the conservatively managed AMLs increased in size or became symptomatic over a —3-year period.[47] Malignant degeneration has not been documented.
Conservative treatment of asymptomatic, non-secreting AML in the elderly is justified. The cost of follow-up in younger patients has to be weighed against the safety and cost of surgery. In the absence of any conclusive evidence, most of the centers worldwide do not operate for an asymptomatic AML less than 4 cm in size.
Being an incidentaloma, in the literature, AML has been reported in association with several diseases like cholelithiasis and malignancies of the kidney, bladder, stomach, lung etc., Interestingly, a case of secondaries from lung carcinoma into an AML has also been reported.[48] Of special interest is its association with hemolytic anemia. There are 11 reported cases in association with thalassemia and one each in association with hereditary spherocytosis and sickle cell anemia. Because the myeloid component of AML represents extramedullary erythropoiesis, hemolytic anemia is implicated in the etiology of AML.[20,49,50] There is an interesting case of AML associated with nephrotic syndrome in one of the publications[51] where the nephrotic syndrome disappeared after the resection of the tumor, which may be coincidental.
CONCLUSION
Management of AI is evolving. Successive guidelines are being issued by various endocrine associations. Widespread use of imaging modalities has resulted in a 20-fold rise in the discovery of AI and AML. Randomized controlled trials and metaanalysis are difficult in rare tumors like AML. The AACE/AAES guidelines for AI state that AMLs are an exception to the mandatory endocrine/metabolic work-up for AI. However, a review of the literature shows that a significant number of AMLs are either secreting hormones or occur in association with either CAH or ACTs. Avoiding metabolic work-up may result in missing out on these conditions (EL 4).
We feel that unequivocal CT/MRI findings suggesting AML should not be a reason to avoid metabolic work-up. A subset of AML patients — those with hypertension, diabetes or pre-diabetes, younger patients and those with bilateral AML – would benefit from endocrine workup (EL 4]. Patients with undiagnosed CAH may present late in life with AML. Such patients benefit by thorough metabolic work-up and steroid replacement (EL 2b]. Several reports of resolution of hypertension and its sequel after the excision of AML suggests that the incidence of hormonal abnormality in AML is underdiagnosed.
Surgeons should be aware of the various presentations of AML, including its occasional presentation as an acute abdomen due to hemorrhage. Larger AMLs measuring more than 6 cm are prone to spontaneous rupture; hence, these require surgical excision even if they are clinically asymptomatic and metabolically inactive. Minimal access surgery is getting acceptance in the treatment of adrenal masses and even larger AMLs are being managed laparoscopically (EL 3b). Of particular interest is retroperitoneoscopic liposuction for AML. However, more evidence is needed before such a procedure can be recommended. The natural history of AML is still unclear — many of those conservatively treated AML may require surgical excision during the follow-up, either due to growth in size or because they become symptomatic.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Plaut A. Myelolipoma in the adrenal cortex; myeloadipose structures. Am J Pathol. 1958;34:487–515. [PMC free article] [PubMed] [Google Scholar]
- 2.Feng C, Jiang H, Ding Q, Wen H. Adrenal myelolipoma: A mingle of progenitor cells. Med Hypotheses. 2013;80:819–22. doi: 10.1016/j.mehy.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Bishop E, Eble JN, Cheng L, Wang M, Chase DR, Orazi A, et al. Adrenal myelolipomas show nonrandom X- chromosome inactivation in hematopoietic elements and fat: Support for a clonal origin of myelolipomas. Am J Surg Pathol. 2006;30:838–43. doi: 10.1097/01.pas.0000202044.05333.17. [DOI] [PubMed] [Google Scholar]
- 4.Schulte KM, Heinze M, Mengel M, Scheuring S, Köhrer K, Röher HD. Complete sequencing and mRNA expression analysis of the MEN-I gene in adrenal myelolipoma. Horm Metab Res. 2000;32:169–73. doi: 10.1055/s-2007-978616. [DOI] [PubMed] [Google Scholar]
- 5.Adleff V, Rácz K, Tóth M, Varga I, Bezzegh A, Gláz E. P 53 protein and its messenger ribonucleic acid in human adrenal tumors. J Endocrinol Invest. 1998;21:753–7. doi: 10.1007/BF03348041. [DOI] [PubMed] [Google Scholar]
- 6.NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”) NIH Consensus State Sci Statements. 2002;19:1–25. [PubMed] [Google Scholar]
- 7.Zeiger MA, Thompson GB, Duh QV, Hamrahian AH, Angelos P, Elaraj D, Fishman E, Kharlip J. American Association of Clinical Endocrinologists; American Association of Endocrine Surgeons. The American Association of Clinical Endocrinologists and American Association of endocrine Surgeons medical guidelines for the management of adrenal incidentalomas Endocr Pract. 2009 Jul-Aug;15(Suppl 1):1–20. doi: 10.4158/EP.15.S1.1. [DOI] [PubMed] [Google Scholar]
- 8.Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi G, et al. AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011;164:851–70. doi: 10.1530/EJE-10-1147. [DOI] [PubMed] [Google Scholar]
- 9.Settakorn J, Sirivanichai, Rangdaeng S, Chaiwun B. Fine-needle aspiration cytology of adrenal myelolipoma: Case report and review of literature. Diagn Cytopathol. 1999;21:409–12. doi: 10.1002/(sici)1097-0339(199912)21:6<409::aid-dc9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29:298–302. doi: 10.1007/BF03344099. [DOI] [PubMed] [Google Scholar]
- 11.Song JH, Chaudhry FS, Mayo-Smith WW. The Incidental Adrenal Mass on CT: Prevalence of Adrenal Disease in 1,049 Consecutive Adrenal Masses in Patients with No Known Malignancy. AJR Am J Roentgenol. 2008;190:1163–8. doi: 10.2214/AJR.07.2799. [DOI] [PubMed] [Google Scholar]
- 12.Bin X, Qing Y, Linhui W, Li G, Yinghao S. Adrenal incidentalomas: Experience from a retrospective study in a Chinese population. Urol Oncol. 2011;29:270–4. doi: 10.1016/j.urolonc.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Montero F, Masini AM, Opocher G, Giovagnetti M, Arnaldi G. Adrenal incidentaloma: An overview of hormonal data from the National Italian Study Group. Horm Res. 1997;47:284–9. doi: 10.1159/000185478. [DOI] [PubMed] [Google Scholar]
- 14.Cardinalli IA, de Oliveira-Filho AG, Mastellaro MJ, Ribeiro RC, Aguiar SS. A unique case of synchronous functional adrenocortical adenoma and myelolipoma within the ectopic adrenal cortex in a child with Beckwith-Wiedemann syndrome. Pathol Res Pract. 2012;208:189–94. doi: 10.1016/j.prp.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Hsu SW, Shu K, Lee WC, Cheng YT, Chiang PH. Adrenal myelolipoma: A 10-year single-center experience and literature review. J Med Sci. 2012;28:377–82. doi: 10.1016/j.kjms.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Amano T, Takemae K, Niikura S, Kouno M, Amano M. Retroperitoneal hemorrhage due to spontaneous rupture of adrenal myelolipoma. Int J Urol. 1999;6:585–8. doi: 10.1046/j.1442-2042.1999.611109.x. [DOI] [PubMed] [Google Scholar]
- 17.Giacinto J, DeMuro JP. Nonoperative management of adrenal myelolipoma hemorrhage resulting from trauma. Am Surg. 2012;78:E463–4. [PubMed] [Google Scholar]
- 18.Nakajo M, Onohara S, Shinmura K, Fujiyoshi F, Nakajo M. Embolization for spontaneous retroperitoneal hemorrhage from adrenal myelolipoma. Radiat Med. 2003;21:214–9. [PubMed] [Google Scholar]
- 19.Chng SM, Lin MB, Ng FC, Chng HC, Khoo TK. Adrenal myelolipoma presenting with spontaneous retroperitoneal hemorrhage demonstrated on computed tomography and angiogram-a case report. Ann Acad Med Singapore. 2002;31:228–30. [PubMed] [Google Scholar]
- 20.Gamss C, Chia F, Chernyak V, Rozenblit A. Giant hemorrhagic myelolipoma in a patient with sickle cell disease. Emerg Radiol. 2009;16:319–22. doi: 10.1007/s10140-008-0740-3. [DOI] [PubMed] [Google Scholar]
- 21.Cyran KM, Kenney PJ, Memel DS, Yacoub I. Adrenal myelolipoma. AJR Am J Roentgenol. 1996;166:395–400. doi: 10.2214/ajr.166.2.8553954. [DOI] [PubMed] [Google Scholar]
- 22.Musante F, Derchi LE, Bazzocchi M, Avataneo T, Gandini G, Pozzi Mucelli RS. MR imaging of adrenal myelolipomas. J Comput Assist Tomogr. 1991;15:111–4. doi: 10.1097/00004728-199101000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Park BK, Kim CK, Kim B, Kwon GY. Adrenal tumors with late enhancement on CT and MRI. Abdom Imaging. 2007;32:515–8. doi: 10.1007/s00261-006-9156-2. [DOI] [PubMed] [Google Scholar]
- 24.Lam KY, Lo CY. Adrenal lipomatous tumours: A 30 year clinicopathological experience at a single institution. J Clin Pathol. 2001;54:707–12. doi: 10.1136/jcp.54.9.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katabathina VS, Flaherty E, Kaza R, Ojili V, Chintapalli KN, Prasad SR. Adrenal collision tumors and their mimics: Multimodality imaging findings. Cancer Imaging. 2013;13:602–10. doi: 10.1102/1470-7330.2013.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takanami K, Kaneta T, Morimoto R, Satoh F, Nakamura Y, Takase K, et al. Characterization of lipid-rich adrenal tumors by FDG PET/CT: Are they hormone-secreting or not? Ann Nucl Med. 2014;28:145–53. doi: 10.1007/s12149-013-0793-6. [DOI] [PubMed] [Google Scholar]
- 27.Castinetti F, Verschueren A, Cassagneau P, Brue T, Sebag F, Daniel L, et al. Adrenal myelolipoma: An unusual cause of bilateral highly 18F-FDG-avid adrenal masses. J Clin Endocrinol Metab. 2012;97:2577–8. doi: 10.1210/jc.2012-1713. [DOI] [PubMed] [Google Scholar]
- 28.Bedrna J, Nozicka Z. Giant myelolipoma of the adrenal gland. Rozhl Chir. 2003;82:403–6. [PubMed] [Google Scholar]
- 29.Osman Y, El-Mekresh M, Gomha AM, Mohsen T, Taha N, Hussein N, et al. Percutaneous adrenal biopsy for indeterminate adrenal lesion: Complications and diagnostic accuracy. Urol Int. 2010;84:315–8. doi: 10.1159/000288235. [DOI] [PubMed] [Google Scholar]
- 30.Castillo OA, Vitagliano G, Cortes O, Sánchez-Salas R, Arellano L. Laparoscopic adrenalectomy for adrenal myelolipoma. Arch Esp Urol. 2007;60:217–21. doi: 10.4321/s0004-06142007000200022. [DOI] [PubMed] [Google Scholar]
- 31.Maestroni U, Ziglioli F, Dinale F, Ferretti S, Frattini A, Cortellini P. Is laparoscopy contraindicated in giant adrenal masses? Surg Laparosc Endosc Percutan Tech. 2010;20:288–90. doi: 10.1097/SLE.0b013e3181ec2ab0. [DOI] [PubMed] [Google Scholar]
- 32.Yin L, Teng J, Zhou Q, Liu Y, Yao Y, Gao Y, et al. A 10-Year Single-Center Experience with Surgical Management of Adrenal Myelolipoma. J Endourol. 2014;28:252–5. doi: 10.1089/end.2013.0283. [DOI] [PubMed] [Google Scholar]
- 33.Shen WT, Kebebew E, Clark OH, Duh QY. Reasons for conversion from laparoscopic to open or hand-assisted adrenalectomy: Review of 261 laparoscopic adrenalectomies from 1993 to 2003. World J Surg. 2004;28:1176–9. doi: 10.1007/s00268-004-7620-0. [DOI] [PubMed] [Google Scholar]
- 34.Gershuni VM, Bittner JG, Moley JF, Brunt LM. Adrenal myelolipoma: Operative indications and outcomes. J Laparoendosc Adv Surg Tech A. 2014;24:8–12. doi: 10.1089/lap.2013.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen X, Qiu Y, Zheng Y, Zhang S. Retroperitoneal laparoscopic liposuction for large adrenal myelolipomas: A report of nine cases. J Laparoendosc Adv Surg Tech A. 2012;22:578–80. doi: 10.1089/lap.2012.0113. [DOI] [PubMed] [Google Scholar]
- 36.Katsimpris JM, Vandoros M, Petropoulos IK, Andrikopoulos P. Central serous chorioretinopathy associated with adrenal myelolipoma. Klin Monbl Augenheilkd. 2003;220:199–203. doi: 10.1055/s-2003-38187. [DOI] [PubMed] [Google Scholar]
- 37.Jinno T, Tago M, Yoshida H, Yamane M. DeBakey IIIb type dissecting aneurysm with adrenal myelolipoma; report of a case. Kyobu Geka. 2002;55:1065–7. [PubMed] [Google Scholar]
- 38.Camarero-Temiño V, Mercado-Ortiz V, Hijazi-Prieto B, Abaigar-Luquin P. Adrenal myelolipoma associated with primary hyperaldosteronism. Nefrologia. 2012;32:124–5. doi: 10.3265/Nefrologia.pre2011.Nov.11195. [DOI] [PubMed] [Google Scholar]
- 39.Ahsan T, Kanwal S, Banu Z, Jabeen R. Virilization with adrenal myelolipoma, adrenal hyperplasia, and fibroadenoma of breast. J Coll Physicians Surg Pak. 2010;20:819–21. [PubMed] [Google Scholar]
- 40.Joy PS, Marak CP, Nashed NS, Guddati AK. Giant adrenal myelolipoma masquerading as heart failure. Case Rep Oncol. 2014;7:182–7. doi: 10.1159/000360981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.John M, Menon SK, Shah NS, Menon PS. Congenital adrenal hyperplasia 11beta-hydroxylase deficiency: Two cases managed with bilateral adrenalectomy. Singapore Med J. 2009;50:e68–70. [PubMed] [Google Scholar]
- 42.Nermoen I, Følling I, Vegge K, Larmo A, Nedrebø BG, Husebye ES, et al. Two adults with adrenal myelolipoma and 21-hydroxylase deficiency. Case Rep Med 2009. 2009 doi: 10.1155/2009/916891. 916891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nermoen I, Rørvik J, Holmedal SH, Hykkerud DL, Fougner KJ, Svartberg J, et al. High frequency of adrenal myelolipomas and testicular adrenal rest tumors in adult Norwegian patients with classical congenital adrenal hyperplasia because of 21-hydroxylase deficiency. Clin Endocrinol (Oxf) 2011;75:753–9. doi: 10.1111/j.1365-2265.2011.04151.x. [DOI] [PubMed] [Google Scholar]
- 44.Terzolo M, Pia A, Alì A, Osella G, Reimondo G, Bovio S, et al. Adrenal incidentaloma: A new cause of the metabolic syndrome? J Clin Endocrinol Metab. 2002;87:998–1003. doi: 10.1210/jcem.87.3.8277. [DOI] [PubMed] [Google Scholar]
- 45.Melck AL, Rosengart MR, Armstrong MJ, Stang MT, Carty SE, Yip L. Immediate laparoscopic adrenalectomy versus observation: Cost evaluation for incidental adrenal lesions with atypical imaging characteristics. Am J Surg. 2012;204:462–7. doi: 10.1016/j.amjsurg.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Imamura R, Kinouchi T, Fujimoto M, Meguro N, Maeda O, Saiki S, et al. Bilateral synchronous adrenal myelolipomas: A case report. Hinyokika Kiyo. 1998;44:801–3. [PubMed] [Google Scholar]
- 47.Han M, Burnett AL, Fishman EK, Marshall FF. The natural history and treatment of adrenal myelolipoma. J Urol. 1997;157:1213–6. [PubMed] [Google Scholar]
- 48.Althoen MC, Siegel A, Tsapakos MJ, Seltzer MA. Lung cancer metastasis to an adrenal myelolipoma detected by PET/CT. Clin Nucl Med. 2011;36:922–4. doi: 10.1097/RLU.0b013e318217ae93. [DOI] [PubMed] [Google Scholar]
- 49.Gamberini MR, Prandini N, Chiodi E, Farneti C, Garani MC. Adrenal incidentaloma in thalassemia: A case report and literature review. Pediatr Endocrinol Rev. 2011;8(Suppl 2):324–30. [PubMed] [Google Scholar]
- 50.Sekido N, Kawai K, Takeshima H, Uchida K, Akaza H, Koiso K. Adrenal myelolipoma associated with hereditary spherocytosis. J Urol. 1996;3:61–3. doi: 10.1111/j.1442-2042.1996.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 51.Alexopoulos E, Kirmizis D, Visvardis G, Grollios G, Leontsini M, Memmos D. Focal segmental glomerulosclerosis in a patient with large bilateral asymptomatic adrenal myelolipomas. Ren Fail. 2003;25:1051–6. doi: 10.1081/jdi-120026041. [DOI] [PubMed] [Google Scholar]


