Abstract
We established a relationship between cognitive deficits and cortical circuits in the LgDel model of 22q11 Deletion Syndrome (22q11DS)—a genetic syndrome with one of the most significant risks for schizophrenia and autism. In the LgDel mouse, optimal acquisition, execution, and reversal of a visually guided discrimination task, comparable to executive function tasks in primates including humans, are compromised; however, there is significant individual variation in degree of impairment. The task relies critically on the integrity of circuits in medial anterior frontal cortical regions. Accordingly, we analyzed neuronal changes that reflect previously defined 22q11DS-related alterations of cortical development in the medial anterior frontal cortex of the behaviorally characterized LgDel mice. Interneuron placement, synapse distribution, and projection neuron frequency are altered in this region. The magnitude of one of these changes, layer 2/3 projection neuron frequency, is a robust predictor of behavioral performance: it is substantially and selectively lower in animals with the most significant behavioral deficits. These results parallel correlations of volume reduction and altered connectivity in comparable cortical regions with diminished executive function in 22q11DS patients. Apparently, 22q11 deletion alters behaviorally relevant circuits in a distinct cortical region that are essential for cognitive function.
Keywords: anterior cingulate cortex, cortical circuit disorders, interneurons, projection neurons, reversal learning
Introduction
22q11 Deletion Syndrome (22q11DS) is one of the most significant genetic risks for autism, schizophrenia, and other cortical circuit disorders thought to arise during development (Geschwind and Levitt 2007; Niklasson et al. 2009; Meechan et al. 2011). Cognitive disabilities are common in 22q11DS; however, not all domains are affected equally: executive functions including working memory and attention are more frequently compromised (Gerdes et al. 1999; Swillen et al. 1999; Woodin et al. 2001; Campbell et al. 2010). Prefrontal and anterior cingulate regions essential for these functions are altered in 22q11DS patients (Schaer et al. 2006; Bearden et al. 2009; Shashi et al. 2010). Nevertheless, it remains unknown if circuits within these regions are disrupted, how disruptions arise, and whether they are related to behavioral deficits. Thus, we asked whether circuit elements are specifically compromised, reflecting altered development, in a distinct cortical region crucial for an executive function-related task in the LgDel mouse (Merscher et al. 2001), a genomically accurate model of 22q11DS.
Current behavioral paradigms assess a variety of cognitive capacities in mice (van der Staay and Steckler 2001), and some tasks apparently depend upon the integrity of distinct cortical regions. Touchscreen mediated visual discrimination/reversal tasks have emerged as a relevant assay for murine executive functions, and these tasks can be associated with distinct areas of the frontal cortex (Bussey et al. 2001; Brigman et al. 2010, 2012). Recent behavioral analyses in 22q11DS patients indicate that distinct aspects of executive function, some of which may engage behavioral mechanisms and frontal cortical areas similar to those used for reversal tasks in mice, are selectively compromised (Shashi et al. 2010; Shapiro et al. 2013). Indeed, the full range of capacities that comprise executive function, including working memory and attention are altered in 22q11DS (Karayiorgou et al. 2010). Thus, in the LgDel 22q11DS mouse model, we focused on visual discrimination/reversal performance as an indication of cognitive capacity relevant to executive functions that rely upon frontal cortical circuits.
There is little evidence that genetic lesions associated with cortical circuit disorders alter cognition in register with developmental changes in relevant cortical regions. We therefore focused on changes that reflect 2 developmental anomalies in the LgDel frontal cortex. First, disrupted migration leads to aberrant laminar position of parvalbumin-labeled GABAergic interneurons. Second, altered proliferation of basal progenitors—subventricular zone precursors that produce projection neurons for all layers (Kowalczyk et al. 2009)—reduces layer 2/3 projection neuron frequency (Meechan et al. 2009, 2012). Previously, we found pronounced changes in area 6 (Caviness 1975), lateral frontal motor/association cortex, lAFC. In 22q11DS patients, prefrontal and anterior cingulate cortices are altered in parallel with executive function (Bearden et al. 2009; Shashi et al. 2010). In mice, aspects of the discrimination/reversal task depend upon the medial frontal/anterior cingulate cortex, medial anterior frontal cortex (mAFC), (areas 24/25; Caviness 1975; Bussey, Everitt, et al. 1997; Brigman and Rothblat 2008) often compared with human prefrontal cortex. Thus, using the LgDel mouse, we asked whether developmental anomalies that alter frontal cortical circuits are related to cognitive capacities that rely upon integrity of those circuits in adults.
We found that LgDel mice, as a group, are cognitively impaired, similar to 22q11DS patient populations. As with variable deficits in individual 22q11DS patients, some LgDel mice are more impaired than others. Cognitive impairment in LgDel mice is paralleled by changes in mAFC GABAergic interneurons and layer 2/3 projection neurons—mature manifestations of developmental disruptions we reported previously (Meechan et al. 2009, 2012). When behavioral performance and these cortical circuit phenotypes are compared, a robust and specific relationship between diminished cognitive capacity and mAFC projection neuron frequency is seen. Apparently, the degree of disrupted circuit construction in a cortical region critical for executive functions, similar to those altered in 22q1DS patients, predicts cognitive impairment in the mouse model of 22q11DS.
Materials and Methods
Mice
The George Washington University Institutional Animal Care and Use Committee (IACUC) reviewed and approved all animal procedures. The LgDel line (Merscher et al. 2001) has been maintained on a C57/Bl6N background in our colony since 2003 (>25 generations). We insure the consistency of the background by routine backcrosses to C57Bl6N stock from the vendor (Charles River Laboratories, Wilmington, MA, USA). Eleven LgDel and 11 wild-type (WT) males, generated from 6 litters in which the LgDel was transmitted paternally, were analyzed. These males were group housed as littermates for ∼10 weeks when the food deprivation schedule for behavioral testing was begun. Each animal was then housed individually on a 12-h light–dark cycle with unlimited access to water. Each individually housed mouse was put on a restricted diet and weighed daily until it reached 85% of its free feeding weight. Behavioral testing was begun immediately thereafter.
Behavioral Testing
The 11 LgDel and 11 WT male mice were trained using an infrared LCD touchscreen apparatus by operators blind to animal genotype (Rutz and Rothblat 2012). Initially, mice were rewarded (at a pellet food dispenser in a central location distal to the screen) for nose-poking the correct visual stimulus, independent of left/right position. Animals are given 20 “first-choice” trials per session (e.g., each trial requires correctly choosing vertical instead of horizontal bars; Fig. 1A, left) with the correct stimulus equally distributed on the left or right over the session. Animals successfully reach criterion when they make the correct discrimination between vertical and horizontal bars 80% of the time in a session (16 of 20 first-choice trials). When an incorrect choice is made during first-choice trials, the same stimulus in the same location is presented until the correct choice is made. For the acquisition phase, we report total number of sessions to criterion, total number of errors made while acquiring the task, and the time spent performing the task per session when criterion is reached.
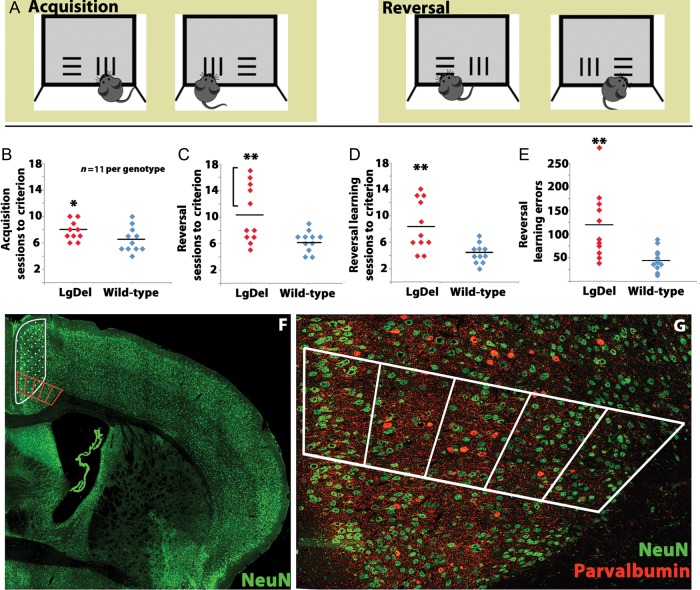
Figure 1.
Behavioral assessment and cortical localization of cognitive functions in LgDel and WT mice. (A) The touchscreen visual discrimination/reversal task assesses murine executive functions. At left: The mouse initially learns to discriminate which touch-sensitive pattern—vertical versus horizontal lines—presented on the LCD screen is associated with food reward, irrespective of screen position. At right: During reversal learning, the pattern associated with food reward is switched. (B) During the initial acquisition phase of the behavioral task, LgDel animals take modestly longer to learn the rule associated with food reward, *P < 0.05 (C) LgDel animals require more reversal sessions to learn that the pattern associated with food reward has been reversed, **P < 0.01. (D) The number of learning sessions required by LgDel animals during the reversal phase was significantly increased, **P < 0.01. (E) The number of learning errors made by LgDel animals during the reversal phase was also greater, **P < 0.01. (F) The medial anterior frontal cortex (mAFC) from behaviorally tested animals was assessed histologically (red counting box). This region overlaps with the lesioned regions (hatched area, based upon Brigman and Rothblat 2008) shown to be important for reversal learning. (G) Parvalbumin-labeled GABAergic interneuron distribution, perisomatic parvalbumin-labeled puncta (presumed to be synaptic endings, not visible in this low power image), and NeuN cell frequency (including presumed projection neurons in layer 2/3) were assessed within the counting box.
Upon successful initial acquisition, the reinforcement contingency was reversed: the mouse must nose-poke the other stimulus (e.g., horizontal lines; Fig. 1A, right) to receive a reward. For this “reversal learning” phase, criterion was reached upon 80% correct first-choice trials. Accordingly, for this phase, we report the number of total sessions required (each session: 20 first-choice trials). We also record the initial number of correct trials for the first reversal session as a baseline indicator of performance. In addition, we report the number of total “perseverative” as well as “learning” sessions for each animal, which records sessions required to reach criterion before (perseverative) and after (learning) a 39% performance level has been reached on first-choice trials during the reversal phase (Rutz and Rothblat 2012). We also assessed the total number of errors made throughout the reversal phase. We categorized these errors as perseverative if they occurred on sessions when performance was <39% and learning if they occurred when performance was >39%.
Tissue Collection and Histology
All behaviorally tested animals were perfusion fixed, and brains were collected and processed as described previously (Meechan et al. 2009). 10/11 LgDel and 6/11 WT behaviorally tested animals were analyzed for parvalbumin neuron distribution and projection neuron frequency blind to genotype. We examined a smaller subset of behaviorally tested LgDel (n = 5) and WT (n = 3) animals that fell within the full range of behavioral performance to quantify perisomatic parvalbumin-labeled synaptic terminals. Histological material from this subset of animals had sufficiently distinct punctate staining to permit reliable quantification of presumed perisomatic synapses. The following primary antibodies were used: rabbit anti-parvalbumin (1:2000; Swant, Marly, Switzerland) and mouse anti-NeuN (1:500 dilution; Millipore, Billerica, MA, USA). Species appropriate Alex Fluor 488, 546 and 647 nm secondary antibodies (Life Technologies Corporation, Carlsbad, CA, USA) were used for fluorescent detection.
Cell Counting
Medial prefrontal cortical areas (i.e., the mAFC) have been implicated in the reversal task (Bussey, Muir et al. 1997; Chudasama and Robbins 2003; Brigman and Rothblat 2008), and are thought to approximate prefrontal and cingulate areas that are compromised in human 22q11 patients with cognitive deficits (Bearden et al. 2009; Shashi et al. 2010). Thus, we focused our cellular analysis on the mAFC. We sampled the mAFC at an anterior–posterior position defined by the anterior commissure crossing the midline ventrally, equivalent to coronal section 209 (Sidman et al. 1971) or Bregma 0.25 mm. This section includes areas 24 and 25 (Caviness 1975). Sections at this level also include lateral frontal motor association cortex (lAFC; area 6; Caviness 1975), which we analyzed as a positive control based upon our previous data (Meechan et al. 2009, 2012). Three adjacent tissue sections were counted from this location. In each section, immunolabeled cells were counted within a box comprised of 5 bins of equivalent areas (Fig. 1) spanning the mAFC or the lAFC. To accommodate mAFC geometry, bin 1 was aligned to the edge of cortical layer 2 and bin 5 to the boundary adjacent to the white matter of the cingulum (see Fig. 1). The percentage of parvalbumin cells per bin as a fraction of total parvalbumin cells in the probe was calculated. The average number of parvalbumin-labeled terminals contacting the soma of NeuN-labeled neurons in each bin was determined. A cell density value per bin was calculated for NeuN-labeled cells. All values were then plotted as a function of bin position to assess differences in distribution between LgDel and WT circuit elements.
Imaging and Analysis
Tiled montages were collected using a Zeiss 710 confocal microscope with automated stage. Parvalbumin-labeled interneurons and NeuN-labeled neurons were identified as described previously (Meechan et al. 2009, 2012). For perisomatic synapses, single optical section montages of 0.8 µm depth were captured with a 63× oil immersion objective. Parvalbumin-labeled puncta touching cell soma of NeuN-labeled neurons were counted.
Statistical Analysis
Behavioral differences (sessions and errors to criterion) were assessed using analysis of variance (ANOVA). Differences in distribution of GABAergic cell bodies and NeuN-labeled neuronal nuclei were analyzed as described previously (Meechan et al. 2009, 2012), and perisomatic parvalbumin puncta were assessed similarly. When we compared means between more than 2 groups, a 1-way ANOVA followed by Tukey's test was used; otherwise, Student's t-test was used. The degree of correlation between behavioral measures and neuronal or synaptic puncta frequency and distribution was assessed by the Pearson product-moment correlation coefficient (r) and Spearman Rank Order Correlation (rs) tests for which 2-tailed probability values (P) were assigned.
Results
Cognitive Capacity in LgDel Mice
Most available data indicate that cognitive capacity, including that for tasks requiring optimal executive function (encompassing working memory and attention), is impaired in 22q11DS patients. In LgDel and other 22q11DS mouse models, there has been little evaluation of cognitive capacity beyond assessments of spatial memory or conditioned fear responses (Long et al. 2006; Stark et al. 2008; Sigurdsson et al. 2010). In addition, basic sensory/motor function has been reported to be indistinguishable from WT, even though prepulse inhibition, a measure of sensory gating, is altered (Paylor et al. 2001, 2006; Long et al. 2006). Thus, to evaluate cognitive capacity in the LgDel mouse model of 22q11DS, we used the visual discrimination/reversal task (Fig. 1A).
LgDel mice have a modest, but statistically significant impairment in initial acquisition of this task: 8.09 ± 0.54 (SEM) sessions for LgDel versus 6.45 ± 0.60 for WT, F1,20 = 4.49, P = 0.047 (Fig. 1B). Nevertheless, the 2 genotypes did not differ significantly in total number of errors committed when acquiring the initial discrimination task: LgDel, 116.91 ± 10.05 errors versus WT 105.45 ± 10.11 errors, P = 0.6 Moreover, during the session in which criterion is met, LgDel and WT mice spend approximately the same amount of time performing the task: 12.64 ± 3.98 min for LgDel versus 11.71 ± 2.39 min for WT, P = 0.515. Thus, while LgDel mice have a modest initial delay, other aspects of acquisition and performance are similar to WT mice.
At the outset of the reversal phase, LgDel and WT mice perform similarly: during the first reversal learning session, LgDel mice make 3.91 ± 0.59 correct responses/20 trials, while WT mice make 3.73 ± 0.60/20 trials, P = 0.82. Similarly, the number of perseverative sessions—which occur at earlier stages of reversal learning—does not differ between the 2 genotypes: 1.91 ± 0.28 for LgDel versus 2.09 ± 0.28 for WT. Finally, the total number of errors is statistically indistinguishable. LgDel mice make 213.00 ± 8.43 errors, while WT mice make 194.82 ± 7.51 prior to reaching criterion, P = 0.60. This number of errors, for both genotypes, is nearly twice the number committed during initial acquisition of the task. Together, these data indicate that the initial phase of the reversal task places equivalent demands on both LgDel and WT mice to which they respond similarly.
The most substantial differences between LgDel and WT mice emerge during the learning phase of the reversal task. LgDel mice complete the reversal task to criterion substantially more slowly than their WT littermate counterparts. They require 10.45 ± 1.39, while WT mice require 6.36 ± 0.49 total sessions, F1,20 = 8.40, P = 0.009 (Fig. 1C). Most of this difficulty reflects a significant increase in learning sessions. LgDel mice need 8.54 ± 1.14 learning sessions, while WT mice need only 4.36 ± 0.4 to reach criterion, F1,20 = 12.39, P = 0.0023) (Fig. 1D). Although the total number of reversal errors is similar between the 2 genotypes, the types of errors differ significantly. There is a modest but significant decrease in the number of perseverative errors made by LgDel mice: 94.4 ± 11.51 for LgDel versus 149.7 ± 24.63 for WT, F1,20 = 4.54, P = 0.045. In contrast, LgDel mice make substantially more learning errors: 118.6 ± 24.66 versus 45.1 ± 7.46 for WT, F1,20 = 8.97, P = 0.007 (Fig. 1E). Apparently, cognitive capacities that underlie distinct phases of the reversal task are differentially altered in LgDel mice, and many changes are seen during the learning phase.
We also recognized substantial differences in variability in individual performance between genotypes. This variability was more pronounced for the total number of reversal sessions (F = 0.006) as well as the number of learning sessions (F = 0.004) and learning errors (F = 0.0007) for LgDel versus WT mice. In contrast, perseverative performance varied less. There was no detectable variability for the number of perseverative sessions (F = 1.0), and only modest variability for the number of perseverative errors (F = 0.024). Accordingly, we defined a “more profoundly impaired” group as those LgDel mice that had a number of reversal sessions 3 standard deviations or greater relative to the average number of WT reversal sessions. This LgDel group (5 of 11) was distributed across 5 of the 6 the litters used in this study. These 5 LgDel mice also required more learning sessions to reach criterion than either the remaining LgDel or WT control mice. Finally, 4 of 5 of these LgDel animals also have the worst learning error scores during reversal. Thus, the degree of cognitive impairment, particularly for aspects related to reversal learning, varies substantially between individual LgDel mice.
Interneuron Distribution and Behavioral Performance in LgDel Mice
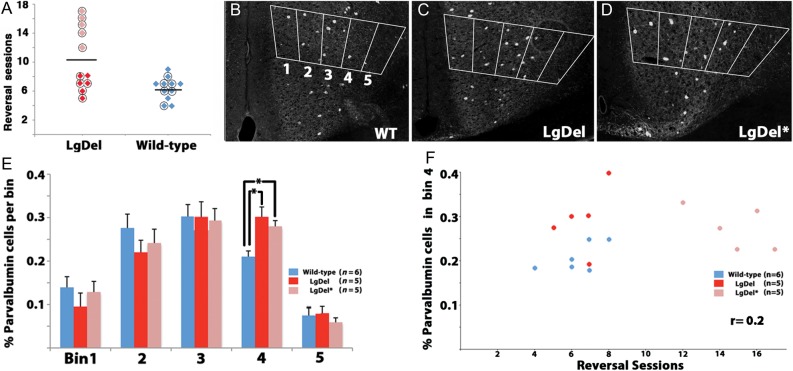
The visual reversal task on which LgDel mice are impaired likely depends on the integrity of the mAFC (Fig. 1F,G; Bussey, Muir, et al. 1997; Chudasama and Robbins 2003); therefore, it seemed possible that mAFC circuit elements might be altered in the LgDel. In the mAFC, parvalbumin interneurons—apparent pathological targets in schizophrenia and other cortical circuit disorders (Lewis et al. 2004, 2005; Volk and Lewis 2005), and disrupted developmentally in LgDel mice (Meechan et al. 2009, 2012)—are particularly abundant (Van De Werd et al. 2010). Their laminar distribution in the mAFC is distinct: they are more abundant in deeper layers (layers 3/4/5) rather than concentrated in layers 2/3 as in lAFC. Thus, in the mAFC from behaviorally tested LgDel and WT animals (Fig. 2A), we asked whether there were changes in parvalbumin interneurons that reflect altered development (Fig. 2B–D), and their correlation with behavioral performance. The distribution of these cells in the LgDel mAFC is significantly altered. Their frequency in deeper layers (bin 4) is significantly increased compared with WT (Fig. 2E). There is a compensatory reduced frequency in superficial layers indicating the same overall stability of parvalbumin cell numbers we have reported previously; however, changes in the upper layers do not reach statistical significance. The altered laminar distribution of parvalbumin interneurons was comparable in the “less” as well as “substantially” impaired LgDel behavioral groups versus WT (Fig. 2E). We also asked whether there was any relationship between the altered distribution of parvalbumin cells in bin 4 of each LgDel or WT animal with divergent measures of behavioral performance; there were no significant correlations (Fig. 2F and data not shown).
Figure 2.
Relationship between variable behavior and mAFC interneuron distribution in the LgDel mouse. (A) Circled data points on the reversal learning sessions performance graph represent behaviorally tested animals that were analyzed histologically (10/11 behaviorally tested LgDel, 6/11 behaviorally tested WT). LgDel animals were split into better performing (darker red points, LgDel) and poor performing (lighter red points, LgDel*) groups for further analysis. (B) Images of parvalbumin interneuron distribution in the mAFC of WT, (C) LgDel, and (D) LgDel* animals. A counting box comprised of 5 bins traversed the mAFC (see Fig. 1 and Materials and Methods). (E) The number of parvalbumin-labeled interneurons is significantly increased in bin4 (layer 5) in LgDel and LgDel * animals, *P < 0.05. (F) There was no significant correlation between the number of parvalbumin-labeled cells in bin 4 and reversal learning sessions.
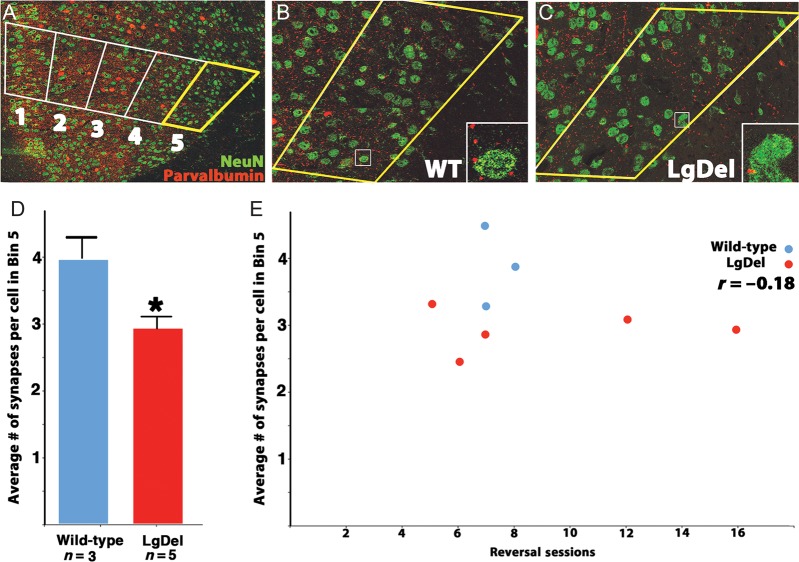
Next, we asked whether the distribution of perisomatic parvalbumin-labeled synaptic terminals, usually made onto projection neuron soma (Fig. 3A) was altered in register with redistributed parvalbumin-labeled cell bodies, and whether changes might be related to variable cognitive capacities in LgDel mice. For this analysis, we examined a smaller subset of behaviorally tested LgDel and WT animals based upon the quality of the immunolabeling of synaptic puncta (see Materials and Methods). We recorded the average number of distinct labeled terminals adjacent to NeuN-labeled neurons within each counting box bin. There is a consistent reduction in perisomatic puncta frequency in the lower cortical layers of LgDel animals (P = 0.04 between WT and LgDel in bin 5; Fig. 3B–D). These values for each WT or LgDel animal, however, did not correlate with behavioral performance (Fig. 3E). Together, these results indicate that the LgDel mAFC is a site of distinctly altered position and synaptic distribution for the parvalbumin subset of cortical GABAergic interneurons. These changes may contribute generally to altered behavior; however, in the mAFC, they are not correlated with any measure of cognitive impairment in LgDel mice.
Figure 3.
The distribution of perisomatic inhibitory synaptic endings is altered in the LgDel mAFC. (A) Low-magnification image of parvalbumin-labeled interneurons and NeuN-labeled neurons in the mAFC. A counting box traversed the mAFC, as described above, and average numbers of perisomatic parvalbumin terminals per cell per bin were determined. (B) Images of perisomatic parvalbumin-labeled synapses in bin 5 of WT and (C) LgDel animals. Insets show individual parvalbumin-labeled puncta (red), and their relationship to NeuN-labeled cell bodies (green). (D) The average number of parvalbumin-labeled perisomatic terminals in bin 5 (layer 6) is significantly reduced in LgDel versus WT animals, *P < 0.05. (E) There was no significant correlation between the number of these terminals in bin 5, where group differences are found, and reversal sessions.
Projection Neuron Frequency and Behavioral Performance in LgDel Mice
We next asked whether diminished projection neuron frequency, previously detected in the LgDel lAFC and associated with aberrant basal progenitor proliferation (Meechan et al. 2009), could be detected in the mAFC, which has been associated with efficient learning of the reversal task (Bussey, Muir, et al. 1997; Brigman and Rothblat 2008). Most mAFC layer 2/3 neurons are projection neurons (Van De Werd et al. 2010); however, specific layer 2/3 projection neuron markers are expressed primarily in fetal and early postnatal cortex (Molyneaux et al. 2007). Thus, in the mature cortex, we counted NeuN-labeled cells (neurons) in the mAFC (Fig. 4A–D) since changes must primarily reflect altered projection neuron frequencies (see above and Fig. 2).
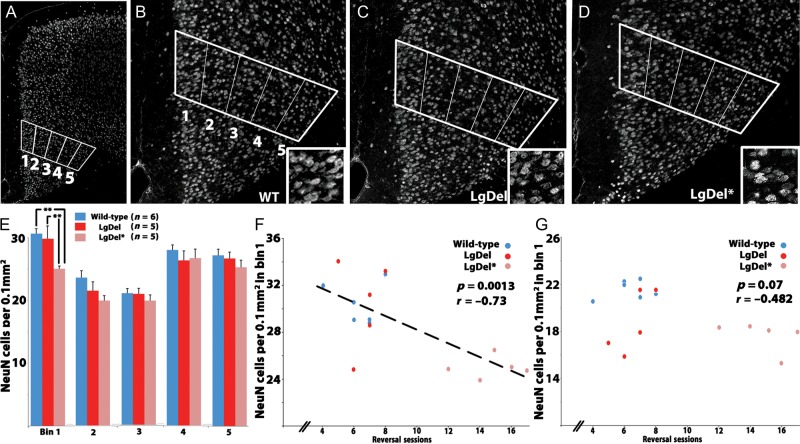
Figure 4.
Projection neuron density in mAFC layer 2/3 predicts reversal learning performance in the LgDel mouse. (A) NeuN-labeled neurons in the mAFC. The counting box was placed over the mAFC as described above. (B) WT, (C) LgDel, and (D) LgDel* animals. Insets show representative differences in cell frequency in bin 1 (layer 2/3) for each group. (E) Poorly performing LgDel animals displayed reduced superficial layer neuronal density (bin 1) relative to normally performing LgDel (**P < 0.01) and WT animals (**P < 0.01). (F) A significant negative correlation between superficial layer (bin 1) neuronal density and reversal learning was seen within the entire behaviorally tested cohort (WT and LgDel combined) and within the LgDel group alone. (G) A correlation between superficial layer (bin 1) neuronal density and reversal sessions in lAFC was not seen within the entire behaviorally tested cohort (WT and LgDel combined) or within the LgDel group alone.
Neuron frequency is reduced in superficial layers of the LgDel mAFC (bin 1 of the counting box, corresponding to layers 2/3). Thus, projection neurons in layer 2/3 are altered in the LgDel mAFC. NeuN neuron frequency in bin 1 is significantly reduced in the poorly performing LgDel animals (Fig. 4D,E). Thus, we assessed the correlation between neuron frequencies in bin 1 from each animal with significantly divergent measures of behavioral performance (Fig. 4F and data not shown). There were no correlations between layer 2/3 projection neuron frequency and any aspect of acquisition (Spearman and Pearson product tests). In contrast, there is a significant relationship between impaired reversal performance and mAFC layer 2/3 projection neuron frequency (Fig. 4F). mAFC layer 2/3 projection neuron frequency is correlated with total number of reversal sessions in the LgDel cohort (Pearson product r = −0.72, P = 0.018; Spearman rs = −0.68, P = 0.04) and for LgDel and WT mice combined (Pearson product r = −0.73; P = 0.001; Spearman rs = −0.6, P = 0.02). Moreover, there is a relationship between reversal learning sessions and mAFC layer 2/3 projection neuron frequency. The number of learning sessions is significantly correlated in LgDel mice (Pearson product r = −0.66, P = 0.03; Spearman rs = −0.72, P = 0.03) as well as LgDel and WT mice combined (Pearson product r = −0.71, P = 0.002; Spearman rs = −0.59, P = 0.02).
To assess whether reversal learning was associated selectively with projection neuron changes in the mAFC or more generally with superficial projection neuron frequency in frontal association cortical regions, we determined lAFC projection neuron frequency in the behaviorally tested animals. lAFC superficial projection neuron frequency (bin 1) was significantly reduced in LgDel animals, in agreement with our earlier observations (Meechan et al. 2009). Nevertheless, we found no significant correlation between this reduction of layer 2/3 projection neurons in lAFC and any measure of performance for acquisition or reversal of the visual discrimination task (Fig. 4G).
Discussion
We have shown that diminished 22q11 gene dosage in the LgDel mouse model of 22q11DS compromises cognitive function as well as cortical circuit elements—interneurons, synaptic terminals, and projection neurons—in the mAFC, a distinct cortical region critical for cognition. The degree of cognitive impairment in LgDel but not WT animals varies substantially, and is correlated with variable frequency of one of the circuit elements we analyzed: mAFC projection neuron frequency. Apparently, layer 2/3 projection neuron frequencies in the mAFC contribute to distinct aspects of behavioral impairment in a visual reversal task that assesses murine cognition. These data, together with our previous observations on regional variability of cortical developmental anomalies in the LgDel mouse (Meechan et al. 2009, 2012), suggest that diminished 22q11 dosage selectively compromises frontal cortical regions and circuits crucial for cognitive capacities. Similar mechanisms may account for individual variability in cognitive impairment as well as regional selectivity in altered cortical structure in 22q11DS patients.
We analyzed the murine equivalent of executive function, distinct from spatial memory and sensory gating known to be impaired in LgDel and other 22q11DS mouse models (Long et al. 2006; Stark et al. 2008; Powell et al. 2009) using a task that is independent of spatial or chemosensory cues (Bussey et al. 2001). The modest delay in initial acquisition in the LgDel is congruent with observations of variable general intellectual impairment in 22q11DS (Gerdes et al. 1999; Swillen et al. 1999; Woodin et al. 2001). Stratified performance deficits during reversal learning are consistent with individually variable impairment of attention, working memory, and other executive functions in 22q11DS patients (Gerdes et al. 1999; Swillen et al. 1999; Woodin et al. 2001; Campbell et al. 2010). In humans, these capacities depend upon the integrity of frontal and anterior cingulate cortical areas. In mice, parallel cognitive capacities engage the mAFC (Jones 2002; Kellendonk et al. 2009; Matzel and Kolata 2010). Reversal learning sessions and errors are significantly increased in LgDel mice. These measures, associated with working memory and visual attention, depend upon mAFC circuits (Bussey, Muir, et al. 1997; Brigman and Rothblat 2008; Glascher et al. 2012). In contrast, perseverative sessions and errors are unchanged or modestly improved in LgDel mice. These measures reflect suppression of the previously rewarded stimulus response at the beginning of the reversal phase, and are associated with orbitofrontal cortex (Chudasama and Robbins 2003). The more pronounced and variable deficits in learning sessions and errors suggest a critical relationship between disrupted executive function and mAFC circuits in LgDel mice.
Based upon these data, we focused cellular analysis of the behaviorally characterized mice on interneuron and projection neuron integrity in the LgDel mAFC. It is likely that the changes we found in interneuron distribution and projection neuron frequency in the mAFC reflect altered development, particularly mechanisms of interneuron migration and cortical projection neurogenesis from basal progenitors. We complemented our analysis of interneuron position with a novel assessment of changes in the distribution of perisomatic parvalbumin synaptic terminals because this aspect of inhibitory circuitry has also been implicated in disease pathology and behavioral change (Gonzalez-Burgos et al. 2011). Our results suggest that local inhibitory synaptic organization is generally altered in the LgDel mAFC; reinforcing the conclusion that disrupted parvalbumin interneuron-dependent inhibitory circuitry (Woo and Lu 2006; Lewis et al. 2012), is a key feature of cortical connectivity disorders that arise during development, including 22q11DS.
The variable impairment in LgDel mice allowed us to determine whether specific circuit phenotypes are selectively related to specific aspects of behavioral change. We found a robust correlation between 2 behavioral deficits and a single aspect of circuit disruption in the mAFC: frequency of layer 2/3 projection neurons. Whether this relationship reflects a continuous distribution of individuals or stratification of 2 populations remains to be determined. Nevertheless, there are few examples in the current literature of behavioral variability related quantitatively to variable circuit organization or function (Kao et al. 2005; Wang et al. 2011), and none have been reported for mouse models of human disorders of cortical circuitry. The specific correlation of some learning measures with layer 2/3 projection neuron frequency in mAFC in LgDel and WT animals suggests that cortico-cortical or other long distance connections made from the mAFC to other forebrain regions may be key for aspects of learning the reversal task. Additional aspects of reversal performance are thought to depend on additional anterior cortical areas. In WT animals, orbitofrontal cortex lesions result in increased perseverative errors. The distinctions between perseverative errors in LgDel and WT mice indicate further circuit changes that result in behavioral differences; however, these issues remain to be addressed.
Our data provide a foundation for exploring mechanisms of regional and individual variability in cortical structure and function in 22q11DS. Regional volumes and presumed axon density of local fiber tracts are reduced in frontal and anterior cingulate cortex in 22q11DS patient in children and in adults (Simon et al. 2005; Campbell et al. 2006; Ottet et al. 2013). These differences are correlated with deficits in sustained attention and working memory—key features of the “schizophrenia cognitive phenotype” (Oram et al. 2005; Abraham et al. 2007)—in 22q11DS subjects (Dufour et al. 2008; Shashi et al. 2010). Changes in LgDel mAFC projection neurons provide a new model to understand the pathogenesis of region-selective, cognitively disruptive changes in 22q11DS patients. Diminished 22q11 dosage may differentially compromise basal progenitors that generate upper-layer projection neurons due to altered capacity to respond to locally available signals (Fukuchi-Shimogori and Grove 2001; Haskell and LaMantia 2005; Siegenthaler et al. 2009), or anterior–posterior, dorso-ventral, and medio-lateral molecular identities established for cortical precursors by additional molecular patterning mechanisms (Hamasaki et al. 2004). Such discontinuities in basal progenitor proliferative or neurogenic capacity could modify cortical parcellation, size, and folding (Elsen et al. 2013; Nonaka-Kinoshita et al. 2013). Characterization of region-specific changes in cortical development, circuit organization, and behavior in 22q11DS mouse models provides an opportunity to determine how disrupted cortical development leads to cellular and behavioral pathology associated with cortical circuit disorders including schizophrenia, ADHD, and autism.
Funding
This work was supported by the National Institutes of Health (R01 HD042182; A.-S.L.).
Notes
We thank Elizabeth Paronett and Thomas Harrigan for technical assistance. Conflict of Interest: None declared.
References
- Abraham A, Windmann S, McKenna P, Gunturkun O. 2007. Creative thinking in schizophrenia: the role of executive dysfunction and symptom severity. Cogn Neuropsychiatry. 12:235–258. 10.1080/13546800601046714 [DOI] [PubMed] [Google Scholar]
- Bearden CE, van Erp TG, Dutton RA, Lee AD, Simon TJ, Cannon TD, Emanuel BS, McDonald-McGinn D, Zackai EH, Thompson PM. 2009. Alterations in midline cortical thickness and gyrification patterns mapped in children with 22q11.2 deletions. Cereb Cortex. 19:115–126. 10.1093/cercor/bhn064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Graybeal C, Holmes A. 2010. Predictably irrational: assaying cognitive inflexibility in mouse models of schizophrenia. Front Neurosci. 4:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Powell EM, Mittleman G, Young JW. 2012. Examining the genetic and neural components of cognitive flexibility using mice. Physiol Behav. 107:666–669. 10.1016/j.physbeh.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Rothblat LA. 2008. Stimulus specific deficit on visual reversal learning after lesions of medial prefrontal cortex in the mouse. Behav Brain Res. 187:405–410. 10.1016/j.bbr.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Everitt BJ, Robbins TW. 1997. Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behav Neurosci. 111:908–919. 10.1037/0735-7044.111.5.908 [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. 1997. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 111:920–936. 10.1037/0735-7044.111.5.920 [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Rothblat LA. 2001. Discrimination of computer-graphic stimuli by mice: a method for the behavioral characterization of transgenic and gene-knockout models. Behav Neurosci. 115:957–960. 10.1037/0735-7044.115.4.957 [DOI] [PubMed] [Google Scholar]
- Campbell LE, Azuma R, Ambery F, Stevens A, Smith A, Morris RG, Murphy DG, Murphy KC. 2010. Executive functions and memory abilities in children with 22q11.2 deletion syndrome. Aust N Z J Psychiatry. 44:364–371. 10.3109/00048670903489882 [DOI] [PubMed] [Google Scholar]
- Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Catani M, Ng V, van Amelsvoort T, Chitnis X, Cutter W, et al. 2006. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 129:1218–1228. 10.1093/brain/awl066 [DOI] [PubMed] [Google Scholar]
- Caviness VS., Jr 1975. Architectonic map of neocortex of the normal mouse. J Comp Neurol. 164:247–263. 10.1002/cne.901640207 [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. 2003. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 23:8771–8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour F, Schaer M, Debbane M, Farhoumand R, Glaser B, Eliez S. 2008. Cingulate gyral reductions are related to low executive functioning and psychotic symptoms in 22q 11.2 deletion syndrome. Neuropsychologia. 46:2986–2992. 10.1016/j.neuropsychologia.2008.06.012 [DOI] [PubMed] [Google Scholar]
- Elsen GE, Hodge RD, Bedogni F, Daza RA, Nelson BR, Shiba N, Reiner SL, Hevner RF. 2013. The protomap is propagated to cortical plate neurons through an Eomes-dependent intermediate map. Proc Natl Acad Sci USA. 110:4081–4086. 10.1073/pnas.1209076110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. 2001. Neocortex patterning by the secreted signaling molecule FGF8. Science. 294:1071–1074. 10.1126/science.1064252 [DOI] [PubMed] [Google Scholar]
- Gerdes M, Solot C, Wang PP, Moss E, LaRossa D, Randall P, Goldmuntz E, Clark BJ, 3rd, Driscoll DA, Jawad A, et al. 1999. Cognitive and behavior profile of preschool children with chromosome 22q11.2 deletion. Am J Med Genet. 85:127–133. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. 2007. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 17:103–111. 10.1016/j.conb.2007.01.009 [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D. 2012. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc Natl Acad Sci USA. 109:14681–14686. 10.1073/pnas.1206608109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. 2011. GABA Neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011:723184 10.1155/2011/723184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki T, Leingartner A, Ringstedt T, O'Leary DD. 2004. EMX2 Regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 43:359–372. 10.1016/j.neuron.2004.07.016 [DOI] [PubMed] [Google Scholar]
- Haskell GT, LaMantia AS. 2005. Retinoic acid signaling identifies a distinct precursor population in the developing and adult forebrain. J Neurosci. 25:7636–7647. 10.1523/JNEUROSCI.0485-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW. 2002. A comparative review of rodent prefrontal cortex and working memory. Curr Mol Med. 2:639–647. 10.2174/1566524023361989 [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. 2005. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 433:638–643. 10.1038/nature03127 [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA. 2010. 22q11.2 Microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 11:402–416. 10.1038/nrn2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Kandel ER. 2009. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 32:347–358. 10.1016/j.tins.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF. 2009. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 19:2439–2450. 10.1093/cercor/bhn260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. 2012. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35:57–67. 10.1016/j.tins.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. 2005. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 6:312–324. 10.1038/nrn1648 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Volk DW, Hashimoto T. 2004. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl). 174:143–150. 10.1007/s00213-003-1673-x [DOI] [PubMed] [Google Scholar]
- Long JM, LaPorte P, Merscher S, Funke B, Saint-Jore B, Puech A, Kucherlapati R, Morrow BE, Skoultchi AI, Wynshaw-Boris A. 2006. Behavior of mice with mutations in the conserved region deleted in velocardiofacial/DiGeorge syndrome. Neurogenetics. 7:247–257. 10.1007/s10048-006-0054-0 [DOI] [PubMed] [Google Scholar]
- Matzel LD, Kolata S. 2010. Selective attention, working memory, and animal intelligence. Neurosci Biobehav Rev. 34:23–30. 10.1016/j.neubiorev.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Maynard TM, Tucker ES, LaMantia AS. 2011. Three phases of DiGeorge/22q11 deletion syndrome pathogenesis during brain development: patterning, proliferation, and mitochondrial functions of 22q11 genes. Int J Dev Neurosci. 29:283–294. 10.1016/j.ijdevneu.2010.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. 2012. Cxcr4 regulation of interneuron migration is disrupted in 22q11.2 deletion syndrome. Proc Natl Acad Sci USA. 109:18601–18606. 10.1073/pnas.1211507109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. 2009. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci USA. 106:16434–16445. 10.1073/pnas.0905696106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu MM, Xavier RJ, Demay MB, Russell RG, Factor S, et al. 2001. TBX1 Is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 104:619–629. 10.1016/S0092-8674(01)00247-1 [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. 2007. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 8:427–437. 10.1038/nrn2151 [DOI] [PubMed] [Google Scholar]
- Niklasson L, Rasmussen P, Oskarsdottir S, Gillberg C. 2009. Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Res Dev Disabil. 30:763–773. 10.1016/j.ridd.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Nonaka-Kinoshita M, Reillo I, Artegiani B, Angeles Martinez-Martinez M, Nelson M, Borrell V, Calegari F. 2013. Regulation of cerebral cortex size and folding by expansion of basal progenitors. EMBO J. 32:1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J, Geffen GM, Geffen LB, Kavanagh DJ, McGrath JJ. 2005. Executive control of working memory in schizophrenia. Psychiatry Res. 135:81–90. 10.1016/j.psychres.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Ottet MC, Schaer M, Cammoun L, Schneider M, Debbane M, Thiran JP, Eliez S. 2013. Reduced fronto-temporal and limbic connectivity in the 22q11.2 deletion syndrome: vulnerability markers for developing schizophrenia? PLoS One. 8:e58429 10.1371/journal.pone.0058429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Glaser B, Mupo A, Ataliotis P, Spencer C, Sobotka A, Sparks C, Choi CH, Oghalai J, Curran S, et al. 2006. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci USA. 103:7729–7734. 10.1073/pnas.0600206103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, McIlwain KL, McAninch R, Nellis A, Yuva-Paylor LA, Baldini A, Lindsay EA. 2001. Mice deleted for the DiGeorge/velocardiofacial syndrome region show abnormal sensorimotor gating and learning and memory impairments. Hum Mol Genet. 10:2645–2650. 10.1093/hmg/10.23.2645 [DOI] [PubMed] [Google Scholar]
- Powell SB, Zhou X, Geyer MA. 2009. Prepulse inhibition and genetic mouse models of schizophrenia. Behav Brain Res. 204:282–294. 10.1016/j.bbr.2009.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz HL, Rothblat LA. 2012. Intact and impaired executive abilities in the BTBR mouse model of autism. Behav Brain Res. 234:33–37. 10.1016/j.bbr.2012.05.048 [DOI] [PubMed] [Google Scholar]
- Schaer M, Schmitt JE, Glaser B, Lazeyras F, Delavelle J, Eliez S. 2006. Abnormal patterns of cortical gyrification in velo-cardio-facial syndrome (deletion 22q11.2): an MRI study. Psychiatry Res. 146:1–11. 10.1016/j.pscychresns.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Shapiro HM, Wong LM, Simon TJ. 2013. A cross-sectional analysis of the development of response inhibition in children with chromosome 22q11.2 deletion syndrome. Front Psychiatry. 4:81 10.3389/fpsyt.2013.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashi V, Kwapil TR, Kaczorowski J, Berry MN, Santos CS, Howard TD, Goradia D, Prasad K, Vaibhav D, Rajarethinam R, et al. 2010. Evidence of gray matter reduction and dysfunction in chromosome 22q11.2 deletion syndrome. Psychiatry Res. 181:1–8. 10.1016/j.pscychresns.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Angevine JB, Pierce ET. 1971. Atlas of the mouse brain and spinal cord. Cambridge: (MA): Harvard University Press; xi, 261 p. (chiefly illus.). [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, et al. 2009. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 139:597–609. 10.1016/j.cell.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. 2010. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 464:763–767. 10.1038/nature08855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Ding L, Bish JP, McDonald-McGinn DM, Zackai EH, Gee J. 2005. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. Neuroimage. 25:169–180. 10.1016/j.neuroimage.2004.11.018 [DOI] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, et al. 2008. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 40:751–760. 10.1038/ng.138 [DOI] [PubMed] [Google Scholar]
- Swillen A, Vandeputte L, Cracco J, Maes B, Ghesquiere P, Devriendt K, Fryns JP. 1999. Neuropsychological, learning and psychosocial profile of primary school aged children with the velo-cardio-facial syndrome (22q11 deletion): evidence for a nonverbal learning disability? Child Neuropsychol. 5:230–241. 10.1076/0929-7049(199912)05:04;1-R;FT230 [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Steckler T. 2001. Behavioural phenotyping of mouse mutants. Behav Brain Res. 125:3–12. 10.1016/S0166-4328(01)00278-9 [DOI] [PubMed] [Google Scholar]
- Van De Werd HJ, Rajkowska G, Evers P, Uylings HB. 2010. Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct Funct. 214:339–353. 10.1007/s00429-010-0247-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. 2005. GABA Targets for the treatment of cognitive dysfunction in schizophrenia. Curr Neuropharmacol. 3:45–62. 10.2174/1570159052773396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. 2011. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 334:693–697. 10.1126/science.1209951 [DOI] [PubMed] [Google Scholar]
- Woo NH, Lu B. 2006. Regulation of cortical interneurons by neurotrophins: from development to cognitive disorders. Neuroscientist. 12:43–56. 10.1177/1073858405284360 [DOI] [PubMed] [Google Scholar]
- Woodin M, Wang PP, Aleman D, McDonald-McGinn D, Zackai E, Moss E. 2001. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genet Med. 3:34–39. 10.1097/00125817-200101000-00008 [DOI] [PubMed] [Google Scholar]