Abstract
Neurobiological theories of memory posit that the neocortex is a storage site of declarative memories, a hallmark of which is the association of two arbitrary neutral stimuli. Early sensory cortices, once assumed uninvolved in memory storage, recently have been implicated in associations between neutral stimuli and reward or punishment. We asked whether links between neutral stimuli also could be formed in early visual or auditory cortices. Rats were presented with a tone paired with a light using a sensory preconditioning paradigm that enabled later evaluation of successful association. Subjects that acquired this association developed enhanced sound evoked potentials in their primary and secondary visual cortices. Laminar recordings localized this potential to cortical Layers 5 and 6. A similar pattern of activation was elicited by microstimulation of primary auditory cortex in the same subjects, consistent with a cortico-cortical substrate of association. Thus, early sensory cortex has the capability to form neutral stimulus associations. This plasticity may constitute a declarative memory trace between sensory cortices.
Keywords: auditory cortex, cross-modal, plasticity, sensory preconditioning, visual cortex
Introduction
The neocortex is widely held to be the storage site of long-term memories, supported by a preponderance of electrophysiological recordings, controlled lesion, pathological cases, and neuroimaging studies (Lashley 1929; Hebb 1949; Thompson et al. 1972; Squire 1992; Nadel and Moscovitch 1997; Frankland and Bontempi 2005). A principle suggested by these findings is that memories are stored in the same cortical regions activated during an experience (McClelland et al. 1995; Wheeler et al. 2000; Weinberger 2004). Understanding how the functional properties of cortical regions change with learning is necessary to explain how we remember, and ultimately exactly what we do remember from any given experience.
Mnemonic plasticity in the inferotemporal and perirhinal cortices have received particular attention, perhaps because they are necessary for the performance of standard tests of declarative memory (Mishkin 1982), and their neurons fire in a manner that reflects a subject's past experience. For example, using a visual paired-associate reward task, Messinger et al. (2001) found that neurons in the perirhinal cortex of monkeys alter their responding in a concordant manner for paired stimuli, either increasing or decreasing their firing to both stimuli. Moreover, this plasticity developed across the training session, tracking correct behavioral performance. These changes are maintained, or even strengthened, with repeated training or time from initial learning, as seen in related reward tasks in perirhinal (Erickson and Desimone 1999), inferotemporal (Sakai and Miyashita 1991), and prefrontal cortex (Freedman et al. 2003).
In contrast to such “higher” cortical regions, early sensory cortical fields have been assumed to function as sensory analyzers rather than as mnemonic substrates (Campbell 1905). However, support for this assumption has been steadily eroding until it is no longer sustainable (Scheich et al. 2011; Weinberger 2011). For example, primary auditory cortex (A1) shifts the frequency tuning of its neurons to emphasize tones that signal explicit reinforcement (reward or punishment) (Bakin and Weinberger 1990; Gao and Suga 1998; Kisley and Gerstein 2001; Yan and Zhang 2005; Blake et al. 2006). Moreover, tuning plasticity possesses the cardinal attributes of associative memory: associativity (Bakin and Weinberger 1990; Bakin et al. 1992), specificity for the physical properties of the stimulus that were behaviorally relevant (Edeline and Weinberger 1993; Kisley and Gerstein 2001; Polley et al. 2006), rapid development (Edeline et al. 1993), consolidation (increased strength over days) (Galván and Weinberger 2002), and long-term retention (for at least 2 months) (Weinberger et al. 1993). These tuning shifts can be sufficiently extended across the tonotopic map of A1 to produce an increase in the area of representation of a signal frequency (Recanzone et al. 1993), where the amount of expansion apparently encodes the level of acquired stimulus importance (Rutkowski and Weinberger 2005) and serves as a substrate for strengthening specific memory (Bieszczad and Weinberger 2010, 2012). Furthermore, directly increasing representational area in A1 by stimulation of the cholinergic nucleus basalis is sufficient to induce de novo specific behavioral memory (Bieszczad et al. 2013). Supporting the necessity of auditory cortex in memory for sounds, post-training lesions of A1 disrupt long-term memory for auditory fear conditioning (Boatman and Kim 2006), and similar deficits occur for secondary sensory cortices in other modalities (Sacco and Sacchetti 2010). Although less extensively studied, other early sensory fields have also been implicated in associative memory, for example, somatosensory cortex (Zhou and Fuster 2000; Siucinska and Kossut 2004; Galvez et al. 2006) and visual cortex (Knight et al. 2004; Shuler and Bear 2006; Gavornik et al. 2009).
Such findings demonstrate that early sensory cortical fields are not restricted to stimulus analysis, but are capable of mnemonic processes like higher cortical areas. However, early sensory fields were tested only with explicit reinforcements, that is, rewards or punishments. Their abilities to form associations between 2 arbitrary neutral stimuli, a hallmark of declarative memory (Eichenbaum 2004), are unknown. Traditional theories would not suggest of cortical functional organization with respect to memory (Mishkin 1982).
To study associative plasticity in the sensory cortex for 2 neutral stimuli, in the absence of motivationally strong reinforcers, we used a sensory preconditioning task (Brogden 1939). This task consists of 2 training phases followed by a test phase. In the first phase, 2 neutral stimuli are paired. In the second phase, one of the stimuli is reinforced with food or shock, which allows it to produce a conditioned response (CR). If an association had been formed between the stimuli in Phase 1, then the CR should also be elicited by the stimulus that was never paired with the reinforcer, presumably via an associative chain (Rizley and Rescorla 1972). This is determined in a final test phase.
Materials and Methods
Subjects and Surgery
Twenty-three adult male albino Sprague–Dawley rats (Charles River Laboratories) were used in this study. Subjects were housed individually on a 12/12 h-light/dark cycle with ad libitum food and water. All procedures were conducted during the light phase. All surgical and experimental treatments were approved by the Institutional Animal Care and Use Committee for the University of California at Irvine.
Subjects weighing between 275 and 350 g were anesthetized with sodium pentobarbital (55 mg/kg, i.p.; Sigma-Aldrich). Once secured in stereotaxic frame (Kopf), stereotaxic coordinates (Paxinos and Watson 2007) guided placement of a rectangular craniotomy over visual cortex (caudal/medial corner AP: –6.5, ML: 3.0; rostral/lateral corner AP: –5.5, ML: 7.0). The site of the auditory cortex craniotomy was chosen based on skull landmarks. Several miniature stainless steel screws (#0-80, Small Parts) were inserted on the dorsal and lateral sides of the skull and dental cement was applied to form a pedestal to hold the connectors for the microwire arrays and electrocardiogram (EKG) electrodes. A screw placed over the left cerebellum, contralateral to the side of array implantation, served as a ground.
Two 2 × 8 microwire arrays (250 μm separation, 50 μm tungsten, California Fine Wire) were inserted into the right hemisphere, one into visual cortex and the other into auditory cortex. Two microwires in each array were 1 mm shorter and served as reference electrodes that rested on the dural surface. For 3 subjects a 16-site linear silicon array (Neuronexus) was implanted in the visual cortex to examine the laminar pattern of activation.
At least 5 days after array implantation subjects were anesthetized and a Teflon insulated stainless steel wire (#793200, A-M Systems) was sutured around the thoracic musculature for detecting the EKG. A ground wire was placed in the back. Both wires terminated in a pin connector cemented to the skull pedestal.
Stimuli and Calibration
Pure tones across a range of frequencies (0.75–48 kHz, quarter octave intervals) were generated by a real-time processor (RP2.1, Tucker-Davis Technologies) and delivered through an electromagnetic speaker (FF1, Tucker-Davis Technologies) parallel to the axis of the left ear canal at a distance of 15 cm. The speaker was calibrated across the entire frequency range used in this study with a calibrated microphone and preamplifier (Brüel and Kjær). Tone pips were used to probe frequency tuning of unit activity in auditory cortex (50 dB SPL, 50 ms duration, 8 ms rise/fall time). Training tones had the same properties as the tone pips, except their duration was lengthened to 10 s). A green light emitting diode (LED) served as a visual stimulus during training and was oriented towards the left eye 45° from the midline at a distance of 25 cm.
Behavioral Training
All phases of the experiment were conducted with animals in a wire mesh alley (30.5 × 5.8 × 6.0 cm) tilted at ∼20° so that the subject's head was above its body (for details see Headley and Weinberger 2011). The alley sat atop a weighted table inside an acoustic isolation chamber (Industrial Acoustics). Elastic bands attached to the cage could be threaded through metal loops in the skullcap to restrict head movements during training and delivery of acoustic stimuli.
Subjects acclimated to the enclosure for 2 days prior to the implant surgery. Acclimation consisted of placement in the cage and 30 min of tone pip presentations with the chamber lights on. Subsequently the chamber lights shut off and subjects remained in the enclosure with silence. The day after implantation of the EKG electrode, they received one more acclimation session, with the addition of head restraint provided. Subjects adjusted to the restriction of head movement within minutes.
Training began the day following the final acclimation session. For each session, subjects were placed in the alley, their heads restrained, and 20 repetitions of tone pips (1.5 s interstimulus interval) were delivered to assess frequency receptive fields at each recording site. Throughout the ∼13 min of receptive field acquisition the chamber lights were kept on. Following this the lights were turned off and training began.
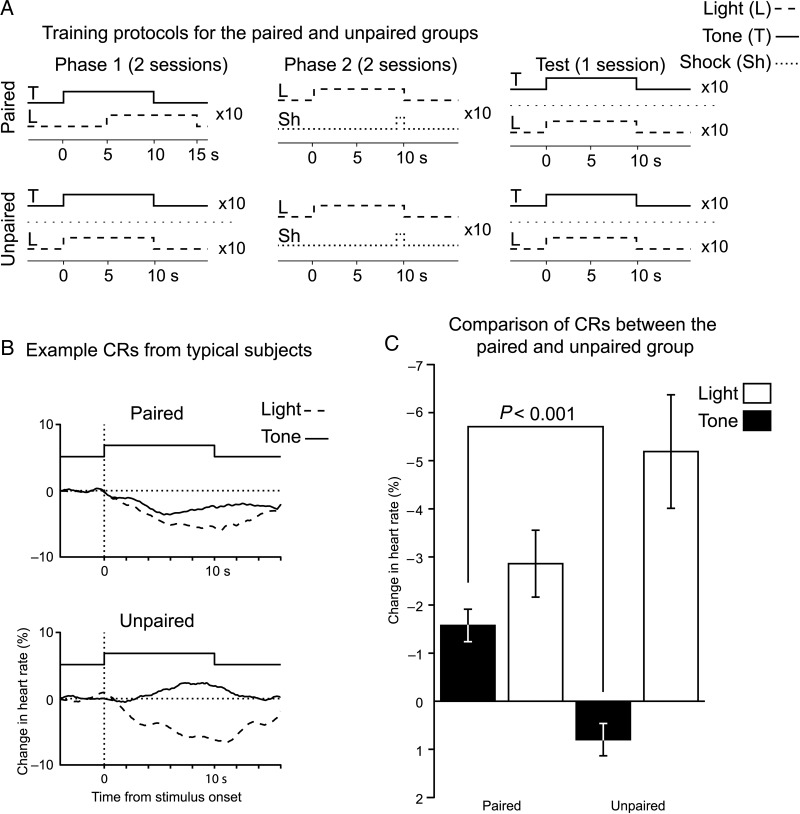
Sensory preconditioning is composed of 2 training phases followed by a test phase (Fig. 1A). Phase 1 gave subjects in the paired group the opportunity to learn that a particular tone (preconditioned stimulus) predicts the appearance of a light. Each trial consisted of pairing a 10 s tone with a 10 s green LED, with 5 s of overlap (tone–light interval = 5 s, 10 trials per day, 2 days, 6 min intertrial interval). Subjects in the unpaired group received separate presentations of the tone and light (10 trials per day, 2 days, 3 min intertrial interval). For Phase 2, both paired and unpaired groups received the same training. The green LED was presented for 10 s and coterminated with a 1 s shock (light–shock interval = 9 s, 10 trials per day, 2 days, 6 min intertrial interval). Shocks were delivered internally through the EKG leads (40 Hz, biphasic 8.3 ms pulses, constant current source, #H13-15, Coulbourn Instruments). The day after the last Phase 2 session, a test session was delivered. Both the tone and the light were presented separately, in an unpaired manner, and heart rate was recorded. The mean heart rate was measured for the 4 s prior to stimulus delivery and for 15 s post stimulus onset. The percent change in mean heart rate between these periods was the CR, which manifested itself as a decrease in heart rate (Teyler 1971; Richardson et al. 1995).
Figure 1.
Subjects undergoing sensory preconditioning acquired a tone→light association. (A) A diagram illustrating the 2 training phases and test phase for the sensory preconditioning and nonassociative control groups. (B) Typical stimulus driven changes in heart rate during the test phase. CRs manifested as a slowing in heart rate, with both groups expressing fear responses to the light. Only sensory preconditioning subjects showed fear to the tone, despite it never being paired with a shock. (C) A group level comparison of the mean change in heart rate to the tone and light during the test phase.
Recording and Data Acquisition
Throughout each training session extracellular potentials were recorded from microwires and linear silicon probes implanted in auditory and visual cortices. Custom scripts written in MATLAB controlled stimulus delivery and recording. The wideband local field potential was recorded with a 64-channel recording setup (Tucker-Davis Technologies). Multiunit activity (MUA) was filtered offline (300–5000 Hz bandpass filter) and detected with a window discriminator and subsequent sorting step using k-means clustering of spike waveform principle components (Lewicki 1998). The low-frequency component of the local field potential was obtained offline by digital lowpass filtering the wideband signal with a Butterworth filter (300 Hz, –3 dB cutoff, filtfilt command in MATLAB). Any trials containing movement or chewing artifacts detected by auditory and visual inspection were excluded from all subsequent analyses.
Microstimulation
Recording sites in auditory cortex were stimulated not <1 week after the test session. Subjects were anesthetized throughout the microstimulation session. Each microwire implanted in auditory cortex received 10 repetitions of a range of bipolar current pulses (24–96 μA, 0.25 ms for each phase, A-M Systems), while simultaneously recording the EPs in V1. Only potentials evoked by the 96 μA pulse were analyzed, since they were the most reliable across subjects.
Histology and Localization of Electrodes
Following all experimental procedures, subjects were anesthetized and microlesions made at each recording site (10 μA for 10 s). Afterward they were euthanized with an overdose of sodium pentobarbital and transcardially perfused with a formalin solution. Brains were stored in formaldehyde until sectioning. Tissue was sectioned at 50 μm intervals on a freezing microtome. Sections were wet mounted on subgelled slides and left to dry for at least 24 h. To visualize cell bodies all slides were thionin stained for Nissl substance and coverslipped.
The division between primary visual cortex (V1) and secondary lateral visual cortex (V2L) was demarcated by changes in the cell density across layers. V1 exhibited a prominent increase in Layer 4, while V2L had relatively even cell density between Layers 2 and 6. A1 was identified by the tonotopic progression of best frequencies across recording sites, which increased in the caudal to rostral direction, and was bordered on the rostral side by an abrupt reversal of this trend, corresponding to the anterior auditory field. Somatosensory cortex was identified by the presence of barrels in Layer 4 and the appearance of the hippocampus (Paxinos and Watson 2007).
Data Analyses
Evoked potentials (EPs) were calculated by averaging the local field potential traces from a particular recording site across all trials during a session. The amplitude of the mean EP was the peak negativity during the period from 10 to 120 ms after the stimulus onset. For linear silicon arrays, current source-density (CSD) profiles were calculated by taking the approximation of the second spatial derivative across the array with the finite difference formula (Freeman and Nicholson 1975; Vaknin et al. 1988),
where z is the electrode depth, n is the differentiation grid (for us n = 1), and φ is the extracellular potential. Because of slight differences in the overall signal levels between recording sites on such arrays, each site was multiplied by a correction factor calculated by the ratio of that site's root mean square amplitude (RMS) and the mean RMS across all other sites.
Frequency receptive fields were calculated from tone-evoked MUA. Responding to each tone pip was defined as the mean firing rate during the 50 ms tone pip. The receptive field was smoothed by a triangular convolution filter ([0.25 0.5 0.25]). The best frequency at a site was the frequency with the maximum response. The best frequency at a particular site during the last acclimation session, prior to training, assigned that site's tuning distance from the training tone frequency.
Statistical analyses began with all sets of samples subjected to a Shapiro–Wilk test for normality. Behavioral data were normally distributed; however, EP amplitudes were not, due to a long-tail on the high side. To mitigate this, we applied a square root transform. Mixed effect ANOVAs with factorial designs tested for experimental effects. The random effect was a subject in the case of behavioral analyses, and electrode for EPs. In the case of the A1 EPs the square root transform did not produce a normal distribution, so a nonparametric Kruskal–Wallis test was run as well to validate the lack of significance for the one-way ANOVA.
If a significant effect was found, then in certain cases these were followed up with planned 2-sample t-tests. The result of a test was deemed significant when P ≤ 0.05. For all charts, error bars (and shaded regions) denote mean ± standard error of the mean.
Results
Behavioral Acquisition of the Tone→Light Association
Sensory preconditioning was composed of 2 training phases, followed by a test session (Fig. 1A). During Phase 1, subjects (n = 13) in the paired group received partially overlapping (5 s) presentations of a tone (50 dB SPL, 10 s) and light (green LED, 10 s). A nonassociative control group was also run (n = 9), the unpaired group, for which presentation of the tone and light occurred on separate trials. To test whether subjects acquired this association, which is behaviorally silent, they received a second training phase that motivated responding. For Phase 2, both groups received pairing of the light with a shock, which inculcated conditioned fear to the light. Twenty-four hours after the last fear conditioning session, we administered a test phase wherein the tone and the light occurred on separate trials without reinforcement. Conditioned fear was measured by a change in heart rate during and shortly after stimulus presentation (Fig. 1B,C). Because our subjects were restrained, conditioned fear manifested as a decrease in heart rate, known as bradycardia. Since both groups received pairing of the light and shock, both should exhibit conditioned fear to the light. On the other hand, only the paired group experienced the tone paired with the light, and so only they should display conditioned fear to the tone.
We tested whether conditioned fear responses to the tone and light differed between the paired and unpaired groups. An ANOVA with factors group (paired and unpaired) and stimulus (tone and light) returned a significant interaction between stimulus and group (F1,45 = 10.1, P = 0.005). A planned comparison of the tone-evoked CR between paired and unpaired subjects showed that paired subjects had significantly greater bradycardia (2-sample 2-tailed t-test; t(23) = −4.86, P < 0.001). Thus, subjects that received paired presentations of the tone and light generalized their conditioned fear to the tone during the test phase. In contrast, unpaired subjects did not exhibit any conditioned fear to the tone, despite trending toward stronger conditioned fear to the light (t(23) = 1.81, P = 0.08).
Development of Enhanced EPs
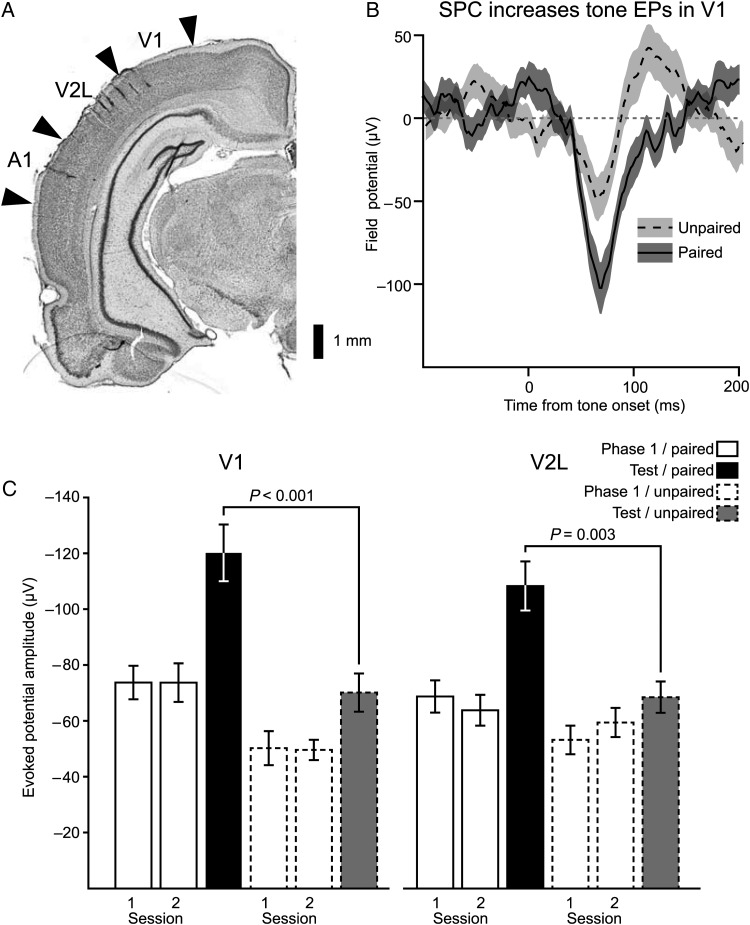
Throughout each of the training phases EPs were recorded from 2 microwire arrays, one targeting A1, and the other the visual cortices V1 and V2L (Fig. 2A). Electrophysiological and anatomical indicators localized recording sites to particular cortical regions (see Materials and methods). During the test session, when conditioned fear to the tone and light was assessed, the paired group had stronger tone onset EPs in V1 and V2L compared with subjects in the unpaired group (Fig 2B,C). The enhanced tone EPs in the paired group could be an effect of receiving tone→light pairing during Phase 1, or result from individual baseline differences in EP strength between the subjects composing the groups. To address this possibility, and examine the development of the tone EP across sensory preconditioning, we compared EP strengths between the paired and unpaired group across Phase 1, when the tone and light were first presented, and during testing following acquisition of the association between the tone and light (Fig. 2C). These responses can be directly compared because the tone onset EP was uncontaminated by the light stimulus across all phases for both groups of subjects; it preceded the light during tone→light pairing trials, and was presented on separate trials during the test phase and Phase 1 of the unpaired group. An ANOVA with the factors region (V1 and V2L), phase (Phase 1 Day 1, Phase 1 Day 2, test), and group (paired and unpaired), returned a significant interaction between group and phase (F2,758 = 17.4, P < 0.001), indicating that across training the magnitude of the tone EPs changed differently between the paired and unpaired groups. Planned comparisons showed that the strength of the tone EP during the test phase was stronger in the paired than the unpaired group for both V1 and V2L (V1, t(106) = 4.8, P < 0.001; V2L, t(143) = 3.4, P = 0.003). Furthermore, post hoc tests (Tukey–Kramer method) retained the significance of these enhancements (P < 0.05), and did not reveal any other significant differences between the paired and unpaired groups during Phase 1. Thus, both groups began training with similar tone responsiveness in their visual cortices.
Figure 2.
Sensory preconditioning strengthened tone EPs in visual cortex. (A) A coronal section illustrating the placement of microwire arrays. The array targeting auditory cortex was aligned along the rostro-caudal axis, while that targeting visual cortex spanned V2L and V1 along the medial-lateral axis. (B) The mean EP waveform to the tone during the test phase from subjects in the paired group is overlaid upon that of group that received unpaired presentations of the tone and light during Phase 1. The shaded region corresponds to the standard error of the mean. (C) EP amplitudes were compared between the paired and unpaired groups across Phase 1 and the test phase. Subjects that received paired presentations of the tone and light developed strengthened tone EPs in both V1 and V2L, while the unpaired subjects did not show significant changes in either visual region. Bar height is the mean EP strength, while error bars reflect standard error of the mean.
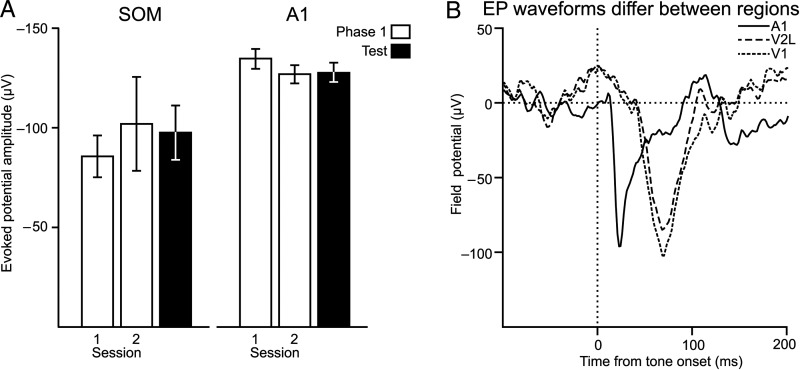
We next characterized the regional specificity of tone EP enhancements by examining changes across sensory regions adjacent to visual cortex. For several subjects, implanted arrays encroached upon somatosensory cortex, while others directly targeted A1, which has robust tone EPs in naïve subjects. If the tone EP enhancements reflected generalized enhancement of cortical activity for stimuli (Bakin et al. 1992), then these regions should also exhibit enhanced tone EPs. However, we did not find changes in tone EPs across training phases in either auditory or somatosensory cortex (Fig. 3A, one-way ANOVA; somatosensory, F2,41 = 0.5, P = 0.61; A1, F2,474 = 0.95, P = 0.39). These results also argue against the hypothesis that the increased amplitude of tone EPs in visual cortex were caused by passive spread of enhanced potentials from adjacent cortical regions, particularly auditory cortex. Underscoring this point, the time course of the tone EP in A1 did not overlap with those in V1 or V2L (Fig. 3B).
Figure 3.
Enhanced tone EPs were specific for to the visual cortex. (A) Neither somatosensory cortex (SOM), nor A1, increased their tone-evoked EP strength in the paired group, despite both exhibiting comparable EP strength to that recorded in visual regions. (B) The tone EP in visual cortex does not reflect passive spread from auditory cortex. A1 exhibits a shorter, more punctuate, EP to the tone than V2L and V1. Each trace is the mean EP recorded from sites in A1 V2L, and V1.
To summarize, only subjects in the paired group developed enhanced tone EPs in visual regions, and this enhancement was only evident after the subjects associated the tone with the light, and the light with the shock.
Laminar Specificity of Enhanced Tone EPs
The neocortex is composed of multiple layers, each receiving a distinct collection of afferents from other cortical regions or subcortical nuclei. Afferents from auditory cortex terminate on pyramidal cells in Layer 5 of V2L, which in turn project to infragranular layers of V1 (e.g., Laramée et al. 2011). If this pathway mediates the tone EP in visual cortex, then it should be constrained to Layers 5 and 6.
To address this, a linear microelectrode array was implanted into V1 (3 subjects). The array spanned all cortical layers, which allowed us to measure the EP strength across cortical layers, and localize the corresponding generators with CSD.
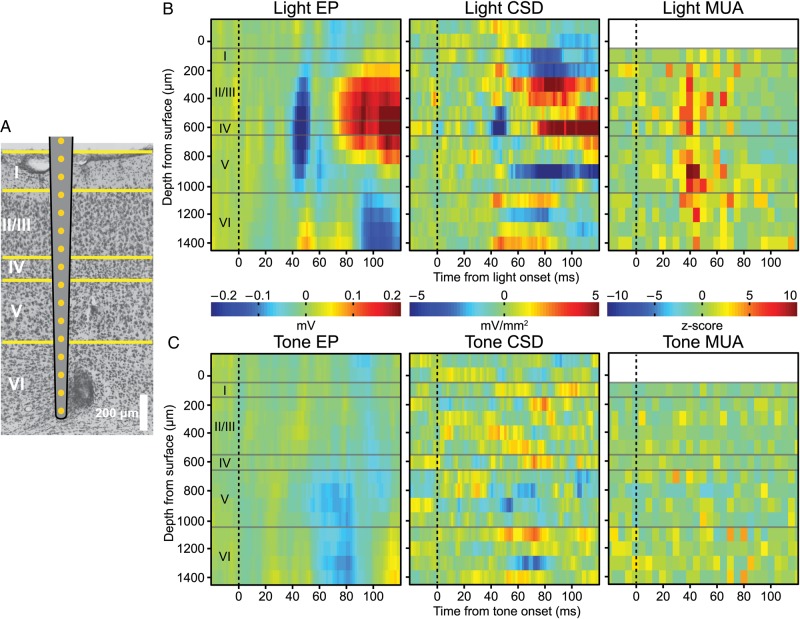
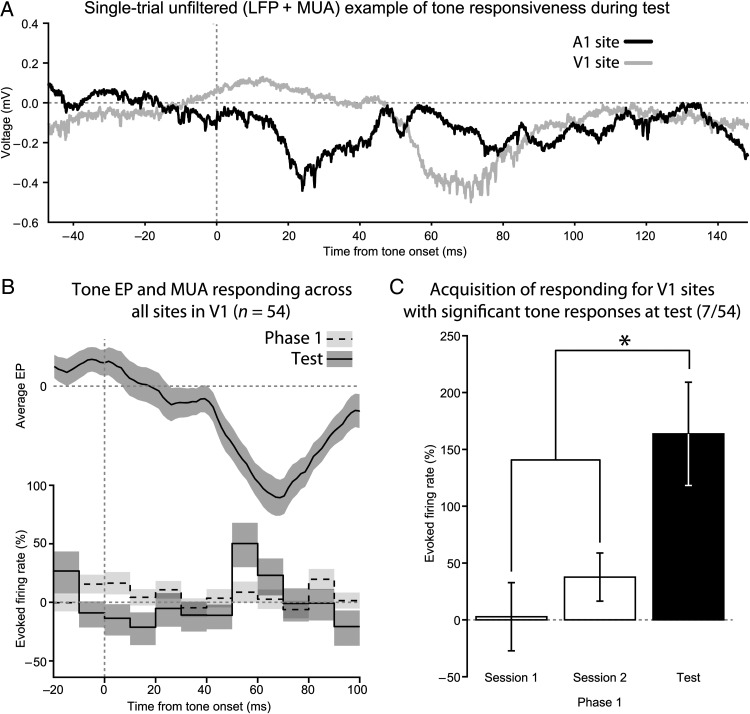
Histological examination revealed that the linear arrays were placed in V1 (Fig. 4A). Furthermore, light presentation evoked a prominent early sink in Layer 4 and MUA distributed throughout Layers 2–6 (Fig. 4B). During the test phase, tone EPs localized to the infragranular Layers 5 and 6, with current sinks in those layers as well (Fig. 4C). Unlike activation by light, no MUA was observed in response to the tone. However, unit activity coincident with the negative peak of the tone EP did occur for some microwire sites in V1 (Fig. 5A), and was only present during the test phase, but not during Phase 1 (Fig. 5B). Only a minority (7/54, Fig. 5C) of microwire sites in V1 exhibited significantly enhanced MUA to the tone with training (Wilcoxon rank-sum test, P ≤ 0.05).
Figure 4.
Tone-evoked EPs in V1 were strongest in infragranular layers. (A) Laminar silicon electrode arrays that encompassed all cortical layers were implanted in V1. Histological validation of electrode placements were performed on Nissl stained sections. A marking lesion was placed on the second to last electrode. (B) Presenting the light produced an EP centered on Layer 4 with an ∼45 ms latency. CSD revealed that the generator of the EP was localized to Layer 4, while the distribution of MUA spanned all layers except Layer 1, which is cell sparse. (C) Tones produced an EP limited to infragranular layers, with current sinks localized to infragranular layers. No substantial changes in MUA occurred to the tone in any of the layers.
Figure 5.
Only limited changes in MUA occurred in V1. (A) Example wideband local field potential recordings from A1 and V1 during the same trial in response to the tone stimulus. Unit activity co-occurs with EP deflections. (B) On average, MUA from sites in V1 exhibited a small, but significant, increase in firing rate during the tone EP. Like the EP, this was not present during Phase 1, only becoming evident during the test phase. Top, the mean tone EP recorded from V1 during the test phase. Bottom, a firing rate histogram comparing the percent change in firing rate between Phase 1 and the test phase. (C) Only those sites in V1 that had significant changes in firing rate to the tone during the test were analyzed. These sites lacked responding during Phase 1, which supports the hypothesis that sensory preconditioning enhanced their tone-evoked MUA.
To establish whether auditory cortex could drive a similar infragranular pattern of activation in V1, we stimulated sites in auditory cortex while recording from V1. To eliminate confounds arising from arousal and cognitive factors these experiments were conducted under anesthesia at least 1 week after training. Stimulation of sites both within or 2 octaves away from the preconditioned tone-evoked infragranular activation (Fig. 6A,B). These responses were substantially stronger compared with naturalistic stimuli (see Fig. 4C), which could be due to the synchrony of activation caused by punctuate delivery of the stimulation current or an increased recruitment of visually projecting neurons in A1. To test whether there was a significant difference in their laminar EP profiles (<125 ms from delivery of microstimulation), we used a multivariate 2-sample test (Székely and Rizzo 2004). The test statistic, ε, is:
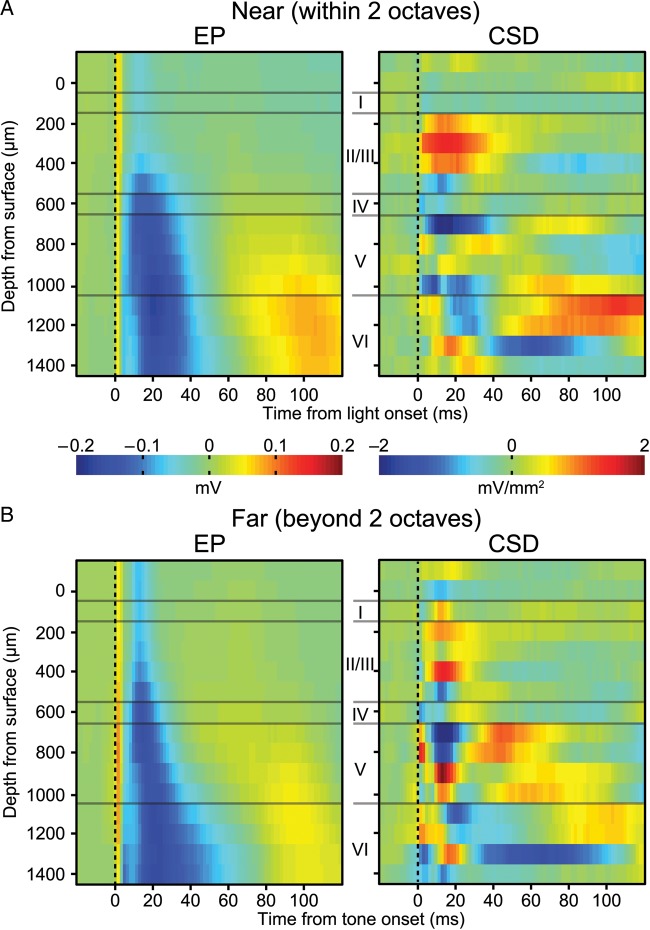
where n is the set of EP profiles evoked by stimulation sites tuned near the tone, f are those far from the tone, n′ and f′ are the respective mean profiles, and E||x|| is the expected value of the Euclidean distance. Significance of ε was determined with a permutation test, wherein the labels for near and far sites were randomly rearranged and the test statistic was recalculated for each new set (repeated 10 000 times), which generated a distribution of ε for the null hypothesis that n = f. This test revealed that the site of stimulation in A1 was not a significant factor for explaining the differences in the laminar EP profiles in V1 (ε(10,10) = 1.05 × 10−7, P = 0.19). The lack of an effect may result from current spread from the site of stimulation, since the current level was on the high side of our range (96 μA).
Figure 6.
Responses in V1 to microstimulation of A1. (A) Laminar EP and CSD profile for responses evoked by stimulation of sites in A1 with a best frequency within 2 octaves of the training tone frequency. (B) Same as (A), except for sites tuned >2 octaves away. On average both EP profiles show an infragranular pattern of activation that is similar to that seen for tone presentation during the test session.
Discussion
Our study identifies an enhancement in the responsiveness of early visual cortex to auditory stimuli in an associative task thought to exploit declarative memory systems. The enhanced tone EP was localized to infragranular layers. A similar potential was evoked by microstimulation of A1 in the same subjects under anesthesia, consistent with a cortico-cortical pathway. These finding demonstrate that early sensory regions are capable of participating in the storage of associations between 2 neutral stimuli.
Validity of the Association Between Neutral Stimuli Using Sensory Preconditioning
Rats in our study exhibited conditioned fear to the tone, despite it never being paired with the shock. Their responses to the tone depended upon it being paired with the light, which was later paired with a shock. An alternative explanation for responding to the tone is that light→shock pairing sensitized the subjects, which caused them to respond in the test phase to any stimulus, irrespective of its associative history with the light. However, this alternative can be ruled out because the control group received the same primary conditioning of light with shock but did not exhibit fear to the tone. Moreover, their only difference from the sensory preconditioning group was that they received unpaired presentations of the tone and light during Phase 1, indicating that the association between the tone and light was the critical factor. These findings agree with past assessments of the nature of sensory preconditioning, which have found that responding to the tone is mediated by its association with the light (Rizley and Rescorla 1972). In that study, extinction of the light also reduced fear to the tone, supporting the view that an associative chain from tone to light to shock mediated conditioned responding to the tone.
Utility of the Sensory Preconditioning Paradigm
Sensory preconditioning, as a means of obtaining associations between neutral stimuli, appears to be a valuable behavioral paradigm for studying the neural basis of declarative memories based on the same type of association. While declarative memories are commonly defined as reflecting conscious recall of experiences and facts, studies in humans effectively and operationally define declarative memories by their ability to be verbally reported; the subject explicitly expresses their recall of the material. This is obviously not possible in infrahuman species. To overcome this difficulty experimenters have taken 2 different approaches. One is to use tasks that require flexible responding, such as a transitive inference task, where the relative weights of stimuli must be evaluated in a novel situation. Success at this task rules out procedural memory, that is, responding produced by unconscious or habitual processes, since such learning is thought to be inflexible (Reber et al. 1996). One limitation of such tasks is that they require subjects to be extensively pretrained to acquire a complex set of rules or schema. The second approach is to use the content of what is learned. Declarative memory is thought to encompass certain kinds of information, such as spatial layouts or associations between arbitrary stimuli, and so tasks that require the subject to use this information should tap into declarative memory processes (Eichenbaum 2004). A disadvantage of such tasks is that control trials are required to exclude the possibility that correct responding is driven by procedural memory. Sensory preconditioning overcomes these problems, while simultaneously tapping into the defining features of declarative memory in animals. The development of responding relies on a nonprocedural associative chain, with one of the associations being between neutral stimuli. Furthermore, in contrast to most other transitive inference tasks, sensory preconditioning does not require extensive training, a practical advantage. Altogether, from both experimental and theoretical standpoints, sensory preconditioning is an ideal task for probing declarative memory.
In addition to the applicability of sensory preconditioning to declarative memory, it also isolates acquisition of an association, in this case between the tone and light, from the reinforcement needed to evoke a behavioral response, for example, a shock. Many learning paradigms, such as fear conditioning and the Morris water maze task, engage both processes simultaneously and thus do not allow the experimenter to study them in isolation, or to examine their interaction across multiple experiences.
Electrophysiological Signatures of Sensory Preconditioning
Our study is the first to demonstrate electrophysiological plasticity in the neocortex following cross-modal sensory preconditioning. Prior investigators have found enhanced responding to preconditioned stimuli in the hippocampus of the rabbit with sensory preconditioning (Port et al. 1987). Subjects in the sensory preconditioning group developed tonic responses to the preconditioned stimuli, while those in the unpaired group exhibited only transient neuronal activity to the same stimuli.
The current study focused on tone EPs and MUA in both early auditory and visual cortices. While auditory cortex showed robust EPs to the tone throughout training, their strength did not substantially change with training. This is in stark contrast to the readily observed enhanced responding to sounds that predict shock in conventional fear conditioning tasks (Gonzalez-Lima and Scheich 1986; Weinberger and Diamond 1987; Bakin and Weinberger 1990; Edeline et al. 1993; Weinberger 1995; Poremba et al. 1998; Ji and Suga 2003; Hennevin and Maho 2005; Bruchey and Gonzalez-Lima 2006). Unlike these tasks, sensory preconditioning separates the formation of the association (Phase 1) from the reinforcement (Phase 2).
Current theories of mnemonic plasticity in auditory cortex depend upon a strong biological reinforcer driving a neuromodulatory system, in conjunction with ascending thalamocortical information about the sound (Weinberger et al. 1990, 2003); a model also adopted by Suga (2008). Neither phase of sensory preconditioning allows for this pairing to take place. During Phase 1, the tone was followed by a neutral visual stimulus, which presumably does not strongly drive neuromodulatory centers. In Phase 2, when neuromodulatory systems would be engaged by the shock, the tone was not present, and so the auditory cortex would not have received any auditory thalamocortical volley representing the conditioned stimulus. Thus, according to these models, plasticity should not develop in A1 during sensory preconditioning, matching the current findings. Therefore, the present results vindicate current theories of auditory cortical plasticity in the face of a situation they never sought to explain, a strong test of their validity (Popper 2002).
Associative Plasticity in Early Visual Cortex
Enhanced associative plasticity was restricted to V1 and V2L. There were no similar changes in putative somatosensory regions, or in auditory cortex, which argues against the possibility that the enhanced tone EPs resulted from a generalized increase in cortical excitability. Area V2L is “early” in the network of visual processing in the cortex, but may have been expected to develop associative plasticity to an auditory stimulus given the integrative role that secondary sensory cortices are thought to play in the cortex. In contrast, the development of associative tone responses in V1 would generally be unexpected, given the longstanding treatment of primary sensory regions as obligatorily unimodal. The current findings reveal that a more complex, multimodal and associative theory of V1 is required.
V1 has already been implicated in perceptual learning both in animals (Schoups et al. 2001) and humans (Jehee et al. 2012; Kok et al. 2012). Perceptual learning differs from associative learning in that it ordinarily requires extensive practice and results in an increase in acuity usually specific to the practiced stimulus dimension. Moreover, the training stimulus does not become a signal for another, arbitrary, sensory stimulus. In contrast, associative learning involves the, often rapid, formation of a link between 2 arbitrary, usually cross-modal stimuli. It occurs continually in experience. The primary visual field has been implicated in aspects of associative learning that involved a biologically strong reinforcer. For example, when rats form an association between a visual stimulus and water reward, cells in V1 shift from responding to the physical attributes of the stimulus to predicting the time of scheduled reward (Shuler and Bear 2006). The present findings reveal that V1 and V2L develop associative plasticity to the signal stimulus (tone) even in the absence of a motivational state or a biological reinforcer. Together, such findings indicate that early visual cortex does not differ qualitatively from “higher” sensory cortical fields in its capability for behavioral associations.
Possible Anatomical Substrates of Associative Plasticity in V1 and V2L
Miller and Vogt (1984) found that V2L receives direct projections from A1, and V2L in turn projects to V1. Such a pathway potentially supports a di-synaptic linkage between primary auditory and visual cortex in the rat. For this to occur neurons in V2L that receive projections from auditory cortex should in turn project to V1. Using a combination of retrograde and anterograde tracers, it has been found that neurons in Layer 5 of V2L receive afferents from auditory cortex, and these same neurons project to V1 (Laramée et al. 2011). Furthermore, activation of Layer 5 neurons in V2L mostly drives infragranular layers of V1 (Domenici et al. 1995; De Pasquale and Sherman 2011). A recent study investigated the circuit that mediates these cross-modal responses (Iurilli et al. 2012). Auditory stimuli produced inhibitory postsynaptic potentials in Layer 2/3 pyramidal cells of mouse V1. These potentials were unreliable, requiring averaging across multiple trials to be readily detected. Suggesting a cortico-cortical route, stimulation of auditory cortex elicited the same potentials, while its inactivation prevented them. The absence of these supragranular potentials in our study may result from methodological shortcomings. In our study, only 10 trials were presented for each session, which may be insufficient for detecting the sporadic hyperpolarizing potential. Also, our recordings were extracellular, which often fails to detect hyperpolarizing potentials (Buzsáki et al. 2012).
The hypothesis that a cortico-cortical connection mediates the enhanced tone response in visual cortex makes another testable prediction, that is, that tone EPs in visual cortex result from activation of infragranular layers. Iurilli et al. (2012) found infragranular EPs that were strongest in Layer 5. Using linear arrays of silicon recording sites, we obtained the laminar distribution of tone EPs in V1. By applying CSD analysis to these recordings, we localized the current sinks and sources that were responsible for the EP. A current sink reflects an area of neuronal depolarization, because cations rush across the membrane and into the cell body, and away from the extracellular region (Rall 1962). As with the microwire arrays, rats with chronically implanted silicon probes had tone EPs in V1 following sensory preconditioning. Most importantly, the current sinks were only situated in infragranular layers, particularly Layer 5, which agrees with the aforementioned cortico-cortical circuit.
Auditory afferents in the visual cortices meet the laminar criteria for feedback projections (Felleman and Van Essen 1991). Principal neurons in infragranular layers of A1 project to the supra- and infragranular layers in V2L (Laramée et al. 2011; Iurilli et al. 2012). Furthermore, V2L sends a feedback projection to V1 (Coogan and Burkhalter 1993). However, the functional interpretation of feedback implies that a connection is reciprocated. This criterion is not met for the auditory to visual pathway, because A1 lacks feedforward type afferents from visual cortices. V1 does not project to A1 in the rat, while in the mouse the projections from V2L and secondary medial visual cortex are both to supra- and infragranular layers (Banks et al. 2011). An alternative formulation is that these cross-modal linkages between visual and auditory cortices are modulatory and not feedback per se (Friedman 1983). Such an interpretation is parsimonious with the electrophysiological phenomenology of the tone responses observed in visual cortex.
Despite the robustness of the enhanced tone EP in visual cortex, there was not an in-kind facilitation of MUA. This disassociation between the EP and spiking activity is frequently reported in studies of cross-modal integration in the cerebral cortex. In primate auditory cortex, somatosensory stimuli can drive infragranular sinks, but do not elicit MUA (Lakatos et al. 2007). Only when an acoustic stimulus accompanies the somatosensory stimulus did it elicit increased MUA, by superadditively increasing the MUA to the bimodal stimulus. Another possible cause of the absence of consistent MUA activity to the tone is that the responses of Layer 5 pyramidal cells in V1 to auditory stimuli tend to be mixed, with an approximately equal chance of either depolarization, hyperpolarization, or no change at all (Iurilli et al. 2012). This would preclude the display of auditory responses at the level of MUA, while sparing changes in the field potential.
The paucity of tone-evoked unit activity in visual cortex does not preclude functional consequences for downstream structures. Under certain conditions, subthreshold activity exerts effects that are subtle, yet consequential (e.g., Lakatos et al. 2007). For the present study, we localized the generator of the tone EP in visual cortex to the infragranular layers. Given that Layer 5 neurons receive auditory information in the visual cortices (Laramée et al. 2011; Iurilli et al. 2012), the infragranular sink most likely corresponds to activation of their basal dendrites. Despite their close proximity to the cell body, the changes in the membrane potential of basal dendrites are highly compartmentalized, with their locally elicited excitatory postsynaptic potentials attenuated by as much as 40 times before reaching the soma (Nevian et al. 2007). This may explain why the V1 and V2L tone EPs, which were comparable in strength to the tone EPs in A1, did not reliably co-occur with spiking.
In addition to their electrotonic isolation from the soma, basal dendrites on Layer 5 neurons can integrate synaptic potentials independent from the soma. They exhibit a sigmoidal transfer function between the number of local synapses that are activated and the resulting peak of the membrane potential: driving a few synapses produces linear summation of their potentials, while a moderate number exceeds the linear sum, and activating a large number evokes a sublinear peak (Polsky et al. 2004). Moreover, the threshold and gain of this sigmoidal relationship is affected by the location of the synapses that are activated, both with respect to each other and their distance from the cell body (Behabadi et al. 2012). This extends the repertoire of functional consequences for synaptic plasticity beyond changes in connection strength and into the interaction between synapses, along with enlarging the space of combinatorial relationships between patterns of synaptic activity and corresponding changes in membrane potential (Poirazi and Mel 2001). From this, Poirazi and Mel theorized that such a scheme radically increases the capacity of cortical circuits to encode memories. Thus, subthreshold changes in synaptic strength could exert substantial effects on the local integration of excitatory postsynaptic potentials from nonauditory sources, which may not be evinced as a change in spiking to the tone alone.
Moving beyond early visual cortices, it is known that behavioral responding to the preconditioned stimulus in sensory preconditioning (i.e., tone in the current study) depends upon the integrity of multimodal regions bordering visual cortex. The first neuroscientific study of sensory preconditioning found that lesioning the association cortices in the cat blocked acquisition of responding to the preconditioning stimulus when a tone and light were used as training stimuli (Thompson and Kramer 1965). In the rat, immediately anterior to V2L is the posterior parietal cortex, which receives afferents from both V1 and V2L (Reep et al. 1994). Lesions of posterior parietal cortex block responding to a preconditioned tone while sparing learning in a standard classical conditioning task with explicit biological reinforcement, that is, light→food association (Robinson and Bucci 2012). Another such region, retrosplenial cortex, borders medial secondary visual cortex. It receives afferents from Layers 5 and 6 of V1, and projects back to V1 (van Groen and Wyss 1992). Retrosplenial lesions also block sensory preconditioning, while again sparing classical conditioning for food reward (Robinson et al. 2011). Both the retrosplenial and posterior parietal lesions were carried out prior to training, and so it is unknown whether the lack of a sensory preconditioning effect arose from a deficit in acquiring the neutral association, retaining it across days, or integrating it with the overtly reinforced association.
Functional Role of Cross-Modal Associative Plasticity in Visual Cortex
Although the present study is the first to our knowledge to find associative plasticity in early sensory cortex based simply on the pairing of 2 neutral stimuli, cross-modal associative plasticity in primary sensory fields is known to develop with standard rewards and punishments. For example, ∼15% of MUA sites in A1 of monkeys develop responses to a cue light during an acoustic detection task (Brosch et al. 2005). Another study investigated cross-modal responses in primary auditory and somatosensory cortex during a discrimination task, and found units in both regions that responded to heteromodal stimuli (Lemus et al. 2010). However, these authors also found that these responses only indicated the presence of the stimulus, and did not encode the particular cross-modal attributes that were to be discriminated. Such results imply that cross-modal responses may instead reflect task events in general, and not necessarily physical characteristics. Consistent with this possibility are the findings (summarized above) of reward-timing related responses in V1 after several training sessions (Shuler and Bear 2006). Altogether, it is apparent that cross-modal unit activity can develop with training, but it is unclear whether it represents the heteromodal stimulus per se (McGurk and MacDonald 1976; Shams et al. 2002), or some task contingency for training with standard reinforcers.
However, the situation appears to be more well-defined in the case of the cross-modal association formed in the present study. The sensory preconditioning paradigm reduces associative training to its bare essentials, merely pairing stimuli from 2 modalities. There are no overt motivational, motor or other task complexities. Therefore, it would appear that the associative plasticity that developed in V1 and V2L does represent the tone itself.
Conclusion and Future Directions
The current results demonstrate that the formation of a behavioral tone→light association, in the absence of standard reinforcers (reward or shock), is accompanied by the development of associative plasticity in early visual cortical fields; specifically EPs to the tone increased significantly in both V2L and most importantly, in V1. Laminar analysis localized this plasticity to Layers 5 and 6, and microstimulation of A1 produced the same pattern of activity, consistent with a cortico-cortical pathway from A1 to V2L and thence to V1. Together with prior studies using standard reinforced training, the results indicate that early visual cortex, particularly V1, has associative capabilities that are not qualitatively different from those of higher visual and associated cortical fields. The findings are consistent with a burgeoning literature showing that primary sensory cortex is involved in associative learning rather than being restricted to perceptual analysis or perceptual learning. To the extent that associations between arbitrary neutral stimuli constitute evidence of declarative memory, these results demonstrate that V1 is involved in such memory. Further investigations are required to establish whether mammals with a more elaborate cortical organization exhibit the same form of plasticity.
The present study demonstrates the advantages of using sensory preconditioning to elucidate neutral substrates of learning and memory in primary sensory cortex. Insofar as this is the first study of sensory preconditioning in early sensory cortex, it raises virtually all of the major issues addressed over the years regarding the neural substrates of strongly reinforced classical and instrumental learning.
Perhaps first and foremost is the need to fully characterize simple bimodal associations in sensory cortex. For example, presumably, the link between tone and light occurred during Phase 1 when they were paired. However, there was no difference between the paired and control groups during this phase. It is possible that the nascent differences were too small to be detected during Phase 1, and that consolidation occurred for the paired group thereafter (Galván and Weinberger 2002). This might be addressed by modifying the sensory preconditioning paradigm to include interphase testing that itself would not affect either the putative association from Phase 1 or associations formed during Phase 2.
Another line of important inquiry concerns devising a testable neural model of sensory preconditioning that explains the nature of the mechanisms underlying sensory cortical multimodal associations between neutral stimuli. Such associations form as a matter of daily interactions with the environment.
Additionally, the findings have implications for conceptions of the functional organization of the cerebral cortex. Primary sensory areas have traditionally been excluded from mnemonic functions. However, that even V1 is involved in neutral stimulus associations, even declarative memory, renders such views untenable. Rather, it seems that even primary sensory zones integrate sensory experience in a multimodal manner, while maintaining the integrity of their dedicated modality. How the brain manages this constitutes a major challenge, as does the formulation of a more ecumenical view of cortical organization.
Funding
This research was supported by the National Institute on Deafness and Other Communication Disorders (DC-02938 to N.M.W.).
Notes
We thank Jacquie Weinberger and Gabriel K. Hui for secretarial assistance, and Tahir Rizvi for assistance in constructing microwire arrays. Conflict of interest: None declared.
References
- Bakin JS, Lepan B, Weinberger NM. 1992. Sensitization induced receptive field plasticity in the auditory cortex is independent of CS-modality. Brain Res. 577(2):226–235. 10.1016/0006-8993(92)90278-H [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. 1990. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 536(1–2):271–286. 10.1016/0006-8993(90)90035-A [DOI] [PubMed] [Google Scholar]
- Banks MI, Uhlrich DJ, Smith PH, Krause BM, Manning KA. 2011. Descending projections from extrastriate visual cortex modulate responses of cells in primary auditory cortex. Cereb Cortex. 21(11):2620–2638. 10.1093/cercor/bhr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behabadi BF, Polsky A, Jadi M, Schiller J, Mel BW. 2012. Location-dependent excitatory synaptic interactions in pyramidal neuron dendrites. PLoS Comput Biol. 8(7):e1002599 10.1371/journal.pcbi.1002599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Miasnikov AA, Weinberger NM. 2013. Remodeling sensory cortical maps implants specific behavioral memory. Neuroscience. 246:40–51. 10.1016/j.neuroscience.2013.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. 2012. Extinction reveals that primary sensory cortex predicts reinforcement outcome. Eur J Neurosci. 35(4):598–613. 10.1111/j.1460-9568.2011.07974.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. 2010. Representational gain in cortical area underlies increase of memory strength. Proc Natl Acad Sci USA. 107(8):3793–3798. 10.1073/pnas.1000159107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Heiser MA, Caywood M, Merzenich MM. 2006. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 52(2):371–381. 10.1016/j.neuron.2006.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman JA, Kim JJ. 2006. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. Eur J Neurosci. 24(3):894–900. 10.1111/j.1460-9568.2006.04965.x [DOI] [PubMed] [Google Scholar]
- Brogden WJ. 1939. Sensory pre-conditioning. J Exp Psychol. 25(4):323–332. 10.1037/h0058944 [DOI] [PubMed] [Google Scholar]
- Brosch M, Selezneva E, Scheich H. 2005. Nonauditory events of a behavioral procedure activate auditory cortex of highly trained monkeys. J Neurosci. 25(29):6797–6806. 10.1523/JNEUROSCI.1571-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchey AK, Gonzalez-Lima F. 2006. Brain activity associated with fear renewal. Eur J Neurosci. 24(12):3567–3577. 10.1111/j.1460-9568.2006.05229.x [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. 2012. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 13(6):407–420. 10.1038/nrn3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AW. 1905. Histological studies on the localization of cerebral function. Cambridge: (UK: ): Cambridge University Press. [Google Scholar]
- Coogan TA, Burkhalter A. 1993. Hierarchical organization of areas in rat visual cortex. J Neurosci. 13(9):3749–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pasquale R, Sherman SM. 2011. Synaptic properties of corticocortical connections between the primary and secondary visual cortical areas in the mouse. J Neurosci. 31(46):16494–16506. 10.1523/JNEUROSCI.3664-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici L, Harding GW, Burkhalter A. 1995. Patterns of synaptic activity in forward and feedback pathways within rat visual cortex. J Neurophysiol. 74(6):2649–2664. [DOI] [PubMed] [Google Scholar]
- Edeline J-M, Pham P, Weinberger NM. 1993. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci. 107(4):539–551. 10.1037/0735-7044.107.4.539 [DOI] [PubMed] [Google Scholar]
- Edeline J-M, Weinberger NM. 1993. Receptive field plasticity in the auditory cortex during frequency discrimination training: selective retuning independent of task difficulty. Behav Neurosci. 107(1):82–103. 10.1037/0735-7044.107.1.82 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. 2004. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 44(1):109–120. 10.1016/j.neuron.2004.08.028 [DOI] [PubMed] [Google Scholar]
- Erickson CA, Desimone R. 1999. Responses of macaque perirhinal neurons during and after visual stimulus association learning. J Neurosci. 19(23):10404–10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1(1):1–47. 10.1093/cercor/1.1.1 [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. 2005. The organization of recent and remote memories. Nat Rev Neurosci. 6(2):119–130. 10.1038/nrn1607 [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. 2003. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J Neurosci. 23(12):5235–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JA, Nicholson C. 1975. Experimental optimization of current source-density technique for anuran cerebellum. J Neurophysiol. 38(2):369–382. [DOI] [PubMed] [Google Scholar]
- Friedman DP. 1983. Laminar patterns of termination of cortico-cortical afferents in the somatosensory system. Brain Res. 273(1):147–151. 10.1016/0006-8993(83)91103-4 [DOI] [PubMed] [Google Scholar]
- Galván VV, Weinberger NM. 2002. Long-term consolidation and retention of learning-induced tuning plasticity in the auditory cortex of the guinea pig. Neurobiol Learn Mem. 77(1):78–108. 10.1006/nlme.2001.4044 [DOI] [PubMed] [Google Scholar]
- Galvez R, Weiss C, Weible AP, Disterhoft JF. 2006. Vibrissa-signaled eyeblink conditioning induces somatosensory cortical plasticity. J Neurosci. 26(22):6062–6068. 10.1523/JNEUROSCI.5582-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, Suga N. 1998. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci USA. 95(21):12663–12670. 10.1073/pnas.95.21.12663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavornik JP, Shuler MG, Loewenstein Y, Bear MF, Shouval HZ. 2009. Learning reward timing in cortex through reward dependent expression of synaptic plasticity. Proc Natl Acad Sci USA. 106(16):6826–6831. 10.1073/pnas.0901835106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Scheich H. 1986. Neural substrates for tone-conditioned bradycardia demonstrated with 2-deoxyglucose. II. Auditory cortex plasticity. Behav Brain Res. 20(3):281–293. 10.1016/0166-4328(86)90228-7 [DOI] [PubMed] [Google Scholar]
- Headley DB, Weinberger NM. 2011. Gamma-band activation predicts both associative memory and cortical plasticity. J Neurosci. 31(36):12748–12758. 10.1523/JNEUROSCI.2528-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. 1949. The organization of behavior: A neuropsychological theory. NY: Wiley. [Google Scholar]
- Hennevin E, Maho C. 2005. Fear conditioning-induced plasticity in auditory thalamus and cortex: to what extent is it expressed during slow-wave sleep? Behav Neurosci. 119(5):1277–1289. 10.1037/0735-7044.119.5.1277 [DOI] [PubMed] [Google Scholar]
- Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, Tonini R, Tucci V, Benfenati F, Medini P. 2012. Sound-driven synaptic inhibition in primary visual cortex. Neuron. 73(4):814–828. 10.1016/j.neuron.2011.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehee JF, Ling S, Swisher JD, van Bergen RS, Tong F. 2012. Perceptual learning selectively refines orientation representations in early visual cortex. J Neurosci. 32(47):16747–16753a. 10.1523/JNEUROSCI.6112-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Suga N. 2003. Development of reorganization of the auditory cortex caused by fear conditioning: effect of atropine. J Neurophysiol. 90(3):1904–1909. 10.1152/jn.00363.2003 [DOI] [PubMed] [Google Scholar]
- Kisley MA, Gerstein GL. 2001. Daily variation and appetitive conditioning-induced plasticity of auditory cortex receptive fields. Eur J Neurosci. 13(10):1993–2003. 10.1046/j.0953-816x.2001.01568.x [DOI] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. 2004. Neural substrates mediating human delay and trace fear conditioning. J Neurosci. 24(1):218–228. 10.1523/JNEUROSCI.0433-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok P, Jehee JF, de Lange FP. 2012. Less is more: expectation sharpens representations in the primary visual cortex. Neuron. 75(2):265–270. 10.1016/j.neuron.2012.04.034 [DOI] [PubMed] [Google Scholar]
- Lakatos P, Chen CM, O'Connell MN, Mills A, Schroeder CE. 2007. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 53(2):279–292. 10.1016/j.neuron.2006.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laramée ME, Kurotani T, Rockland KS, Bronchti G, Boire D. 2011. Indirect pathway between the primary auditory and visual cortices through layer V pyramidal neurons in V2L in mouse and the effects of bilateral enucleation. Eur J Neurosci. 34(1):65–78. 10.1111/j.1460-9568.2011.07732.x [DOI] [PubMed] [Google Scholar]
- Lashley KS. 1929. Brain mechanisms and intelligence: a quantitative study of injuries to the brain. Chicago: University of Chicago Press. [Google Scholar]
- Lemus L, Hernández A, Luna R, Zainos A, Romo R. 2010. Do sensory cortices process more than one sensory modality during perceptual judgments? Neuron. 67(2):335–348. 10.1016/j.neuron.2010.06.015 [DOI] [PubMed] [Google Scholar]
- Lewicki MS. 1998. A review of methods for spike sorting: the detection and classification of neural action potentials. Network. 9(4):R53–R78. 10.1088/0954-898X/9/4/001 [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. 1995. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 102(3):419–457. 10.1037/0033-295X.102.3.419 [DOI] [PubMed] [Google Scholar]
- McGurk H, MacDonald J. 1976. Hearing lips and seeing voices. Nature. 264(5588):746–748. 10.1038/264746a0 [DOI] [PubMed] [Google Scholar]
- Messinger A, Squire LR, Zola SM, Albright TD. 2001. Neuronal representations of stimulus associations develop in the temporal lobe during learning. Proc Natl Acad Sci USA. 98(21):12239–12244. 10.1073/pnas.211431098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Vogt BA. 1984. Direct connections of rat visual cortex with sensory, motor, and association cortices. J Comp Neurol. 226(2):184–202. 10.1002/cne.902260204 [DOI] [PubMed] [Google Scholar]
- Mishkin M. 1982. A memory system in the monkey. Philos Trans R Soc Lond B Biol Sci. 298(1089):83–95. 10.1098/rstb.1982.0074 [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. 1997. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 7(2):217–227. 10.1016/S0959-4388(97)80010-4 [DOI] [PubMed] [Google Scholar]
- Nevian T, Larkum ME, Polsky A, Schiller J. 2007. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat Neurosci. 10(2):206–214. 10.1038/nn1826 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates. 6th ed Boston: Elsevier. [DOI] [PubMed] [Google Scholar]
- Poirazi P, Mel BW. 2001. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 29(3):779–796. 10.1016/S0896-6273(01)00252-5 [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. 2006. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 26(18):4970–4982. 10.1523/JNEUROSCI.3771-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsky A, Mel BW, Schiller J. 2004. Computational subunits in thin dendrites of pyramidal cells. Nat Neurosci. 7(6):621–627. 10.1038/nn1253 [DOI] [PubMed] [Google Scholar]
- Popper KR. 2002. The logic of scientific discovery. NY: Routledge. [Google Scholar]
- Poremba A, Jones D, Gonzalez-Lima F. 1998. Classical conditioning modifies cytochrome oxidase activity in the auditory system. Eur J Neurosci. 10(10):3035–3043. 10.1046/j.1460-9568.1998.00304.x [DOI] [PubMed] [Google Scholar]
- Port RL, Beggs AL, Patterson MM. 1987. Hippocampal substrate of sensory associations. Physiol Behav. 39(5):643–647. 10.1016/0031-9384(87)90167-3 [DOI] [PubMed] [Google Scholar]
- Rall W. 1962. Electrophysiology of a dendritic neuron model. Biophys J. 2(2 Pt 2):145–167. 10.1016/S0006-3495(62)86953-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber PJ, Knowlton BJ, Squire LR. 1996. Dissociable properties of memory systems: differences in the flexibility of declarative and nondeclarative knowledge. Behav Neurosci. 110(5):861–871. 10.1037/0735-7044.110.5.861 [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. 1993. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 13(1):87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. 1994. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp Brain Res. 100(1):67–84. [DOI] [PubMed] [Google Scholar]
- Richardson R, Wang P, Campbell BA. 1995. Delayed development of conditioned heart rate responses to auditory stimuli in the rat. Dev Psychobiol. 28(4):221–238. 10.1002/dev.420280404 [DOI] [PubMed] [Google Scholar]
- Rizley RC, Rescorla RA. 1972. Associations in second-order conditioning and sensory preconditioning. J Comp Physiol Psychol. 81(1):1–11. 10.1037/h0033333 [DOI] [PubMed] [Google Scholar]
- Robinson S, Bucci DJ. 2012. Damage to posterior parietal cortex impairs two forms of relational learning. Front Integr Neurosci. 6:45 10.3389/fnint.2012.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Keene CS, Iaccarino HF, Duan D, Bucci DJ. 2011. Involvement of retrosplenial cortex in forming associations between multiple sensory stimuli. Behav Neurosci. 125(4):578–587. 10.1037/a0024262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. 2005. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci USA. 102(38):13664–13669. 10.1073/pnas.0506838102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco T, Sacchetti B. 2010. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science. 329(5992):649–656. 10.1126/science.1183165 [DOI] [PubMed] [Google Scholar]
- Sakai K, Miyashita Y. 1991. Neural organization for the long-term memory of paired associates. Nature. 354(6349):152–155. 10.1038/354152a0 [DOI] [PubMed] [Google Scholar]
- Scheich H, Brechmann A, Brosch M, Budinger E, Ohl FW, Selezneva E, Stark H, Tischmeyer W, Wetzel W. 2011. Behavioral semantics of learning and crossmodal processing in auditory cortex: the semantic processor concept. Hear Res. 271(1–2):3–15. 10.1016/j.heares.2010.10.006 [DOI] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Qian N, Orban G. 2001. Practising orientation identification improves orientation coding in V1 neurons. Nature. 412(6846):549–553. 10.1038/35087601 [DOI] [PubMed] [Google Scholar]
- Shams L, Kamitani Y, Shimojo S. 2002. Visual illusion induced by sound. Cogn Brain Res. 14(1):147–152. 10.1016/S0926-6410(02)00069-1 [DOI] [PubMed] [Google Scholar]
- Shuler MG, Bear MF. 2006. Reward timing in the primary visual cortex. Science. 311(5767):1606–1609. 10.1126/science.1123513 [DOI] [PubMed] [Google Scholar]
- Siucinska E, Kossut M. 2004. Experience-dependent changes in cortical whisker representation in the adult mouse: a 2-deoxyglucose study. Neuroscience. 127(4):961–971. 10.1016/j.neuroscience.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Squire LR. 1992. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 99(2):195–231. 10.1037/0033-295X.99.2.195 [DOI] [PubMed] [Google Scholar]
- Suga N. 2008. The neural circuit for tone-specific plasticity in the auditory system elicited by conditioning. Learn Mem. 15(4):198–201. 10.1101/lm.791408 [DOI] [PubMed] [Google Scholar]
- Székely GJ, Rizzo ML. 2004. Testing for equal distributions in high dimension. InterStat. Nov(5):1–16. [Google Scholar]
- Teyler TJ. 1971. Effects of restraint on heart-rate conditioning in rats as a function of US location. J Comp Physiol Psychol. 77(1):31–37. 10.1037/h0031579 [DOI] [PubMed] [Google Scholar]
- Thompson RF, Kramer RF. 1965. Role of association cortex in sensory preconditioning. J Comp Physiol Psychol. 60(2):186–191. 10.1037/h0022298 [DOI] [PubMed] [Google Scholar]
- Thompson RF, Patterson MM, Teyler TJ. 1972. The neurophysiology of learning. Annu Rev Psychol. 23:73–104. 10.1146/annurev.ps.23.020172.000445 [DOI] [PubMed] [Google Scholar]
- Vaknin G, DiScenna PG, Teyler TJ. 1988. A method for calculating current source density (CSD) analysis without resorting to recording sites outside the sampling volume. J Neurosci Methods. 24(2):131–135. 10.1016/0165-0270(88)90056-8 [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. 1992. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol. 315(2):200–216. 10.1002/cne.903150207 [DOI] [PubMed] [Google Scholar]
- Weinberger NM. 2003. The nucleus basalis and memory codes: auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiol Learn Mem. 80(3):268–284. 10.1016/S1074-7427(03)00072-8 [DOI] [PubMed] [Google Scholar]
- Weinberger NM. 2011. Reconceptualizing the primary auditory cortex: learning, memory and specific plasticity. In: Winer JA, Schreiner CE, editors. The auditory cortex. Chapter 22 NY: Springer; p. 465–491. [Google Scholar]
- Weinberger NM. 1995. Retuning the brain by fear conditioning. In: Gazzaniga MS, editor. The cognitive neurosciences. Chapter 71 Cambridge: (MA: ): MIT Press; p. 1071–1089. [Google Scholar]
- Weinberger NM. 2004. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 5(4):279–290. 10.1038/nrn1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin JS, Lennartz RC, Cassady JM. 1990. Neural adaptive information processing: a preliminary model of receptive-field plasticity in auditory cortex during Pavlovian conditioning. In: Gabriel M, Moore J, editors. Learning and computational neuroscience: Foundations of adaptive networks. Chapter 3 Cambridge: (MA: ): MIT Press; p. 91–138. [Google Scholar]
- Weinberger NM, Diamond DM. 1987. Physiological plasticity in auditory cortex: rapid induction by learning. Prog Neurobiol. 29(1):1–55. 10.1016/0301-0082(87)90014-1 [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Javid R, Lepan B. 1993. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proc Natl Acad Sci USA. 90(6):2394–2398. 10.1073/pnas.90.6.2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. 2000. Memory's echo: vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci USA. 97(20):11125–11129. 10.1073/pnas.97.20.11125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Zhang Y. 2005. Sound-guided shaping of the receptive field in the mouse auditory cortex by basal forebrain activation. Eur J Neurosci. 21(2):563–576. 10.1111/j.1460-9568.2005.03878.x [DOI] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM. 2000. Visuo-tactile cross-modal associations in cortical somatosensory cells. Proc Natl Acad Sci USA. 97(17):9777–9782. 10.1073/pnas.97.17.9777 [DOI] [PMC free article] [PubMed] [Google Scholar]