Abstract
Objective
Exaggerated cardiovascular (CV) reactivity to laboratory challenge has been shown to predict future CV morbidity and mortality. CV recovery, has been less studied, and has yielded inconsistent findings, possibly due to presence of moderators. Reviews on the relationship between CV recovery and CV outcomes have been limited to cross-sectional studies and have not considered methodological factors. We performed a comprehensive meta-analytic review of the prospective literature investigating CV recovery to physical and psychological challenge and adverse cardiovascular outcomes.
Methods
We searched PsycINFO and PubMed for prospective studies investigating the relationship between CV recovery and adverse CV outcomes. Studies were coded for variables of interest and for effect sizes (ES). We conducted a random effects weighted meta-analysis. Moderators were examined with ANOVA-analog and meta-regression analyses.
Results
Thirty seven studies met inclusion criteria (N=125386). Impaired recovery from challenge predicted adverse cardiovascular outcomes (summary effect, r = .17, p < .001). Physical challenge was associated with larger predictive effects than psychological challenge. Moderator analyses revealed that recovery measured at 1 minute post-exercise, passive recovery, use of mortality as an outcome measure, and older sample age were associated with larger effects.
Conclusions
Poor recovery from laboratory challenges predicts adverse CV outcomes, with recovery from exercise serving as a particularly strong predictor of CV outcomes. The overall ES for recovery and CV outcomes is similar to that observed for CV reactivity and suggests that the study of recovery may have incremental value for understanding adverse CV outcomes.
Keywords: cardiovascular recovery, exercise, cardiovascular challenge, cardiovascular disease, mortality, longitudinal
INTRODUCTION
Cardiovascular diseases (CVDs) are the foremost cause for mortality worldwide, responsible for nearly 30% of all deaths (1). Their high prevalence, mortality rate, and economic burden highlight the importance of better understanding mechanisms leading to CVDs. This aligns with the American Heart Association’s 2020 strategic plan to improve CV health and diminish death from CVD by 20% through primordial and secondary prevention, respectively (2). To this end, CV reactivity has received considerable attention and empirical support as one potent CVD risk indicator. Reactivity is often conceptualized as the magnitude of change in one or more CV parameters (3) in response to demanding conditions. Complementary to CV reactivity, CV recovery is usually defined as the response to the cessation of a challenging stimulus, generally involving a return to pre-challenge resting levels, although definitions for and methodologies to capture recovery vary greatly. Despite the interdependence between CV recovery and CV reactivity, recovery is typically investigated separately from reactivity or not assessed (4). This oversight is unfortunate as the duration of challenge-related CV activation may better predict CVD risk than the absolute magnitude of activation. As such, aberrant CV recovery patterns may also contribute to CVD. In fact, CV recovery patterns have been found to independently predict variance in CV health indices beyond those predicted by reactivity (5,6,7).
CV reactivity and recovery and their relationship to development of CVD have been commonly measured in the context of physiological (e.g., exercise) and psychological (e.g., anger recall) laboratory challenges (8). These practices are rooted in convincing evidence that psychological factors, such as hostility, have tight links to CVD formation (9) through cardiovascular mechanisms (10) and exercise electrocardiography, focusing on reactivity to physical exercise, is routinely used in clinical work as a diagnostic tool for detecting CVD (11,12). Recovery from exercise has received less clinical focus (13,14) despite evidence that recovery from exercise predicts future CV and all-cause mortality (13). Similarly, Chida and Steptoe’s meta-analysis (5) of studies of psychological challenges, found that heightened CV reactivity and slow CV recovery were associated with prospective CVD risk (r = 0.13, p < 0.001; r = 0.08, p < 0.01, respectively).
Despite these important early indications, the field has lacked in-depth analyses of recovery and CVD outcomes. For example, no work has contrasted the predictive power of psychological and physiological challenges for health outcomes or considered the presence of moderating variables. Indeed, several authors have speculated on the idea that robust effects of CV recovery and CVD might be masked by the presence of moderating variables or methodological factors (15). The background and rationale for our meta-analysis is explained in the following sections.
During challenge the sympathetic and parasympathetic systems are engaged and lead to robust CV activation during challenge and recovery post challenge. For this reason, timing of CV recovery measurement has implications for observed activation of these two systems (3) and potentially for sensitivity to predict CV outcomes. For example, initial HR decay during recovery from exercise may result from vagal reactivation (3,16) while longer recovery times would include involvement of the relatively-slower sympathetic nervous system (17). Impaired vagal reactivation leading to delayed recovery during the initial recovery minute has been consistently linked to CV mortality (18), warranting an investigation of other CV risk outcomes such as CVD. Similarly, delayed recovery after psychological challenges has been linked to low vagal tone which can lead to vascular (versus cardiac) control of blood pressure (BP) resulting in high BP variability. Over the short term, such changes lead to adaptive responses, however chronic slow recovery from psychological challenges, such as anger, can lead to CV pathological changes and ultimately CVD development.
One obstacle to interpreting existing data on recovery is the heterogeneity of studies, which also may speak to presence of moderators. For example, recovery protocols are highly variable (19) in duration, and in the demands placed on participants (e.g., active versus passive recovery), as well as in what CV outcomes are assessed. One possibility, then, is that in-depth investigation of potential moderators will help to clarify the relationship between CV recovery and CV health.
Time of assessment of CV recovery (e.g. 1, 2, or more minutes post challenge) is also potentially important given differences in the speed of recovery in different CV parameters. This is particularly relevant to CV recovery from exercise: vagal reactivation takes place during the first 30 seconds post exercise and pre-challenge levels can occur within 1–2 minutes post exercise, even after more intense bouts of exercise, making long assessments of CV recovery after exercise potentially futile (20). Conversely, psychological challenges (e.g., frustration; anger) may have longer activation and deactivation periods, making measurement of recovery over short periods of time ineffective.
One distinction in the recovery literature concerns demands placed on participants. Some studies use active recovery periods, including lower intensity demands (e.g., walking, reading), while others use passive recovery periods which typically involve quiet sitting. The impact of this variability on the prognostic or diagnostic value of CV recovery from exercise, in particular, is not well understood. Upon cessation of strenuous exercise, the cardiovascular system goes through rapid changes, such as vagal rebound (21,22), and the potential for post-exercise incidents such as arrhythmias, hypotension, and syncope increases (23,24). Active or supine recovery, however, tends to facilitate venous blood flow to the heart and diminish such incidents (25–28). In trying to further disentangle effects of active versus passive recovery from exercise, Crisafulli and colleagues (29) found passive recovery caused lower cardiac output relative to active recovery. While this could indicate CV impairment, such differences are merely due to decreased muscular engagement during passive recovery and hence faster recovery after exercise (29). Similarly, low intensity tasks after psychological challenge may shorten recovery periods; convincing evidence stems from an investigation of the role of distracters versus passive waiting on recovery from psychological challenge (30). In sum, extant findings suggest it would be useful to examine whether type of recovery (active versus passive) moderates CV outcomes.
Finally, the literature also suggests that the strength of the relationship between CV recovery and CV outcomes may depend on the type of CV outcome studied. Outcomes range from established risk factors for CVD (i.e., hypertension) to mortality. Prior studies have reported inconsistent relationships between CV recovery and hypertension (15), but more consistent relationships with mortality (13). Severity of disease and medication use (31) likely affect the relationship between recovery and CV outcomes. However, despite the apparent variation in strength of the relationship between CV recovery and CV adverse outcomes, systematic quantitative analyses have ignored CV outcome as a moderator.
The current meta-analytic review investigated the predictive value of CV recovery on adverse CV outcomes for both physical challenges (exercise) and psychological challenges. Since neglect of moderators in previous reviews and meta-analyses may account for weak or inconsistent relations between impaired recovery and adverse CV outcomes (5,15), we conducted systematic moderator analyses on the larger exercise literature, including analysis of the length of recovery assessment, passive versus active recovery, and CV health outcome. Finally, although studies used a variety of CV indicators, exercise studies often exclusively examine HR. Thus, for direct comparison on a single measure across all studies we report secondary findings specifically on HR recovery as a predictor, a measure that reflects the concurrent actions of the sympathetic and parasympathetic nervous systems.
METHODS
Literature Search
To obtain relevant published articles, the first and third authors conducted an electronic literature search for English language studies using the PsycINFO and PubMed online databases using various keywords: cardiovascular recovery AND (longitudinal OR prospective). Searches were not restricted by year of publication. In searching for psychological challenge studies, we also used the terms proposed by Chida and Steptoe (5). Furthermore, the reference sections of retrieved articles were examined to identify additional candidate studies. Altogether, this literature search generated 2521 articles. Most of these studies could be easily excluded on the basis of reading the titles and abstracts from a lack of appropriate predictors, design, and/or outcome. Sixty two studies were retrieved for a closer inspection. Of these: 12 used a cross-sectional design, 8 investigated reactivity only, and 5 did not have a relevant predictor. Finally, 37 articles were deemed adequate for the current meta-analysis and met full inclusion criteria described below. Notably, our list of studies of psychological challenge as a predictor of CV outcomes replicated that of Chida and Steptoe (5).
Study Inclusion Criteria
Inclusion criteria required that studies:
included participants ≥ 12 years mean age;
used one or more physical (e.g., exercise) or psychological challenges (e.g., anger recall) known to activate the CV system;
reported CV recovery with acceptable cardiovascular indices (HR, BP) and defined CV recovery as the time in minutes to attain BP/HR levels at or below CV baseline levels following a challenge, rate of decrease from peak HR, or rate of decrease during a predetermined time post task (% decrease during the first 1 or 2 minutes or to attainment of baseline CV levels);
evaluated and reported the association between CV recovery from challenge and CV health status (e.g., changes in CV functioning, clinical changes in CV functioning and development of a CVD, or CV and all cause mortality).
Study Coding
Studies included in the final meta-analysis were coded by two independent raters for the above inclusion criteria and potential moderators, as well as descriptive characteristics. Coding discrepancies were double-checked by each coder and any remaining disagreements were resolve through discussion and consensus. As seen in Table 1, variables extracted from the studies were: author, mean age, sample gender composition, type of exercise, length of recovery measurement, passive (i.e., sitting) vs. active recovery (i.e., walking slowly after maximal exercise), follow-up period, CV predictor, CV outcome, and the computed mean ES. Gender was coded to indicate whether a female, male or mixed sample was used. The first and third author coded all articles to ensure that studies used physical and/or psychological challenges only and to identify the CV challenge protocol: since all physical challenge studies used exercise, we were interested to identify the strength of the challenge (e.g., use of maximal exercise) given the potential impact it has on recovery speed. Additionally, recovery protocol, length of the recovery measurement period, and whether authors had controlled for reactivity levels were all recorded. Finally, studies were likewise coded to represent the type of baseline CV predictor and CV outcome that was prospectively assessed: non-clinical CV changes, clinical CV changes, and development of CVD, and mortality.
Statistical Methods
To estimate our omnibus, secondary, and moderator effects we used the SAS statistical software package Version 9.4. We used Comprehensive Meta-Analysis Version 2.2.064 to generate plots and figures.
Primary Meta-Analysis
Our omnibus analyses included one summary ES per independent sample to minimize bias due to over representation of samples. Specifically, when a study reported multiple ESs for various outcomes, the main meta-analysis included the mean of all the ESs reported for one sample. If studies reported ESs for separate and independent samples (i.e., gender), independent ESs were included in the main analysis for the given study (32,33) to increase number of independent ESs in the analysis, and hence increase power. The overall ES computed for each study was r, which represents the correlation between predictor and outcome. Most studies reported correlations or t-tests and regression coefficients that were transformed into rs. For articles reporting either cardiovascular mortality or all-cause mortality, odds ratios and hazard risk ratios were transformed into r to ensure a common ES metric. Fixed-effects and random-effects models are both commonly used in meta-analysis. The more conservative random effects model was preferred for all analyses, given the probability that studies were drawn from different populations (e.g. healthy vs. at risk). We also tested for heterogeneity of the ESs using the heterogeneity statistic Q.
Secondary Meta-Analysis
We performed a secondary meta-analysis of HR as the main predictor of CV outcomes. This was possible because nearly all exercise studies (36 out of 39) reported HR. Similarly to the main meta-analysis, a random effects model was preferred for this analysis and heterogeneity of the ESs was tested using the heterogeneity statistic Q.
Moderator Analyses
Previous empirical findings guided selection of key moderators: length of the recovery measurement, whether the protocol used passive or active recovery, and CV outcome. We also conducted descriptive exploratory analyses of other potential moderators: gender composition, mean age of the sample, health status of the sample at the time of the testing, and duration of follow-up period. Given the much smaller number of psychological challenge studies, which constrained power, our moderator analyses focused on exercise challenge studies. However, we did perform a moderator analysis on the predictive strength of the type of challenge (physical versus psychological) for CV outcomes. Categorical moderators were tested using procedures analogous to analysis of variance (ANOVA) to test moderating effects. To test whether the difference between groups was statistically significant, we computed the between-groups homogeneity statistic (Qb). Continuous moderators were tested using an analog to regression analysis. When a study reported multiple ESs within a moderator, a separate ES was entered for each group represented by the moderator; however, to reduce overrepresentation of a study, the total sample of the study was divided by the number of ESs included in the analysis.
RESULTS
Summary of Study Characteristics
Thirty seven studies qualified for the current meta-analysis. Over half the studies (73%) included males and females. Two studies reported separate results for men and women and results for these two samples were entered separately, resulting in 39 entries for the main analysis (total sample size N = 125386). Sample sizes ranged from 44 to 29244 participants (see Table 1). Sample mean ages ranged from 19 to 69 years with a mean age of 50.1 (SD = 11.7). Given the large age range, we explored whether age group (≤45 versus >45) moderated the recovery-CV outcome relationship. Indeed, we found that studies with a mean age over 45 showed a stronger relationship between recovery and CV outcomes (Qb = 96.11, p < .001; r (>45) = .20) than studies with a mean age below 45 (r (<45) = .12).
TABLE 1.
Characteristics of studies included in the meta-analysis.
| Study | N | Age | Gender | Health Status | Challenge | Recovery | Time of recovery assessment (minutes) | Cardiovascular predictor | Follow-up (years) | Cardiovascular outcome | Mean ES (r) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arena (50) | 87 | 50 | Mixed | HX | EXS | Active | 1 | HR | 1 | Mortality/HF | .34 |
| Arena (51) | 520 | 58.4 | Mixed | HX | EXS | Active | 1 | HR | 4 | CV Mortality | .39 |
| Borghi (52) | 44 | 24.6 | Mixed | HX | PSY (CT) | Passive | 5 | DBP | 5 | HTN | .25 |
| Cahalin (53) | 258 | 61.8 | Mixed | HX | EXS | Active | 1 | HR | 1.9 | CV Mortality | .46 |
| Chen (54) | 8861 | 58.2 | Mixed | HX | EXS | Passive | 1 | HR | 8 | Mortality | .30 |
| Cheng (55) | 2333 | 49.4 | Males | H | EXS | Passive | 5 | HR | 14.9 | CV Mortality | .05 |
| Cole (18) | 2428 | 56.6 | Mixed | FHX | EXS | Active | 1 | HR | 6 | Mortality | .20 |
| * Cole (32) | 3197 | 44.3 | Males | H | EXS | Passive | 2 | HR | 12 | Mortality | ..27 |
| * Cole (32) | 2034 | 44.3 | Females | H | EXS | Passive | 2 | HR | 12 | Mortality | .20 |
| Dhoble (56) | 6546 | 49 | Mixed | H | EXS | Passive | 1 | HR | 8.1 | Mortality | .01 |
| Diaz (57) | 7163 | 60 | Mixed | HX | EXS | Active | 1 | HR | 6.7 | Mortality | .13 |
| Frolkis (58) | 29244 | 56.3 | Mixed | H | EXS | - | 1 | HR | 5.3 | Mortality | .33 |
| Gayda (59) | 4097 | 51.1 | Mixed | HX | EXS | Passive | 3 | HR | 14.7 | Mortality, CV Mortality | .03 |
| Gorelik (60) | 2193 | 59 | Males | HX | EXS | Passive | 1,2 | HR | 10.2 | Mortality | .24 |
| Heponiemi (7) | 66 | 30.7 | Mixed | H | PSY (CT/PS) | Passive | 6.8 | HR, PEP, HRV | 2.25 | IMT | .21 |
| Kizilbash (61) | 3633 | 24.8 | Mixed | H | EXS | Active | 2 | HR | 15 | SBP, DBP | .001 |
| Kokkinos (62) | 5974 | 57 | Males | HX | EXS | Active | 2 | HR | 6.2 | Mortality | .26 |
| Leino (63) | 1972 | 57 | Mixed | HX | EXS | Passive | 1 | HR | 4 | Mortality, CV Mortality | .23 |
| Lipinski (20) | 2193 | 69.5 | Males | HX | EXS | Passive | 2 | HR | 7 | Mortality | −.003 |
| Maddox (64) | 9519 | 63 | Mixed | HX | EXS | Active | 1 | HR | 3 | Mortality+MI | .09 |
| Mora (65) | 2994 | 45.7 | Females | H | EXS | Passive | 2 | HR | 20 | CV Mortality | .05 |
| Morshedi (66) | 2967 | 43 | Mixed | H | EXS | Passive | 1,2 | HR | 15 | Mortality | .02 |
| Moseley (6) | 117 | 26.9 | Mixed | H | PSY (CT/AR) | Active | 5 | HP, BP | 10 | IMT | .00 |
| Myers (67) | 1910 | 57 | Males | HX | EXS | Passive | 2 | HR | 5 | Mortality | .19 |
| Nanas (68) | 92 | 51 | Mixed | HX | EXS | Passive | 1 | HR | 1.7 | Mortality | −.06 |
| Nishime (69) | 9454 | 53.2 | Mixed | H | EXS | Active | 1 | HR | 5.2 | CV Mortality | .20 |
| Nissinen (70) | 229 | 59.4 | Mixed | HX | EXS | Passive | 1 | HR | 2.6 | Mortality | .37 |
| Pitsavos (71) | 581 | 41.2 | Mixed | FHX | EXS | Active | 1 | HR | 6 | CHD (fatal+non-fatal) | −.03 |
| Savonen (72) | 1102 | 51 | Males | H | EXS | Active | 2 | HR | 18 | Mortality | .04 |
| Shetler (73) | 2193 | 59 | Males | HX | EXS | Passive | 1,2 | HR | 7 | Mortality | .25 |
| Shinoda (74) | 79 | 52.6 | Mixed | HX | EXS | Active | 1 | HR | 1.6 | CVD | .42 |
| * Singh (33) | 1026 | 42.2 | Males | H | EXS | Passive | 3 | BP | 8 | HTN | .23 |
| * Singh (33) | 1284 | 41.9 | Females | H | EXS | Passive | 3 | BP | 8 | HTN | .13 |
| Steptoe (75) | 209 | 52.2 | Mixed | H | PSY (CT) | Passive | 45 | HR, SBP, DBP, CI, HRV | 3 | SBP, DBP | .11 |
| Steptoe (76) | 125 | 55.3 | Mixed | H | PSY (CT) | Active | 45 | HR, SBP, DBP | 3 | IMT | .04 |
| Stewart (77) | 73 | 19 | Mixed | FHX | EXS | Passive | 5 | HR, SBP, DBP | 3 | SBP | .32 |
| Stewart (78) | 216 | 60.1 | Mixed | H | PSY (CT/PS) | Active | 5 | HR, SBP, DBP | 3 | SBP, DBP | .09 |
| Vivekananthan (79) | 2935 | 60.1 | Mixed | HX | EXS | Active | 1 | HR | 6 | Mortality | .13 |
| Watanabe (80) | 5438 | 57.3 | Mixed | HX | EXS | Passive | 1 | HR | 3 | Mortality | .20 |
Note. Status: HX = known cardiovascular disease, FHX = Family history of cardiovascular disease, H = healthy; Challenge: EXS = exercise, PSY = psychological (CT = cognitive task/math, PS = public speaking, AR = anger recall); Cardiovascular predictor/outcome: HTN = hypertension, IMT = intima-media thickness, HR = hear rate, HRV = heart rate variability, CI = cardiac index, SBP = systolic blood pressure, DBP = diastolic blood pressure.
This study reported separate results for males and females.
The sampled studies were diverse in health status at baseline. Of the 39 study entries, 19 were composed of individuals with a known history of CV conditions (HX), 3 studies had samples of individuals at high familial risk for CVD (FHX) and, finally, 17 studies recruited healthy individuals only (H). When we explored health status as a potential moderator of the relationship between CV recovery and development of CVD we found that status of the sample was a significant moderator. Healthy individuals showed the strongest relationship between recovery and CV outcomes (Qb = 26.79, p < .001; r (H) = .21), followed by those with a known CV diagnosis (r(HX) = .18) and those at familial risk (r (FHX) = .16).
From a methodological stand point, time-to-follow-up (TTF) period was recorded for descriptive purposes. Within this sample of studies, participants were followed-up for 1–20 years, with a mean TTF period of 7.7 years (median = 6.7, SD = 5). Given the high variability in the TTF period, we explored TTF period as a potential moderator. Since TTF is a continuous variable, we examined the moderating effect of TTF using meta-regression procedure, under the random-effects model via method of moments; there was a negative relationship between TTF length and CV outcome (Qmodel = 9.92, p < .001), with longer TTFs generally showing smaller ESs, possibly due to other risks possibly developing over longer periods of time.
Recovery protocols varied considerably; for example, twenty reported recovery at 1 minute, eleven at 2 minutes post peak HR, three at 3 minutes, five at 5 minutes, and three at 6.8. and 45 minutes. The context of recovery varied among studies as well, with two thirds (n = 21) using passive recovery and 16 employing an active recovery protocol. Finally, CV outcomes were disparate. More than half the studies (n = 21, 54%) examined all-cause mortality and nine reported findings specifically for CV mortality. Eight studies examined clinical CV changes and disease (i.e. development of hypertension) and four studies reported non-clinical CV changes in functioning (i.e. changes in BP or HR). Despite large differences in recovery protocols, some characteristics were common across studies. The majority of studies (n = 29) used maximal exercise as CV activator (i.e., 85–90% age adjusted HR per AHA guidelines). More importantly, given the role of reactivity on subsequent recovery patters, nearly all studies (with the exception of three) controlled for CV reactivity either by looking at change from maximal HR or by statistically controlling for reactivity. Finally, the majority of the studies controlled for age, gender, BMI, smoking; however, covariates (with the exception of reactivity) were not always specified (i.e., authors merely provided a verbal statement that analyses controlled for attributes that differed among groups).
For the main analyses one mean ES was reported for each study. For moderator analyses, some studies reported two separate ESs that mapped onto our established moderators. The rest of the studies had one representation in each moderator analysis.
Omnibus Analysis
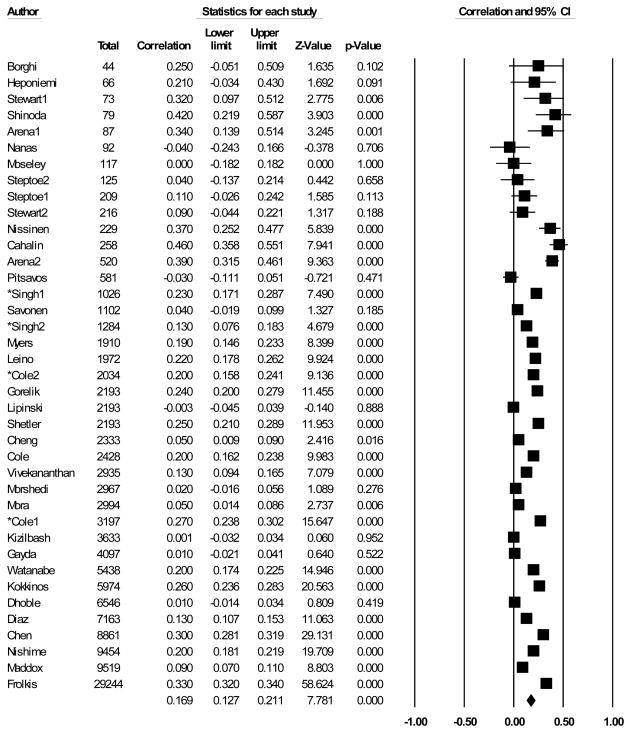
An overall test examining the predictive relationship between CV recovery from exercise and adverse cardiovascular outcomes revealed a significant summary effect: r = .17 with a 95% confidence interval of .12 – .20 (see Figure 1). The confidence interval for the summary effect did not include 0 and p < .001, which indicates that CV recovery from exercise was related to adverse CV outcomes. A positive r indicates that impaired recovery is predictive of increased risk for adverse CV events. A heterogeneity test revealed a significant Q statistic, (Q = 1725.71, p < .001), which indicates considerable variability in the ESs. Given that the meta-analysis included 37 studies (39 entries) with a total sample size of 125386 participants, power was more than adequate to detect heterogeneity among the ESs (29). The I2 statistic was 97.79, which indicates that 97.79% of the total variance may be due to differences in the ESs. Also, the estimate of between-studies variance was T2 = .01. Overall, there was strong evidence for heterogeneity among ESs. The omnibus effect remained unchanged when analyses were repeated with the sample of studies only using HR as a CV predictor for our secondary meta-analysis.
Figure 1.
Omnibus meta-analysis: ESs and 95% CI for the association between recovery and CV outcomes.
Moderator Analyses
Recovery Protocol: Time of Assessment
One moderator analysis tested the length of time allotted for recovery. The between-groups homogeneity statistic was significant (Qb = 97.99, p < .001) indicating that the length of recovery period had a moderating effect. The mean ES for studies examining recovery at 1 minute (r = .22) was significantly larger than recovery at 2 minutes (r = .15).
Recovery Protocol: Active vs. Passive Recovery
In analyses of the moderating effect of recovery protocol (active versus passive), the between-groups homogeneity statistic was significant, (Qb = 127.92, p < .001) indicating that recovery activity after exercise had a moderating effect. The mean ES for studies examining active recovery (r = .15) was smaller than passive recovery (r = .22).
CV Outcome
In moderator analyses on CV outcome, the between-groups homogeneity statistic was significant (Qb = 311.49, p < .001) indicating that the type of CV outcome had a moderating effect. CV recovery was a more robust predictor of all-cause mortality (r = .21) and CV mortality (r = .15) than of clinical changes and CVD (r = .08) and non-clinical CV changes (r = .01).
Type of Challenge
Finally, type of challenge (exercise versus psychological challenge) was examined as a potential moderator in predicting adverse CV outcomes. The between-groups homogeneity statistic of this meta-analysis was significant (Qb = 7.67, p = .006), with both exercise (r = .19) and psychological challenge (r = .09) exhibiting significant individual effect sizes (ps < .05). Therefore, type of challenge had a moderating effect, and recovery from exercise was a more robust predictor of adverse CV outcomes than recovery from psychological challenge.
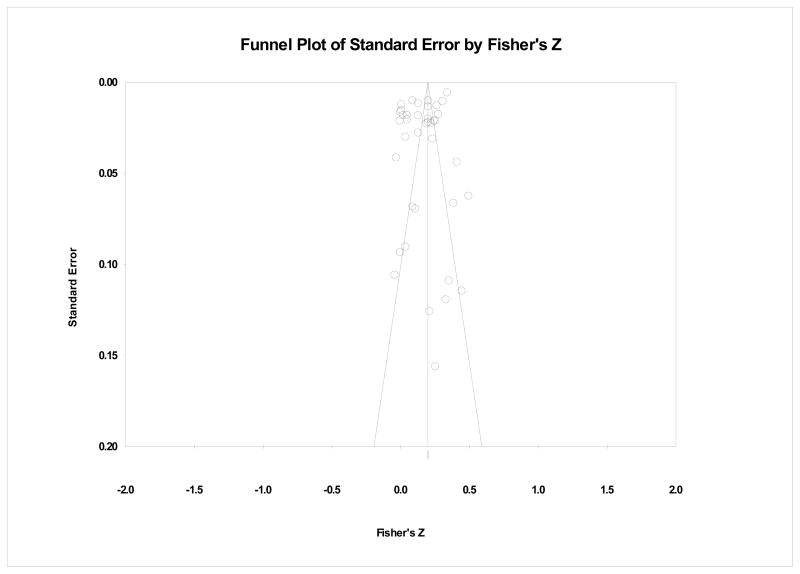
File Drawer Analyses
Since our review considered only published sources, we conducted file-drawer analyses to assess the reliability of the omnibus effects in the face of additional unpublished null results. For the probability for the omnibus analysis to become non-significant (p>.05), there would have to be over 706.6 unpublished studies with non-significant results for each of the observed studies (N = 23317). To reinforce the trustworthiness of our omnibus analysis, our funnel plot in Figure 2 shows that overall, studies are relatively well distributed around the combined ES.
Figure 2.
Publication bias funnel plot.
DISCUSSION
This meta-analysis was a comprehensive investigation of the relationship between impaired CV recovery and adverse CV outcomes. We obtained two main results. First, in omnibus tests, we found a reliable relationship between impaired recovery and adverse CV outcomes, including CVD risk at follow-up and mortality. Second, we also found evidence for several moderating factors. We discuss each group of results in turn.
There has been longstanding interest in the role of CV responses to laboratory challenge as a risk factor for CVD but most of this work has concentrated on exaggerated CV reactivity (4). Our overall ES for CV recovery (r = .17) was comparable to that for CV reactivity (r = .13; (5)), and accounted for a similar amount of variance in adverse CV outcomes as other established risk factors, such as BMI, smoking, cholesterol or diabetes (rs = .14–.30; (35)). Since virtually all of our studies controlled for CV reactivity, our overall results reinforce the position that CV recovery is an important aspect of CV functioning (4) that provides incremental data over CV reactivity in predicting CVD.
Though our moderator analyses of exercise recovery cannot directly identify responsible mechanisms, they pointed out specific contexts in which recovery has a stronger relation to negative CV outcomes. Moderators have been relatively neglected (5,15) so the current meta-analysis investigated several substantive and methodological moderators in studies using exercise as a CV challenge. For instance, within exercise physiology, timing of CV recovery measurement has implications for observed activation of the sympathetic and parasympathetic systems (3). Our moderator analyses showed that CV recovery at 1 minute post exercise was a stronger predictor of adverse CV outcomes than CV recovery at 2 minutes post exercise, suggesting faster vagal control of recovery may be more important than slower sympathetic mechanisms for CVD risk. Thus, our results would seem to counter the more recent proposition that CV recovery is better understood as a coordination of parasympathetic reactivation and sympathetic withdrawal (36).
Importantly, we found that passive recovery was a better context to determine prospective CV risk than active recovery, at least for exercise protocols. Others have been interested in better understanding the importance of recovery context (29). Passive recovery generally results in a faster decrease of cardiac output in healthy individuals. Therefore, impairment in CV recovery in a context that would support faster recovery (i.e., passive recovery) may indicate higher CV impairment, hence leading to stronger relationships to prospective CV outcomes (37). Although we were not able to test this hypothesis for recovery from psychological challenges, Neumann and colleagues (30) have shown that distraction leads to faster CV recovery after an anger recall by limiting development of ruminative cognitions. More generally, one way in which exercise may differ from psychological challenges is that psychological challenge recovery periods may be longer due to involvement of emotional and cognitive systems that prolong recovery via processes, such as rumination (10). Future work should test the idea that slower active recovery after psychological challenges is a stronger predictor of CVD.
Type of outcome moderated the relationship between recovery and adverse CV outcomes; CV recovery was a more robust predictor of all-cause and CV mortality compared to clinical CV incidence, which in turn was a more potent predictor than non-clinical CV changes. Although seemingly counterintuitive, this may be because the non-clinical CV changes typically measured (BP, HR) focus on only one of the pathways to CVD and mortality. Impaired recovery may signal not only changes in hemodynamic CV function, but changes in other unmeasured disease processes such as endothelial function, coagulation factors, and even immune processes. Also, the stability of the measures may be an issue, with more stable changes showing more reliable associations with CV recovery than changes in BP or HR. For example, while IMT was shown to be reversible in children (38), if untreated, CV changes become more durable and more likely to lead to fatal outcomes (39). Although the present results are in line with previous findings suggesting a robust relationship linking CV recovery and mortality (13,40), the best explanation for this finding remains unclear. However, on a related note, one exploratory analysis in the current study uncovered a stronger relationship between CV recovery and adverse CV outcomes in samples with a mean age over 45. Taken together, these results possibly indicate that CV recovery from exercise is a stronger predictor among more impaired individuals.
Although the number of independent study samples using recovery after psychological challenge as a predictor of CV outcomes was too modest to allow for an investigation of moderators, we did examine the type of challenge (physical versus psychological) in a moderator analysis. Here we found that while CV recovery from exercise and psychological challenges both significantly predicted CV outcomes, the strength of the relationship was stronger for exercise recovery than that for recovery from psychological challenges. These results, at first glance, point to the conclusion that recovery from exercise is a “better” predictor of adverse CV outcomes. We believe such a sweeping conclusion is unwarranted at this time, however, because of the many differences between the physical and psychological challenge studies. These differences include the studies not matched on factors such as the degree of challenge intensity, the type of activity during active recovery, or the time allowed for recovery. Indeed, it is clear that it is much easier to control the “dose” of the physical challenge intensity (3) than the intensity of a psychological challenge, a factor which makes physical challenge studies more precise. Generally, while both types of challenges have been linked to development of disease, identifying the active ingredients of psychological challenges is more difficult. This is not only because the body of psychological challenges is too small for a sound moderator analysis, but because psychological challenges invoke a greater number of complex regulatory processes than exercise challenges. Relatedly, mechanisms involved in psychological challenges may be subject to more delayed and uneven recovery than physical processes alone (4). More specifically, it is likely that psychological challenges are likely subject to a wider number of individual differences (anger (10); worry (41); anxiety (42) and depressed affect (43), perseverative cognition (44) and hostility (10), that may contribute both to slower recovery from challenge and to greater variability in recovery. All of these factors should temper our interpretations of observed differences between physical and psychological challenge studies.
Limitations and Future Directions
Although this was the first comprehensive meta-analysis of the association between CV recovery and development of future CV adverse outcomes, results should be interpreted in light of limitations. First, a relatively modest number of articles met all inclusion criteria. This was particularly true for psychological challenge studies, where there were too few to allow an adequately powered moderator analyses. The paucity of studies may reflect the (understandably) slow development of longitudinal work in this area. Longitudinal studies are relatively expensive and time consuming, and as we report, often require long periods of follow-up for reliable adverse CV changes to develop. Even so, this meta-analytic review was still the largest to date. Second, outcomes varied in severity and reliability in predicting future development of a CVD and very few studies represented non-clinical CV changes. Therefore, the weak association between recovery and non-clinical CV changes should not be regarded as definitive. Third, the current analyses did not afford an investigation of the role of individual characteristics in the observed association between recovery and CV outcomes, as individual differences were largely controlled for in the analyzed studies. Fourth, although results suggest that CV recovery and CV reactivity are both important, we did directly examine variability explained by recovery adjusting for variance explained by reactivity. Our analyses only indirectly suggest incremental variance in CV outcomes, given that our sample of studies focused on and reported findings primarily for CV recovery and adjusted for CV reactivity. Fifth, reflecting continued debate about the meta-analytic practice of combining various effect sizes, one could argue against combining dichotomous and continuous measures that pertain to CV outcomes (i.e., CV changes (continuous) vs. mortality (dichotomous)) because these might be considered different constructs. Nevertheless, to enable investigation of moderators, given the overall size of the literature, we followed recommendations to transform various measures into r for simplicity of interpretation. Additionally, there are counter indications about meta-analyzing results of multivariate statistics due to frequent variation and inconsistency of covariates included in the analysis. However, we would argue that the current analyses were ultimately better served by multivariate results, given that these covariates represented clear established risks for CVD, such as age, gender, or smoking.
While this review does not speak directly to the mechanisms linking recovery and CVD, results are consistent with several possible mechanisms that could explain why poor CV recovery predicts adverse CV outcomes. Impaired vagal control after exercise is one possible mechanism linking recovery and CVD. Vagal reactivation is proposed to have a primary function in recovery from exercise (16) and inflexible vagal control was noted to link other health concerns, such as depression (47) or obesity (48). Other possible mechanisms include psychological processes that may delay CV recovery, such as poor emotion regulation (45) or negative cognitive styles, such as excessive worry (44). These processes may contribute to prolonged activation after challenge, such as increased blood pressure or inflammation, among others, which may contribute to artherosclerosis and CVD (46). The application of such mechanisms to exercise is uncertain, since studies have that exercise has variable affects on affect, which depend on individual differences (49). The dual mode theory proposes that intensity and duration will influence affect after exercise, with more intense bouts of exercise possibly triggering negative affect. Although the level of negative affect during exercise may not reach that triggered by psychological challenges, processes related to negative affect may nonetheless contribute to prolonged activation after challenge, as mentioned above (46). Overall, prolonged activation after exposure to a challenge may be an overarching theme among these proposed mechanisms.
Clearly, the current meta-analytic review underscores the need for additional research to better understand mechanisms that impair CV recovery. Our review of the exercise literature possibly implicates vagal control in recovery from physical challenge. Studies investigating CV recovery from psychosocial challenge have identified perseverative cognition and other psychological characteristics to be implicated in risk for CVD. Despite findings linking vagal control to perseverative cognition, for example (10,41), research has yet to test models linking vagal control and perseverative cognition to prospective risk for CVD. Such an approach would help elucidate physiological and psychological mechanisms, and the identification of factors unique to CV recovery that could be targeted in prevention efforts.
Glossary
- BMI
body mass index
- BP
blood pressure
- COG
cognitive challenge (math exercise)
- CV
cardiovascular
- CVD
cardiovascular disease
- CI
confidence interval
- DBP
diastolic blood pressure
- EMO
emotional induction
- EXS
exercise stress test
- HR
heart rate
- HTN
hypertension
- IMT
intima-media thickness
- PS
public speaking
- SBP
systolic blood pressure
- WHO
World Health Organization
REFERENCES*
*Articles included in the meta-analysis
- 1.World Health Organization. 2011 fact sheet on cardiovascular disease. http://www.who.int/mediacentre/factsheets/fs317/en/index.html.
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction. The American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.Pierpont GL, Stolpman DR, Gornick CC. Heart rate recovery post-exercise as an index of parasympathetic activity. J Auton Nerv Syst. 2000;80:169–174. doi: 10.1016/s0165-1838(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 4.Linden W, Earle TL, Gerin W, Christenfeld N. Physiological stress reactivity and recovery: conceptual siblings separated at birth? J PsychosomRes. 1997;42:117–135. doi: 10.1016/s0022-3999(96)00240-1. [DOI] [PubMed] [Google Scholar]
- 5.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status. A meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 6*.Moseley JV, Linden W. Predicting blood pressure and heart rate change with cardiovascular reactivity and recovery: results from 3-year and 10-year follow-up. Psychosom Med. 2006;68:833–843. doi: 10.1097/01.psy.0000238453.11324.d5. [DOI] [PubMed] [Google Scholar]
- 7*.Heponiemi T, Elovainio M, Pulkki L, Puttonen S, Raitakari O, Keltikangas-Järvinen L. Cardiac autonomic reactivity and recovery in predicting carotid atherosclerosis: The cardiovascular risk in Young Finns Study. Health Psychology. 2007;26:13–21. doi: 10.1037/0278-6133.26.1.13. [DOI] [PubMed] [Google Scholar]
- 8.Gerin W, Pickering TG, Glynn L, Christenfeld N, Schwartz A, Carroll D, Davidson K. An historical context for behavioral models of hypertension. J Psychosom Res. 2000;48:369–377. doi: 10.1016/s0022-3999(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 9.Whooley MA, Wong JM. Depression and cardiovascular disorders. Annual review of clinical psychology. 2013;9:327–354. doi: 10.1146/annurev-clinpsy-050212-185526. [DOI] [PubMed] [Google Scholar]
- 10.Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: A model of the link between hostility and cardiovascular disease. Ann Behav Med. 1998;20:1–8. doi: 10.1007/BF02886382. [DOI] [PubMed] [Google Scholar]
- 11.Kwok Y, Kim C, Grady D, Segal M, Redberg R. Meta-analysis of exercise testing to detect coronary artery disease in women. Am J Cardiol. 1999;83:660–666. doi: 10.1016/s0002-9149(98)00963-1. [DOI] [PubMed] [Google Scholar]
- 12.Gianrossi R, Detrano R, Mulvihill D, Lehmann K, Dubach P, Colombo A, Froelicher V. Exercise-induced ST depression in the diagnosis of coronary artery disease. A meta-analysis. Circulation. 1989;80:87–98. doi: 10.1161/01.cir.80.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Lauer MS. Exercise testing, part 2: The value of heart rate recovery. Cardiology Rounds. 2002;6:1–6. [Google Scholar]
- 14.Metz LD, Beattie M, Hom R, Redberg RF, Grady D, Fleischmann KE. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: A meta-analysis. J Am Coll Cardiol. 2007;49:227–237. doi: 10.1016/j.jacc.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 15.Hocking Schuler JL, O’Brien WH. Cardiovascular recovery from stress and hypertension risk factors: a meta-analytic review. Psychophysiology. 1997;34:649–59. doi: 10.1111/j.1469-8986.1997.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 16.Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Kamada T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 17.Sears CE, Choate JK, Paterson DJ. Inhibition of nitric oxide synthase slows heart rate recovery from cholinergic activation. J Appl Physiol. 1998;84:1596–1603. doi: 10.1152/jappl.1998.84.5.1596. [DOI] [PubMed] [Google Scholar]
- 18*.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–7. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 19.Gibbons RJ. Abnormal heart-rate recovery after exercise. The Lancet. 2002;359:1536–1537. doi: 10.1016/S0140-6736(02)08525-2. [DOI] [PubMed] [Google Scholar]
- 20*.Lipinski MJ, Vetrovec GW, Froelicher VF. Importance of the first two minutes of heart rate recovery after exercise treadmill testing in predicting mortality and the presence of coronary artery disease in men. Am J Cardiol. 2004;93:445–449. doi: 10.1016/j.amjcard.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Arai Y, Saul JP, Albrecht P, Hartley LH, Lilly LS, Cohen RJ, Colucci WS. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol Heart Circ Physiol. 1989;256:H132–H141. doi: 10.1152/ajpheart.1989.256.1.H132. [DOI] [PubMed] [Google Scholar]
- 22.Paterson DJ. Antiarrhythmic mechanisms during exercise. J Appl Physiol. 1996;80:1853–1862. doi: 10.1152/jappl.1996.80.6.1853. [DOI] [PubMed] [Google Scholar]
- 23.Crisafulli A, Melis F, Orrù V, Lener R, Lai C, Concu A. Hemodynamics during a postexertional asystolia in a healthy athlete: a case study. Med Sci Sports Exerc. 2000;32:4–9. doi: 10.1097/00005768-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Fleg JL, Lakatta EG. Prevalence and significance of postexercise hytotension in apparently healthy subjects. Am J Cardiol. 1986;57:1380–1384. doi: 10.1016/0002-9149(86)90222-5. [DOI] [PubMed] [Google Scholar]
- 25.Carter R, III, Watenpaugh DE, Wasmund WL, Wasmund SL, Smith ML. Muscle pump and central command during recovery from exercise in humans. J Appl Physiol. 1999;87:1463–1469. doi: 10.1152/jappl.1999.87.4.1463. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg DI, Shephard RJ. Stroke volume during recovery from upright bicycle exercise. J Appl Physiol. 1980;48:833–837. doi: 10.1152/jappl.1980.48.5.833. [DOI] [PubMed] [Google Scholar]
- 27.Johnson EC, Hudson TL, Greene ER. Left ventricular hemodynamics during exercise recovery. J Appl Physiol. 1990;69:104–111. doi: 10.1152/jappl.1990.69.1.104. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Okada A, Saitoh T, Hayano J, Miyamoto Y. Difference in human cardiovascular response between upright and supine recovery from upright cycle exercise. Eur J Appl Physiol. 2000;81:233–239. doi: 10.1007/s004210050036. [DOI] [PubMed] [Google Scholar]
- 29.Crisafulli A, Orru V, Melis F, Tocco F, Concu A. Hemodynamics during active and passive recovery from a single bout of supramaximal exercise. Eur J Appl Physiol. 2003;89:209–216. doi: 10.1007/s00421-003-0796-4. [DOI] [PubMed] [Google Scholar]
- 30.Neumann SA, Waldstein SR, Sellers JJ, III, Thayer JF, Sorkin JD. Hostility and distraction have differential influences on cardiovascular recovery from anger recall in women. Health Psychology. 2004;23(6):631. doi: 10.1037/0278-6133.23.6.631. [DOI] [PubMed] [Google Scholar]
- 31.Desai MY, De la Peña-Almaguer E, Mannting F. Abnormal heart rate recovery after exercise as a reflection of an abnormal chronotropic response. Am J Cardiol. 2001;87:1164–1169. doi: 10.1016/s0002-9149(01)01487-4. [DOI] [PubMed] [Google Scholar]
- 32*.Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132:552–555. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- 33*.Singh JP, Larson MG, Manolio TA, O’Donnell CJ, Lauer M, Evans JC. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension: The Framingham Heart Study. Circulation. 1999;99:1831–1836. doi: 10.1161/01.cir.99.14.1831. [DOI] [PubMed] [Google Scholar]
- 34.Higgins J, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 36.Pierpont GL, Voth EJ. Assessing autonomic function by analysis of heart rate recovery from exercise in healthy subjects. Am J Cardiol. 2004;94:64–68. doi: 10.1016/j.amjcard.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Gerin W, Davidson KW, Christenfeld NJ, Goyal T, Schwartz JE. The role of angry rumination and distraction in blood pressure recovery from emotional arousal. Psychosom Med. 2006;68:64–72. doi: 10.1097/01.psy.0000195747.12404.aa. [DOI] [PubMed] [Google Scholar]
- 38.Wunsch R, de Sousa G, Toschke AM, Reinehr T. Intima-media thickness in obese children before and after weight loss. Pediatrics. 2006;118:2334–2340. doi: 10.1542/peds.2006-0302. [DOI] [PubMed] [Google Scholar]
- 39.Nichols WW, Pepine CJ, O’Rourke MF. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke. N Engl J Med. 1999;340:1762–1763. [PubMed] [Google Scholar]
- 40.Schwartz PJ. The autonomic nervous system and sudden death. Eur Heart J. 1998;19(Suppl F):F72–80. [PubMed] [Google Scholar]
- 41.Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- 42.Carrillo E, Moya-Albiol L, González-Bono E, Salvador A, Ricarte J, Gómez-Amor J. Gender differences in cardiovascular and electrodermal responses to public speaking task: the role of anxiety and mood states. International Journal of Psychophysiology. 2001;42(3):253–264. doi: 10.1016/s0167-8760(01)00147-7. [DOI] [PubMed] [Google Scholar]
- 43.Key BL, Ross KM, Bacon SL, Lavoie KL, Campbell T. Depressed affect is associated with poorer cardiovascular recovery in young women following a mental stressor. Annals of Behavioral Medicine. 2009;38(2):154–159. doi: 10.1007/s12160-009-9104-9. [DOI] [PubMed] [Google Scholar]
- 44.Brosschot JF, Pieper S, Thayer JF. Expanding stress theory: Prolonged activation and perseverative cognition. Psychoneuroendocrinology. 2005;30:1043–9. doi: 10.1016/j.psyneuen.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Penley JA, Tomaka J, Wiebe JS. The association of coping to physical and psychological health outcomes: A meta-analytic review. J Behav Med. 2002;25:551–603. doi: 10.1023/a:1020641400589. [DOI] [PubMed] [Google Scholar]
- 46.Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370:1089–100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- 47.Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biol Psychol. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Karason K, Mølgaard H, Wikstrand J, Sjöström L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242–1247. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 49.Ekkekakis P, Acevedo EO. Affective responses to acute exercise: Toward a psychobiological dose-response model. Psychobiology of physical activity. 2006:91–109. [Google Scholar]
- 50*.Arena R, Guazzi M, Myers J, Peberdy MA. Prognostic value of heart rate recovery in patients with heart failure. Am Heart J. 2006;151:851–7. doi: 10.1016/j.ahj.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 51*.Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M. The prognostic value of the heart rate response during exercise and recovery in patients with heart failure: influence of beta-blockade. Int J Cardiol. 2010;138:166–173. doi: 10.1016/j.ijcard.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 52*.Borghi C, Costa FV, Boschi S, Mussi A, Ambrosioni E. Predictors of stable hypertension in young borderline subjects: a five-year follow-up study. J Cardiovasc Pharmacol. 1986;8:S138–41. doi: 10.1097/00005344-198608005-00030. [DOI] [PubMed] [Google Scholar]
- 53*.Cahalin LP, Arena R, Labate V, Bandera F, Lavie CJ, Guazzi M. Heart rate recovery after the 6 min walk test rather than distance ambulated is a powerful prognostic indicator in heart failure with reduced and preserved ejection fraction: a comparison with cardiopulmonary exercise testing. European journal of heart failure. 2013;15(5):519–527. doi: 10.1093/eurjhf/hfs216. [DOI] [PubMed] [Google Scholar]
- 54*.Chen MS, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery and impact of myocardial revascularization on long-term mortality. Circulation. 2004;110:2851–2857. doi: 10.1161/01.CIR.0000147539.39775.F4. [DOI] [PubMed] [Google Scholar]
- 55*.Cheng YJ, Lauer MS, Earnest CP, Church TS, Kampert JB, Gibbons LW, Blair SN. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care. 2003;26:2052–2057. doi: 10.2337/diacare.26.7.2052. [DOI] [PubMed] [Google Scholar]
- 56*.Dhoble A, Lahr BD, Allison TG, Kopecky SL. Cardiopulmonary Fitness and Heart Rate Recovery as Predictors of Mortality in a Referral Population. Journal of the American Heart Association. 2014;3(2):e000559. doi: 10.1161/JAHA.113.000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Diaz LA, Brunken RC, Blackstone EH, Snader CE, Lauer MS. Independent contribution of myocardial perfusion defects to exercise capacity and heart rate recovery for prediction of all-cause mortality in patients with known or suspected coronary heart disease. J Am Coll Cardiol. 2001;37:1558–1564. doi: 10.1016/s0735-1097(01)01205-0. [DOI] [PubMed] [Google Scholar]
- 58*.Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. 2003;348:781–790. doi: 10.1056/NEJMoa022353. [DOI] [PubMed] [Google Scholar]
- 59*.Gayda M, Bourassa MG, Tardif JC, Fortier A, Juneau M, Nigam A. Heart rate recovery after exercise and long-term prognosis in patients with coronary artery disease. Canadian Journal of Cardiology. 2012;28(2):201–207. doi: 10.1016/j.cjca.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 60*.Gorelik DD, Hadley D, Myers J, Froelicher V. Is there a better way to predict death using heart rate recovery? Clin Cardiol. 2006;29:399–404. doi: 10.1002/clc.4960290906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Kizilbash MA, Carnethon MR, Chan C, Jacobs DR, Sidney S, Liu K. The temporal relationship between heart rate recovery immediately after exercise and the metabolic syndrome: the CARDIA study. Eur Heart J. 2006;27:1592–1596. doi: 10.1093/eurheartj/ehl043. [DOI] [PubMed] [Google Scholar]
- 62*.Kokkinos P, Myers J, Doumas M, Faselis C, Pittaras A, Manolis A, et al. Heart rate recovery, exercise capacity, and mortality risk in male veterans. Eur J Prev Cardiol. 2012;19(2):177–184. doi: 10.1177/1741826711398432. [DOI] [PubMed] [Google Scholar]
- 63*.Leino J, Minkkinen M, Nieminen T, Lehtimäki T, Viik J, Lehtinen R, Kähönen M. Combined assessment of heart rate recovery and T-wave alternans during routine exercise testing improves prediction of total and cardiovascular mortality: the Finnish Cardiovascular Study. Heart Rhythm. 2009;6(12):1765–1771. doi: 10.1016/j.hrthm.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 64*.Maddox TM, Ross C, Ho PM, Masoudi FA, Magid D, Daugherty SL, Rumsfeld JS. The prognostic importance of abnormal heart rate recovery and chronotropic response among exercise treadmill test patients. Am Heart J. 2008;156:736–744. doi: 10.1016/j.ahj.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 65*.Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, Blumenthal RS. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women. JAMA. 2003;290:1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 66*.Morshedi-Meibodi A, Larson MG, Levy D, O’Donnell CJ, Vasan RS. Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study) Am J Cardiol. 2002;90:848–852. doi: 10.1016/s0002-9149(02)02706-6. [DOI] [PubMed] [Google Scholar]
- 67*.Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehabil. 2007;14:215–221. doi: 10.1097/HJR.0b013e328088cb92. [DOI] [PubMed] [Google Scholar]
- 68*.Nanas S, Anastasiou-Nana M, Dimopoulos S, Sakellariou D, Alexopoulos G, Kapsimalakou S, Nanas J. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. Int J Cardiol. 2006;110:393–400. doi: 10.1016/j.ijcard.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 69*.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 70*.Nissinen SI, Mäkikallio TH, Seppänen T, Tapanainen JM, Salo M, Tulppo MP, Huikuri HV. Heart rate recovery after exercise as a predictor of mortality among survivors of acute myocardial infarction. The American journal of cardiology. 2003;91(6):711–714. doi: 10.1016/s0002-9149(02)03410-0. [DOI] [PubMed] [Google Scholar]
- 71*.Pitsavos CH, Chrysohoou C, Panagiotakos DB, Kokkinos P, Skoumas J, Papaioannou I, Stefanadis CI. Exercise capacity and heart rate recovery as predictors of coronary heart disease events, in patients with heterozygous familial hypercholesterolemia. Atherosclerosis. 2004;173:345–350. doi: 10.1016/j.atherosclerosis.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 72*.Savonen KP, Kiviniemi V, Laaksonen DE, Lakka TA, Laukkanen JA, Tuomainen TP, et al. Two-minute heart rate recovery after cycle ergometer exercise and all-cause mortality in middle-aged men. J Intern Med. 2011;270(6):589–596. doi: 10.1111/j.1365-2796.2011.02434.x. [DOI] [PubMed] [Google Scholar]
- 73*.Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, Myers J. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980–1987. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- 74*.Shinoda N, Hirashiki A, Okumura T, Okamoto R, Wu Cheng X, Kono Y, et al. Predictive value of heart rate recovery after exercise testing in addition to brain natriuretic peptide levels in ambulatory patients with nonischemic dilated cardiomyopathy. Ann Noninvasive Electrocardiol. 2012;17(4):378–386. doi: 10.1111/j.1542-474X.2012.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75*.Steptoe A, Marmot M. Impaired cardiovascular recovery following stress predicts 3-year increases in blood pressure. J Hypertens. 2005;23:529–536. doi: 10.1097/01.hjh.0000160208.66405.a8. [DOI] [PubMed] [Google Scholar]
- 76*.Steptoe A, Donald AE, O’Donnell K, Marmot M, Deanfield JE. Delayed blood pressure recovery after psychological stress is associated with carotid intima-media thickness. Whitehall psychobiology study. Arterioscler Thromb Vasc Biol. 2006;26:2547–2551. doi: 10.1161/01.ATV.0000242792.93486.0d. [DOI] [PubMed] [Google Scholar]
- 77*.Stewart JC, France CR. Cardiovascular recovery from stress predicts longitudinal changes in blood pressure. Biol Psychol. 2001;58:105–20. doi: 10.1016/s0301-0511(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 78*.Stewart JC, Janicki DL, Kamarck TW. Cardiovascular reactivity to and recovery from psychological challenge as predictors of 3-year change in blood pressure. Health Psychol. 2006;25:111–118. doi: 10.1037/0278-6133.25.1.111. [DOI] [PubMed] [Google Scholar]
- 79*.Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42:831–838. doi: 10.1016/s0735-1097(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 80*.Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality the case of stress echocardiography. Circulation. 2001;104:1911–1916. [PubMed] [Google Scholar]