Abstract
The Drosophila mushroom bodies are critical association areas whose role in olfactory associative learning has been well characterized. Recent behavioral studies using a taste association paradigm revealed that gustatory conditioning also requires the mushroom bodies (Masek and Scott, 2010; Keene and Masek, 2012). Here, we examine the representations of tastes and the neural sites for taste associations in the mushroom bodies. Using molecular genetic approaches to target different neuronal populations, we find that the gamma lobes of the mushroom bodies and a subset of dopaminergic input neurons are required for taste associative learning. Monitoring responses to taste compounds in the mushroom body calyx with calcium imaging reveals sparse, taste-specific and organ-specific activation in the Kenyon cell dendrites of the main calyx and the dorsal accessory calyx. Our work provides insight into gustatory representations in the mushroom bodies, revealing the essential role of gustatory inputs not only as rewards and punishments but also as adaptive cues.
Keywords: Drosophila, gustatory, learning, memory, taste aversion
Introduction
Learning allows animals to alter their behavior in response to previous experience. Even innate behavioral drives elicited by sensory detection, such as the decision to initiate feeding, may be modified if associated previously with a noxious stimulus.
A central site for experiential learning in Drosophila is the mushroom body (MB) (Heisenberg, 2003; Davis, 2005; Keene and Waddell, 2007). The dendrites of the MB principle cells, the Kenyon cells (KCs), receive sparse, random inputs from olfactory projection neurons (PNs). Activity in different ensembles of KCs encodes unique odor blends, enabling associations between a vast array of odor combinations and learned behaviors (Murthy et al., 2008; Caron et al., 2013; Gruntman and Turner, 2013). In contrast to odor inputs at the KC dendrites, aminergic neural processes wrap around KC axonal lobes to convey unconditioned stimuli (US), such as reward or punishment (Claridge-Chang et al., 2009; Burke et al., 2012; Liu et al., 2012). The coincident detection of an odor [conditioned stimulus (CS)] and a US is thought to alter synapse strength at the MB outputs (Cassenaer and Laurent, 2012), producing a long-lasting association between the odor and the unconditioned response.
Although odors act as CS and tastes can act as US, the diversity of sensory information that the MB integrates is unresolved. Anatomical studies of insect MB inputs suggest that visual, tactile, and gustatory cues are processed in different MB calyx compartments as CS (Menzel, 2014). In addition, behavioral studies in Drosophila argue that the MB receives multimodal inputs, because they are required for taste conditioning, courtship conditioning, and some forms of visual learning (Zars, 2000; Masek and Scott, 2010).
To gain insight into sensory processing in the MB, we examined how taste information is represented in this brain region. Drosophila taste with gustatory neurons on the proboscis labelum, internal mouthpart organs, legs, and wings (Stocker, 1994). Gustatory neurons express different gustatory receptors, detect different taste modalities, including sugar, bitter, and water tastes (Liman et al., 2014), and send axons to the subesophageal zone (SEZ) of the brain (Stocker, 1994). Pathways from the SEZ to higher brain regions have not yet been identified in Drosophila.
Evidence that the MB processes tastes as CS and US comes from behavioral taste conditioning experiments (Masek and Scott, 2010; Keene and Masek, 2012). A simple taste behavior is the proboscis extension response (PER): when leg gustatory neurons detect sucrose, the fly extends its proboscis to eat. Pairing sucrose stimulation to the leg (CS) with an aversive stimulus (US) causes short-term inhibition of proboscis extension. This learned behavior requires the MB, but the neural processing in the MB that underlies taste conditioning is unknown.
Here, we used behavioral and calcium imaging studies to examine taste representations in the MB and the role of these structures in aversive taste conditioning. These studies reveal that taste inputs into the main calyx are segregated by taste modality and taste organ. In combination with aminergic lobe inputs, this organization may allow tastes to serve as both sensory inputs to be contextualized and rewards or punishments.
Materials and Methods
Fly strains.
The following fly lines were used: 247–Gal4 (Schulz et al., 1996; Zars et al., 2000), c772–Gal4 and H24–Gal4 (Zars et al., 2000), c739–Gal4 (O'Dell et al., 1995; Yang et al., 1995), c320–Gal4 (Martini and Davis, 2005; Krashes et al., 2007), c305a–Gal4, MB–Gal80 (Krashes et al., 2007), NP1131–Gal4 and NP3061–Gal4 (Tanaka et al., 2008; Aso et al., 2009), Tdc2–Gal4 (Cole et al., 2005), Trh–Gal4 (Sitaraman et al., 2012), tyrosine hydroxylase (TH)–Gal4 (Friggi-Grelin et al., 2003), HL9–Gal4 (Claridge-Chang et al., 2009), UAS–shibirets (Kitamoto, 2001), OK107–Gal4 (Connolly et al., 1996), UAS–GCaMP5 (Akerboom et al., 2012), UAS–GCaMP6s (Chen et al., 2013), LexAop–dTRPA1 (Hamada et al., 2008), Orco–LexA (Lai and Lee, 2006), and NP3208–Gal4 (Tanaka et al., 2008). Flies were grown on standard fly food.
Learning assay.
Five- to 7-d-old female flies were starved for 24 h in a vial on a wet Kimwipe (Kimberly-Clark). Flies were then anesthetized with CO2 and affixed individually to a glass slide with clear nail polish. After recovering in a humid chamber for 2 h, flies were water satiated before training and were presented with 500 mm sucrose (CS) three times on the tarsi before testing. Any fly that did not show a robust PER to each sucrose stimulation was removed from the study. Each learning trial consisted of a presentation of the CS on the tarsi, quickly followed by a stimulation of 50 mm quinine (US) on the proboscis. A single trial was used for 5 min memory tests and 25 trials for 30 min memory tests. In multiple-trial studies, the US was only presented when the fly extended its proboscis to the CS. The intertrial interval was 2 min, during which flies were rinsed with, and were able to consume, water. After training, flies were presented with only the CS on their tarsi once every 5 or 15 min. Flies that did not perform a PER to sucrose, and hence showed successful learned suppression, were counted and compared with the number of flies that extended (either partially or fully). For shibirets (Shits) experiments, the flies' temperature was raised to 32°C by placing them in a heated chamber 20 min before the assay in which they were maintained at that temperature throughout the experiment for conditional neural inactivation, whereas the same genotype control flies were kept at 22°C. In single-leg experiments, the CS was presented to either the right or left tarsi exclusively. Percentages of PER are reported on the graphs, and the 95% confidence intervals (CIs) were calculated based on the population mean and SD using the Wald method. Differences in PER frequency between experimental and control groups were compared using Fisher's exact test (2 × 2 matrix: low temperature, high temperature, PER yes, PER no), appropriate for categorical data.
Calcium imaging.
For imaging of the calyx, 2- to 3-d-old female flies were affixed to a piece of packing tape in an imaging chamber with a fine strand of tape securing their necks. A small hole was cut in the top of the tape, exposing the fly's head, which was stabilized with dental wax and dissected in cold artificial hemolymph to expose the dorsal region of the brain. Tarsi and proboscis were waxed into extended positions to allow for tastant stimulation. For imaging of the paired posterior lateral 1 (PPL1) cluster of neurons, flies were prepared as described previously (Marella et al., 2006). Flies were imaged on a 3i spinning-disk confocal microscope with a 40× water-immersion objective and a 488 nm laser. A piezo drive allowed for imaging of z-stacks of 18–20 1-μm slices at a z-stack rate of ∼1 Hz, making it possible to scan an approximate volume of 150 × 150 × 20 μm across time.
For all calcium imaging experiments, tastants were presented to the fly via a glass capillary for 1–2 s. For PPL1 imaging, 1 m sucrose and a mix of 50 mm caffeine and 60 mm denatonium were used as tastants. Both were diluted in 20% polyethylene glycol to eliminate activation of the water sensory cells. Noxious heat shock was performed by touching a custom-built heat probe to the abdomen of the fly, which rose to ∼40°C and decayed back to room temperature over ∼3 s. For MB calyx imaging, the following tastants were used: (1) 1 m sucrose, 100 mm quinine (high-concentration tastants were used to eliminate possible confounds attributable to water cell activation), and water (for across-modality experiments); (2) 200 mm fructose and 300 mm glucose [for within-modality experiments, tastants chosen based on preferential equivalence (Masek and Scott, 2010)]; (3) 1 m sucrose (for taste organ experiments); and (4) 1 m sucrose, 7 mm quinine, and 300 mm glucose (images in Fig. 6). Transient receptor potential A1 (TRPA1) stimulation during imaging was performed as described previously (Mann et al., 2013). In general, tastants were presented twice during each scan, with two to three scans per condition.
Figure 6.
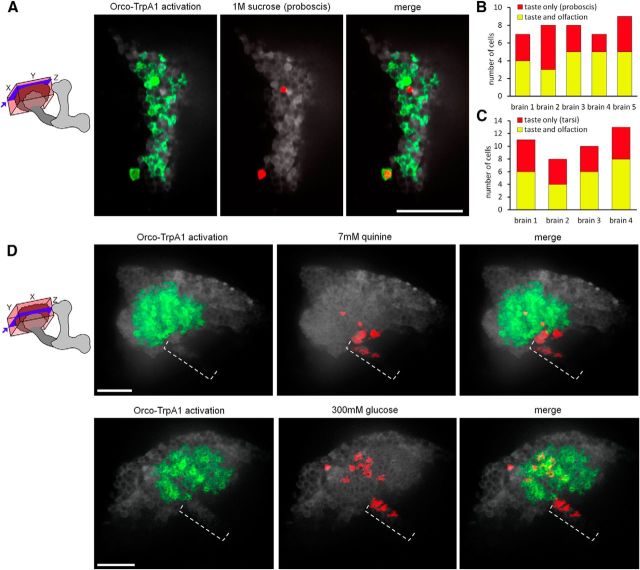
Comparison of gustatory and olfactory representations. A, Many MB calyx cell bodies were activated by heat-induced activation of dTRPA1 expressed in Orco olfactory neurons. Stimulation with a single taste compound (1 m sucrose) activated very few cell bodies. Schematics show the region of the MB imaged and analyzed (box) and the approximate depth of each representative slice (blue plane). Scale bars, 20 μm. B, C, Comparisons of KCs responsive to exogenous Orco activation and taste stimulation [of the proboscis (B) or front tarsi (C)] versus those responsive to taste alone. D, Two representative images showing taste (red) and Orco–dTrpA1 (green) activation in the main calyx and taste activity alone in the dorsal accessory calyx (dotted bracket).
To analyze the data, the ΔF/F activation of each plane was compared across four to six stimulations of a single tastant to determine repeatable, nonrandom activity. Active claws or cells were then identified manually and compared across conditions to determine overlap. To generate comparison figures, ΔF images were thresholded by intensity and size and then overlaid on an single-plane image of the background intensity. Comparisons between groups were performed using Fisher's exact test on the summed data (2 × 2 matrix: overlapping claws, non-overlapping claws, Group 1, Group 2) and were corrected for multiple comparisons using the Bonferroni's method. PPL1 calcium traces were calculated by manually drawing ROIs and then dividing the change in fluorescence by the average intensity of the first five frames. Traces for each ROI were averaged across five flies.
Results
The gamma lobes are required for short-term taste memories
The KCs of the MB are divided into three main classes based on axonal arborizations in the α/β, α′/β′, and γ lobes. Previous studies have identified functional specializations among and within the classes, with different subsets playing different roles in the phase, type, and length of associative memory (van Swinderen, 2009). In general, the α/β neurons are essential for olfactory long-term memory (LTM), the α′/β′ neurons are necessary for medium-term memory and LTM consolidation, and the γ neurons are required for short-term memory (STM) and courtship conditioning (Zars et al., 2000; McGuire et al., 2001; Keleman et al., 2007; Krashes et al., 2007; Blum et al., 2009; Trannoy et al., 2011).
To study the role of the MB classes in aversive taste conditioning, we modified a learning assay in which two different taste compounds are used as the CS and the US (Keene and Masek, 2012). Flies were stimulated on the tarsi with the appetitive CS, 500 mm sucrose, causing the PER, whereupon the bitter US, 50 mm quinine, was applied briefly to the proboscis (Fig. 1B). Memory was assessed after training by presenting the CS and recording the PER. Flies formed a rapid, one-trial conditioned aversion that was retained 5 min after training and decayed rapidly (Fig. 1C).
Figure 1.
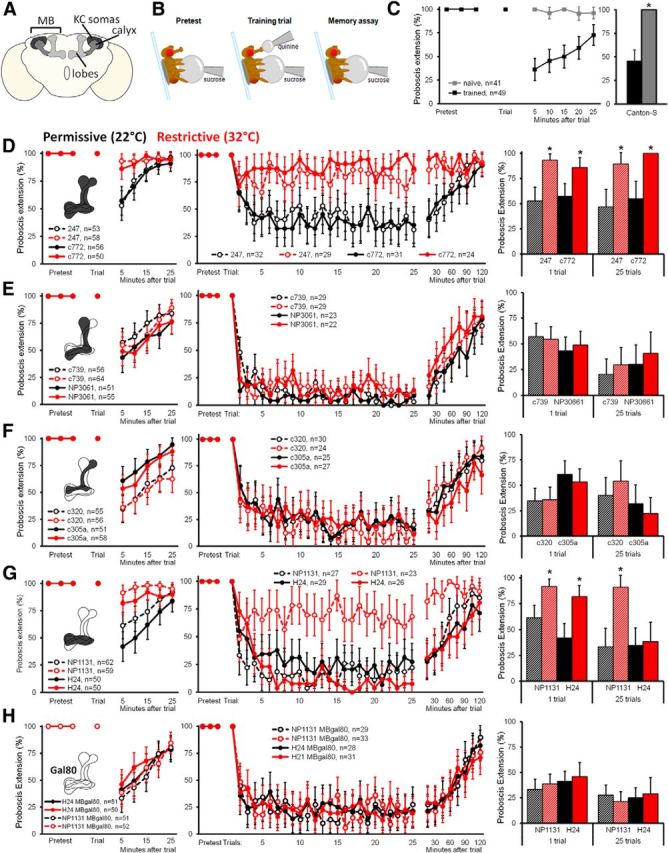
Aversive taste conditioning requires the MB γ lobes. A, Schematic showing the basic structure of the MB in the Drosophila brain. B, Schematic showing the aversive taste memory paradigm. Flies are screened for full extension to the CS (Pretest). Training trials consist of a quinine stimulation on the proboscis after extension. After training, memory is assayed by observing suppression of the PER in response to sucrose stimulation at 5 min intervals. C, Wild-type (Canton-S) flies that have received training (left, black lines) show PER suppression to the CS, assessed at 5 min intervals after training, whereas naive flies show a strong PER throughout (gray). Five minutes after single-trial learning, trained flies show significantly reduced PER compared with wild type (left). Data show percentage PER ± 95% CI (*p < 0.001, Fisher's exact test). D–H, Aversive taste learning requires the γ lobes. Two Gal4 lines were used for each MB subset: D, whole MB (247 and c772); E, α/β (NP3061 and c739); F, α'/β' (c320 and c305a); G, γ (NP1131 and H24); and H, γ lines with MB expression silenced (NP1131/MBgal80 and H24/MBgal80). Flies expressing shits in subsets of KCs (MBsubset–Gal/+;UAS–shits/+) at permissive temperature (22°C) are shown in black, and those at restrictive temperature (32°C) are shown in red. Data indicate percentage PER ± 95% CI. Flies of genotype MBsubset–Gal4, UAS–shits were used. First column, Single-trial conditioning requires γ MB neurons. After pretest, a single pairing of 500 mm sucrose on the tarsi and 50 mm quinine on the proboscis was performed, after which memory was monitored at 5 min intervals. Insets show schematic of the MB neurons silenced in each experiment. Second column, Twenty-five trial conditioning requires γ MB. Similar to single-trial conditioning, except flies were presented with the pairing after PER 25 times, and memory was monitored in 15 min intervals. Third column, PER suppression was compared between groups at 5 min (1 trial) or 30 min (25 trials) after training. Bars show percentage PER ± 95% CI (*p < 0.001, Fisher's exact text).
To determine the role of each class of MB cells in the formation of taste memories, we transiently blocked the synaptic output of KCs using the temperature-sensitive, dominant-negative dynamin shibirets (UAS–shits; Kitamoto, 2001) while flies underwent aversive taste conditioning. All Gal4/UAS–shits lines showed normal memory formation at permissive temperature (Fig. 1D–H). When output was blocked in two Gal4 lines that label the majority of MB KCs (Fig. 1D, 247–Gal4 and c772–Gal4), memory was severely disrupted. No changes in memory performance were observed when synaptic output was blocked from the α/β or the α′/β′ lobes (Fig. 1E,F, using c739–Gal4, NP3061–Gal4 and 320–Gal4, c305–Gal4, respectively). However, blocking synaptic output of the γ lobe neurons (using NP1131–Gal4 and H24–Gal4) completely abolished conditioned aversion (Fig. 1G). To ensure that the block of conditioned aversion was attributable to silencing γ lobe neurons rather than other neurons in the Gal4 lines, we repeated the experiments including MB–Gal80 to prevent expression of shits in MB lobes (Krashes et al., 2007). Under these conditions, flies showed normal conditioned aversion (Fig. 1H), arguing that silencing γ lobe neurons in these lines is required to block conditioned aversion. These experiments show that the γ lobe is the site of aversive taste memory formation in the MB.
Because the γ lobes are known to participate in STM, we wondered whether additional lobes would contribute to taste conditioning if we altered the learning paradigm to generate memories of a longer duration. Increasing the trial number from a single pairing to 25 pairings of the US and CS increased the length of memory retention, with a robust memory after 30 min (Figure 1D–H). The conditioned behavior required the MB, with a requirement for the γ lobes but not the α/β or α′/β′ lobes. Interestingly, only one of the two γ lobe lines was required for multi-trial memory. Inclusion of MB–Gal80 restored conditioned aversion, arguing that silencing MB neurons is required to prevent conditioned aversion. The differences in the requirement of the two γ lobe lines for conditioned aversion could be attributable to the higher number of γ lobe neurons labeled in NP1131–Gal4 compared with H24–Gal4 (Aso et al. 2009), the small number of non-γ ΜΒ neurons labeled, or differences in expression levels of the reporters. These results demonstrate the requirement of MB neurons for taste conditioning.
PPL1 dopaminergic neurons encode bitter punishment signals
Gustatory stimuli act as rewards and punishments in olfactory associative learning, suggesting that these same pathways may also be used for taste conditioning. For example, a subset of dopamine (DA) neurons, the paired anterior medial (PAM) neurons, responds to sensory detection of sucrose and signals sucrose reward (Burke et al., 2012; Liu et al., 2012). Other aminergic neurons that convey reward or punishment signals include DA PPL1 neurons that carry an aversive signal (Claridge-Chang et al., 2009), octopamine (OA) neurons that participate in reward (Burke et al., 2012), and seroton (5-HT) neurons that have been implicated in both punishment and reward (Sitaraman et al., 2008, 2012).
To test whether taste conditioning acts via these aminergic neurons, we inhibited the synaptic output of different classes of aminergic neurons during taste conditioning. No memory deficit was observed when 5-HT neurons or OA neurons were silenced with UAS–shits, showing that neither population has an essential function in taste memory (Fig. 2). To investigate the role of DA, we used two Gal4 driver lines, TH–Gal4 and HL9–Gal4, that label partially overlapping sets of DA neurons. Of the DA neurons that innervate the MB, TH–Gal4 preferentially labels PPL1 neurons, whereas HL9–Gal4 preferentially labels the majority of PAM neurons (Claridge-Chang et al., 2009), allowing us to differentiate between the DA neurons implicated in aversive and appetitive reinforcement, respectively. Silencing TH–Gal4 resulted in complete abolition of aversive taste memory, whereas HL9–Gal4/UAS–shits flies showed no effect (Fig. 2). Although TH–Gal4 labels many neurons, the only TH–Gal4 neurons that innervate the MB and are not found in HL9–Gal4 are PPL1 neurons (Claridge-Chang et al., 2009), suggesting that the PPL1 cluster, but not the PAM cluster, may be required for aversive taste learning.
Figure 2.
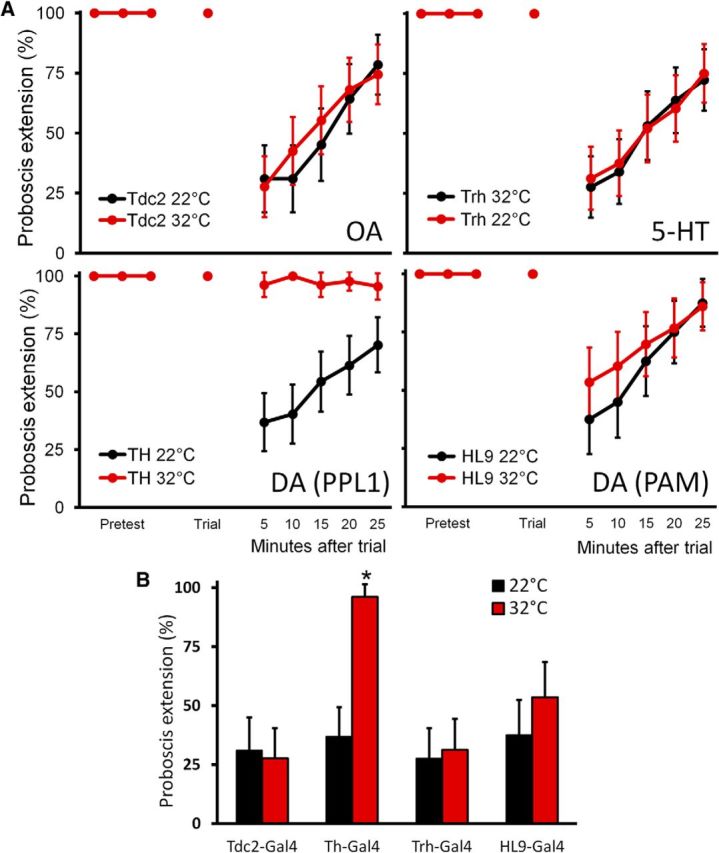
PPL1 neurons are required for taste conditioning. A, Single-trial aversive taste conditioning assays were performed on flies with different classes of aminergic neurons silenced with shits. Flies with OA neurons (Tdc2), 5-HT neurons (Trh), and DA subsets (TH and HL9) expressing shits were conditioned at permissive (22°C; black) and restrictive (32°C; red) temperatures. Flies of genotype aminergic subset–Gal4, UAS–shits were used. Data show percentage PER ± 95% CI. B, Only flies with silenced TH–Gal4 neurons showed an inability to form aversive taste memory at 5 min (data from A). Data show percentage PER ± 95% CI (*p < 0.00001, Fisher's exact test).
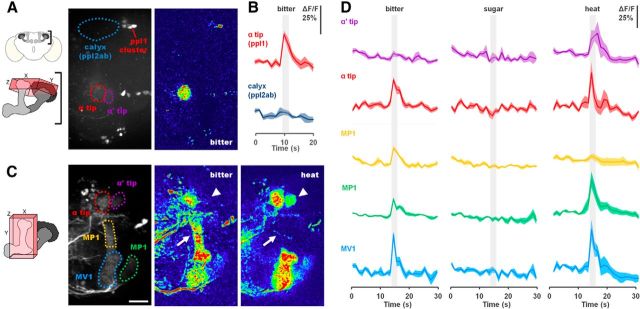
Previous studies of PPL1 neurons showed that they are necessary for shock-induced olfactory aversive conditioning and that they respond to temperature decreases (Tomchik, 2013) and the chemical repellent DEET (Das et al., 2014), suggesting that PPL1 encodes multiple types of aversive US. We next used calcium imaging to test whether DA neurons respond to bitter stimuli and signal the bitter US by expressing GCaMP6s in TH–Gal4 neurons. Using a spinning-disk microscope equipped with a piezo drive, we were able to record calcium signals in a three-dimensional volume over time in live flies. No calcium activity was observed in the calyx (Fig. 3A,B), ruling out the PPL2ab cluster, so analysis was focused on five subsets of PPL1 neurons that innervate the MB lobes: Medial lobe pedunculus 1 (MP1), medial vertical lobes 1 (MV1), vertical lobe 1 (V1), and the neurons that innervate the tips of the α and α′ lobes (Fig. 3C). PPL1 neurons showed strong responses to bitter stimulation of the proboscis and to a noxious heat shock to the abdomen but did not respond to sugar sensory stimulation, arguing that PPL1 carries the bitter aversive signal (Fig. 3C,D). Interestingly, bitter and heat show slightly different activation patterns, with MB–MP1 neurons responding to bitter but not heat and the α′ tip neurons responding to heat but not bitter. These data suggest that the PPL1 cluster encodes a general aversive signal, but individual neurons within the cluster may have more specific response properties.
Figure 3.
Bitter and heat, but not sugar, activate the PPL1 neurons. A, Schematic shows the brain region imaged in these experiments. z-projection of background fluorescence of TH–Gal4; UAS–GCaMP6s flies, showing TH–Gal4 innervation that innervates the MB calyx and lobes (dotted circles). Representative ΔF heat maps of the maximum responses to bitter in the α lobe tip and calyx. B, Average ΔF/F responses of the TH–Gal4 processes in the α lobe tip and calyx to bitter (50 mm caffeine and 60 mm denatonium) over time. Mean (bold line) ± SEM (light surrounds). Gray bars indicate the presence of the stimulus. n = 5 for both conditions. C, Schematic shows the brain region imaged in these experiments. z-projection of background fluorescence of TH–Gal4; UAS–GCaMP6s flies, showing the five subsets of PPL1 neurons (dotted circles) that innervate the MB lobes. Representative ΔF heat maps of the maximum responses to bitter and noxious heat in the PPL1 neurons. Arrowhead indicates the α′ tip region (only active for noxious heat), and arrow indicates V1 region (only active for bitter). Scale bar, 20 μm. D, Average ΔF/F responses of the five PPL1 subsets to bitter (50 mm caffeine and 60 mm denatonium), sugar (1 m sucrose), and noxious heat over time. Mean (bold line) ± SEM (light surrounds). Gray bars indicate the presence of the stimulus. n = 5 for all conditions.
Interestingly, the neurons implicated previously in relaying sugar information to the MB, OA neurons, and PAM DA neurons (Burke et al., 2012; Liu et al., 2012) played no role in aversive taste conditioning, despite sugar stimulation serving as the CS in this paradigm. This suggests that taste information is relayed to the MB via multiple pathways.
Representations of taste modalities in the main calyx
If information about the sugar CS is not reaching the axonal MB lobes indirectly through the aminergic reinforcement circuitry, it might be directly conveyed to the MB dendrites in a manner similar to olfactory CS. The dendrites of KCs form a dense cloud of neuropil called the calyx. Olfactory PNs, which relay information from the antennal lobes, extend large boutons into this region, in which each bouton is wrapped individually in dendrites from multiple KCs, forming microglomeruli known as synaptic claws (Leiss et al., 2009).
To examine taste activation in the calyx, we drove expression of the genetically encoded calcium indicator UAS–GCaMP5 with OK107–Gal4, which drives strong expression in KCs (Aso et al., 2009). Calcium responses in the calyx were monitored as tastants were presented to the tarsi or the proboscis of the fly. The entire volume of the calyx was analyzed for each stimulation. Olfactory organs were removed to prevent possible confounds.
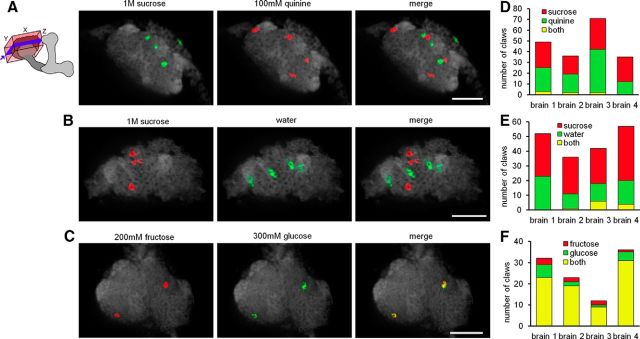
Tastants produced sparse activation of claws in the main calyx (Fig. 4). To examine how taste information is encoded in the MB, multiple tastants were presented to each fly and active claws were counted and compared. Tastants that activate different sensory cells in the periphery, such as sugar and bitter (Fig. 4A,D) or sugar and water (Fig. 4B,E), activated different populations of claws, with only 4 ± 2.8 and 6 ± 6.2% (mean ± SD) of claws responding to both stimuli, respectively. However, tastants that activate the same peripheral cells matched for perceived intensity (Masek and Scott, 2010), such as the two sugars fructose and glucose (Fig. 4C,F), activated primarily overlapping populations (78.9 ± 6.6%). The difference in overlap between the tastants within a modality (the two sugars fructose and glucose) and those across modalities (sugar and water, or sugar and bitter) is highly significant (p < 0.0001, Fisher's exact test with Bonferroni's correction). These data reveal that activation of different gustatory cells in the periphery is represented as activation of different MB calyx claws and provide a potential mechanism for discrimination across, but not within, modalities (Masek and Scott, 2010).
Figure 4.
Taste inputs into the main calyx. A–C, Representative slices from calcium imaging experiments in OK107–Gal 4, UAS–GCaMP5 flies presented successively with 1 m sucrose and 100 mm quinine (A), 1 m sucrose and water (B), and 200 mm fructose and 300 mm glucose (C). Thresholded ΔF images of active claws (red, green) overlaid on background fluorescence (gray). Schematics show the region of the MB imaged and analyzed (box) and the approximate depth of each representative slice (blue plane). Scale bars, 20 μm. D–F, Summary counts of the taste-responsive claws in the entire calyx of the four animals in each condition.
Different taste organs activate partially overlapping populations of KC claws
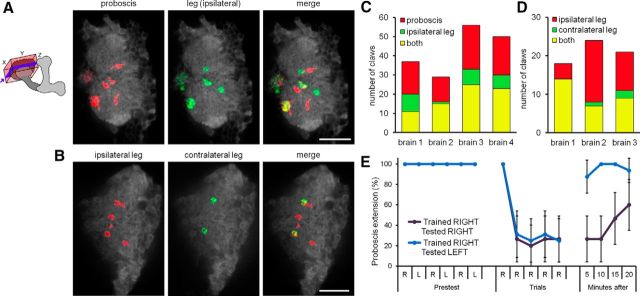
In insects, gustatory neurons are found on the proboscis, legs, and wings (Stocker, 1994; Wang et al., 2004). Different taste organs project to distinct regions of the SEZ and ventral nerve cord (Stocker, 1994; Wang et al., 2004), but it is unknown whether this organotopic segregation is maintained in the higher brain. To examine whether MB receives inputs from multiple taste organs, we monitored calcium responses in the main calyx while presenting the same tastant to the legs or the proboscis. Proboscis and leg stimulation activated partially overlapping claw populations, with both singular and shared representations (14 ± 8.5% for leg only, 43 ± 2.9% for proboscis only, and 43 ± 9.4% for both; Fig. 5A,C). Similarly, comparing stimulation of the left foreleg and the right foreleg revealed different leg representations, with the representation of the contralateral leg primarily subsumed within the larger ipsilateral leg representation (4.6 ± 4.5% for contralateral only, 45.4 ± 22.3% for ipsilateral only, and 50 ± 25.1% for both; Fig. 5B,D). Thus, both different taste modalities and different taste organs have unique representations in the MB calyx, suggesting that these inputs have the potential to be differentially and independently modified by learning.
Figure 5.
Representation of taste organs in the MB calyx. A–D, Representative slices from the MB calyx, showing claws responding to 1 m sucrose stimulation on the proboscis or ipsilateral leg (A) and ipsilateral or contralateral leg (B), as well as summaries of the taste-responsive claws from four animals (C, D). Schematics show the region of the MB imaged and analyzed (box) and the approximate depth of each representative slice (blue plane). Scale bars, 20 μm. E, Conditioned taste aversion is leg specific. Pairing sucrose on the right leg with bitter delivery to the proboscis (5-trial conditioning) caused conditioned aversion to right leg stimulation (R) but not to left leg stimulation (L). Data represent percentage PER ± 95% CI. Significant difference 10 min after learning (n = 15; p < 0.0001, Fisher's exact test).
To test whether the different representations translate into independent learned associations, we asked whether taste conditioning was leg specific. We paired five trials of sucrose on the right tarsi with bitter punishment on the proboscis and then tested whether flies would extend the proboscis to sucrose stimulation on the right or left legs. Remarkably, flies only suppressed the PER when the sucrose was presented to the trained leg and showed normal a PER when they sensed sugar on the untrained leg (Fig. 5E). This demonstrates that associations are taste organ specific and suggests that different representations in the MB calyx enable independent associations.
Integration and segregation of gustatory and olfactory inputs
Our imaging studies revealed that gustatory stimuli activate the MB calyx, suggesting similarities between processing of tastes and odors as CS. Previous studies have shown that each KC extends dendrites into several synaptic claws and receives inputs from five to seven different olfactory PNs (Leiss et al., 2009). To examine whether the same KC receives both gustatory and olfactory inputs, we monitored activity at the KC body in response to tastants and artificial activation of olfactory neurons. We sequentially stimulated the proboscis or the tarsi with tastants and then activated a large number of olfactory sensory classes by expressing the heat-activated dTRPA1 channel (Hamada et al., 2008) in many olfactory neurons using Orco–LexA (Lai and Lee, 2006) and presenting a heat probe to the antenna. The vast majority of KCs responded to exogenous olfactory activation, and a small number responded to tastants, with ∼50% of the taste-responsive KCs also responsive to odors (Fig. 6A,C). Flies expressing LexAop–dTRPA1 without the Orco–LexA driver showed no calcium activity in KCs in response to heat, confirming that the activity was driven by Orco activation not heat alone (numbers of KCs responsive to sugar and heat in flies with and without Orco–LexA are significantly different; U = 0; p < 0.01, Mann–Whitney U test). This argues that a single KC can integrate inputs across sensory modalities and organs, enabling multimodal associations with US.
Although tastants have a sparse representation in the main calyx, similar to the activation pattern of single odorants, we observed a clear difference in the response of the dorsal accessory calyx. Tastants, but not olfactory stimulation, activated the dorsal accessory calyx, a region that has been implicated in gustatory processing in many insects based on anatomy (Farris, 2008). Bitter compounds and sucrose both activated the dorsal accessory calyx, providing a gustatory MB representation distinct from olfactory cues (Fig. 6D). We tested the role of the dorsal accessory calyx in aversive taste conditioning, using NP3208–Gal4 and UAS–Shits for acute silencing, and found that it was not required (permissive and restrictive temperature groups not significantly different; mean of 30 and 26.1%, respectively; n = 46–50; p = 0.821, Fisher's exact test). Although the function of the dorsal accessory calyx is unknown, gustatory activation of this region demonstrates a compartmentalization of sensory inputs into the MB. Thus, gustatory inputs activate both the dorsal accessory calyx and the main calyx, with representations distinct from odor representations in the dorsal accessory calyx and sparse representations in the main calyx, similar to that of single odors.
Discussion
Our studies provide insight into sensory representations in the MB, demonstrating multiple independent entries for gustatory cues. Here, we show that tastes activate the MB not only via DA neurons signaling reward and punishment but also at the KC dendrites, providing a neural basis that enables tastes to signal innate value while also allowing taste responses to be modified by learning. These studies extend and broaden our understanding of the MB as a sensory integration center in the fly brain.
Previous studies of olfactory associative learning have established a pathway for rewards and punishments that is distinct from contextualized cues. For example, it has been shown that PAM neurons signal sucrose reward (Burke et al., 2012; Liu et al., 2012) and PPL1 neurons signal aversive stimuli, including shock and the insect repellent DEET (Mao and Davis, 2009; Das et al., 2014). We find that PPL1 neurons are also required for conditioned taste aversion and respond to bitter taste stimulation of the proboscis, arguing that PPL1 neurons signal multiple aversive cues.
In addition to taste inputs at the MB axons, we also find that gustatory information, like olfactory information, triggers activity in the dendrites of the MB calyx. Elegant studies have shown that each KC receives inputs from a small, random subset of second-order olfactory neurons, providing the capacity to enable thousands of unique olfactory associations (Murthy et al., 2008; Caron et al., 2013; Gruntman and Turner, 2013). Although the main calyx is primarily olfactory, we show here that gustatory information is also processed in the main calyx, with separate inputs for different taste modalities and both separate and shared inputs for different taste organs. Gustatory representations are sparse and represent ∼3% of calyx inputs (based on bouton estimation in the study by Turner et al., 2008). Some KC bodies respond to both odors and tastes, which may enable coding of multimodal CS. An additional representation in the dorsal calyx may enable associations to be categorized based on sensory modality or may allow for convergence of tastes with the nonchemical senses.
Because taste projections to higher brain centers have not yet been characterized, questions regarding the circuitry providing gustatory inputs to the MB remain. However, our current study provides direct evidence of gustatory inputs into the main calyx, with different representations for tastants of different modalities and different representations for different taste organs. Here, we show multimodal inputs into the MB, broadening our understanding of the neural coding underlying conditioned learning and providing a basis for examining taste circuitry in the higher brain.
Footnotes
This work was supported by a National Science Foundation predoctoral fellowship (C.K.) and a grant from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (K.S.). K.S. is a Howard Hughes Medical Institute Early Career Scientist. Members of the Scott laboratory provided advice and comments on this manuscript.
The authors declare no competing financial interests.
References
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SS, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Grübel K, Busch S, Friedrich AB, Siwanowicz I, Tanimoto H. The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet. 2009;23:156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- Blum AL, Li W, Cressy M, Dubnau J. Short- and long-term memory in Drosophila require cAMP signaling in distinct neuron types. Curr Biol. 2009;19:1341–1350. doi: 10.1016/j.cub.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron SJ, Ruta V, Abbott LF, Axel R. Random convergence of olfactory inputs in the Drosophila mushroom body. Nature. 2013;497:113–117. doi: 10.1038/nature12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassenaer S, Laurent G. Conditional modulation of spike-timing-dependent plasticity for olfactory learning. Nature. 2012;482:47–52. doi: 10.1038/nature10776. [DOI] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenböck G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- Connolly JB, Roberts IJ, Armstrong JD, Kaiser K, Forte M, Tully T, O'Kane CJ. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- Das G, Klappenbach M, Vrontou E, Perisse E, Clark CM, Burke CJ, Waddell S. Drosophila learn opposing components of a compound food stimulus. Curr Biol. 2014;24:1723–1730. doi: 10.1016/j.cub.2014.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Farris SM. Tritocerebral tract input to the insect mushroom bodies. Arthropod Struct Dev. 2008;37:492–503. doi: 10.1016/j.asd.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Gruntman E, Turner GC. Integration of the olfactory code across dendritic claws of single mushroom body neurons. Nat Neurosci. 2013;16:1821–1829. doi: 10.1038/nn.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Leiss F, Groh C, Butcher NJ, Meinertzhagen IA, Tavosanis G. Synaptic organization in the adult Drosophila mushroom body calyx. J Comp Neurol. 2009;517:808–824. doi: 10.1002/cne.22184. [DOI] [PubMed] [Google Scholar]
- Keene AC, Masek P. Optogenetic induction of aversive taste memory. Neuroscience. 2012;222:173–180. doi: 10.1016/j.neuroscience.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- Keleman K, Krüttner S, Alenius M, Dickson BJ. Function of the Drosophila CPEB protein Orb2 in long-term courtship memory. Nat Neurosci. 2007;10:1587–1593. doi: 10.1038/nn1996. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- Liman ER, Zhang YV, Montell C. Peripheral coding of taste. Neuron. 2014;81:984–1000. doi: 10.1016/j.neuron.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Plaçais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- Mann K, Gordon MD, Scott K. A pair of interneurons influences the choice between feeding and locomotion in Drosophila. Neuron. 2013;79:754–765. doi: 10.1016/j.neuron.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Martini SR, Davis RL. The dachshund gene is required for the proper guidance and branching of mushroom body axons in Drosophila melanogaster. J Neurobiol. 2005;64:133–144. doi: 10.1002/neu.20130. [DOI] [PubMed] [Google Scholar]
- Masek P, Scott K. Limited taste discrimination in Drosophila. Proc Natl Acad Sci U S A. 2010;107:14833–14838. doi: 10.1073/pnas.1009318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- Menzel R. The insect mushroom body, an experience-dependent recoding device. J Physiol Paris. 2014;108:84–95. doi: 10.1016/j.jphysparis.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Murthy M, Fiete I, Laurent G. Testing odor response stereotypy in the Drosophila mushroom body. Neuron. 2008;59:1009–1023. doi: 10.1016/j.neuron.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell KM, Armstrong JD, Yang MY, Kaiser K. Functional dissection of the Drosophila mushroom bodies by selective feminization of genetically defined subcompartments. Neuron. 1995;15:55–61. doi: 10.1016/0896-6273(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Schulz RA, Chromey C, Lu MF, Zhao B, Olson EN. Expression of the D-MEF2 transcription in the Drosophila brain suggests a role in neuronal cell differentiation. Oncogene. 1996;12:1827–1831. [PubMed] [Google Scholar]
- Sitaraman D, Zars M, Laferriere H, Chen YC, Sable-Smith A, Kitamoto T, Rottinghaus GE, Zars T. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci U S A. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D, LaFerriere H, Birman S, Zars T. Serotonin is critical for rewarded olfactory short-term memory in Drosophila. J Neurogenet. 2012;26:238–244. doi: 10.3109/01677063.2012.666298. [DOI] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- Tomchik SM. Dopaminergic neurons encode a distributed, asymmetric representation of temperature in Drosophila. J Neurosci. 2013;33:2166–2176a. doi: 10.1523/JNEUROSCI.3933-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trannoy S, Redt-Clouet C, Dura JM, Preat T. Parallel processing of appetitive short- and long-term memories in Drosophila. Curr Biol. 2011;21:1647–1653. doi: 10.1016/j.cub.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol. 2008;99:734–746. doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- van Swinderen B. Fly memory: a mushroom body story in parts. Curr Biol. 2009;19:R855–R857. doi: 10.1016/j.cub.2009.07.064. [DOI] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Yang MY, Armstrong JD, Vilinsky I, Strausfeld NJ, Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995;15:45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Zars T. Behavioral functions of the insect mushroom bodies. Curr Opin Neurobiol. 2000;10:790–795. doi: 10.1016/S0959-4388(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]