Abstract
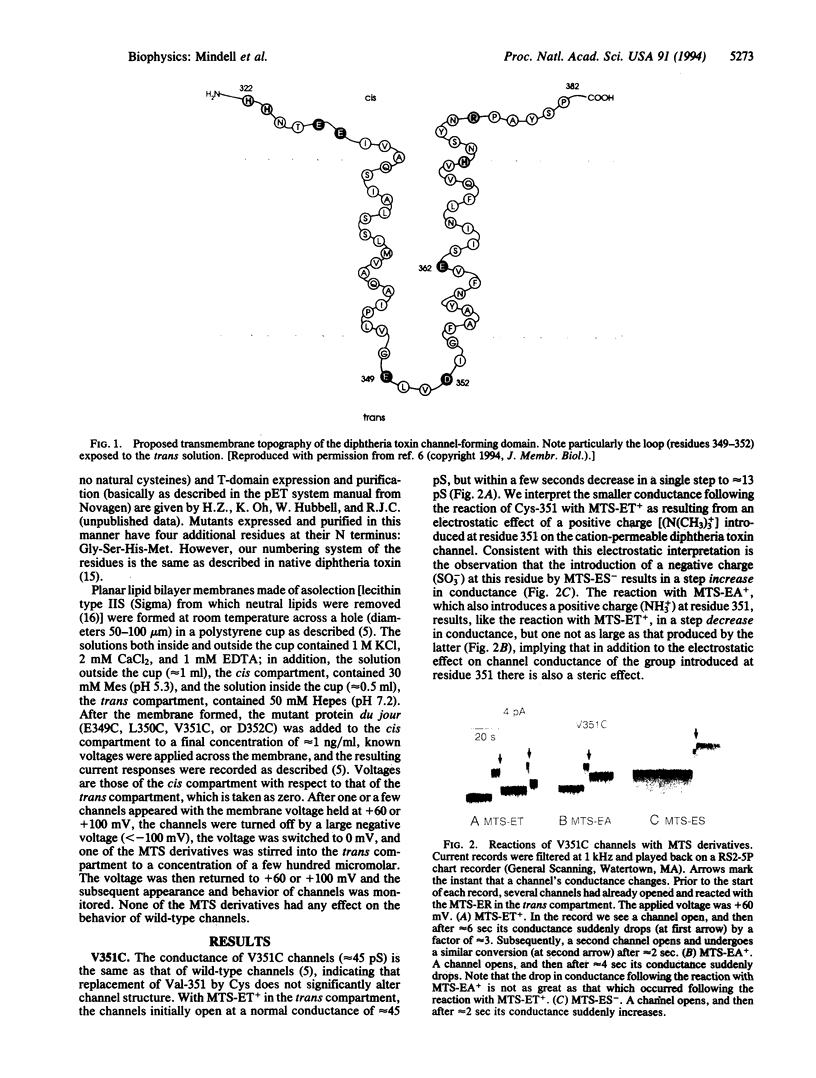
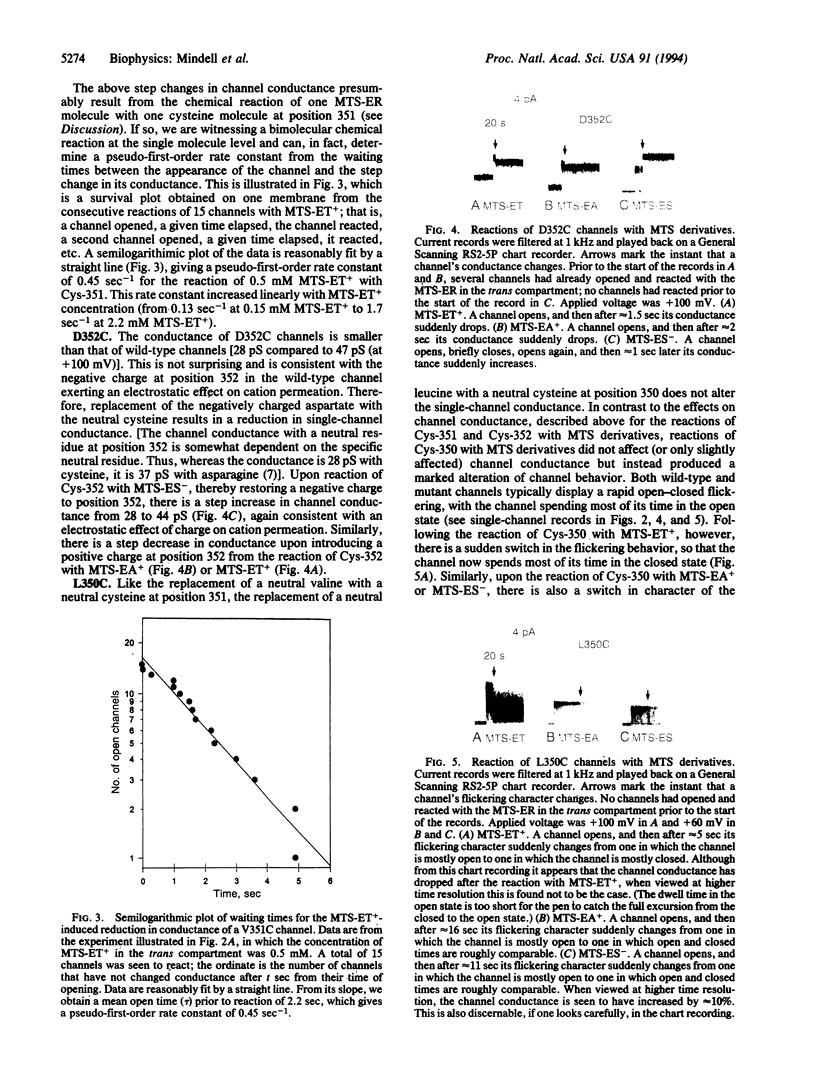
The diphtheria toxin channel is believed to be a homooligomer of its T domain in which each subunit consists of two alpha-helices, lying within the membrane, connected by a short interhelical loop of four amino acids (residues 349-352). To investigate the validity and implications of this model, we singly mutated each of these amino acids to cysteines, formed channels with the mutant T-domain proteins in planar lipid bilayers, and added to the trans compartment sulfhydryl-specific reagents [methanethiosulfonate derivatives (MTS-ER)] that introduce a positive or negative charge to reacted cysteines. The introduction of a positive charge at residue 351 or 352 (through the MTS-ER reactions) resulted in a step decrease in single-channel conductance, whereas the introduction of a negative charge resulted in a step increase. The opposite sign of these effects indicates the predominantly electrostatic nature of the phenomenon and implies that residues 351 and 352 lie close to the channel entrance. The same reactions at residue 350 resulted in very little change in channel conductance but instead changed the character of the natural rapid flickering of the channel between open and closed states to one in which the channel spent more time in the closed state; this may have resulted from the group introduced at position 350 acting as a tethered channel blocker. The MTS derivatives had no effect on channels containing a cysteine at position 349, suggesting that this residue faces away from the channel entrance. We propose that the step changes in conductance or flickering pattern result from the chemical reaction of one MTS-ER molecule with one cysteine, and thus a bimolecular chemical reaction is being witnessed at the single molecule level. From the distribution of waiting times between the appearance (i.e., the opening) of a channel and the step change in its conductance or flickering pattern, we can calculate a pseudo-first-order rate constant, which can then be converted to a second-order rate constant, for the chemical reaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akabas M. H., Stauffer D. A., Xu M., Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992 Oct 9;258(5080):307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Altenbach C., Marti T., Khorana H. G., Hubbell W. L. Transmembrane protein structure: spin labeling of bacteriorhodopsin mutants. Science. 1990 Jun 1;248(4959):1088–1092. doi: 10.1126/science.2160734. [DOI] [PubMed] [Google Scholar]
- Blaustein R. O., Lea E. J., Finkelstein A. Voltage-dependent block of anthrax toxin channels in planar phospholipid bilayer membranes by symmetric tetraalkylammonium ions. Single-channel analysis. J Gen Physiol. 1990 Nov;96(5):921–942. doi: 10.1085/jgp.96.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S., Bennett M. J., Fujii G., Curmi P. M., Kantardjieff K. A., Collier R. J., Eisenberg D. The crystal structure of diphtheria toxin. Nature. 1992 May 21;357(6375):216–222. doi: 10.1038/357216a0. [DOI] [PubMed] [Google Scholar]
- Falke J. J., Dernburg A. F., Sternberg D. A., Zalkin N., Milligan D. L., Koshland D. E., Jr Structure of a bacterial sensory receptor. A site-directed sulfhydryl study. J Biol Chem. 1988 Oct 15;263(29):14850–14858. [PubMed] [Google Scholar]
- Greenfield L., Bjorn M. J., Horn G., Fong D., Buck G. A., Collier R. J., Kaplan D. A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes K. S., Abrams C. K., Finkelstein A., Slatin S. L. Alteration of the pH-dependent ion selectivity of the colicin E1 channel by site-directed mutagenesis. J Biol Chem. 1990 Apr 25;265(12):6984–6991. [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H., Stenmark H. Entry of ADP-ribosylating toxins into cells. Curr Top Microbiol Immunol. 1992;175:1–26. doi: 10.1007/978-3-642-76966-5_1. [DOI] [PubMed] [Google Scholar]
- Mindell J. A., Silverman J. A., Collier R. J., Finkelstein A. Structure function relationships in diphtheria toxin channels: II. A residue responsible for the channel's dependence on trans pH. J Membr Biol. 1994 Jan;137(1):29–44. doi: 10.1007/BF00234996. [DOI] [PubMed] [Google Scholar]
- Mindell J. A., Silverman J. A., Collier R. J., Finkelstein A. Structure-function relationships in diphtheria toxin channels: III. Residues which affect the cis pH dependence of channel conductance. J Membr Biol. 1994 Jan;137(1):45–57. doi: 10.1007/BF00234997. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E., Garber S. S., Miller C. Batrachotoxin-activated Na+ channels in planar lipid bilayers. Competition of tetrodotoxin block by Na+. J Gen Physiol. 1984 Nov;84(5):665–686. doi: 10.1085/jgp.84.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakula A. A., Simon M. I. Determination of transmembrane protein structure by disulfide cross-linking: the Escherichia coli Tar receptor. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4144–4148. doi: 10.1073/pnas.89.9.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin-induced channels in Vero cells selective for monovalent cations. J Biol Chem. 1988 Sep 5;263(25):12352–12359. [PubMed] [Google Scholar]
- Silverman J. A., Mindell J. A., Zhan H., Finkelstein A., Collier R. J. Structure-function relationships in diphtheria toxin channels: I. Determining a minimal channel-forming domain. J Membr Biol. 1994 Jan;137(1):17–28. doi: 10.1007/BF00234995. [DOI] [PubMed] [Google Scholar]
- Todd A. P., Cong J., Levinthal F., Levinthal C., Hubbell W. L. Site-directed mutagenesis of colicin E1 provides specific attachment sites for spin labels whose spectra are sensitive to local conformation. Proteins. 1989;6(3):294–305. doi: 10.1002/prot.340060312. [DOI] [PubMed] [Google Scholar]
- Xu M., Akabas M. H. Amino acids lining the channel of the gamma-aminobutyric acid type A receptor identified by cysteine substitution. J Biol Chem. 1993 Oct 15;268(29):21505–21508. [PubMed] [Google Scholar]