Abstract
Background:
Optimizing cardiovascular function to ensure adequate tissue oxygen delivery is a key objective in the care of critically ill patients with burns. Hemodynamic monitoring may be necessary to optimize resuscitation in serious burn patients with reasonable safety. Invasive central venous pressure (CVP) monitoring has become the corner stone of hemodynamic monitoring in patients with burns but is associated with inherent risks and technical difficulties. Previous studies on perioperative patients have shown that measurement of peripheral venous pressure (PVP) is a less invasive and cost-effective procedure and can reliably predict CVP.
Objective:
The aim of the present prospective clinical study was to determine whether a reliable association exists between changes in CVP and PVP over a long period in patients admitted to the Burns Intensive Care Unit (BICU).
Subjects and Methods:
The CVP and PVP were measured simultaneously hourly in 30 burns patients in the BICU up to 10 consecutive hours. The predictability of CVP by monitoring PVP was tested by applying the linear regression formula and also using the Bland–Altman plots of repeated measures to evaluate the agreement between CVP and PVP.
Results:
The regression formula revealed a reliable and significant association between CVP and PVP. The overall mean difference between CVP and PVP was 1.628 ± 0.84 mmHg (P < 0.001). The Bland–Altman diagram also showed a perfect agreement between the two pressures throughout the 10 h period.
Conclusion:
Peripheral venous pressure measured from a peripheral intravenous catheter in burns patients is a reliable estimation of CVP, and its changes have good concordance with CVP over a long period of time.
Keywords: Burns, central venous pressure, monitoring, peripheral venous pressure
Introduction
The focus of hemodynamic monitoring is moving away from invasive monitoring. This has been attributed to procedure time, cost, and the known risks, which include arterial puncture, pneumothorax, and infection. Numerous studies done in the last few years have paid attention to peripheral venous pressure (PVP) and more specifically its pressure waveform.[1] Although controversy still exists concerning the role of peripheral veins and their contribution to the central volume in face of blood loss, many studies in the late 1990s and early 2000s have shown a consistent correlation between central venous pressure (CVP) and PVP.[2,3] This implies that in emergency conditions and situations where anatomical sites are inaccessible for central venous catheterization as seen in burns patients, the estimation of CVP is possible via measurement of peripheral intravenous (IV) catheter. Hemodynamic monitoring has been shown to provide valuable additional information if burn resuscitation is not proceeding as planned or volume therapy guided by the typical vital signs is not attaining the desired effect.[4,5]
The goal of this study was to determine a reliable association between changes in CVP and PVP in patients with burns and to assess the long-term correlation in varied hemodynamic status during the first 10 h.
Subjects and Methods
After obtaining Institutional Ethical Committee approval and informed consent from each patient, 30 consecutive patients admitted to our Burns Intensive Care Unit (BICU) from June to August 2014 were included in our study. The exclusion criteria were patients on the mechanical ventilator and untimely death during the study period. The sample size was decided after doing a pilot study for a month in our BICU and based on power of analysis.
Central venous pressure access was obtained using a 7 French double-lumen, Arrow International catheter placed via the left or right internal jugular or subclavian vein. Tip of central venous catheter was inserted at the junction of the superior vena cava and right atrium confirmed by chest X-ray. The peripheral measurement of CVP was obtained from a peripheral IV site (dorsum of the hand, forearm or antecubital region) using a standard IV catheter (18, 20 or 22 gauges). CVP was measured from both the central venous catheter and the peripheral IV catheters using Philips and Spacelab monitors equipped with invasive blood pressure monitoring transducers, which were zeroed at the phlebostatic axis. Simultaneous measurements of CVP from central and peripheral venous catheters were made hourly for 10 consecutive hours. Age, weight, height, site of CVP and PVP and peripheral IV catheter size for each patient were recorded. The differences between the CVP and PVP were evaluated using paired t-test. The predictability of CVP by PVP was examined using linear regression analysis at a P ≤ 0.05. The analysis was performed using SPSS software.
Results
Among the 30 patients in this study, there were 20 females and 10 males. The age range was from 18 to 65 years, and their weight ranged from 45 to 60 kg. The percentage of burns varied from 30% to 60%. The predictability of CVP by PVP was tested by applying the linear regression which is shown in Figure 1. This regression formula shows a reliable and significant association between CVP and PVP (P < 0.001). The overall mean difference between CVP and PVP was 1.628 ± 0.84 mmHg. The mean difference between CVP and PVP in each hour is shown in Figure 2. We used the Bland-Altman diagram for estimation of agreement between CVP and PVP during the 10 h period. This showed a perfect agreement (difference of − 1.2 with an SD of + 1.96) as seen in Figure 3.
Figure 1.
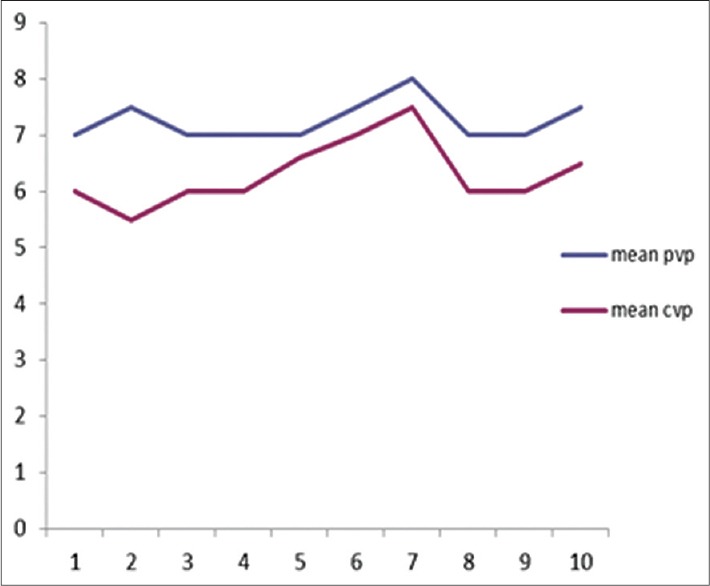
The predictability of central venous pressure by measuring peripheral venous pressure tested by applying linear regression
Figure 2.
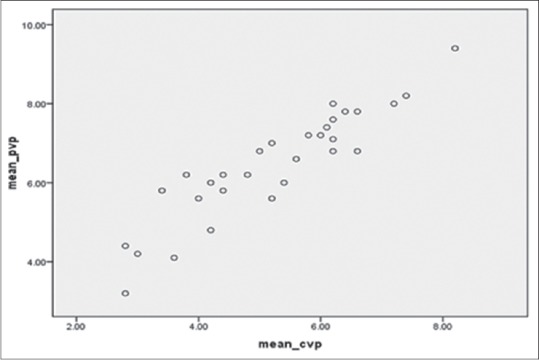
The hourly mean difference between central venous pressure and peripheral venous pressure
Figure 3.
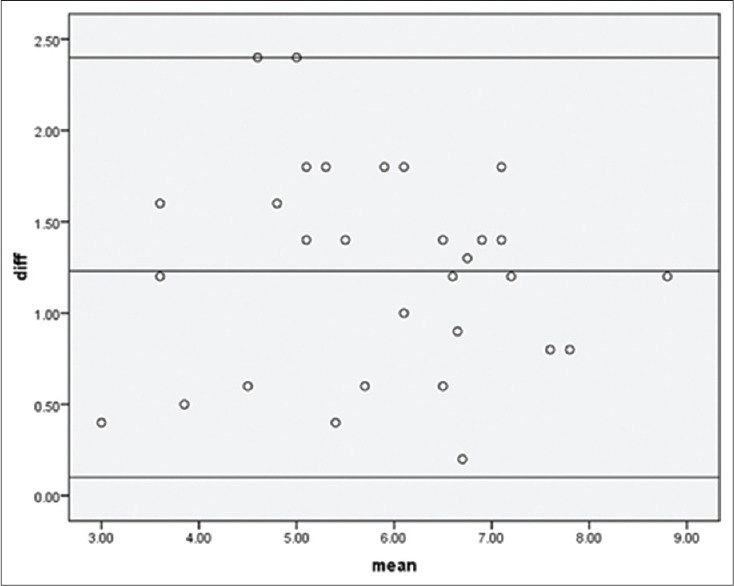
Agreement between central venous pressure and peripheral venous pressure during the 10 h period using Bland–Altman diagram
Discussion
The present study demonstrated reliable agreement between CVP and PVP over a 10 h period, suggesting that PVP monitoring can be used as a simple, cost-effective and less invasive substitute for CVP monitoring in patients admitted to the BICU. Previous studies comparing CVP measured from central and peripheral access have shown a consistent correlation, but most of these studies were done in surgical patients,[6,7,8,9,10] and we could not find any similar study in burns patients despite extensive literature search. CVP monitoring in the critically ill patients is usually performed by catheterization of either the subclavian or internal jugular veins. In burns patients, the site for catheterization of the central veins maybe inaccessible and in such patients monitoring the CVP via a peripheral vein is definitely an attractive option. The results of our study show that we can estimate CVP through simultaneous measurement of PVP and the difference between CVP and PVP measurements remain almost in a constant range over a period of time. Hence, evaluation of hemodynamic changes occurring with dehydration or volume overload can be made by measuring PVP. A similar study by Amoozgar et al., showed that PVP measured from a peripheral IV catheter in infants and children with congenital heart disease was an accurate estimation of CVP and its changes had good concordance with CVP over a long period of time.[11] Charalambous et al., however, reported that PVP measurement in critically ill patients did not give an accurate estimate of absolute value of CVP.[3] Tugrul et al., also reported that PVP showed strong correlation with CVP and mean difference between PVP and CVP was 2 + 1.8 mmHg, but the upper limit of agreement of PVP-CVP (5.6 mmHg) indicated the difference between two pressures might reach a clinically unacceptable value.[12] However, in our study, the overall mean difference between CVP and PVP was 1.628 ± 0.84 mmHg and the upper limit of agreement correlated to the clinically acceptable limits and were comparable with data described in other studies. Amar et al., found the mean difference between PVP and CVP to be 1.6 mmHg in all intraoperative patients and 2.2 mmHg in the postoperative period.[6] Munis et al.,reported a PVP-CVP difference of 3 mmHg.[7] Hoftman et al., showed that PVP correlated with CVP even under adverse hemodynamic conditions in patients undergoing liver transplantation.[10] Studies have also shown that neither the peripheral IV catheter size nor the site of catheter placement interfered with the agreement of PVP and CVP.[6,12] In our study, we used three different sites in the upper limb and three sizes of the peripheral catheter but did not find any statistically significant variations in the agreement of PVP and CVP values. Although CVP waveforms characteristically showed a-waves, c-waves, and v-waves, PVP waveforms appeared as a more dampened sinusoidal pattern. We also noticed that the PVP tracing had more typical CVP waveforms when the peripheral catheter diameter was of a larger gauge and when the site was antecubital but since our subgroup sample size were small, we could not stratify this. Although by placing the peripheral IV catheter more distally and decreasing its diameter increased the PVP-CVP gradient, these failed to reach statistical significance in the study done by Tugrul et al.[12] Several studies have also demonstrated that the difference between CVP and PVP varies with the value of CVP. In nine patients undergoing orthotopic liver transplantation, Hoftman et al., reported a weaker correlation between PVP and CVP at lower CVP values.[10] Another study by Cave and Harvey showed that when CVP increased, the difference between PVP and CVP tended to decrease.[13] There are some limitations to our current investigation. The number of patients was too small for subgroup analysis with regard to the percentage of burns and to the site and size of peripheral catheters affecting the PVP-CVP gradient. We also did not have a protocol for fluid management and its effect on our data.
Conclusion
The changes in CVP and PVP are strongly correlated and consistent over time. Hence, the trends in PVP may be useful as an alternative for hemodynamic monitoring in the BICU during emergencies, in situations where central venous site is inaccessible and also to avoid the complications of central venous catheterization in critically ill burns patients. Further studies are needed to determine the clinical usefulness of PVP as a trend monitor for evaluating the intravascular volume in this patient population.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Wardhan R, Shelley K. Peripheral venous pressure waveform. Curr Opin Anaesthesiol. 2009;22:814–21. doi: 10.1097/ACO.0b013e328332a343. [DOI] [PubMed] [Google Scholar]
- 2.Weingarten TN, Sprung J, Munis JR. Peripheral venous pressure as a measure of venous compliance during pheochromocytoma resection. Anesth Analg. 2004;99:1035–7. doi: 10.1213/01.ANE.0000130853.58560.5D. [DOI] [PubMed] [Google Scholar]
- 3.Charalambous C, Barker TA, Zipitis CS, Siddique I, Swindell R, Jackson R, et al. Comparison of peripheral and central venous pressures in critically Ill patients. Anaesth Intensive Care. 2003;31:34–9. doi: 10.1177/0310057X0303100106. [DOI] [PubMed] [Google Scholar]
- 4.He L, Guo Z, Chai J. Hemodynamic monitoring in 52 serious burn patients in ten years. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1999;15:117–9. [PubMed] [Google Scholar]
- 5.Czermak C, Hartmann B, Scheele S, Germann G, Küntscher MV. Burn shock fluid resuscitation and hemodynamic monitoring. Chirurg. 2004;75:599–604. doi: 10.1007/s00104-004-0859-z. [DOI] [PubMed] [Google Scholar]
- 6.Amar D, Melendez JA, Zhang H, Dobres C, Leung DH, Padilla RE. Correlation of peripheral venous pressure and central venous pressure in surgical patients. J Cardiothorac Vasc Anesth. 2001;15:40–3. doi: 10.1053/jcan.2001.20271. [DOI] [PubMed] [Google Scholar]
- 7.Munis JR, Bhatia S, Lozada LJ. Peripheral venous pressure as a hemodynamic variable in neurosurgical patients. Anesth Analg. 2001;92:172–9. doi: 10.1097/00000539-200101000-00033. [DOI] [PubMed] [Google Scholar]
- 8.Anter AM, Bondok RS. Peripheral venous pressure is an alternative to central venous pressure in paediatric surgery patients. Acta Anaesthesiol Scand. 2004;48:1101–4. doi: 10.1111/j.1399-6576.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 9.Sahin A, Salman MA, Salman AE, Aypar U. Effect of catheter site on the agreement of peripheral and central venous pressure measurements in neurosurgical patients. J Clin Anesth. 2005;17:348–52. doi: 10.1016/j.jclinane.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Hoftman N, Braunfeld M, Hoftman G, Mahajan A. Peripheral venous pressure as a predictor of central venous pressure during orthotopic liver transplantation. J Clin Anesth. 2006;18:251–5. doi: 10.1016/j.jclinane.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Amoozgar H, Ajami GH, Borzuoee M, Amirghofran AA, Ebrahimi P. Peripheral venous pressure as a predictor of central venous pressure in continuous monitoring in children. Iran Red Crescent Med J. 2011;13:342–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Tugrul M, Camci E, Pembeci K, Al-Darsani A, Telci L. Relationship between peripheral and central venous pressures in different patient positions, catheter sizes, and insertion sites. J Cardiothorac Vasc Anesth. 2004;18:446–50. doi: 10.1053/j.jvca.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Cave G, Harvey M. The difference between peripheral venous pressure and central venous pressure (CVP) decreases with increasing CVP. Eur J Anaesthesiol. 2008;25:1037–40. doi: 10.1017/S0265021508004742. [DOI] [PubMed] [Google Scholar]


