Abstract
The last decades have been characterized by a continuous evolution of hemodynamic monitoring techniques from intermittent toward continuous and real-time measurements and from an invasive towards a less invasive approach. The latter approach uses ultrasounds and pulse contour analysis techniques that have been developed over the last 15 years. During the same period, the concept of prediction of fluid responsiveness has also been developed and dynamic indices such as pulse pressure variation, stroke volume variation, and the real-time response of cardiac output to passive leg raising or to end-expiration occlusion, can be easily obtained and displayed with the minimally invasive techniques. In this article, we review the main hemodynamic monitoring devices currently available with their respective advantages and drawbacks. We also present the current viewpoint on how to choose a hemodynamic monitoring device in the most severely ill patients and especially in patients with circulatory shock.
Keywords: Cardiac output, fluid responsiveness, hemodynamics, monitoring, pulse contour analysis, pulse pressure variation, stroke volume variation, thermodilution
Introduction
The last decades have been characterized by a continuous evolution of hemodynamic monitoring techniques from intermittent toward continuous and real-time measurements and from an invasive towards a less invasive approach. Initially, the pulmonary artery catheter (PAC) provided clinicians with measurements of cardiac output (CO) and derived variables according to the intermittent thermodilution technique calculated from the Stewart-Hamilton principle. Few years later, a modified PAC allowing continuous monitoring of CO and of mixed venous oxygen saturation was developed. However, two major drawbacks are still charged to this modified PAC: (1) The so-called continuous measurement of CO is not a real-time measurement since it tracks the changes in CO with some delay; (2) the technique is invasive although it has clearly been demonstrated that the use of PAC does not alter the outcome of critically ill patients.[1] In addition, the appropriate use of the PAC is difficult since it requires a perfect knowledge of how to measure and interpret the provided variables. For all these reasons, less invasive and simpler to use techniques such as the pulse contour analysis technique have been developed over the last 15 years. During the same period, the concept of prediction of fluid responsiveness has been developed.[2] This concept has emerged since it was evidenced that only 50% of patients respond to fluid administration[2] and that fluid overload is associated with increased mortality.[3] The concept of prediction of fluid responsiveness has gained popularity with implementation of the arterial pulse contour analysis to CO monitoring devices for the routine managements of critically ill patients or surgical patients. These techniques allow clinicians to assess fluid responsiveness using dynamic indices such as pulse pressure variation (PPV), stroke volume variation (SVV), and real-time response of CO to passive leg raising (PLR) or to end-expiration occlusion.[4] Hundreds of clinical studies repeatedly documented the superiority of such dynamic tests over static measures of preload such as cardiac filling pressures to predict fluid responsiveness.[4] Three categories of pulse contour CO techniques have been successively developed: (1) Calibrated CO monitors using an indicator dilution CO measurement for calibration, (2) uncalibrated CO monitors and (3) noninvasive CO monitors. These two latter techniques, however, gain in safety what they loose in precision. The indication of each of these monitoring methods will depend on the clinical situation and, in particular, the degree of acceptability of the risks versus the need of a perfect precision of measurements [Table 1].
Table 1.
Compared analysis of advantages and drawbacks of hemodynamic monitoring devices
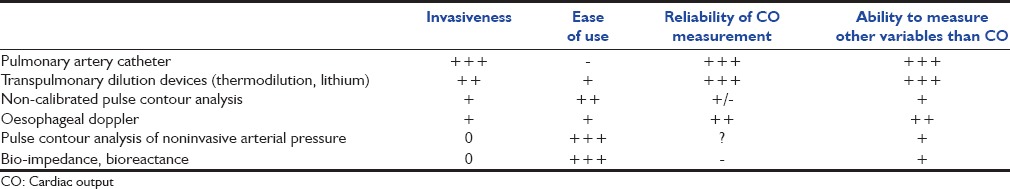
What Hemodynamic Devices are Currently Available?
Calibrated cardiac output monitors
Transpulmonary thermodilution devices
These devices contain two distinct techniques for measuring CO: Transpulmonary thermodilution and pulse contour analysis.
The transpulmonary thermodilution method performs intermittent CO measurements by applying the indicator dilution principles with temperature as the indicator. A known amount of the cold solution with a known temperature is injected rapidly into the circulation through a central venous catheter (superior vena cava territory). This cold solution mixes with the surrounding blood, and the temperature is measured downstream at the level of the femoral artery through a thermistor-tipped arterial catheter. The mathematical analysis of the thermodilution curve (blood temperature vs. time) recorded by the device allows calculation of CO and of other relevant hemodynamic variables.
Two commercially available devices can afford transpulmonary thermodilution-derived parameters. Most of the experimental and clinical experience of the literature about transpulmonary thermodilution involves the PiCCO monitor (Pulsion Medical systems, Germany). The VolumeView monitor (Edwards Lifesciences, USA) has been more recently commercialized. Both systems use the two following technologies: Transpulmonary thermodilution and pulse contour analysis. The main transpulmonary thermodilution-derived variables intermittently obtained after cold bolus injection are CO, global end-diastolic volume (GEDV) considered as a marker of preload, cardiac function index an indicator of cardiac contractility, extravascular lung water (EVLW) a marker of pulmonary edema and pulmonary vascular permeability index (PVPI) assumed to reflect the permeability of the lung capillary membrane. A good agreement between transpulmonary thermodilution and intermittent bolus pulmonary artery thermodilution was reported in critically ill patients.[5] Although theoretically the conventional thermodilution method should allow more accurate measurements of CO (less indicator loss between the injection and the sampling sites), the transpulmonary thermodilution method has the advantages of being less influenced by the respiration.
The pulse contour method estimates the CO from the arterial pressure waveform analyzed at the level of the femoral artery. With the PiCCO monitor, the pulse contour CO algorithm is derived from the initial Wesseling algorithm[6] and calculates stroke volume by measuring the area under the systolic portion of the arterial pressure waveform and dividing it by the aortic impedance determined at the calibration time. Compliance and resistance are updated beat-to-beat according to a proprietary algorithm taking into account the shape of the pressure wave, the position of the dicrotic notch, the systemic vascular resistance, and the arterial compliance.[7] With the VolumeView monitor, another proprietary algorithm is used.[8] Importantly, with both monitors, the pulse contour-derived estimation of CO is not reliable anymore if the device has not been recalibrated since a long time. This does not mean that recalibration should be made systematically every hour in every patient, but rather that during a punctual hemodynamic assessment, one should carefully recalibrate pulse contour analysis if the last calibration was done more than 1 h ago. This is particularly the case if doses of vasoactive drugs have been changed since the last calibration.[9]
One major clinical interest of the transpulmonary thermodilution/pulse contour analysis monitors is to follow in real-time the short-term CO changes induced by therapeutic trials such as fluid challenge, dobutamine trial, etc., This method also provides the clinicians with dynamic indices of fluid responsiveness. The PiCCO monitor automatically calculates PPV and SVV and can display in real-time their values on its screen whereas the VolumeView monitor displays SVV only. The use of PPV and SVV is based on the concept of marked heart-lung interactions during mechanical ventilation in case of cardiac preload-dependency[10] and thus of fluid responsiveness.[11] In clinical circumstances where PPV or SVV are not valid, monitoring pulse contour CO during short tests as PLR and end-expiratory occlusion tests can be used to accurately predict fluid responsiveness.[4,12]
Lithium dilution device
The LiDCOplus monitor (LiDCO Ltd., London, UK) provides CO measurements using two distinct technologies: The lithium dilution and the pulse power analysis.
The lithium dilution method provides intermittent CO measurements. A small amount of lithium chloride (0.002–0.004 mmoL/kg) is injected as a bolus through a central vein catheter. The change in lithium levels is detected by blood being drawn out of a radial artery catheter over a lithium-selective sensor. The CO is then measured from analysis of the lithium dilution curve utilizing a method of integrating the changes in lithium levels over time. This technique has been validated against pulmonary artery thermodilution in humans.[13] To achieve a good precision with this technique, three lithium dilution measurements should be averaged. This allows changes in CO of more than 14% to be reliably detected.[14] The inconvenience of this system is the need of lithium bolus injection, which cannot be repeated infinitely.
The pulse power analysis provides a beat-to-beat measurement of CO using a radial artery catheter. As the PiCCO and the VolumeView systems, the LiDCOplus monitor contains a proprietary algorithm (PulseCO) for converting a pressure-based signal into a flow measurement. The PulseCO algorithm is based on the physics of conservation of mass and energy. Manual calibration of the PulseCO is performed using the lithium dilution technique. The LiDCOplus also allows monitoring of PPV and SVV, markers of fluid responsiveness.[15] Unlike transpulmonary thermodilution devices, the LidCO plus has the advantage to be used with a radial artery catheter. However, it cannot provide thermodilution-derived variables such as GEDV or EVLW.
Uncalibrated arterial pressure waveform analysis cardiac output monitors
The FloTrac/Vigileo system
The FloTrac/Vigileo technology (Edwards Lifesciences, USA) also allows real-time CO measurements by deriving the arterial pressure waveform recorded from an artery catheter (radial or femoral). The FloTrac has a proprietary software algorithm that analyses characteristics of the arterial pressure waveform and uses this analysis along with patient-specific demographic information[16] to determine continuous CO and SVV (but not PPV). Since the FloTrac algorithm continuously adjusts for the patient's ever changing vascular tone, it does not require manual calibration and thus a central venous line for performing calibration. The FloTrac software provides clinicians with reliable CO measurements in the operating room setting.[17] However, CO measurements are less reliable in the Intensive Care Unit (ICU) setting, especially in cases of septic shock and/or of vasopressors use.[18,19,20]
ProAQT/PulsioFlex
The ProAQT/PulsioFlex (Pulsion Medical Systems, Munich, Germany) is a newer pulse contour analysis device. Like the FloTrac/Vigileo system, the ProAQT/PulsioFlex does not need any external calibration of pressure waveform analysis. Nevertheless and according to constructor's indications, it differs from the FloTrac/Vigileo in two main aspects. First, the pressure waveform analysis software is different. Second, the initial CO value from which the pulse contour analysis is started is not estimated by pulse contour analysis itself but by an innovative proprietary algorithm that performs an “auto-calibration.” The algorithm uses the biometric values (age, height, and weight) as well as mean arterial pressure and heart rate. It is possible to reset CO measurement with this auto-calibration at any time. In addition, it is also possible to enter manually a value for CO measured by another technique (e.g., echocardiography). The pulse contour analysis then starts from this external calibration CO value. In practice, the device works with a standard arterial catheter, which is connected to a specific disposable pressure transducer (ProAQT), itself connected to the monitor (PulsioFlex). With the ProAQT/PulsioFlex device, PPV and SVV can also be calculated and displayed in real-time. In patients undergoing off-pump coronary artery bypass grafting, CO measured with this method was shown to be accurate and precise but the ability of this method to follow trends in CO was poor.[21] Opposite results have been reported in critically ill patients where an acceptable concordance between changes in fluid-induced and norepinephrine-induced CO changes measured with the ProAQT/PulsioFlex and those measured by transpulmonary thermodilution was found.[20] Further studies are necessary to know in which patients and in which specific situations this method can be used.
LiDCOrapid
This device is an evolution of the LiDCOplus technology that does not require any calibration. Stroke volume is calculated from the analysis of the stroke volume-induced pulsatile change in the pressure waveform. It needs a radial catheter only. The proprietary algorithm uses the biometric patient's characteristics to determine the starting CO value, which is then continuously updated according to the pulse power algorithm. Thus, the LiDCOrapid system cannot measure accurate CO values but can only display trends. However, as every uncalibrated system, there is a risk of drift of CO values, especially in cases of marked changes in arterial impedance. In this regard, only moderate concordance was reported between LiDCOrapid CO changes and thermodilution CO changes after liver transplantation[22] or cardiac surgery.[23] There is still a need for validation studies showing that the LiDCOrapid system can provide reliable changes in CO over time.
This system also calculates and displays PPV and SVV values in real-time. However, prediction of fluid responsiveness with these LiDCOrapid dynamic variables has been seriously questioned even during surgery.[24,25]
Pressure recording analytical method
The pressure recording analytical method (PRAM) implemented in the MostCare device (Vytech, Padova, Italy) is based on the complex theory of perturbations when analyzing the arterial pressure waveform. The MostCare is the only currently available pulse contour monitor for which neither calibration nor adjustments based on user-entered data are required. This system uses a proprietary algorithm taking into account the area under the systolic part of the arterial pressure curve and the mean arterial pressure.[26] The Mostcare device measures and displays real-time CO and SVV values. Validation studies (against thermodilution) have reported divergent results.[27,28,29]
The ability of SVV calculated by the PRAM technology to predict fluid responsiveness has been questioned in ICU patients.[30] Further studies are required to make sure that this system is valid in the ICU or in the operating room.
Oesophageal Doppler
The use of esophageal Doppler is aimed at monitoring CO by continuously measuring the blood flow in the descending thoracic aorta. Basically, the esophageal Doppler device continuously calculates the aortic blood flow value from the aortic blood velocity and the aortic diameter values. From the value of aortic blood flow, the esophageal Doppler devices infer the value of CO, based on the hypothesis that there is a constant distribution of the systemic blood flow between the upper territories and the descending aorta. It is generally considered that blood flow in the descending thoracic aorta represents around 70% of the systemic blood flow.[31] Numerous studies demonstrated that esophageal Doppler aortic blood flow measurements are reliable and confirmed the validity of the CO estimation by esophageal Doppler.[32] However, many limitations to the routine use of esophageal Doppler can be encountered. A first limitation is related to the fact that the distribution of CO between the upper and the lower parts of the arterial tree can change with spontaneous or therapeutic changes in hemodynamic status especially when they are associated with changes in the sympathetic tone. A second limitation of the technique is that most devices estimate but do not directly measure the diameter of the descending aorta. The estimation is based on the patient's characteristics such as age and body surface. Obviously, the diameter of the descending aorta cannot be constant in every condition since the aorta at this level is compliant enough to change its diameter with its transmural pressure. In this regard, a correlation between changes in mean arterial pressure and in the diameter of the descending aorta was found in critically ill patients.[33] Therefore, during shock resuscitation where mean arterial pressure can change widely, changes in CO cannot be tracked by changes in such estimated descending aorta blood flow. Finally, a practical limitation of the technique is that when the patient is moving, the probe can easily move into the esophagus resulting in signal loss. Thus, the place of esophageal Doppler during complex hemodynamic resuscitation of critically ill patients is questionable, although it seems a valuable tool during the perioperative fluid management of high-risk surgical patients.[34] In this regard, numerous studies demonstrated that using esophageal Doppler for goal-directed perioperative hemodynamic optimization could reduce patient's morbidity after surgery.[35,36,37] In sedated critically ill patients, esophageal Doppler can be helpful for assessing short-term changes in CO, such as those induced by PLR, in order to predict fluid responsiveness.[38]
In addition to the estimation of CO, esophageal Doppler measures other parameters derived from the aortic blood velocity signal [Table 1]. The flow time corrected (FTc) is often used as a marker of left ventricular preload in fluid resuscitation protocols in high-risk surgical patients, although it also depends on left ventricular afterload. Nevertheless, as a static parameter, FTc cannot reliably predict fluid responsiveness.[39] The mean acceleration and the peak of velocity are quite reliable markers of left ventricular systolic function.[40] Finally, the respiratory variability of aortic blood flow is a reliable marker of fluid responsiveness in mechanically ventilated patients.[39]
Noninvasive techniques
The Nexfin device
This is a totally noninvasive continuous blood pressure and CO monitor based on finger arterial pressure pulse contour analysis by an inflatable cuff around the middle phalange of a finger. The pulsating finger artery is clamped to a constant volume by applying a varying counter pressure equivalent to the arterial pressure using a built-in photoelectric plethysmograph and an automatic algorithm. The resulting finger arterial pressure waveform is reconstructed into a brachial artery pressure waveform by a specific algorithm. The CO is calculated by a pulse contour analysis method using the measured systolic pressure time integral and the cardiac afterload determined from the Windkessel model.[41] It also affords a beat-to-beat estimation of arterial pressure, and hence a noninvasive estimation of PPV. Recent studies have investigated the reliability of the Nexfin device to monitor CO. In the context of the perioperative period of cardiac surgery, divergent results have been reported, although trends in CO measured by the Nexfin monitor seem to reliably reflect trends in thermodilution CO.[42,43] If further studies confirm the validity of this technique to monitor the trends in CO, the Nexfin device could rapidly become a popular noninvasive CO monitoring in the perioperative period. However, its interest for monitoring patients with circulatory shock is less valuable since such patients often need an arterial catheter for their routine management. In addition, the reliability of this device to track the changes in CO during a fluid challenge in critically ill patients has been seriously questioned.[44] Other CO monitoring devices using noninvasive blood pressure curves will be available soon.
Impedance cardiography
This is a noninvasive technique allowing beat-to-beat estimation of CO from analysis of cyclic changes in thoracic electrical impedance induced by changes in thoracic blood volume that occur at each heartbeat. The impedance cardiography technique is based on Ohm's law and applies a constant, low-amplitude, high-frequency, alternating electrical current through the thorax and measures the corresponding voltage to detect changes in thoracic impedance.[45] Four skin electrodes placed on the patient's neck and chest allow application of the electric current and four other electrodes allow measurement of the changes in voltage that result from changes in impedance. Obesity, increased thoracic fluid volume (pulmonary edema, pleural effusion), changes in thoracic blood volume induced by mechanical ventilation and cardiac arrhythmias represent the main limitations of this technique. Reliability of this technique has been questioned in spite of many refinements of the mathematical algorithms.[46]
Bioreactance
In contrast to thoracic bioimpedance, which is based on measurements of voltage amplitude changes in response to a high-frequency current, bioreactance measures change in the frequency of the electrical currents traversing the thorax. This is supposed to result in a higher signal-to-noise ratio and thus in an improved performance of the device. During postcardiac surgery, the bioreactance device (NICOM; Cheetah Medical, Vancouver, WA, USA) has been compared to continuous thermodilution PAC with quite good results in terms of accuracy and precision of CO measurements.[47] However, in critically ill patients disappointing results were reported.[48,49] Further validation studies are on-going and are necessary to establish the real place of such a technique.
How to Choose a Hemodynamic Monitoring Device in Intensive Care Unit Patients?
In cases of critical situations such as circulatory shock, at least three different cardiovascular abnormalities (hypovolemia, depressed vascular tone and myocardial depression) can be involved and sometimes coexist such that it is important to assess the degree of each abnormality to select the appropriate treatment (fluids, vasopressors and inotropes). The place of echocardiography is essential to determine the type of shock and in complex patients, it is suggested to additionally use PAC or transpulmonary thermodilution.[50] Although routine measurement of CO is not recommended for patients with shock responding to initial therapy, it is recommended to evaluate the CO response to fluids or inotropes in those not responding to initial therapy.[50] In patients with refractory shock and right ventricular dysfunction, PAC can be used.[50] in order to measure pulmonary artery pressure. In patients with severe shock especially in the case of associated acute respiratory distress syndrome, the use of PAC or transpulmonary thermodilution devices is suggested.[50] The major strength of transpulmonary thermodilution is its ability to both predict the benefits of fluid administration by using dynamic tests of preload responsiveness (PPV, SVV, response of pulse contour CO to PLR or to end-expiratory occlusion) and assess the risks of fluid administration by measuring EVLW and PVPI. The superiority of dynamic indices of preload responsiveness over static markers of preload is now well-recognized.[50] Less invasive devices such as uncalibrated pulse contour CO monitors are not recommended in the context of patients with shock unless they have been validated,[50] which is not yet the case today.
Conclusion
Although, hemodynamic monitoring has been continuously evolving towards real-time and noninvasive methods, the ideal hemodynamic monitoring device is not yet available.[50,51] The choice of the appropriate technique varies from patient to patient and depends on: (1) The precise clinical questions that should be answered, (2) the current clinical situation that the ICU physician is facing to, and in particular the presence of lung injury and of cardiac dysfunction, (3) a history of a cardiovascular disease, (4) the degree of acceptability of the risks versus the need of a perfect precision of measurements, and (5) the ICU physician's experience with either technique. The most invasive methods (PAC and transpulmonary thermodilution monitors) are these providing the greatest number of relevant parameters. Such an advanced hemodynamic monitoring should be reserved to the most severe patients with acute circulatory failure and especially those with associated lung injury and/or cardiac dysfunction as recently recommended by a consensus conference.[50] The uncalibrated CO monitors and esophageal Doppler should find their place in the operating room rather than in the ICU since these devices can be favorably used in a goal-directed therapy approach during and after surgery.[34] Finally, the noninvasive techniques would find their places in the emergency ward and/or the operating room.
Footnotes
Source of Support: Nil
Conflict of Interest: Profs. Jean-Louis Teboul and Xavier Monnet are members of the Medical Advisory Board of Pulsion Medical Systems. Dr Olfa Hamzaoui has not conflict of interest.
References
- 1.Shah MR, Hasselblad V, Stevenson LW, Binanay C, O'Connor CM, Sopko G, et al. Impact of the pulmonary artery catheter in critically ill patients: Meta-analysis of randomized clinical trials. JAMA. 2005;294:1664–70. doi: 10.1001/jama.294.13.1664. [DOI] [PubMed] [Google Scholar]
- 2.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: A critical analysis of the evidence. Chest. 2002;121:2000–8. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 4.Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1:1. doi: 10.1186/2110-5820-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakka SG, Reinhart K, Meier-Hellmann A. Comparison of pulmonary artery and arterial thermodilution cardiac output in critically ill patients. Intensive Care Med. 1999;25:843–6. doi: 10.1007/s001340050962. [DOI] [PubMed] [Google Scholar]
- 6.Wesseling KH, Dewitt B, Weber AP, Smith NT. A simple device for the continuous measurement of cardiac output. Adv Cardiovasc Phys. 1983;5:16–52. [Google Scholar]
- 7.Gödje O, Höke K, Goetz AE, Felbinger TW, Reuter DA, Reichart B, et al. Reliability of a new algorithm for continuous cardiac output determination by pulse-contour analysis during hemodynamic instability. Crit Care Med. 2002;30:52–8. doi: 10.1097/00003246-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Bendjelid K, Marx G, Kiefer N, Simon TP, Geisen M, Hoeft A, et al. Performance of a new pulse contour method for continuous cardiac output monitoring: Validation in critically ill patients. Br J Anaesth. 2013;111:573–9. doi: 10.1093/bja/aet116. [DOI] [PubMed] [Google Scholar]
- 9.Hamzaoui O, Monnet X, Richard C, Osman D, Chemla D, Teboul JL. Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6-hour calibration-free period. Crit Care Med. 2008;36:434–40. doi: 10.1097/01.CCM.OB013E318161FEC4. [DOI] [PubMed] [Google Scholar]
- 10.Michard F, Teboul JL. Using heart-lung interactions to assess fluid responsiveness during mechanical ventilation. Crit Care. 2000;4:282–9. doi: 10.1186/cc710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–8. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 12.Monnet X, Osman D, Ridel C, Lamia B, Richard C, Teboul JL. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med. 2009;37:951–6. doi: 10.1097/CCM.0b013e3181968fe1. [DOI] [PubMed] [Google Scholar]
- 13.Linton RA, Band DM, Haire KM. A new method of measuring cardiac output in man using lithium dilution. Br J Anaesth. 1993;71:262–6. doi: 10.1093/bja/71.2.262. [DOI] [PubMed] [Google Scholar]
- 14.Cecconi M, Dawson D, Grounds RM, Rhodes A. Lithium dilution cardiac output measurement in the critically ill patient: Determination of precision of the technique. Intensive Care Med. 2009;35:498–504. doi: 10.1007/s00134-008-1292-4. [DOI] [PubMed] [Google Scholar]
- 15.Cecconi M, Monti G, Hamilton MA, Puntis M, Dawson D, Tuccillo ML, et al. Efficacy of functional hemodynamic parameters in predicting fluid responsiveness with pulse power analysis in surgical patients. Minerva Anestesiol. 2012;78:527–33. [PubMed] [Google Scholar]
- 16.Langewouters GJ, Wesseling KH, Goedhard WJ. The static elastic properties of 45 human thoracic and 20 abdominal aortas in vitro and the parameters of a new model. J Biomech. 1984;17:425–35. doi: 10.1016/0021-9290(84)90034-4. [DOI] [PubMed] [Google Scholar]
- 17.Mayer J, Suttner S. Cardiac output derived from arterial pressure waveform. Curr Opin Anaesthesiol. 2009;22:804–8. doi: 10.1097/ACO.0b013e328332a473. [DOI] [PubMed] [Google Scholar]
- 18.Monnet X, Anguel N, Jozwiak M, Richard C, Teboul JL. Third-generation FloTrac/Vigileo does not reliably track changes in cardiac output induced by norepinephrine in critically ill patients. Br J Anaesth. 2012;108:615–22. doi: 10.1093/bja/aer491. [DOI] [PubMed] [Google Scholar]
- 19.Meng L, Tran NP, Alexander BS, Laning K, Chen G, Kain ZN, et al. The impact of phenylephrine, ephedrine, and increased preload on third-generation Vigileo-FloTrac and esophageal doppler cardiac output measurements. Anesth Analg. 2011;113:751–7. doi: 10.1213/ANE.0b013e31822649fb. [DOI] [PubMed] [Google Scholar]
- 20.Monnet X, Vaquer S, Anguel N, Jozwiak M, Cipriani F, Richard C, et al. Comparison of pulse contour analysis by Pulsioflex and Vigileo to measure and track changes of cardiac output in critically ill patients. Br J Anaesth. 2015;114:235–43. doi: 10.1093/bja/aeu375. [DOI] [PubMed] [Google Scholar]
- 21.Smetkin AA, Hussain A, Kuzkov VV, Bjertnæs LJ, Kirov MY. Validation of cardiac output monitoring based on uncalibrated pulse contour analysis vs transpulmonary thermodilution during off-pump coronary artery bypass grafting. Br J Anaesth. 2014;112:1024–31. doi: 10.1093/bja/aet489. [DOI] [PubMed] [Google Scholar]
- 22.Costa MG, Chiarandini P, Scudeller L, Vetrugno L, Pompei L, Serena G, et al. Uncalibrated continuous cardiac output measurement in liver transplant patients: LiDCOrapid™ system versus pulmonary artery catheter. J Cardiothorac Vasc Anesth. 2014;28:540–6. doi: 10.1053/j.jvca.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Phan TD, Kluger R, Wan C, Wong D, Padayachee A. A comparison of three minimally invasive cardiac output devices with thermodilution in elective cardiac surgery. Anaesth Intensive Care. 2011;39:1014–21. doi: 10.1177/0310057X1103900606. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald N, Ahmad T, Mohr O, Kirk-Bayley J, Moppett I, Hinds CJ, et al. Dynamic preload markers to predict fluid responsiveness during and after major gastrointestinal surgery: An observational substudy of the OPTIMISE trial. Br J Anaesth. 2015;114:598–604. doi: 10.1093/bja/aeu398. [DOI] [PubMed] [Google Scholar]
- 25.Nordström J, Hällsjö-Sander C, Shore R, Björne H. Stroke volume optimization in elective bowel surgery: A comparison between pulse power wave analysis (LiDCOrapid) and oesophageal Doppler (CardioQ) Br J Anaesth. 2013;110:374–80. doi: 10.1093/bja/aes399. [DOI] [PubMed] [Google Scholar]
- 26.Romano SM, Pistolesi M. Assessment of cardiac output from systemic arterial pressure in humans. Crit Care Med. 2002;30:1834–41. doi: 10.1097/00003246-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 27.Franchi F, Silvestri R, Cubattoli L, Taccone FS, Donadello K, Romano SM, et al. Comparison between an uncalibrated pulse contour method and thermodilution technique for cardiac output estimation in septic patients. Br J Anaesth. 2011;107:202–8. doi: 10.1093/bja/aer123. [DOI] [PubMed] [Google Scholar]
- 28.Paarmann H, Groesdonk HV, Sedemund-Adib B, Hanke T, Heinze H, Heringlake M, et al. Lack of agreement between pulmonary arterial thermodilution cardiac output and the pressure recording analytical method in postoperative cardiac surgery patients. Br J Anaesth. 2011;106:475–81. doi: 10.1093/bja/aeq372. [DOI] [PubMed] [Google Scholar]
- 29.Gopal S, Do T, Pooni JS, Martinelli G. Validation of cardiac output studies from the Mostcare compared to a pulmonary artery catheter in septic patients. Minerva Anestesiol. 2014;80:314–23. [PubMed] [Google Scholar]
- 30.Biais M, Cottenceau V, Stecken L, Jean M, Ottolenghi L, Roullet S, et al. Evaluation of stroke volume variations obtained with the pressure recording analytic method. Crit Care Med. 2012;40:1186–91. doi: 10.1097/CCM.0b013e31823bc632. [DOI] [PubMed] [Google Scholar]
- 31.Boulnois JL, Pechoux T. Non-invasive cardiac output monitoring by aortic blood flow measurement with the Dynemo 3000. J Clin Monit Comput. 2000;16:127–40. doi: 10.1023/a:1009902517388. [DOI] [PubMed] [Google Scholar]
- 32.Dark PM, Singer M. The validity of trans-esophageal Doppler ultrasonography as a measure of cardiac output in critically ill adults. Intensive Care Med. 2004;30:2060–6. doi: 10.1007/s00134-004-2430-2. [DOI] [PubMed] [Google Scholar]
- 33.Monnet X, Chemla D, Osman D, Anguel N, Richard C, Pinsky MR, et al. Measuring aortic diameter improves accuracy of esophageal Doppler in assessing fluid responsiveness. Crit Care Med. 2007;35:477–82. doi: 10.1097/01.CCM.0000254725.35802.17. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–402. doi: 10.1213/ANE.0b013e3181eeaae5. [DOI] [PubMed] [Google Scholar]
- 35.Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: Randomised controlled trial. BMJ. 1997;315:909–12. doi: 10.1136/bmj.315.7113.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth. 2002;88:65–71. doi: 10.1093/bja/88.1.65. [DOI] [PubMed] [Google Scholar]
- 37.Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97:820–6. doi: 10.1097/00000542-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402–7. doi: 10.1097/01.CCM.0000215453.11735.06. [DOI] [PubMed] [Google Scholar]
- 39.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, et al. Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med. 2005;31:1195–201. doi: 10.1007/s00134-005-2731-0. [DOI] [PubMed] [Google Scholar]
- 40.Monnet X, Robert JM, Jozwiak M, Richard C, Teboul JL. Assessment of changes in left ventricular systolic function with oesophageal Doppler. Br J Anaesth. 2013;111:743–9. doi: 10.1093/bja/aet212. [DOI] [PubMed] [Google Scholar]
- 41.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566–73. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 42.Fischer MO, Avram R, Cârjaliu I, Massetti M, Gérard JL, Hanouz JL, et al. Non-invasive continuous arterial pressure and cardiac index monitoring with Nexfin after cardiac surgery. Br J Anaesth. 2012;109:514–21. doi: 10.1093/bja/aes215. [DOI] [PubMed] [Google Scholar]
- 43.Broch O, Renner J, Gruenewald M, Meybohm P, Schöttler J, Caliebe A, et al. A comparison of the Nexfin® and transcardiopulmonary thermodilution to estimate cardiac output during coronary artery surgery. Anaesthesia. 2012;67:377–83. doi: 10.1111/j.1365-2044.2011.07018.x. [DOI] [PubMed] [Google Scholar]
- 44.Monnet X, Picard F, Lidzborski E, Mesnil M, Duranteau J, Richard C, et al. The estimation of cardiac output by the Nexfin device is of poor reliability for tracking the effects of a fluid challenge. Crit Care. 2012;16:R212. doi: 10.1186/cc11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yancy C, Abraham WT. Noninvasive hemodynamic monitoring in heart failure: Utilization of impedance cardiography. Congest Heart Fail. 2003;9:241–50. doi: 10.1111/j.1751-7133.2003.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 46.de Waal EE, Konings MK, Kalkman CJ, Buhre WF. Assessment of stroke volume index with three different bioimpedance algorithms: Lack of agreement compared to thermodilution. Intensive Care Med. 2008;34:735–9. doi: 10.1007/s00134-007-0938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Squara P, Denjean D, Estagnasie P, Brusset A, Dib JC, Dubois C. Noninvasive cardiac output monitoring (NICOM): A clinical validation. Intensive Care Med. 2007;33:1191–4. doi: 10.1007/s00134-007-0640-0. [DOI] [PubMed] [Google Scholar]
- 48.Fagnoul D, Vincent JL, Backer de D. Cardiac output measurements using the bioreactance technique in critically ill patients. Crit Care. 2012;16:460. doi: 10.1186/cc11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kupersztych-Hagege E, Teboul JL, Artigas A, Talbot A, Sabatier C, Richard C, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111:961–6. doi: 10.1093/bja/aet282. [DOI] [PubMed] [Google Scholar]
- 50.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B, et al. Clinical review: Update on hemodynamic monitoring - a consensus of 16. Crit Care. 2011;15:229. doi: 10.1186/cc10291. [DOI] [PMC free article] [PubMed] [Google Scholar]


