Abstract
Colorectal cancer metastasis is believed to be associated with microRNA dysregulation. However, little is known as to how microRNAs regulate colorectal cancer proliferation, invasion and metastasis. In the present study, we compared the microRNA expression profiles between patients of colorectal cancer at diagnosis with and without liver metastasis. MicroRNA-320b was found to be among those up-regulated in the patient group with metastasis. We subsequently found that microRNA-320b, opposite of its homolog, microRNA-320a that differs by only a single nucleotide, functions in promoting colorectal cancer cell proliferation and invasion. Moreover, we found that overexpression of exogenous microRNA-320b can up-regulate the target genes of microRNA-320a including β-catenin, Neuropilin-1 and Rac-1, which are all known to promote tumor proliferation, invasion and metastasis. These results suggest that microRNA-320b may function in competing with microRNA-320a. Thus, our study has proposed one novel mechanism for controlling colorectal cancer proliferation and invasion through homologous competition between microRNAs. This mechanism may be important for colorectal cancer metastasis.
Keywords: microRNA-320b, microRNA-320a, Colorectal cancer, Invasion, Metastasis
Introduction
Colorectal cancer (CRC) is the third most common cause of cancer death worldwide [1]. As many as one third of the CRC patients have either synchronous or metachronous liver metastases [2]. Approximately 50% of patients who are diagnosed with CRC patients die of the distant metastasis [3]. Due to poor prognosis of metastatic CRC, understanding the underlying mechanisms for the metastatic process is highly critical. Such knowledge is essential for early detection of metastasis and more effective treatment.
Colorectal cancer metastasis is associated with epigenetic regulation, such as microRNAs dysregulation [4–6]. Some microRNAs such as miR-21, were found to be preferentially expressed in colorectal tumors at more advanced TNM stages and associated with poorer survival [7]. Other microRNAs such as miR-135b were found to be significantly associated with more favorable prognosis in CRC patients [8]. A number of microRNAs have been shown to functionally participate in the tumorigenesis and metastasis process. MicroRNAs such as miR-31, miR-21 and miR-9 promote CRC migration and invasion whereas miR-200 and miR-320a suppress colorectal cancer [9–11]. Moreover, studies have indicated that microRNAs regulate the mechanistic pathways of CRC metastasis, such as epithelial–mesenchymal transition (EMT) and angiogenesis. Downstream targets of these microRNAs that are involved in these pathways were also identified. For example, the miR-200 family repressed EMT by targeting ZEB1/2β-catenin [12,13]. In a similar manner, miR-107 was shown to function as a suppresser of HIF-1 and VEGF expression [14]. Additionally, Neuropilin-1 and Rac-1 were found to be targeted by microRNA-320a [15–18]. Like many other microRNAs, microRNA-320a has been shown to be also involved in multiple cancer diseases including colorectal cancer, breast cancer, and prostate cancer.
To obtain a comprehensive knowledge of how microRNAs control CRC metastasis, we performed microRNA microarray analysis to compare microRNA expression profiles between primary colorectal adenocarcinomas with metastasis and those without metastasis found at diagnosis. In this study, microRNA-320b was identified as one of the microRNAs up-regulated in primary colorectal adenocarcinomas with metastasis. Intriguingly, microRNA-320b was found to promote CRC proliferation and invasion by competing with its homolog microRNA-320a.
Materials and methods
Patients and tissue samples
Tumor tissue samples were obtained by surgical resection and stored at −80 °C from 2009 to 2013 at the Second Affiliated Hospital of Zhejiang University School of Medicine. Two pathologists confirmed these tissue samples as colorectal adenocarcinoma. Liver metastases were identified by CT scans, MRI and/or biopsy preoperatively and confirmed intra-operatively.
This project was approved by the ethical committee of the Second Affiliated Hospital of Zhejiang University, School of Medicine and informed consent was obtained from all the patients.
RNA extraction and microRNA microarray
Total RNA, including microRNA used for microarray, was extracted using TRIZOL (Invitrogen), according to the manufacturer's protocol. The quality of RNA was assessed by the Agilent 2100 Bioanalyzer (Agilent Technologies). 100 ng of total RNA was labeled using the Agilent miRNA Complete and Hyb Kit. The samples were hybridized to the Agilent Human miRNA Microarrays that were formatted with eight high-definition 60K arrays, based on miRBase Release 19.0. Hybridization was carried out at 20 RPM at a temperature of 55 °C for 20 hours. Scanning and image analysis were performed using the Agilent High Resolution C-scanner equipped with extended dynamic range software. Feature Extraction Software (Version 10.5) was used for data extraction from raw microarray image files. Data visualization and analysis were performed with GeneSpring GX (Version 10.0).
Cell culture and transient infection
The SW620 human colon cancer cells were cultured in Leibovitz's L-15 medium (Gibco, Life Technology) with 10% fetal bovine serum. The RKO human colon cancer cells were cultured in RPIM1640 medium (Gibco, Life Technology) containing 10% fetal bovine serum. The LoVo human colon cancer cells were cultured in F-12 medium (Gibco, Life Technology). All the cell lines were originally obtained from the American Type Culture Collection (ATCC). For overexpression and inhibition of microRNAs, microRNA-320a mimics (micrON™ hsa-miR-320a mimic, miR10000510, RiboBio, China), microRNA-320b mimics (micrON™ hsa-miR-320b mimic, miR10005792, RiboBio, China), microRNA-320a inhibitor (micrOFF™ hsa-miR-320a inhibitor, miR20000510, RiboBio, China), and microRNA-320b inhibitor (micrOFF™ hsa-miR-320b inhibitor, miR20005792, RiboBio, China) were transiently transfected to mammalian cells by Lipofectamine2000 (Invitrogen, Life Technology). Real-time PCR TaqMan assays (Applied Biosystems, Life Technologies) were used to assess the expression level of microRNA after transfection.
Cell proliferation assay
Cell proliferation assay was conducted using the Cell Counting Kit-8 (CCK-8, Dojindo). Cells were plated into the 96-well plates, and cell proliferation was evaluated at 24 h, 48 h and 72 h after plating. 10 μl of CCK-8 solution was added to each well and the plate was incubated with cells in the 37 °C incubator for 4 hours. Cell proliferation was measured at an absorbance of 450 nm.
Trans-well invasion assay
Matrigel (BD Biosciences) coated 8 μm-pore trans-wells (Corning Inc.) were used for the invasion assay. Assays were conducted according to the manufacturer's protocol. SW620 or RKO cells after transient infection were seeded in the upper chamber with serum-free Leibovitz's L-15 or RPMI 1640 medium, and the lower chamber was prepared with the medium containing 10% fetal bovine serum. In 48 h or 72 h, non-invaded cells in the upper chamber were removed with cotton swabs, and crystal violet stain solution was then used to stain the invaded cells. Images were taken at 10× magnification. For enumeration of the invaded cells, the invaded cells were trypsinized for cell calculation after removing the non-invaded cells.
Protein extraction and western blotting
Cells were lysed in RIPA buffer (Beyotime, Jiangsu, China) containing protease inhibitor cocktail (Beyotime). Total protein was quantified by the Bradford protein assay (Bio-Rad, Hercules, CA). Samples were denatured at 100 °C for 5 min. Equal amounts of total protein were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting using polyvinylidene fluoride microporous membranes (Bio-Rad). Membranes were incubated with primary antibody overnight at 4 °C, followed by incubation with horseradish peroxidase-linked secondary antibody at room temperature for 1 hour and detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo). The primary antibodies, anti-Rac1 and anti-Neuropilin1 were purchased from Abcam (Cambridge, UK), while anti-β-catenin and anti-GAPDH were purchased from Cell Signaling Technology (Danvers, MA).
Statistics analysis
Statistical analysis was performed with SPSS16.0. Statistical significance was tested by two-tailed Student's t-test and differences were considered statistically significant with a p-value < 0.05.
Results
Microarray analysis of microRNAs in primary colorectal adenocarcinomas of patients with liver metastasis compared to those without metastasis
To identify the microRNAs that correlated to colorectal cancer with metastasis, we conducted a microRNA microarray analysis of RNA obtained from primary colorectal adenocarcinomas of twenty patients with liver metastasis and of twenty-seven patients without metastasis at the time of diagnosis. There is no statistically significant difference in basic demographic or clinicopathologic features between the patients with or without metastasis (Supplementary Table S1). However, as anticipated, slightly more patients with metastasis possess the factors known to be associated with poor prognosis including T4 stage, N2 stage, lymphatic invasion and perineural invasion. Ten microRNAs were found to be significantly up-regulated and three were found to be significantly down-regulated for more than 1.40 folds in primary colorectal adenocarcinomas of patients with liver metastasis at the time of diagnosis compared to those of patients without metastasis (Fig. 1A). Among them, microRNA-320b was found to be up-regulated by 1.42 folds in primary colorectal adenocarcinomas of patients with liver metastasis at the time of diagnosis compared to those of patients without metastasis (p = 0.0036) (Fig. 1B). Interestingly, microRNA-320a has been reported to be down-regulated in the liver metastasis tissues of the colorectal cancer. Therefore, microRNA-320b appears to functionally different from microRNA-320a in colorectal cancer. However, there is only one base difference at the 3′-terminus between microRNA-320a and microRNA-320b (Fig. 1C). The probe sequence that detected microRNA-320a (probe ID: A_25_P00012261) in the microarray analysis is 5′-TCGCCCTCTCAA-3′; and that for microRNA-320b (probe ID: A_25_P00015035) is 5′-TTGCCCTC TCAACCC-3′. Therefore, probe sequences for microRNA-320a and microRNA-320b are different and explained how microarray analysis may distinguish microRNA-320b from microRNA-320a. We then found that the current quantitative real-time RT-PCR assay can only partially distinguish the expression of microRNA-320a from that of microRNA-320b (Supplementary Fig. S1).
Fig 1.
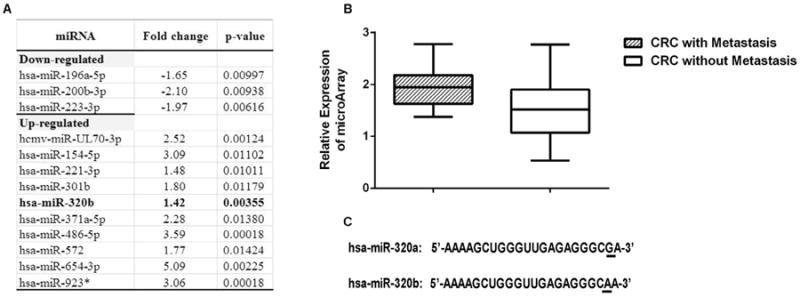
A. A list of microRNAs differentially expressed in CRC with metastasis vs. without metastasis with fold changes (Metastasis/Non-metastasis) ≥ 1.4 and p values ≤ 0.01 in the unpaired t-test. * indicates a dead microRNA entry in the miRBase (v.21) database. B. Microarray analysis of relative expression levels of microRNA-320b in primary colorectal adenocarcinomas of patients with liver metastasis at the time of diagnosis compared to those of patients without metastasis (Fold change = 1.42, p = 0.0036). C. Sequences of microRNA-320a and microRNA-320b.
MicroRNA-320a and microRNA-320b have distinct functions in colorectal cancer cell proliferation
As microRNA-320b is up-regulated in primary colorectal adenocarcinomas of patients with liver metastasis compared to those of patients without metastasis, we hypothesized that microRNA-320b functions in promoting colorectal cancer growth and invasion. To this end, we first assessed its role in tumor cell proliferation. The SW620 colon cancer cells have low expression level of microRNA-320 (Supplementary Fig. S2) and therefore were used to study if transfection of microRNA-320 would affect cell proliferation. As shown in Fig. 2A measured by the CCK8 assay and in Fig. 2B measured by the cell numbers, transfection of the microRNA-320b mimic increased the proliferation rate of SW620 cells whereas transfection of the microRNA-320a mimic decreased the proliferation of SW620 cells. By contrast, RKO colon cells show high expression levels of microRNA-320 (Supplementary Fig. S2) and therefore were used to study if inhibition of microRNA would affect cell proliferation. As shown in Fig. 2C measured by the CCK8 assay and in Fig. 2D measured by the cell confluence, transfection of the microRNA-320b inhibitor decreased the proliferation rate of RKO cells, whereas transfection of the microRNA-320a inhibitor increased the proliferation of RKO cells. These results indicate that microRNA-320a and microRNA-320b have distinct functions in controlling the proliferation of the colorectal cancer cells.
Fig 2.
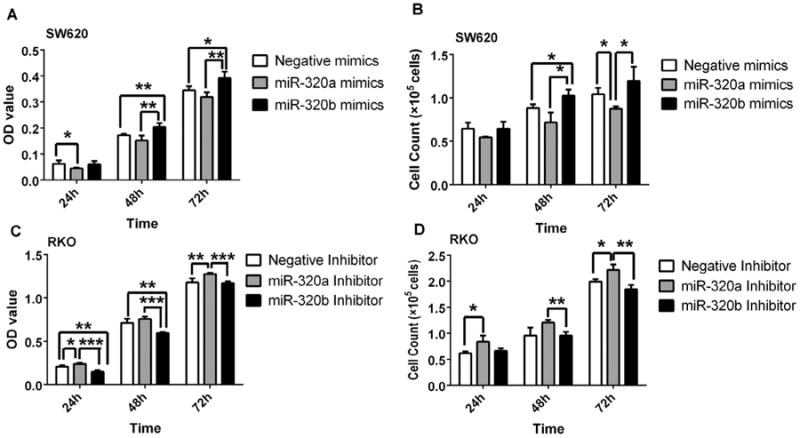
MicroRNA-320a and microRNA-320b have different functions in colorectal cancer cell proliferation. SW620 cells transfected with 40 nM mimics of microRNA-320a and microRNA-320b, respectively, or with the control microRNA were measured by the CCK8 assay (A) and by counting the cell number (B) at 24, 48, 72 hours following transfection. RKO cells transfected with 40 nM inhibitors of microRNA-320a and microRNA-320b, respectively, or with the control microRNA inhibitor were measured by the CCK8 assay (C) and by counting the cell number (D) at 24, 48, 72 hours following transfection. (*p < 0.05, **p < 0.01, ***p < 0.001).
MicroRNA-320a and microRNA-320b have opposite functions in colorectal cancer cell invasion
Next, we examined the role of microRNA-320 in colorectal cancer cell invasion by the trans-well assay. As shown in Fig. 3A, B and D demonstrating the invaded cells, transfection of the microRNA-320b mimic increased the invasion of both SW620 cells and RKO cells whereas transfection of the microRNA-320a mimic decreased the invasion of both SW620 cells and RKO cells. Similarly, RKO colon cells that have high expression level of microRNA-320 (Supplementary Fig. S2) were used to study if inhibition of microRNA will affect cell invasion. As shown in Fig. 3C and E demonstrating invaded cells, transfection of the microRNA-320b inhibitor decreased the invasion of RKO cells whereas transfection of the microRNA-320a inhibitor increased the invasion of RKO cells. This indicates that microRNA-320a and microRNA-320b have opposite functions in controlling the invasion of the colorectal cancer cells.
Fig 3.
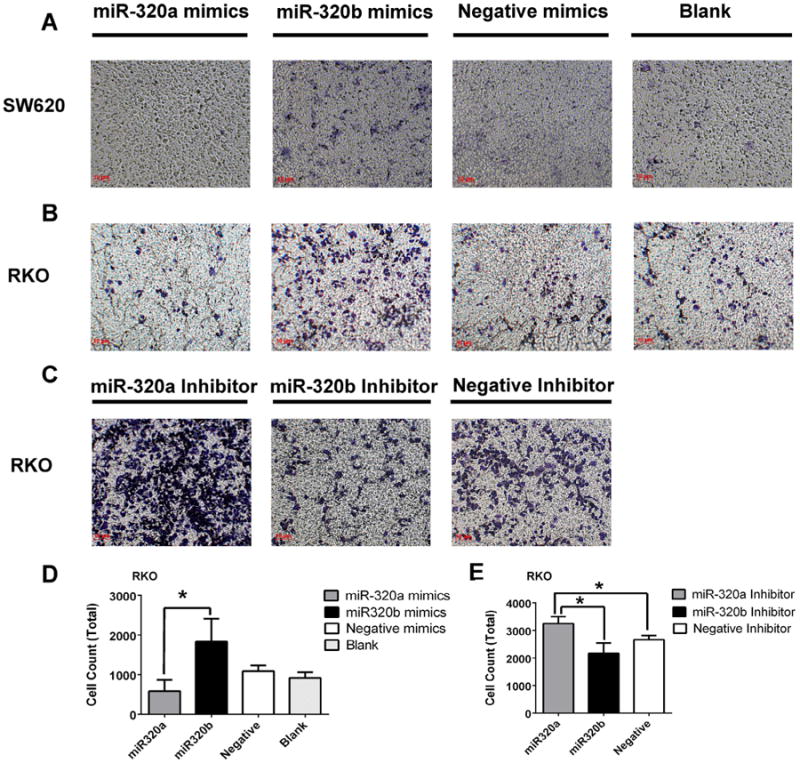
MicroRNA-320a and microRNA-320b have opposite functions in colorectal cancer cell invasion. The invasion of SW620 cells and RKO cells transfected with the mimics of microRNA-320a and microRNA-320b, respectively, or with the control microRNA was measured by the trans-well assay at 48 hours following transfection. A and B show invaded SW620 cells and invaded RKO cells stained by crystal violet. Invaded RKO cells were counted and shown in the histogram in D. The invasion of RKO cells transfected with the inhibitors of microRNA-320a and microRNA-320b, respectively, or with the control microRNA were measured by the trans-well assay at 48 hours following transfection. Invaded RKO cells stained by crystal violet shown in C. Invaded RKO cells were counted and shown in the histogram in E. (*p < 0.05).
MicroRNA-320b competes with microRNA-320a in targeting β-catenin, Neurophilin-1 and Rac-1
Based on the observation that microRNA-320a and microRNA-320b, homologs with one nucleotide difference, have opposite functions in controlling the proliferation and invasion of the colorectal cancer cells, we hypothesized that microRNA-320b competes with microRNA-320a. To test this hypothesis, we overexpressed microRNA-320a and microRNA-320b in RKO cells, SW620 cells, and LoVo cells, respectively. RKO cells express low levels of β-catenin, Neuropilin-1 and Rac-1, which, as described above, are well-characterized targets of microRNA-320a. Thus, low expression of β-catenin, Neuropilin-1 and Rac-1 in RKO cells may be caused by a high expression of microRNA-320a. We confirmed that β-catenin, Neuropilin-1 and Rac-1 are targets of mciroRNA-320a in RKO cells as transfection of microRNA-320a was able to further inhibit the expression of all three targets (Fig. 4A). By contrast, overexpression of microRNA-320b can enhance the expression of β-catenin, Neuropilin-1 and Rac-1 (Fig. 4B), suggesting that micro-320b may counteract the function of microRNA-320a by competing with microRNA-320a in RKO cells. Thus, β-catenin, Neuropilin-1 and Rac-1 appear to be also the in vivo targets of microRNA-320b.
Fig 4.
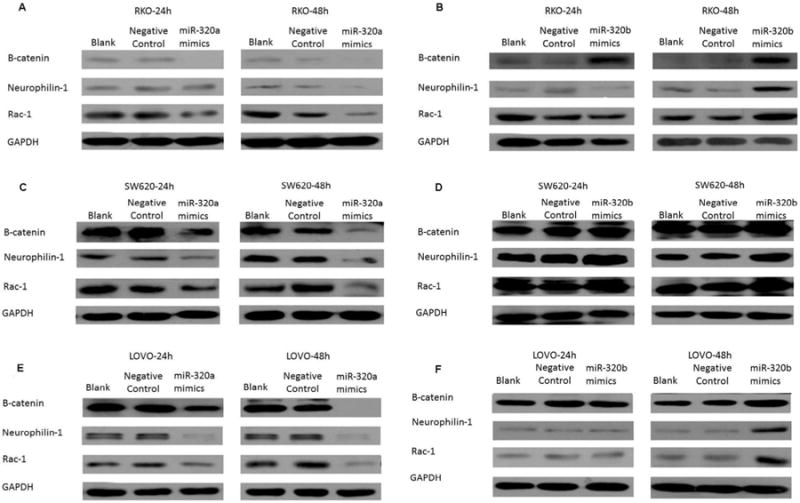
Competition between microRNA-320a and microRNA-320b in targeting β-catenin, Neuropilin-1 and Rac-1. At 24 hours and 48 hours following transfection, the expression levels of β-catenin, Neuropilin-1 and Rac-1 in RKO (A, B), SW620 (C, D) and LOVO (E, F) cells transfected with the mimics of microRNA-320a (A, C, E) and microRNA-320b (B, D, F), respectively, transfected with the Lipo2000 negative control, or untransfected (blank) were measured by Western Blot. GAPDH was used as an internal control. It should be noted that the expression of Neuropilin-1 and Rac-1 was not enhanced at 24 hours, but was substantially enhanced at 48 hours following the transfection of exogenous microRNA-320 mimics.
SW620 cells express high levels of β-catenin, Neuropilin-1 and Rac-1(Fig. 4C&D). High expression of β-catenin, Neuropilin-1 and Rac-1 may be a result of microRNA-320b, whose expression may exceed that of microRNA-320a in SW620 cells. As shown in Fig. 4C, overexpression of microRNA-320a can suppress the expression of β-catenin, Neuropilin-1 and Rac-1, confirming that β-catenin, Neuropilin-1 and Rac-1 are the targets of microRNA-320a in SW620 cells and suggesting that overexpression of microRNA-320a may overcome the competing effect of microRNA-320b in SW620 cells. By contrast, transfection of microRNA-320b in SW620 cells was still able to enhance the expression of β-catenin, Neuropilin-1 and Rac-1 (Fig. 4D).
To further confirm that β-catenin, Neuropilin-1 and Rac-1 are targets of both microRNA-320a and microRNA-320b, we examined their effects in LoVo cells, which express moderate levels of β-catenin, Neuropilin-1 and Rac-1 compared to either RKO or SW620 cells. As anticipated, we found that microRNA-320a inhibits the expression of all three targets (Fig. 4E) whereas microRNA-320b enhances the expression of all three targets (Fig. 4F). Taken together, these results suggest that microRNA-320a and microRNA-320b targets β-catenin, Neuropilin-1 and Rac-1 in colon cancer cells in two opposite directions, thus providing an explanation for the distinct biological functions of microRNA-320a and -320b.
Discussion
Until now, this study was the largest one to identify microRNAs involved in CRC metastasis by comparing the expression of microRNAs between primary colorectal adenocarcinomas with vs. without metastasis. For the first time, our study suggested that the one nucleotide difference between microRNA-320a and microRNA-320b results in opposite functions in controlling cell proliferation and invasion of colorectal cancer. In contrast to the previously established role of microRNA-320a in suppressing CRC proliferation and invasion, microRNA-320b was identified in our study as a biomarker that is up-regulated in primary colorectal adenocarcinomas with liver metastasis compared to those without metastasis and was found to promote CRC proliferation and invasion. Furthermore, our study suggested that microRNA-320b targets the same genes of microRNA-320a including β-catenin, Neuropilin-1 and Rac-1 by competing with microRNA-320a.
However, we were unable to distinguish the expression of microRNA-320b from that of microRNA-320a, by using quantitative real-time PCR. With only one nucleotide difference between microRNA-320a and microRNA-320b, it would be difficult for PCR-based techniques to distinguish between microRNA-320a and microRNA-320b. The current hybridization-based technology of the TaqMan assay was not able to accurately distinguish between microRNA-320a and microRNA-320b, either. In the current TaqMan assay, although the probe specific for microRNA-320a binds to microRNA-320a stronger than to microRNA-320b, it is still able to bind microRNA-320b to a significant degree [19]. Similarly, the probe specific for microRNA-320b is able to bind microRNA-320a to a significant degree. The subtlety of the one nucleotide difference between microRNA-320b and microRNA-320a presents a challenge in the utilization of a number of techniques to characterize expression.
It has been well established that, after cleaving the pre-microRNA into mature microRNA by Dicer, the single strand mature microRNAs will be incorporated into Argonaute (Ago) proteins to form the RNA-induced silencing complex (RISC). Guided by sequence complementarity between microRNA and targeting mRNA, the RISC plays its silencing functions through site-specific cleavage or translational inhibition [20,21]. The site-specific cleavage process requires a perfect match or near-perfect match between microRNA and target mRNA, while the translational inhibition is more commonly mediated by mismatched microRNA and targeting mRNA sequences [20]. Therefore, it is possible that microRNA-320b can bind to the targeting mRNAs of microRNA-320a with a partially matched sequence, particularly where there is only one single nucleotide difference. In addition, many studies have suggested that a 7–8 mer length of nucleotide sequence at the 5′ seed area is sufficient for a microRNA to select its specific mRNA target. It is common that homologous microRNAs share the same 5′ seed area but have distinct 3′ compensatory sites, and thus have different functions [22]. Here, it is possible that microRNA-320a and microRNA-320b share the same 5′ seed area which determines the binding to target mRNAs whereas the single nucleotide mismatch in the 3′ compensatory sites determines the interaction with the Ago proteins. The literature suggests that the function of microRNAs can be determined by a single nucleotide in the region that is associated with Ago [23–25]. Thus, microRNA-320b, having the same 5′ seed area, would compete with microRNA-320a in binding to target mRNAs, but may fail to form RISC with Ago due to a single nucleotide difference in the 3′ region. Nevertheless, the exact mechanism underlying the different functions of microRNA-320a and microRNA-320b remains to be explored.
Our comparison of microRNA microarrays between primary tumors of CRC with and without liver metastasis has identified microRNA-320b as a facilitator of colorectal cancer proliferation and invasion. Historically, metastasis-associated genes were often uncovered by comparing primary tumors to their matched metastases; and many of them were differentially regulated as a consequence of metastasis. In the past, only one study compared the microRNA expression profiles between primary colorectal adenocarcinomas with vs. without metastasis in three samples in each patient group [26]. As microRNA-320b was identified by screening the biomarkers that are up-regulated in 20 primary colorectal adenocarcinomas with liver metastasis compared to 27 primary colorectal adenocarcinomas without liver metastasis, microRNA-320b may more likely be a cause of metastasis. Therefore, further analysis of the mechanistic role of microRNA-320b in CRC development and metastasis is warranted. MicroRNA-320b may also become a target for colorectal cancer therapies.
Supplementary Material
Acknowledgments
We thank Jennifer Kleponis for her careful editing of the written language and acknowledge Dr. Hong Xutao of Zhejiang-California International Nanosystems Institute, for providing us useful advice in analyzing the microRNA microarray data. This study was supported by the Foundation of the National High Technology Research and Development Program of China (863 Program) (Grant No. 2012AA02A506). The Design of the study was approved by the Ministry of Science and Technology of the People's Republic of China.
Footnotes
Conflict of interest:The authors have declared no conflicts of interest.
This work has not been previously presented in any forms.
Appendix: Supplementary material: Supplementary data to this article can be found online at doi:10.1016/j.canlet.2014.10.014.
References
- 1.American Cancer Society. Cancer Facts & Figs 2013. American Cancer Society; Atlanta: 2013. [Google Scholar]
- 2.Ismaili N. Treatment of colorectal liver metastases. World J Surg Oncol. 2011;9:154. doi: 10.1186/1477-7819-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemeny EN. Treatment of metastatic colon cancer: “The times they are A-changing”. J Clin Oncol. 2013;31:1913–1916. doi: 10.1200/JCO.2013.49.4500. [DOI] [PubMed] [Google Scholar]
- 4.Meijer GA. What makes CRCs metastasise? Gut. 2010;59:1164–1165. doi: 10.1136/gut.2010.212241. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen XD, Bos DP, Massagué J. Metastasis from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 6.Schetter AJ, Okayama H, Harris CC. The role of microRNAs in colorectal cancer. Cancer J. 2012;18:244–252. doi: 10.1097/PPO.0b013e318258b78f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaedcke J, Grade M, Camps J, Sokilde R, Kaczkowski B, Schetter AJ, et al. The rectal cancer microRNAome – microRNA expression in rectal cancer and matched normal mucosa. Clin Cancer Res. 2012;18:4919–4930. doi: 10.1158/1078-0432.CCR-12-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottonham CL, Kaneko S, Xu L. miR-21 and miR-31 converge on TIAM1 to regulate migration and invasion of colon carcinoma cells. J Biol Chem. 2010;285:35293–35302. doi: 10.1074/jbc.M110.160069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, Carneiro F, et al. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene. 2012;31:2062–2074. doi: 10.1038/onc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu L, Chen H, Zhou D, Li D, Bai R, Zheng S, et al. MicroRNA-9 up-regulation is involved in colorectal cancer metastasis via promoting cell motility. Med Oncol. 2012;29:1037–1043. doi: 10.1007/s12032-011-9975-z. [DOI] [PubMed] [Google Scholar]
- 12.Chen ML, Liang LS, Wang XK. miR-200c inhibits invasion and migration in human colon cancer cells SW480/620 by targeting ZEB1. Clin Exp Metastasis. 2012;29:457–469. doi: 10.1007/s10585-012-9463-7. [DOI] [PubMed] [Google Scholar]
- 13.Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, Carneiro F, et al. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene. 2012;31:2062–2074. doi: 10.1038/onc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamakuchi M, Lotterman CD, Bao C, Hruban RH, Karim B, Mendell JT, et al. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad Sci U S A. 2010;107:6334–6339. doi: 10.1073/pnas.0911082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ, Lee KH, et al. MicroRNA-320 suppresses the stem cell-like characteristics of prostate cancer cells by downregulating the Wnt/beta-catenin signaling pathway. Carcinogenesis. 2013;34:530–538. doi: 10.1093/carcin/bgs371. [DOI] [PubMed] [Google Scholar]
- 16.Sun JY, Huang Y, Li JP, Zhang X, Wang L, Meng YL, et al. MicroRNA-320a suppresses human colon cancer cell proliferation by directly targeting beta-catenin. Biochem Biophys Res Commun. 2012;420:787–792. doi: 10.1016/j.bbrc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, He X, Liu Y, Ye Y, Zhang H, He P, et al. microRNA-320a inhibits tumor invasion by targeting neuropilin 1 and is associated with liver metastasis in colorectal cancer. Oncol Rep. 2012;27:685–694. doi: 10.3892/or.2011.1561. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H, Dong T, Zhou H, Wang L, Huang A, Feng B, et al. miR-320a suppresses colorectal cancer progression by targeting Rac1. Carcinogenesis. 2014;35:886–895. doi: 10.1093/carcin/bgt378. [DOI] [PubMed] [Google Scholar]
- 19.Haroutunian V, Katsel P, Dracheva S, Davis LK. The human homolog of the QKI gene affected in the severe dysmyelination “Quaking” mouse phenotype: downregulated in multiple brain region in schizophrenia. Am J Psychiatry. 2006;163:1834–1837. doi: 10.1176/ajp.2006.163.10.1834. [DOI] [PubMed] [Google Scholar]
- 20.Gu S, Kay MA. How do miRNAs mediate translational repression? Silence. 2010;1:11. doi: 10.1186/1758-907X-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 25.Jalvy-Delvaille S, Maurel M, Majo V, Pierre N, Chabas S, Combe C, et al. Molecular basis of differential target regulation by miR-96 and miR-182: the Glypican-3 as a model. Nucleic Acids Res. 2012;40:1356–1365. doi: 10.1093/nar/gkr843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin M, Chen W, Huang J, Gao H, Ye Y, Song Z, et al. MicroRNA expression profiles in human colorectal cancers with liver metastases. Oncol Rep. 2011;25:739–747. doi: 10.3892/or.2010.1112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


