Abstract
Background
During lower limb lengthening, poor bone regeneration is a devastating complication. Several local or systemic applications have been used to promote osteogenesis, and biologic stimulations are gaining attention, but their utility has not been proven in this setting.
Questions/purposes
In patients undergoing bilateral tibial lengthening, we compared those receiving an osteotomy site injection of autologous bone marrow aspirate concentrate (BMAC) plus platelet-rich plasma (PRP) with those not receiving such an injection in terms of external fixator index (time in external fixation divided by amount of lengthening), full weightbearing index (time until a patient was permitted to do full weightbearing divided by amount of lengthening), four cortical healing indexes (time until each cortical union divided by amount of lengthening), and callus shape and type.
Methods
Twenty-two patients (44 tibias) undergoing bilateral tibial lengthening enrolled in this randomized trial. Two patients were excluded, one due to insufficient radiographic evaluation and one who was lost to followup, leaving 20 patients (40 segments) for inclusion. Ten patients (20 segments) received BMAC combined with PRP injection (treatment group) and 10 patients (20 segments) received no injection (control group). All patients underwent stature lengthening for familial short stature with the lengthening over nail technique. Autologous BMAC combined with PRP was injected at the tibial osteotomy site at the end of the index surgery. Mean distraction rates were similar between groups (0.75 mm/day in the treatment group versus 0.72 mm/day in the control group; p = 0.24). Full weightbearing was permitted when we observed radiographic evidence of healing at two cortices; this assessment was made by the surgeon who was blinded to the treatment each patient received. Minimum followup was 24 months (mean, 28 months; range, 24–34 months).
Results
There was no difference in mean external fixator index between groups. However, mean cortical healing indexes (anterior/posterior/medial/lateral) were 1.14/0.81/0.96/0.88 months/cm in the treatment group and 1.47/1.26/1.42/1.22 months/cm in the control group (all p < 0.001), showing faster healing in the treatment group at each cortex. Full weightbearing was permitted earlier in the treatment group than in the control group (index: 0.99 months/cm and 1.38 months/cm, respectively, p < 0.001). Callus shape and type were not different between groups.
Conclusions
Autologous BMAC combined with PRP injection at the osteotomy site helped improve bone healing in distraction osteogenesis of the tibia, although the effect size was small.
Level of Evidence
Level I, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Distraction osteogenesis has been an important technique in limb lengthening and reconstructive surgery. The investigation of experimental and clinical research on the influence of the tension-stress effect by Ilizarov [21–24] has led to the recognition of the importance of stability, preservation of soft tissue and marrow, and the distraction rate/frequency during limb lengthening. Although he established biomechanical principles for successful bone formation, poor bone regeneration has been one of the most devastating complications in distraction osteogenesis [16, 29, 54, 58]. The enhancement of bone regeneration enables avoidance of critical complications such as nonunion or malunion and removal of the bulky external fixator earlier. It also allows a patient to bear weight and return to normal daily activities earlier.
Several factors that may influence bone formation have been described: (1) host factors, including age, limb segment, and systemic illness; (2) local factors, including poor soft tissue conditions and concomitant infection; and (3) surgeon factors, including poor soft tissue handling, poor selection of osteotomy, poor osteotomy technique, mechanically unstable frame, and improper rate and rhythm of distraction [46]. In addition, several methods for enhancement of bone formation in distraction osteogenesis have been reported, including physical stimulations and local or systemic applications [9, 16, 17, 28, 30, 33, 42]. Several physical stimulations have been considered effective in enhancing bone formation in distraction osteogenesis, such as the axial compression (weightbearing) method, the accordion maneuver (repeated overlengthening and then compression), and low-intensity pulsed ultrasound [20, 22, 40, 41, 50], which has been reported to be especially effective in acute fracture, nonunion, and distraction osteogenesis but still lacks controlled studies in distraction osteogenesis [16, 32, 50].
Recently, there has been a growing interest in biologic stimulations to enhance bone regenerate [6, 9, 28, 30, 33, 36, 43, 44, 60]. Local application of bone marrow cells, platelet-rich plasma (PRP), demineralized bone matrix, and BMP have been tried in experimental distraction osteogenesis models [9, 30, 33, 60]. Furthermore, systemic applications of parathyroid hormone, growth hormone, and bisphosphonate have been investigated [6, 36, 45, 46]. Among the locally applied substances, bone marrow cells are rich in mononucleated cells, which are reported to have high potentiality in bone healing [26, 45, 53]. Also, PRP has many osteoinductive growth factors that are released from platelets [7, 11, 39], although some controversy exists as to whether it really helps bone healing. However, there have been only very limited clinical reports on their local injection in distraction osteogenesis [30, 55].
We therefore performed a randomized controlled trial to evaluate the clinical efficacy of local injections of autologous bone marrow aspirate concentrate (BMAC) combined with autologous PRP at the osteotomy site during the lengthening surgery. Our hypothesis was that this treatment would enhance callus regeneration.
Patients and Methods
Patients
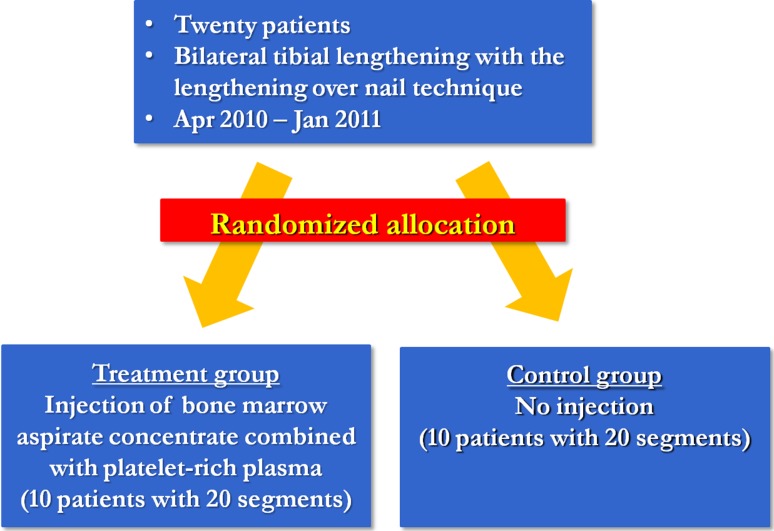
Twenty-two patients with bilateral tibial lengthening (44 segments) using the lengthening over nail technique from April 2010 to January 2011 under the same surgeon (DHL) enrolled in this study. Inclusion criteria were as follows: (1) skeletally mature patient, (2) no history of medical illness, fracture, soft tissue compromise, bony deformities, or infections of the lower extremity, (3) bilateral tibias with similar amount of lengthening, and (4) use of the lengthening over nail technique for both tibias. Two patients were excluded because one had insufficient radiographic evaluation and the other one was lost to followup, leaving 20 patients (40 segments) for evaluation. All patients were divided into two groups preoperatively by computer-generated randomization: the BMAC combined with PRP injection group (treatment, 10 patients with 20 segments) and the noninjection group (control, 10 patients with 20 segments) (Fig. 1). The randomization was performed by patient (each patient got the same treatment in both limbs). The random numbers were placed in sealed, opaque envelopes and opened one at a time and the group assignment took place in the clinic at the time of consent. There were no differences in demographics between the two groups (Table 1). Each patient agreed to participate in the study and had sufficient preoperative, postoperative, and followup clinical and radiographic evaluations. There was no crossover between the groups, so all patients were analyzed in the groups to which they were randomized. Minimum followup was 24 months (mean, 28 months; range, 24–34 months). This study was approved by the institutional review board of the author’s institution (BD2011-025D).
Fig. 1.
Twenty patients who underwent bilateral tibial lengthening were preoperatively divided into two groups by computer-generated randomization: the BMAC plus PRP injection group (treatment group) and the noninjection group (control group).
Table 1.
Demographic data of patients undergoing bilateral limb tibial lengthening
| Variable | Treatment group (injection) | Control group (noninjection) | p value |
|---|---|---|---|
| Number of patients | 10 | 10 | |
| Number of tibial segments | 20 | 20 | |
| Male:female (number of tibia) | 16:4 | 14:6 | 0.10 |
| Age (years)* | 20 (16–28) | 23 (18–30) | 0.12 |
| Preoperative height (cm)* | 158 (145–169) | 159 (149–168) | 0.72 |
| BMI (kg/cm2)* | 22 (18–26) | 20 (19–22) | 0.06 |
| Smoking history (yes:no) (number of tibia) | 8:12 | 13:7 | 0.20 |
| Followup (months)* | 27 (24–33) | 29 (25–34) | 0.45 |
* Values are expressed as mean, with range in parentheses.
Preparation of BMAC and PRP
At the end of the index surgery, the autologous BMAC and PRP were prepared for those patients who were randomized to receive it. Fifty millimeters of autologous bone marrow was aspirated from the right anterior superior iliac spine. The aspiration was performed at 10 different spots on and around the anterior superior iliac spine, punctured by a 11-gauge vertebroplasty needle to get pure bone marrow (5 mL aspirated at each spot), because the aspiration of more than 10 mL at a single spot may raise the risk of collecting surrounding peripheral blood. A 60-mL syringe already containing 10 mL anticoagulant citrate dextrose A was used. It is stable in the anticoagulated state for 8 hours. After completion of the aspirations, it was inserted into a GPS® tube (Biomet, Inc, Warsaw, IN, USA) for centrifugation. The centrifugation started with no time delay after the aspiration and continued for 15 minutes at 3200 rpm on a Clinispin Horizon 755VES centrifuge (Woodley Laboratory Diagnostics, Lancashire, UK). In a similar way, 55 mL peripheral blood was sampled from a patient’s arm with a similar syringe containing 5 mL anticoagulant citrate dextrose A and then centrifuged under the same conditions.
Handling and Application of BMAC and PRP
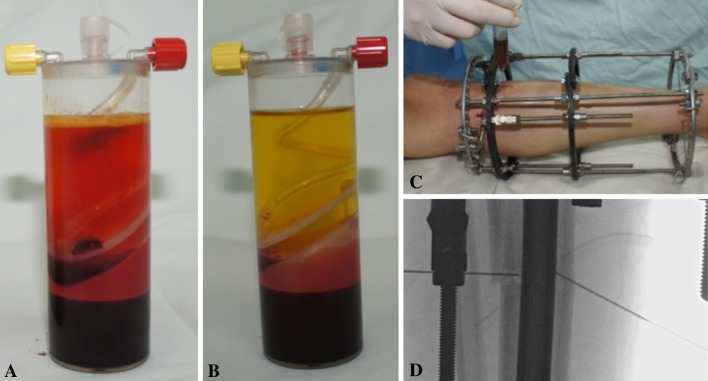
After the 15 minutes’ centrifugation, 6 mL was aspirated from the middle layer of each GPS® tube and inserted at the tibial osteotomy site (3 mL BMAC and 3 mL PRP for each tibial segment). The combined injection of BMAC and PRP was conducted using a 22-gauge spinal needle after confirmation of the exact needle placement by C-arm. The injection was performed after closure of both periosteum and skin of the osteotomy site and slowly as possible to prevent possible washout by injection pressure (Fig. 2). The osteotomy gap made using a multiple drill hole technique was wide enough to allow the insertion of a 22-gauge needle for injection.
Fig. 2A–D.
Combined injection of BMAC and PRP at the osteotomy site enhanced bone regeneration in distraction osteogenesis. (A) BMAC is shown in the GPS® tube after centrifugation. (B) PRP is shown in the GPS® tube after centrifugation. (C) Three milliliters of the aspirate sampled from the middle layer in each tube was injected at the osteotomy site using a 22-gauge spinal needle. (D) The exact placement of the spinal needles was confirmed by the C-arm.
Followup and Radiographic Evaluations
After surgery, every patient had a 7- to 9-day latent period and then entered the distraction period at a target distraction rate of 1 mm/day. However, the actual distraction rate was determined by case-dependent adjustment (going slower or faster than the target rate, as ascertained by the surgeon based on clinical and radiographic followup evaluations) and the mean actual distraction rate was not different between groups (0.75 mm/day [range, 0.55–1.13 mm/day] in the treatment group versus 0.72 mm/day [range, 0.52–1.20 mm/day] in the control group; p = 0.24) (Table 2). The mean desired total length gain was not different between groups, nor was the mean achieved total length gain (58 mm in the treatment group versus 66 mm in the control group; p = 0.08). Insertion of distal interlocking screws and removal of the external fixator followed after the desired length and acceptable limb alignment were achieved. Every patient performed physical exercises as tolerated under the supervision of a physical therapist (JHP). The patients were allowed full weightbearing any time while the external fixator was applied or after the external fixator was removed when radiographic evidence of two cortical healings was observed; this assessment was made by the surgeon who was blinded to the treatment each patient received. Patients were followed up every 2 weeks during the distraction phase and every 1 month thereafter until the end of the consolidation phase.
Table 2.
Radiographic comparison between the two groups
| Variable | Treatment group (injection) | Control group (noninjection) | p value |
|---|---|---|---|
| Latent period (days)* | 7.4 (7–8) | 7.6 (7–9) | 0.45 |
| Distraction rate (mm/day)* | 0.75 (0.55–1.13) | 0.72 (0.52–1.20) | 0.24 |
| Final length gain (mm)* | 58 (43–70) | 66 (46–73) | 0.08 |
| External fixator index (months/cm)* | 0.53 (0.37–0.63) | 0.49 (0.43–0.53) | 0.12 |
| Intramedullary nail diameter (cm)* | 9.2 (8–10) | 8.9 (8–10) | 0.30 |
| Intramedullary nail length (cm)* | 290 (270–300) | 290 (285–300) | 0.14 |
| Mean cortical healing index (months/cm) | |||
| Anterior cortex | 1.14 | 1.47 | < 0.01 |
| Posterior cortex | 0.81 | 1.26 | < 0.01 |
| Medial cortex | 0.96 | 1.42 | < 0.01 |
| Lateral cortex | 0.88 | 1.22 | < 0.01 |
| Mean full weightbearing index (months/cm) | 0.89 | 1.38 | < 0.01 |
* Values are expressed as mean, with range in parentheses.
Radiographic evaluations included final length achieved, the external fixator index (the time in external fixation divided by the amount of lengthening), cortical healing index per each cortex (the time until cortical union divided by the amount of lengthening), and full weightbearing index (the time until a patient was permitted to do full weightbearing divided by the amount of lengthening). Radiographic callus shape and type were also classified according to the system of Li et al. [34], where callus shape is classified as fusiform, cylindrical, concave, lateral, or central and callus type as normal, intermediate, or low density. These radiographic parameters were assessed and compared by evaluators who were blinded to the treatment each patient received.
Statistics and Sample Size Calculation
All radiographic parameters were tested for normality using the Shapiro-Wilk test and none violated normal distribution assumption. At the beginning of the study, we performed an a prior power analysis for the external fixator index and found that a minimum sample size of 20 limbs was required to achieve a statistical significance of 0.05 with 80% power and an effect size of 0.8, meaning it could detect a 80% of difference between treatment and control groups. We had 20 legs in each group, which is equal to the minimum sample size. Student’s t-test was performed to find any significant differences between groups. For comparison of discrete variables, such as callus shape and type and number of complications, a chi-square test was used. A p value of less than 0.05 was considered statistically significant. The statistical software R (Version 2.12; The R Project for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
Results
The mean external fixator index was not different between groups (0.53 months/cm [range, 0.37–0.63 months/cm] in the treatment group and 0.49 months/cm [range, 0.43–0.53 months/cm] in the control group; p = 0.12).
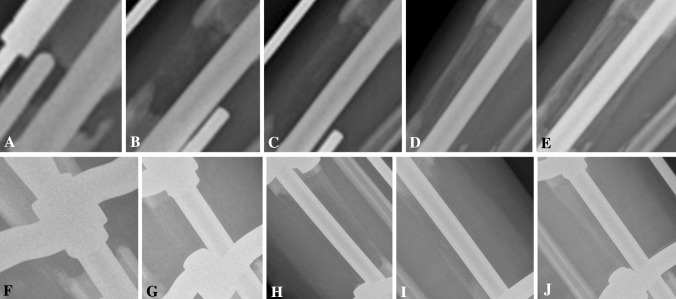
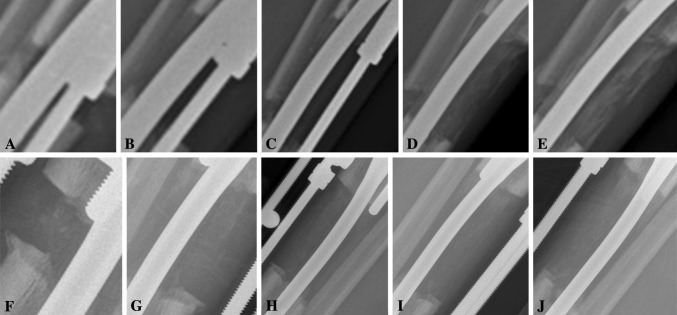
However, there was faster cortical consolidation in the treatment group than in the control group. The mean cortical healing indexes (anterior/posterior/medial/lateral) were 1.14/0.81/0.96/0.88 months/cm in the treatment group and 1.47/1.26/1.42/1.22 months/cm in the control group (all p < 0.001) (Table 2). There was more callus regeneration in the treatment group than in the control group, as seen on serial AP (Fig. 3) and lateral (Fig. 4) plain radiographs. The mean full weightbearing index was 0.99 months/cm in the treatment group and 1.38 months/cm in the control group, suggesting earlier full weightbearing was achieved in the treatment group (p < 0.001) (Table 2).
Fig. 3A–J.
Serial AP plain radiographs show a tibia undergoing lengthening in (A–E) the treatment group and (F–J) the control group. Radiographs are from (A, F) 1 month to (E, J) 5 months postoperatively with 1-month increments between each radiograph. More callus regeneration is seen in the treatment group.
Fig. 4A–J.
Serial lateral plain radiographs show a tibia undergoing lengthening in (A–E) the treatment group and (F–J) the control group. Radiographs are from (A, F) 1 month to (E, J) 5 months postoperatively with 1-month increments between each radiograph. More callus regeneration is seen in the treatment group.
The callus shape according to the classification of Li et al. [34] was either fusiform or cylindrical. The other shapes, such as concave, lateral, or central, were not found. The callus type was either normal or intermediate density. There was no low-density callus type. There were no differences in callus shape and type between groups (Table 3).
Table 3.
Comparison of callus shape and type according to Li et al. [34] between the two groups
| Classification | Number of tibias | p value | |
|---|---|---|---|
| Treatment group (injection) | Control group (noninjection) | ||
| Callus shape | 0.66 | ||
| Fusiform | 2 | 4 | |
| Cylindrical | 18 | 16 | |
| Concave | 0 | 0 | |
| Lateral | 0 | 0 | |
| Central | 0 | 0 | |
| Callus type | 0.66 | ||
| Normal density | 16 | 18 | |
| Intermediate density | 4 | 2 | |
| Low density | 0 | 0 | |
Complications included one impending compartment syndrome in each group, which recovered completely with emergent fasciotomy. Superficial infections occurred in nine patients (45%) in the treatment group and six patients (30%) in the control group (p = 0.51), which were treated with intravenous or oral antibiotics. There were no deep infections, regenerate fractures, or nonunions in either group. No injection-related adverse events were found, such as injection site pain or bone marrow aspiration site infection (Table 4).
Table 4.
Comparison of complications between groups
| Complication | Number of tibias | p value | |
|---|---|---|---|
| Treatment group (injection) | Control group (noninjection) | ||
| Impending compartment syndrome | 1 | 1 | |
| Deep infection | 0 | 0 | |
| Superficial infection | 9 | 6 | 0.51 |
| Peroneal nerve irritation | 0 | 0 | |
| Fibula related | 0 | 0 | |
| Fracture of regenerate | 0 | 0 | |
| Hardware problem | 0 | 0 | |
| Nonunion | 0 | 0 | |
| Injection related | 0 | ||
Discussion
Among a number of investigations to enhance bone formation in distraction osteogenesis, biologic stimulations have been gaining attention recently. Among these trials, the applications of autologous bone marrow cells and PRP have several potential advantages in stimulating bone regenerate in distraction osteogenesis. First, bone marrow cells have enriched mononuclear cells. The differentiation of osteoblasts from bone marrow cells is well described and standardized [26]. The PRP is rich in various growth factors released from platelets, which have been reported to increase vascular ingrowth and mitogenic effects on bone-forming cells [4, 11, 28, 57]. Second, because of the autogenous properties, their application can avoid possible complications, such as immunogenic reactions and disease transmissions, which can come from allogenic or synthetic materials. Various experimental researches have shown considerable evidence of the efficacy of the use of autologous mononuclear cells from bone marrow in combination with various scaffolds in promoting bone formation in the area of bone defects [8, 10, 11, 20, 26, 37]; however, there have been no studies evaluating these treatments in humans undergoing distraction osteogenesis. We found no difference in the external fixation index, but some benefits in terms of cortical consolidation that permitted earlier weightbearing in patients treated with BMAC plus PRP compared to patients who were not thus treated.
Our study has several limitations. First, the study consisted of a relatively small number of patients. A larger trial is necessary, both to confirm efficacy and also importantly to get a more representative look at safety. Second, we performed injection of BMAC and PRP together during surgery. This protocol does not tell us which one played a more important role in enhancing bone healing or whether they had a synergistic or antagonistic effect. Third, the number of cells in BMAC and PRP were not counted. PRP has been known to have different potentiality according to the quantity of platelets, leukocytes, or fibrinogen concentrations [14, 39]. Fourth, the group assignment took place in the clinic at the time of consent and the surgeons knew the treatment before the surgery started. This may have caused them to inadvertently perform the surgery differently. Fifth, the lack of difference in the rate of superficial infection may be due to the small sample size of each group. A larger-sized study may produce a different result. Sixth, there were differences in patient demographics, age, and amount of lengthening. Although not statistically different, these factors can influence the results, especially if the cohort is small. Seventh, we followed all patients every 1 month during the consolidation period. Therefore, the indexes we evaluated are susceptible to error compared to the precision that could have been achieved if more frequent followup had been possible.
There have been limited reports on the effect of bone marrow cells in distraction osteogenesis [30, 45, 55]. In 2007, Richards et al. [47] observed marrow-derived progenitor cell injections enhanced new bone formation in a femoral distraction model of rat. In 2007, Kitoh et al. [30, 31] showed transplantation of culture-expanded bone marrow cells combined with PRP accelerated new bone formation during the distraction osteogenesis, especially in the femur, but with many disadvantages [47]. As for PRP, limited reports could be found [13, 28, 30, 33]. Latalski et al. [33] compared a PRP injection group (nine segments) with a control group (10 segments) retrospectively and reported a shorter healing index in the PRP group. Kawasumi et al. [28] observed a high platelet concentration combined with osteoblastic cells in PRP could accelerate new bone formation during distraction osteogenesis in a rat model. However, the role of PRP in bone regeneration is still a controversial issue and its mechanism is not fully understood yet [1–3, 5, 13, 15, 18, 27, 38, 48].
We found that some parameters related to bone healing, including the cortical healing index and the full weightbearing index were improved using BMAC plus PRP injections, while others, such as the external fixator index and the shape and type of callus, were not improved with treatment. However, our study raises several questions that need to be discussed and investigated. First, what is the best time for injection? There is no clear evidence to guide us in terms of the timing of injection to achieve better bone regeneration. Several immunohistochemical analyses have shown high expression of growth factors receptors during the distraction phase [19, 25, 28, 52, 56, 59]. In addition, Siwicka et al. [52] showed rapid decline of the expression of the receptors for growth factors shortly after the termination of distraction. This result suggests that the administration of growth factors during the distraction phase could be more effective in bone healing. Richards et al. [45] concluded that the timing of osteoprogenitor cell injection appeared to have no effect on experimental new bone formation. In the current study, we transplanted just once into the osteotomy site as the last step of the surgery and the results support that early administration of BMAC combined with PRP can promote osteogenesis effectively during the distraction phase. However, a comparative study that evaluated injection timing would be important to perform. Another question that calls for future work is: Can the enhanced bone healing promoted by injection of both BMAC and PRP speed up the bone distraction rate? We do not recommend increasing the rate of distraction because lengthening is not a matter of bone alone. Although a faster distraction can make a faster removal of the external fixator, it results in greater burden to the surrounding muscles, consequently leaving more fibrosis or degeneration [12, 35, 49, 51].
Injection-related complications can occur, and deep infection, if it occurs, can be devastating. It can result from contamination of the transplanted cells or from the instruments such as aspiration syringe, GPS® tube, injection needles, or during centrifugation. In our series, there were no deep infections. To our knowledge, there has been no case of deep infection among the reports using BMAC or PRP [33, 45]. However, we do discuss this possibility with our patients because it can occur and it is severe [26, 53]. Other potential drawbacks of using BMAC and PRP include additional cost, additional incision on the iliac crest, increased operating time, and possibility of leakage around the osteotomy site.
In conclusion, the combined injection of BMAC and PRP during surgery seems to enhance some parameters related to bone regeneration in human tibial distraction osteogenesis, although the effect size was small. However, further investigations are needed to evaluate whether BMAC or PRP contributes more to bone formation (or if the effect is synergistic), the optimal timing of injections, the effect of repeated injections, the potential for complications including infections, and the ideal concentrations and cell counts for these kinds of injections.
Acknowledgments
We thank Dr. Hae Ryong Song for his valuable comments on human tibial lengthening, Jung Ho Park, our physical therapist, for his enthusiastic and cooperative work for our patients.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that the informed consent for participation in the study was obtained.
References
- 1.Aghaloo TL, Moy PK, Freymiller EG. Investigation of platelet rich plasma in rabbit cranial defects: a pilot study. J Oral Maxillofac Surg. 2002;60:1176–1181. doi: 10.1053/joms.2002.34994. [DOI] [PubMed] [Google Scholar]
- 2.Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–535. [PubMed] [Google Scholar]
- 3.Anitua E, Andia I, Ardanza B. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 4.Arora NS, Ramanayake T, Ren YF, Romanos GE. Platelet-rich plasma in sinus augmentation procedures: a systematic literature review. Part II. Implant Dent. 2010;19:145–157. doi: 10.1097/ID.0b013e3181cd706d. [DOI] [PubMed] [Google Scholar]
- 5.Arpornmaeklong P, Kochel M, Depprich R, Kubler NR, Wurzler KK. Influence of platelet rich plasma (PRP) on osteogenic differentiation of rat bone marrow stromal cells: an in vitro study. Int J Oral Maxillofac Surg. 2004;33:60–70. doi: 10.1054/ijom.2003.0492. [DOI] [PubMed] [Google Scholar]
- 6.Bail H, Raschke MJ, Kolbeck SF, Krummrey G, Windhagen HJ, Weiler A, Raun K, Mosekilde LI, Haas NP. Recombinant species-specific growth hormone increases hard callus formation in distraction osteogenesis. Bone. 2002;30:117–124. doi: 10.1016/S8756-3282(01)00628-7. [DOI] [PubMed] [Google Scholar]
- 7.Batista MA, Leivas TP, Rodrigues CJ, Arenas GC, Belitardo DR, Guarniero R. Comparison between the effects of platelet-rich plasma and bone marrow concentrate on defect consolidation in the rabbit tibia. Clinics. 2011;66:1787–1792. doi: 10.1590/S1807-59322011000100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker S, Maissen O, Ponomarev I, Stoll T, Rahn B, Wilke I. Osteopromotion by a beta-tricalcium phosphate/bone marrow hybrid implant for use in spine surgery. Spine (Phila Pa 1976). 2006;31:11–17. [DOI] [PubMed]
- 9.Burkhart KJ, Rommens PM. Intramedullary application of bone morphogenetic protein in the management of a major bone defect after an Ilizarov procedure. J Bone Joint Surg Br. 2008;90:806–809. doi: 10.1302/0301-620X.90B6.20147. [DOI] [PubMed] [Google Scholar]
- 10.Cancedda R, Mastrogiacomo M, Bianchi G, Derubeis A, Muraglia A, Quarto R. Bone marrow stromal cells and their use in regenerating bone. Novartis Found Symp. 2003;249:133–143. doi: 10.1002/0470867973.ch10. [DOI] [PubMed] [Google Scholar]
- 11.Dallari D, Fini M, Stagni C, Torricelli P, Nicoli Aldini N, Giavaresi G. In vitro study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. J Orthop Res. 2006;24:877–888. doi: 10.1002/jor.20112. [DOI] [PubMed] [Google Scholar]
- 12.De Deyne PG, Hayatsu K, Meyer R, Paley D, Herzenberg JE. Muscle regeneration and fiber-type transformation during distraction osteogenesis. J Orthop Res. 1999;17:560–570. doi: 10.1002/jor.1100170415. [DOI] [PubMed] [Google Scholar]
- 13.Fang TD, Salim A, Xia W. Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res. 2005;20:1114–1124. doi: 10.1359/JBMR.050301. [DOI] [PubMed] [Google Scholar]
- 14.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi A, Doumas C, O’Connor JP. The effects of local platelet rich plasma delivery on diabetic fracture healing. Bone. 2006;38:540–546. doi: 10.1016/j.bone.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Gebauer D, Correl J. Pulsed low-intensity ultrasound: a new salvage procedure for delayed unions and nonunions after leg lengthening in children. J Pediatr Orthop. 2005;25:750–754. doi: 10.1097/01.bpo.0000173245.12184.7e. [DOI] [PubMed] [Google Scholar]
- 17.Greenwald JA, Luchs JS, Mehrara BJ, Spector JA, McCarthy JG, Longaker MT. Pumping the regenerate: an evaluation of oscillating distraction osteogenesis in the rodent mandible. Ann Plast Surg. 2000;44:516–521. doi: 10.1097/00000637-200044050-00010. [DOI] [PubMed] [Google Scholar]
- 18.Gruber R, Karreth F, Fischer MB, Watzek G. Platelet released supernatants stimulate formation of osteoclast-like cells through a prostaglandin/RANKL dependent mechanism. Bone. 2002;30:726–732. doi: 10.1016/S8756-3282(02)00697-X. [DOI] [PubMed] [Google Scholar]
- 19.Haque T, Amako M, Nakada S, Lauzier D, Hamdy RC. An immunohistochemical analysis of the temporal and spatial expression of growth factors FGF 1, 2 and 18, IGF 1 and 2, and TGFbeta1 during distraction osteogenesis. Histol Histopathol. 2007;22:119–128. doi: 10.14670/HH-22.119. [DOI] [PubMed] [Google Scholar]
- 20.Hisatome T, Yasunaga Y, Yanada S, Tabata Y, Ikada Y, Ochi M. Neovascularization and bone regeneration by implantation of autologous bone marrow mononuclear cells. Biomaterials. 2005;26:4550–4556. doi: 10.1016/j.biomaterials.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 21.Ilizarov GA. The tension-stress effect on the genesis and growth of tissue. Part I. The influence of stability of fixation and soft-tissue prevention. Clin Orthop Relat Res. 1989;238:249–281. [PubMed] [Google Scholar]
- 22.Ilizarov GA. The tension-stress effect on the genesis and growth of tissue. Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;239:263–285. [PubMed] [Google Scholar]
- 23.Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res. 1990;250:8–26. [PubMed] [Google Scholar]
- 24.Ilizarov GA. The principles of the Ilizarov method. 1988. Bull Hosp Jt Dis. 1997;56:49–53. [PubMed] [Google Scholar]
- 25.Jacobsen KA, Al-Aql ZS, Wan C. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res. 2008;23:596–609. doi: 10.1359/jbmr.080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jager M, Jelinek EM, Wess KM, Scharfstadt A, Jacobson M, Kevy SV, Krauspe R. Bone marrow concentrate: a novel strategy for bone defect treatment. Curr Stem Cell Res Ther. 2009;4:34–43. doi: 10.2174/157488809787169039. [DOI] [PubMed] [Google Scholar]
- 27.Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Platelet-rich plasma enhances human osteoblast-like cell proliferation and differentiation. J Oral Maxillofac Surg. 2005;63:362–369. doi: 10.1016/j.joms.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Kawasumi M, Kitoh H, Siwicka KA, Ishiguro N. The effect of the platelet concentration in platelet-rich plasma gel on the regeneration of bone. J Bone Joint Surg Br. 2008;90:966–972. doi: 10.1302/0301-620X.90B7.20235. [DOI] [PubMed] [Google Scholar]
- 29.Kenawey M, Krettek C, Liodakis E, Meller R, Hankemeier S. Insufficient bone regenerate after intramedullary femoral lengthening. Clin Orthop Relat Res. 2011;469:264–273. doi: 10.1007/s11999-010-1332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma. J Pediatr Orthop. 2007;27:629–634. doi: 10.1097/BPO.0b013e318093f523. [DOI] [PubMed] [Google Scholar]
- 31.Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the lone bone. Bone. 2007;40:522–528. doi: 10.1016/j.bone.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound: a multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am. 1997;79:961–973. doi: 10.2106/00004623-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Latalski M, Elbatrawy YA, Thabet AM, Gregosiewicz A, Raganowicz T, Fatyga M. Enhancing bone healing during distraction osteogenesis with platelet-rich plasma. Injury. 2011;42:821–824. doi: 10.1016/j.injury.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Li R, Saleh M, Yang L, Couton L. Radiographic classification of osteogenesis during bone distraction. J Orthop Res. 2006;24:339–347. doi: 10.1002/jor.20026. [DOI] [PubMed] [Google Scholar]
- 35.Lindsey CA, Makarov MR, Shoemaker S, Birch JG, Buschang PH, Cherkashin AM, Welch RD, Samchukov ML. The effect of the amount of limb lengthening on skeletal muscle. Clin Orthop Relat Res. 2002;402:278–287. doi: 10.1097/00003086-200209000-00028. [DOI] [PubMed] [Google Scholar]
- 36.Little DG, Smith NC, Williams PR, Briody JN, Bilston LE, Smith EJ, Gardiner EM, Cowell CT. Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J Bone Miner Res. 2003;18:1300–1307. doi: 10.1359/jbmr.2003.18.7.1300. [DOI] [PubMed] [Google Scholar]
- 37.Malard O, Guicheux J, Bouler JM. Calcium phosphate scaffold and bone marrow for bone reconstruction in irradiated area: a dog study. Bone. 2005;36:323–330. doi: 10.1016/j.bone.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Mehta S, Watson T. Platelet rich concentrate: basic science and current clinical applications. J Orthop Trauma. 2008;225:433–438. doi: 10.1097/BOT.0b013e31817e793f. [DOI] [PubMed] [Google Scholar]
- 40.Mizuta H, Nakamura E, Kudo S, Maeda T, Takagi K. Greater frequency of distraction accelerates bone formation in open-wedge proximal tibial osteotomy with hemicallotasis. Acta Orthop Scand. 2004;75:588–593. doi: 10.1080/00016470410001475. [DOI] [PubMed] [Google Scholar]
- 41.Mofid MM, Inoue N, Atabey A, Marti G, Chao E, Manson PN, Vander Kolk CA. Callus stimulation in distraction osteogenesis. Plast Reconstr Surg. 2002;109:1621–1629. doi: 10.1097/00006534-200204150-00020. [DOI] [PubMed] [Google Scholar]
- 42.Popkov D, Popkov A, Haumont T, Journeau P, Lascombes P. Flexible intramedullary nail use in limb lengthening. J Pediatr Orthop. 2010;30:910–918. doi: 10.1097/BPO.0b013e3181f0eaf9. [DOI] [PubMed] [Google Scholar]
- 43.Ramune A, Thomsen JS, Eckardt H, Bundgaard KG, Lind M, Hvid I. Parathyroid hormone PTH (1–34) increases the volume, mineral content, and mechanical properties of regenerated mineralizing tissue after distraction osteogenesis in rabbits. Acta Orthop. 2009;80:716–723. doi: 10.3109/17453670903350032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raschke MJ, Bail H, Windhagen HJ, Kolbeck SF, Weiler A, Raun K, Kappelgard A, Skiaerbaek C, Haas NP. Recombinant growth hormone accelerates bone regenerate consolidation in distraction osteogenesis. Bone. 1999;24:81–88. doi: 10.1016/S8756-3282(98)00158-6. [DOI] [PubMed] [Google Scholar]
- 45.Richards M, Huibregtse BA, Caplan AT, Goulet JA, Goldstein SA. Marrow-derived progenitor cell injections enhance new bone formation during distraction. J Orthop Res. 1999;17:900–908. doi: 10.1002/jor.1100170615. [DOI] [PubMed] [Google Scholar]
- 46.Sabharwal S. Enhancement of bone formation during distraction osteogenesis: pediatric applications. J Am Acad Orthop Surg. 2011;19:101–111. doi: 10.5435/00124635-201102000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Sauerbier S, Rickert D, Gutwald R, Nagursky H, Oshima T. Bone marrow concentrate and bovine bone mineral for sinus floor augmentation: a controlled, randomized, single-blinded clinical and histological trial-per-protocol analysis. Tissue Eng Part A. 2001;17:2187–2197. doi: 10.1089/ten.tea.2010.0516. [DOI] [PubMed] [Google Scholar]
- 48.Shanaman R, Filstein MR, Danesh-Meyer MJ. Localized ridge augmentation using GBR and platelet-rich plasma: case reports. Int J Periodontics Restorative Dent. 2001;21:345–355. [PubMed] [Google Scholar]
- 49.Shen XC, Aronson J. Changes in biomechanical properties of muscle following tibial lengthening in rat. Trans Orthop Res Soc. 1993;18:379. [Google Scholar]
- 50.Shimazaki A, Inui K, Azuma Y, Nishimura N, Yamano Y. Low-intensity pulsed ultrasound accelerates bone maturation in distraction osteogenesis in rabbit. J Bone Joint Surg Br. 200;7:1077–1082. [DOI] [PubMed]
- 51.Simpson AH, Williams PE, Kyberd P, Goldspink G, Kenwright J. The response of muscle to leg lengthening. J Bone Joint Surg Br. 1995;77:630–636. [PubMed] [Google Scholar]
- 52.Siwicka KA, Kitoh H, Kawasumi M, Ishiguro N. Spatial and temporal distribution of growth factors receptors in the callus: implications for improvement of distraction osteogenesis. Nagoya J Med Sci. 2011;73:117–127. [PMC free article] [PubMed] [Google Scholar]
- 53.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 54.Sun XT, Easwar TR, Stephen M, Kim SJ, Song HR. Comparative study of callus progression in limb lengthening with or without intramedullary nail with reference to the pixel value ratio and the Ru Li’s classification. Arch Orthop Trauma Surg. 2011;131:1333–1340. doi: 10.1007/s00402-011-1302-9. [DOI] [PubMed] [Google Scholar]
- 55.Takamine Y, Tsuchiya H, Kitakoji T. Distraction osteogenesis enhanced by osteoblast-like cells and collagen gel. Clin Orthop Relat Res. 2002;399:240–246. doi: 10.1097/00003086-200206000-00029. [DOI] [PubMed] [Google Scholar]
- 56.Tavakoli K, Yu Y, Shahidi S, Bonar F, Walsh WR, Poole MD. Expression of growth factors in the mandibular distraction zone: a sheep study. Br J Plast Surg. 1999;52:434–439. doi: 10.1054/bjps.1999.3157. [DOI] [PubMed] [Google Scholar]
- 57.Thor A, Wannfors K, Sennerby L, Rasmusson L. Reconstruction of the severely resorbed maxilla with autogenous bone, platelet rich plasma and implant: 1-year results of a controlled prospective 5-year study. Clin Implant Dent Relat Res. 2005;7:209–220. doi: 10.1111/j.1708-8208.2005.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 58.Wang K, Edwards E. Intramedullary skeletal kinetic distractor in the treatment of leg length discrepancy: a review of 16 cases and analysis of complications. J Orthop Trauma. 2013;26:138–144. doi: 10.1097/BOT.0b013e318238b5b1. [DOI] [PubMed] [Google Scholar]
- 59.Weiss S, Zimmermann G, Baumgart R, Kasten P, Bidlingmaier M, Henle P. Systemic regulation of angiogenesis and matrix degradation in bone regeneration—distraction osteogenesis compared to rigid fracture healing. Bone. 2005;37:781–790. doi: 10.1016/j.bone.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 60.Yoshitaka E, Wakitani S, Naka Y, Nakamura H, Takaoka K. An injectable composite material containing bone morphogenetic protein-2 shortens the period of distraction osteogenesis in vivo. J Orthop Res. 2011;29:452–456. doi: 10.1002/jor.21225. [DOI] [PubMed] [Google Scholar]