Abstract
Background
Patients with congenital limb shortening can present with joint instability, soft tissue contractures, and significant leg length discrepancy. Classically, lengthening is done with external fixation, which can result in scarring, pin site infection, loss of motion, and pain. We therefore developed an alternative to this approach, a new, controllable, internal lengthening device for patients with congenital limb shortening.
Questions/purposes
We evaluated this device in terms of (1) healing index, (2) complications, (3) accuracy of the device’s external controller, and (4) adjacent-joint ROM.
Methods
Between January 2012 and May 2013, we treated 66 patients for congenital limb shortening, of whom 21 were treated using this device. During this period, general indications for using the device were patients with leg length discrepancies of 2 cm or more, with intramedullary canals able to withstand rods of at least 12.5-mm diameter and 230-mm length, without active infection in the affected bone, able to comply with the need for frequent lengthening, and without metal allergies or an implanted pacemaker. We included only those patients who had completed their course of treatment and were currently fully weightbearing, leaving 18 patients (21 bone segments) available for followup at a minimum of 6 months after limb lengthening (mean, 14 months; range, 6–22 months). Mean age was 19 years (range, 9–49 years). Sixteen femurs and five tibias were lengthened a mean of 4.4 cm (range, 2.1–6.5 cm). Mean distraction index was 1.0 mm/day (range, 0.5–1.8 mm/day). Healing index, complications, device accuracy, and ROM were recorded. To date, 10 of the 21 devices have been removed. This was typically done 12–24 months after insertion when the bone was solidly healed on all four cortices.
Results
Mean healing index was 0.91 months/cm (range, 0.2–2.0 months/cm). There were seven complications requiring an additional unplanned surgery, including one hip flexion contracture, three femurs with delayed healing, one tibia with delayed healing, one hip subluxation/dislocation, and one knee subluxation. The external controller was accurate as programmed and actual lengthening amounts were consistent. ROMs of the hip, knee, and ankle were essentially maintained.
Conclusions
This device is completely internal, allowing for satisfactory joint motion during treatment in most patients. Lengthening was achieved in an accurate, controlled manner, and all patients reached their goal length. Complications remain a concern, as is the case with all approaches to this complex patient population. Both future comparative studies and longer-term followup are needed.
Level of Evidence
Level IV, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Congenital femoral deficiency and fibular hemimelia are rare and complex congenital disorders of the lower limb, with an incidence of approximately one in 50,000 live births for congenital femoral deficiency [12, 19, 30] and between 7.4 to 20 per million live births [6, 11, 33] for fibular hemimelia. Congenital femoral deficiency and fibular hemimelia are found to be associated in the same limb in about 68% of cases [32]. Both can present with a broad spectrum of severity, from mild cases to severe manifestations such as acetabular dysplasia, femoral shortening, genu valgus, anteromedial bowing of the tibia, absent ACL, ball-and-socket ankle, tarsal coalition, and absence of lateral toes [4, 38]. Lengthening in congenitally short limbs is challenging, characterized by a high number of surgical procedures and associated with many complications, such as residual leg length discrepancy (LLD), delayed union, poor regenerate formation, bone preconsolidation, ankle and knee stiffness, refractures, joint subluxation/dislocation, and residual sagittal and coronal deformity [1, 3, 5, 7, 8, 15, 23, 26]. Additionally, the rates are as high as 96% for superficial pin site infection and 20% for deep infection [2, 13].
The use of intramedullary lengthening nails has gained popularity, as they reduce common complications associated with external fixators, including infection, joint stiffness, bone regenerate deformity, scarring, late fracture, and patient implant acceptance [10, 17, 25, 27]. For the past 10 years, the only FDA-approved intramedullary lengthening device has been the Intramedullary Skeletal Kinetic Distractor (ISKD) (Orthofix Inc, Lewisville, TX, USA). However, intramedullary lengthening is associated with complications, including premature or delayed consolidation with difficulty in controlling the rate of distraction, implant breakage, and mechanical failure [9, 16, 20–22, 35].
The PRECICE® nail (Ellipse Technologies, Inc, Irvine, CA, USA), which is FDA and Conformité Européenne approved, incorporates magnet technology with a hand-held external remote controller allowing for noninvasive, controlled lengthening. The nail is also reversible if shortening is desired. Physiotherapy can continue throughout treatment to maintain joint ROM without concern of an uncontrolled runaway nail. The lengthening occurs in an axial direction with no need of rotational forces to distract the rod, reducing shear/torsional forces on the regenerate bone. However, to our knowledge, there are no reports on the accuracy or safety of this device in patients with congenital limb shortening.
We therefore evaluated this new internal lengthening device in terms of (1) healing index, (2) complications, (3) accuracy of the device’s external controller, and (4) adjacent-joint ROM.
Patients and Methods
Between January 2012 and May 2013, we conducted an institutional review board-approved, nonrandomized, prospective study. During this time, we treated 66 patients for congenital limb shortening, of whom 21 (32%) were treated using the PRECICE® internal lengthening device. Two patients were excluded due to their diagnosis of clubfoot rather than congenital femoral deficiency or fibular hemimelia, and one was excluded because she was not fully weightbearing at the conclusion of the study. During this period, general indications for using the new device were patients with an LLD of 2 cm or more, patients with an intramedullary canal able to withstand a rod size of at least 12.5-mm diameter and 230-mm length, patients without active infection in the affected bone, patients able to comply with the need for frequent lengthening, and patients without metal allergies or an implanted pacemaker. Of the 21 treated with the device, 18 patients (21 bone segments; 100% of those treated for congenital limb shortening who met the other inclusion criteria) were available for followup at a minimum of 6 months (mean, 14 months; range, 6–22 months) after lengthening. Only patients with a preoperative diagnosis of congenital femoral deficiency, fibular hemimelia, or both and only those who completed both the lengthening and consolidation phases of treatment were included. Ten female and eight male patients with a mean age of 19 years (range, 9–49 years) were included in the study (Table 1).
Table 1.
Demographic data
| Patient | Sex | Age (years) | Diagnosis | Bone | AIM index | Prior operations | Followup (months) | Length obtained (cm) | Healing index (months/cm) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 49 | CFD + FH | Femur | 4 | FL | 22 | 3.6 | 3.5 |
| 2 | Female | 15 | FH | Femur | 4 | AR, FL (2), TL (2) | 19 | 4.5 | 1.5 |
| 3 | Female | 27 | CFD | Femur | 4 | FL, TL | 18 | 5.3 | 4.4 |
| 4A | Female | 14 | CFD + FH | Femur | 4 | HR, KR, AR, TL | 20 | 3.7 | 3.0 |
| 4B | Tibia | 20 | 3.0 | 3.7 | |||||
| 5 | Male | 15 | CFD | Femur | 4 | HR, FL (2) | 17 | 5.5 | 4.7 |
| 6 | Female | 16 | CFD | Femur | 4 | HR, FL | 16 | 6.5 | 1.9 |
| 7 | Female | 10 | CFD + FH | Femur | 4 | HR, KR, FL | 16 | 6.1 | 4.5 |
| 8 | Male | 15 | CFD + FH | Tibia | 3 | FL, TL (2) | 17 | 4.3 | 1.5 |
| 9 | Female | 14 | CFD | Femur | 4 | HR, KR. AR, FL (3), TL (2) | 15 | 4.7 | 6.0 |
| 10 | Male | 16 | CFD + FH | Femur | 2 | None | 16 | 4.8 | 1.4 |
| 11 | Female | 20 | CFD + FH | Femur | 2 | None | 13 | 4.5 | 3.8 |
| 12A | Male | 23 | CFD + FH | Femur | 2 | None | 14 | 4.2 | 0.93 |
| 12B | Tibia | 14 | 3.5 | 5.2 | |||||
| 13 | Male | 9 | CFD + FH | Femur | 3 | FL, Dega | 13 | 6.1 | 1.9 |
| 14 | Male | 14 | CFD + FH | Femur | 2 | None | 10 | 6.0 | 1.8 |
| 15A | Female | 15 | CFD + FH | Tibia | 3 | None | 9 | 2.1 | 2.6 |
| 15B | Femur | 9 | 2.9 | 1.9 | |||||
| 16 | Female | 28 | CFD + FH | Femur | 2 | None | 6 | 5.0 | 0.82 |
| 17 | Male | 19 | CFD + FH | Femur | 2 | None | 6 | 3.9 | 0.9 |
| 18 | Female | 18 | CFD | Femur | 2 | None | 7 | 2.6 | 2.3 |
| Mean (range) | 19 (9–49) | 14 (6–22) | 4.4 (2.1–6.5) | 0.91 (0.2–2.0) |
CFD = congenital femoral deficiency; FH = fibular hemimelia; FL = femoral lengthening; AR = ankle reconstruction; TL = tibia lengthening; HR = hip reconstruction (including Dega acetabuloplasty); KR = knee reconstruction; Dega = Dega acetabuloplasty.
To quantify the severity of the limb deformity of each patient, we used the Limb Lengthening and Reconstruction Society AIM index [24] before the lengthening procedure. All patients were classified as minimal complexity at the moment of the lengthening procedure. We should note that nine of the 18 patients underwent complex hip, knee, and ankle reconstruction to provide joint stability before lengthening surgery and as such would have scored higher on the AIM index had these preliminary procedures not been performed. Preparatory surgery consisted of a Dega osteotomy or hip stabilization to prevent hip subluxation, while surgery to stabilize the ankle prevented equinovalgus deformity. This preparatory surgery was typically performed before the age of 4 years. Three patients underwent simultaneous lengthening of both the femur and tibia. Sixteen femurs and five tibias were lengthened a mean of 4.4 cm (range, 2.1–6.5 cm). The device itself has a maximum stroke of 6.5 cm. The patient who required a 2.1-cm lengthening had a total LLD of 5.0 cm, divided between the femur and tibia. Because the patient required a simultaneous correction of genu valgum in the tibia, a 2.1-cm lengthening in the femur and 2.9 cm lengthening in the tibia were performed. Nineteen bone segments had a 10.7-mm-diameter rod, while two had a 12.5-mm-diameter rod. The intramedullary bone was reamed between 1.5 and 2 mm over the diameter of the rod. Intraoperative acute lengthening of 1 to 3 mm was performed to ensure that the device was working properly. Prophylactic soft tissue releases of the iliotibial band and injection of Botulinum Toxin A (10–15 U/kg) in the quadriceps muscle were standard in all femoral implantations to avoid joint contractures and subluxation. The maximal dose for the muscle belly was 200 U. In patients with concurrent distal femoral knee valgus correction, the peroneal nerve was prophylactically decompressed. In the tibia, patients received gastrocsoleus release and prophylactic anterior fasciotomy to prevent equinus contractures and compartment syndrome. Postoperative deep vein thrombosis prophylaxis was most commonly performed in patients 16 years and older. This typically consisted of a subcutaneous 40-mg dose of enoxaparin sodium once a day or a 325-mg dose of aspirin once a day for 2 to 4 weeks.
Patients began lengthening 5 to 7 days after surgery, at a mean rate of 0.75 mm/day in the tibia and 1.0 mm in the femur. Patients and their caregivers were trained to use the external remote controller, which was placed on the leg several times throughout the day and used powerful magnets to lengthen the rod. Patients were provided with a log to document daily frequency and amount of lengthening. This was then compared to actual lengthening obtained at their weekly or biweekly followup visits. Bone regeneration and ROM were assessed and used to determine whether the lengthening rate was increased, decreased, or left the same. Lengthening was complete in a mean of 53 days (range, 22–91 days). The mean distraction index was 1.0 mm/day (range, 0.5–1.8 mm/day).
Physical therapy was administered five times a week for a minimum of 1 hour/day. The goals of physical therapy were to increase or maintain joint ROM and muscle strength. Minimal patient requirements included hip abduction of 20°, full knee extension, knee flexion of 45°, and dorsiflexion of the ankle to neutral. Patients were instructed to weightbear 30 to 50 pounds (14–23 kg) until good bone consolidation was observed. For femoral lengthening, patients were asked to wear a dynamic custom knee device at night and 2 hours during the day. Patients undergoing tibial lengthening were asked to wear an ankle brace at night and 2 hours during the day. All patients were instructed to take between 1200 and 1500 mg calcium/day and 1500 to 3000 IU vitamin D3/day to promote bone healing. If knee extension became restricted, a customized knee device was used to provide constant resistance to the knee at night and several times during the day. Once full healing was noted on three of four cortices of bone, patients were allowed to fully weightbear without assistive devices.
All patients achieved their planned lengthening (Fig. 1). In three patients, additional future lengthenings are planned. We removed the device in 10 patients (48%). This was typically done at a minimum of 12 months after insertion with four intact cortices of bone.
Fig. 1A–C.
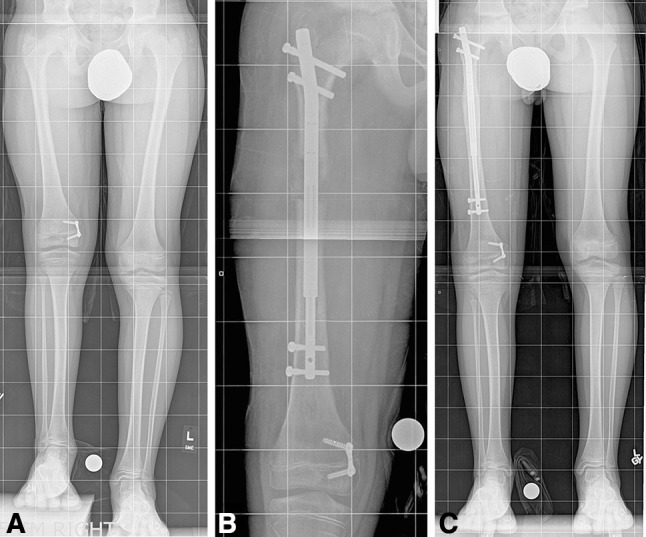
Images illustrate the case of a 9-year-old boy with both congenital femoral deficiency and fibular hemimelia. This young patient underwent prior Dega osteotomy, femoral lengthening, and valgus deformity correction with a tension band plate. (A) A preoperative image shows an LLD of 5.5 cm. (B) Lengthening 3 months after surgery is shown. (C) Consolidation 9 months after initial surgery is shown. The length achieved was 6.1 cm. He will likely need further lengthening in the future. (All images printed with permission of Sinai Hospital of Baltimore).
Distraction and healing indexes, complications, and device accuracy were recorded by research staff during biweekly distraction and monthly consolidation clinic visits. We defined healing index as time divided by lengthening amount achieved until healing on three of four cortices was noted. We defined distraction index as lengthening amount divided by time until the goal length was achieved. Preoperative, postlengthening, and recent hip, knee, and ankle ROM data were obtained for all patients by the attending surgeon with a goniometer.
Statistical Methods
SPSS® software (Version 18.0; SPSS Inc, Chicago, IL, USA) was used to detect differences between preoperative and postoperative measurements. A p value of less than 0.05 was considered significant.
Results
Healing Index
The mean healing index was 0.91 months/cm (range, 0.2–2.0 months/cm) (Table 1). With the numbers available, there was no difference in healing times between femurs and tibias (0.81 months/cm versus 1.1 months/cm, respectively; p = 0.54) (Fig. 2). When we took into account prior lengthening, femurs that had never had a prior lengthening healed faster, at a mean rate of 0.56 months/cm, than those that had a tibia or prior femur lengthening, at a mean rate of 1.1 months/cm (p = 0.01).
Fig. 2A–B.
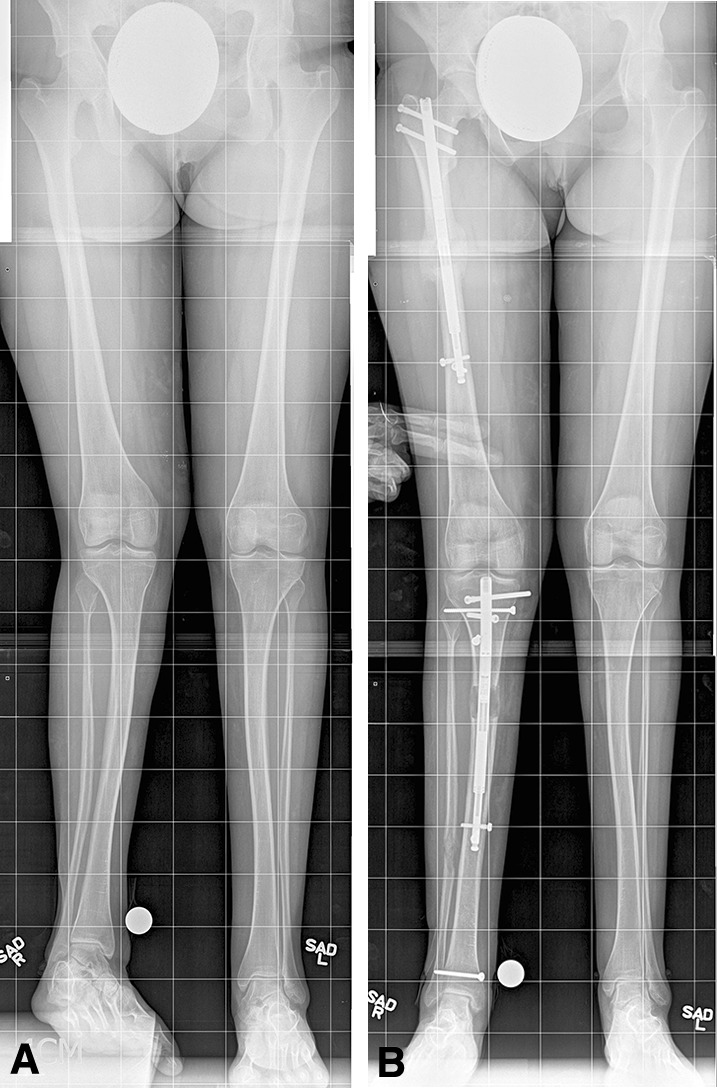
Images illustrate the case of a 15-year-old girl with both congenital femoral deficiency and fibular hemimelia. (A) A preoperative image shows an LLD of 5 cm. (B) A postoperative radiograph of the femur/tibia shows abundant healing in the femur with slower healing in the tibia. The length achieved was 5 cm. (Both images printed with permission of Sinai Hospital of Baltimore).
Complications
Seven of 21 limbs required additional surgery, including two patients with joint subluxation (Table 2). The first patient had hip subluxation and was treated with joint reduction and stabilization with an external fixator and the second patient had knee subluxation and was treated with soft tissue release and ligament reconstruction (Fig. 3). Three patients had delayed healing of the femur, while one experienced delayed healing in the tibia. All patients underwent bone grafting and are now fully healed and weightbearing. One patient developed a hip flexion contracture during lengthening, which resolved after iliotibial band and hip flexor release. Additionally, two patients were rehospitalized for swelling in the calf, one occurring 1 week after rod insertion and the other immediately after rod removal. Ultrasound showed no deep vein thrombosis in either patient, and the swelling and pain resolved.
Table 2.
Complications
| Patient | Complication | Treatment |
|---|---|---|
| 1 | Calf swelling (after rod removal) | Readmission for calf swelling; no DVT or infection found |
| 5 | (A) Hip subluxation/dislocation | Joint reduction and stabilization with external fixation |
| (B) Delayed femoral healing (anterior cortex) | RIA autogenous bone graft | |
| 6 | Knee rotatory subluxation | Soft tissue release with ligament reconstruction |
| 7 | Delayed femoral healing (medial cortex) | Removal 2 proximal screws and bone stem cell injection |
| 9 | Delayed femoral healing (lateral cortex) | Bone graft with bone stem cell injection |
| 12 | (A) Calf swelling | Readmission for calf swelling; no DVT of infection found |
| (B) Delayed tibial healing | Autologous bone graft (proximal tibia) | |
| 15 | Hip flexion contracture | ITB and hip flexor release |
RIA = reamer irrigator aspirator; DVT = deep vein thrombosis; ITB = iliotibial band.
Fig. 3A–C.
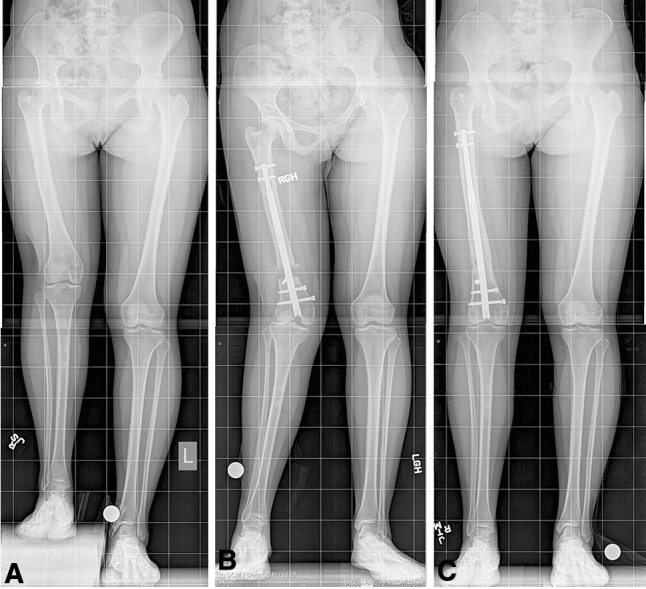
Images illustrate the case of a 16-year-old girl with congenital femoral deficiency. This patient previously underwent hip reconstruction and femoral lengthening with external fixation. (A) A preoperative radiograph shows an LLD of 6.5 cm. (B) The development of rotary knee subluxation was noted 1 month postsurgery. (C) After soft tissue release and ligament reconstruction, the patient was able to obtain 6.5 cm of length without further complication. (All images printed with permission of Sinai Hospital of Baltimore).
External Remote Controller Accuracy
Patient distraction logs were compared to radiographs and were consistent in all patients. When comparing weekly or biweekly radiographs to what was programmed into the controller, no more than 2 mm of discrepancy was noted in any case, which could be due to measurement error or magnification issues. There were no mechanical failures of the device.
ROM
The mean preoperative hip flexion was 114° (range, 90°–135°), which changed to 102° (range, 60°–135°) immediately after the distraction and 114° (range, 95°–135°) at most recent followup (Table 3). The mean preoperative hip extension was 1° (range, −10° to 15°), which changed to −2° (range, −30° to 25°) after the distraction and 5° (range, −20° to 25°) at most recent followup.
Table 3.
ROM
| Patient | ROM (pre/post) (°) | |||||
|---|---|---|---|---|---|---|
| Hip flexion | Hip extension | Knee flexion | Knee extension | Ankle dorsiflexion | Ankle plantarflexion | |
| 1 | 110/– | 0/– | 125/130 | −5/0 | 10/20 | 20/20 |
| 2 | 130/95 | 0/10 | 130/140 | 0/0 | 5/2 | 0/0 |
| 3 | 110/100 | 15/10 | 130/142 | 0/−5 | 0/5 | 45/35 |
| 4A | 110/135 | 0/25 | 130/115 | 0/0 | 5/−10 | 5/15 |
| 4B | 110/110 | 0/25 | 115/130 | 0/0 | 10/10 | 5/60 |
| 5 | 130/110 | 0/0 | 160/115 | 0/0 | 25/20 | 5/−10 |
| 6 | 130/120 | 0/0 | 120/120 | 0/0 | 0/10 | 15/30 |
| 7 | 120/110 | 0/0 | 150/125 | 0/0 | 10/10 | 15/55 |
| 8 | 110/110 | 0/0 | 140/125 | 0/0 | 15/10 | 35/55 |
| 9 | 90/110 | 0/0 | 100/135 | 0/0 | 35/10 | 15/20 |
| 10 | 90/95 | −10/−20 | 100/95 | 5/0 | 15/10 | 50/70 |
| 11 | 135/115 | 15/20 | 140/120 | 0/0 | 20/20 | 30/35 |
| 12A | 120/135 | 0/25 | 140/112 | 0/5 | 15/16 | 5/60 |
| 12B | 120/125 | 0/0 | 115/130 | 0/0 | 15/10 | 45/40 |
| 13 | 110/110 | 0/0 | 135/120 | 0/0 | 10/10 | 30/20 |
| 14 | 110/110 | 0/15 | 115/120 | 0/0 | 10/15 | 20/50 |
| 15A | 110/110 | 0/−3 | 115/135 | 0/0 | 10/7 | 20/30 |
| 15B | 110/110 | 0/−3 | 115/135 | 0/0 | 10/7 | 20/30 |
| 16 | 110/130 | 0/0 | 120/115 | 0/0 | 10/15 | 20/20 |
| 17 | 110/120 | 0/0 | 115/140 | 0/0 | 10/10 | 35/20 |
| 18 | 120/120 | 0/0 | 120/130 | 0/0 | 15/15 | 20/30 |
| Mean (range) | Pre: 114 | Pre: 1 | Pre: 125 | Pre: 0 | Pre: 13 | Pre: 22 |
| (90–135) | (−10 to 15) | (100–160) | (−5 to 5) | (0–35) | (0–50) | |
| Post: 114 | Post: 5° | Post: 125 | Post: 0 | Post: 13 | Post: 31 | |
| (95–135) | (−20 to 25) | (95–142) | (−5 to 5) | (−10 to 20) | (−10 to 70) | |
| (p = 0.94) | (p = 0.08) | (p = 0.99) | (p = 0.99) | (p = 0.99) | (p = 0.05) | |
Pre = preoperative; post = postoperative (most recent followup).
The mean preoperative knee flexion was 125° (range, 100°–160°), which was changed to 105° (range, 60°–140°) after the distraction and 125° (range, 95°–142°) at most recent followup (Table 3). The mean preoperative knee extension was 0° (range, −5° to 5°), which changed to −1° (range, −10° to 0°) after the distraction and 0° (range, −5° to 5°) at the most recent clinic visit.
The mean preoperative ankle dorsiflexion was 13° (range, 0°–35°), which changed to 7° (range, −10° to 20°) after the distraction and 13° (range, −10° to 20°) at most recent followup (Table 3). The mean preoperative plantarflexion was 22° (range, 0°–50°), which changed to 21° (range, −10° to 60°) after the distraction and 31° (range, −10° to 70°) at the most recent clinic visit (p = 0.05).
Pre- and postoperative (most recent followup) hip, knee, and ankle ROM values were not significantly different, with the exception of plantarflexion, which improved slightly at the most recent clinical visit (Table 3).
Discussion
Limb lengthening in patients with congenital limb deficiency has been a challenge for surgeons; most studies to date have used external fixation for this purpose, which is associated with many complications such as pin tract infection, poor regenerate formation, preconsolidation, pain and refractures [3, 7, 8, 15, 23, 26, 39]. To our knowledge, the safety and effectiveness of using an intramedullary lengthening device in patients with congenital shortening have not been previously studied. We therefore developed and tested a new internal device for this indication. In the present report, in a small series of 18 patients (21 limbs) at a mean followup of 14 months (range, 6–22 months), we found reliable healing, a low frequency of complications, consistent lengthening to the desired amount, and maintenance of adjacent-joint ROM.
One limitation of our study is the length of followup (mean, 14 months). This length does not permit us to see whether deformity recurs, a finding described in Radler et al. [31]. It also does not allow us to identify late complications related to the hardware itself. Another limitation of this study is that the surgeon authors (JEH, SCS) both were involved in the design of the nail and its instrumentation. Because of this, these results will need to be replicated by others to determine whether they generalize well to others. It is very important to recognize the complications associated with patients with congenital malformations. Daily physiotherapy and frequent followup with good AP radiographs of the joints are required for success. The cost of the PRECICE® nail is approximately double the cost of a six-axis deformity correction external fixator. The minimal scarring with this nail does negate the need for subsequent scarplasty, which is sometimes needed after treatment with the external fixator. Additionally, we recommend against trochanteric entry for patients younger than 7 years for the reason that growth arrest could lead to coxa valga. We recommend against lengthening in the tibia for anyone with an open growth plate. Femoral retroversion, common in patients with congenital femoral deficiency, can be addressed with hemiepiphysiodesis in the skeletally immature or by retrograde femoral fixator-assisted nailing in patients who are skeletally mature. As this was a case series, it is impossible to determine whether the frequency of complication was greater or less than lengthening with conventional methods such as external fixation.
The mean lengthening for our patients was 4.4 cm. When we examined the healing rate, we found that bones with prior lengthening healed slower than native bones, which is comparable to other results in the literature [14]. This is especially important when discussing mean healing times with patients in an attempt to ensure reasonable postoperative healing expectations. The mean healing index in our study was 0.91 months/cm, which is better than other results in the literature. Kenawey et al. [20] reported a mean healing index of 1.2 months/cm using the ISKD and Catagni et al. [7] reported a mean healing index of 1.5 months/cm using the Ilizarov.
One common complication in this group was joint subluxation of the knee or hip due to underlying joint dysplasia. Current recommendations advocate protecting the hip with preparatory femoral/pelvic osteotomies, which maintain hip extension and abduction during lengthening. The knee is protected from subluxation by extending the fixation to the tibia with hinges to stabilize the joint [3, 28, 29]. This was thought to prevent stiffness of the joint by permitting knee motion. However, the knee has been known to subluxate despite this, as shown by Aston et al. [3] who reported knee subluxation in nine of 27 patients in their series. Lengthening with an internal rod does not afford a hinge across the knee and cannot protect the knee during the distraction phase, theoretically putting the joint at risk of subluxation. Therefore, to help avoid knee and hip subluxation in our patients and maintain mobility, we required daily physiotherapy and frequent followup with good AP radiographs of the regenerate bone. If clinical signs of joint contracture or subluxation appeared, the lengthening rate was slowed, stopped, or reversed until the issue resolved. Prophylactic static or dynamic bracing was employed for patients with significant joint instability. In this study, two of 18 patients developed joint subluxation. One presented with rotatory subluxation of the knee and was treated with soft tissue release and ligament reconstruction. This was the only case in which prophylactic soft tissue release was not performed. The second developed hip subluxation, which was stabilized and reduced with external fixation. We found that it was best to initiate early and frequent physical therapy and to react aggressively to any warning sign of decreased ROM or radiographic evidence of joint subluxation.
When comparing the rates of complications among methods of lengthening using intramedullary nails, the lengthening over nail method, which was developed to reduce fixator wearing time and the likelihood of fracture of the regenerate bone, allows the fixator to extend across the knee with hinges and allows patients to regain ROM more quickly as the external fixator is removed after the lengthening is complete [22, 34]. However, this method does not eliminate the complications associated with external fixation and has a relatively high risk of deep infection [37]. The other method that has been previously used is the ISKD, which is the only intramedullary device for lengthening that has been in general use in United States. This method is associated with several complications, such as premature or delayed consolidation with difficulties in controlling the distraction rate rhythm, implant breakage, and mechanical failure [9, 20, 22, 35, 36]. Mahboubian et al. [22] reported complications requiring additional surgeries such as exchange nailing or removal of the ISKD in six of 12 patients. Schiedel et al. [35] reported seven failures requiring premature removal of the device and secondary implant failure (blockage, break) in up to 36%; almost ½ of the difficulties were implant related. In our study, there were no implant-related complications. Seven of 21 (33%) bone segments required additional surgery for a complication, consistent with other studies in the literature [3, 7, 15, 22, 23, 26, 35]; future comparative trials are needed to determine which approach will be associated with the fewest complications.
The external remote controller demonstrated excellent accuracy, with patient logs and radiographs detailing a consistent rate of lengthening. When comparing the accuracy of other methods of lengthening, we found that other intramedullary devices presented with more difficulty in controlling the rate of distraction, resulting in premature or delayed consolidation [16, 20–22, 35]. Kenawey et al. [20] reported an overall incidence of complications of 33%, of which the most important was insufficient bone regenerate (21%) and nine runaway nails. One patient had accidental acute lengthening of 3 cm during manipulation under anesthesia to achieve only 3 to 4 mm. Simpson et al. [36] reported difficulty in achieving length in eight femurs (24%) and uncontrolled lengthening in seven (21%).
The most important advantage of the intramedullary lengthening device is improving ROM. This improved ROM during the distraction process prevents muscle contractures, joint stiffness, and most importantly joint subluxation or dislocation [27]. Studies demonstrate that there is a rapid return of knee flexion on removal of the external fixator [18, 28]. In our study, the ROM at latest followup was not different from the ROM obtained before the index procedure.
In conclusion, the PRECICE® device provides accurate and controlled lengthening, making it a potential option for patients with congenital shortening. Complications remain a concern, as is the case with all approaches to this complex patient population; both future comparative studies and longer-term followup are needed. It is critical to remember to follow all of the recognized tenets in this population, including preoperative stabilizing of the hip and knee joints and vigorous physical therapy and splinting after surgery.
Acknowledgments
The authors thank Kristina Kotze BS for her help with data collection and entry.
Footnotes
The institution of the authors has received, during the study period, funding from Ellipse Technologies, Inc (Irvine, CA, USA), the company who makes the telescopic intramedullary rods used in study patients. The institution of the authors also receives funding for our annual fundraiser (Save-A-Limb ride) from Stryker Orthopaedics (Mahwah, NJ, USA), Medtronic (Minneapolis, MN, USA), Integra LifeSciences Corp (Plainsboro, NJ, USA), Medevations LLC (Bel Air, MD, USA), Supreme Orthopedic Systems, Inc (Marriottsville, MD, USA), Biomet, Inc (Warsaw, IN, USA), Metro Prosthetics Inc (Landover Hills, MD, USA), and OHK Medical Devices Ltd (Grandville, MI, USA) and for our annual course (Baltimore Limb Deformity Course) from Smith & Nephew, Inc (Memphis, TN, USA), BrainLab Inc (Westchester, IL, USA), Orthofix Inc (Lewisville, TX, USA), Synthes, Inc (West Chester, PA, USA), Stryker Orthopaedics, Wright Medical Technology, Inc (Arlington, TN, USA), Biomet, Inc, and The MHE Coalition.
One of the authors (SCS) certifies that he, or a member of his immediate family, has received or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000, from Ellipse Technologies, Inc.
One of the authors (JEH) certifies that he, or a member of his immediate family, has received or may receive payments or benefits, during the study period, an amount of les than USD 10,000, from Ellipse Technologies, Inc.
The remaining authors certify that they, or a member of their immediate family, have no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangement, etc) that might post a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Alman BA, Krajbich JI, Hubbard S. Proximal femoral focal deficiency: results of rotationplasty and Syme amputation. J Bone Joint Surg Am. 1995;77:1876–1882. doi: 10.2106/00004623-199512000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Antoci V, Ono CM, Antoci V, Jr, Raney EM. Pin-tract infection during limb lengthening using external fixation. Am J Orthop (Belle Mead NJ). 2008;37:E150–E154. [PubMed] [Google Scholar]
- 3.Aston WJ, Calder PR, Baker D, Hartley J, Hill RA. Lengthening of the congenital short femur using the Ilizarov technique: a single-surgeon series. J Bone Joint Surg Br. 2009;91:962–967. doi: 10.1302/0301-620X.91B7.21304. [DOI] [PubMed] [Google Scholar]
- 4.Birch JG, Lincoln TL, Mack PW, Birch CM. Congenital fibular deficiency: a review of thirty years’ experience at one institution and a proposed classification system based on clinical deformity. J Bone Joint Surg Am. 2011;93:1144–1151. doi: 10.2106/JBJS.J.00683. [DOI] [PubMed] [Google Scholar]
- 5.Birch JG, Walsh SJ, Small JM, Morton A, Koch KD, Smith C, Cummings D, Buchanan R. Syme amputation for the treatment of fibular deficiency: an evaluation of long-term physical and psychological functional status. J Bone Joint Surg Am. 1999;81:1511–1518. doi: 10.2106/00004623-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Boakes JL, Stevens PM, Moseley RF. Treatment of genu valgus deformity in congenital absence of the fibula. J Pediatr Orthop. 1991;11:721–724. doi: 10.1097/01241398-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Catagni MA, Radwan M, Lovisetti L, Guerreschi F, Elmoghazy NA. Limb lengthening and deformity correction by the Ilizarov technique in Type III fibular hemimelia: an alternative to amputation. Clin Orthop Relat Res. 2011;469:1175–1180. doi: 10.1007/s11999-010-1635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Changulani M, Ali F, Mulgrew E, Day JB, Zenios M. Outcome of limb lengthening in fibular hemimelia and a functional foot. J Child Orthop. 2010;4:519–524. doi: 10.1007/s11832-010-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole JD, Justin D, Kasparis T, DeVlught D, Knobloch C. The Intramedullary Skeletal Kinetic Distractor (ISKD): first clinical results of a new intramedullary nail for lengthening of the femur and tibia. Injury. 2001;32(suppl 4):SD129–SD139. [DOI] [PubMed]
- 10.Dahl MT, Gulli B, Berg T. Complications of limb lengthening: a learning curve. Clin Orthop Relat Res. 1994;301:10–18. [PubMed] [Google Scholar]
- 11.Froster UG, Baird PA. Congenital defects of lower limbs and associated malformations: a population based study. Am J Med Genet. 1993;45:60–64. doi: 10.1002/ajmg.1320450116. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie R, Torode IP. Classification and management of congenital abnormalities of the femur. J Bone Joint Surg Br. 1983;65:557–568. doi: 10.1302/0301-620X.65B5.6643558. [DOI] [PubMed] [Google Scholar]
- 13.Green SA. Complications of external skeletal fixation. Clin Orthop Relat Res. 1983;180:109–116. [PubMed] [Google Scholar]
- 14.Griffith SI, McCarthy JJ, Davidson RS. Comparison of the complication rates between first and second (repeated) lengthening in the same limb segment. J Pediatr Orthop. 2006;26:534–536. doi: 10.1097/01.bpo.0000226275.70706.d5. [DOI] [PubMed] [Google Scholar]
- 15.Grill F, Dungl P. Lengthening for congenital short femur: results of different methods. J Bone Joint Surg Br. 1991;73:439–447. doi: 10.1302/0301-620X.73B3.1670446. [DOI] [PubMed] [Google Scholar]
- 16.Guichet JM, Deromedis B, Donnan LT, Peretti G, Lascombes P, Bado F. Gradual femoral lengthening with the Albizzia intramedullary nail. J Bone Joint Surg Am. 2003;85:838–848. doi: 10.2106/00004623-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Guidera KJ, Hess WF, Highhouse KP, Ogden JA. Extremity lengthening: results and complications with the Orthofix system. J Pediatr Orthop. 1991;11:90–94. doi: 10.1097/01241398-199101000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Herzenberg JE, Scheufele LL, Paley D, Bechtel R, Tepper S. Knee range of motion in isolated femoral lengthening. Clin Orthop Relat Res. 1994;301:49–54. [PubMed] [Google Scholar]
- 19.Kalamchi A, Cowell HR, Kim KI. Congenital deficiency of the femur. J Pediatr Orthop. 1985;5:129–134. doi: 10.1097/01241398-198505020-00001. [DOI] [PubMed] [Google Scholar]
- 20.Kenawey M, Krettek C, Liodakis E, Meller R, Hankemeier S. Insufficient bone regenerate after intramedullary femoral lengthening: risk factors and classification system. Clin Orthop Relat Res. 2011;469:264–273. doi: 10.1007/s11999-010-1332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieg AH, Lenze U, Speth BM, Hasler CC. Intramedullary leg lengthening with a motorized nail. Acta Orthop. 2011;82:344–350. doi: 10.3109/17453674.2011.584209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahboubian S, Seah M, Fragomen AT, Rozbruch SR. Femoral lengthening with lengthening over a nail has fewer complications than intramedullary skeletal kinetic distraction. Clin Orthop Relat Res. 2012;470:1221–1231. doi: 10.1007/s11999-011-2204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy JJ, Glancy GL, Chang FM, Eilert RE. Fibular hemimelia: comparison of outcome measurements after amputation and lengthening. J Bone Joint Surg Am. 2000;82:1732–1735. doi: 10.2106/00004623-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy JJ, Iobst CA, Rozbruch SR, Sabharwal S, Eismann EA. Limb Lengthening and Reconstruction Society AIM index reliably assesses lower limb deformity. Clin Orthop Relat Res. 2013;471:621–627. doi: 10.1007/s11999-012-2609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noonan KJ, Leyes M, Forriol F, Cañadell J. Distraction osteogenesis of the lower extremity with use of monolateral external fixation: a study of two hundred and sixty-one femora and tibiae. J Bone Joint Surg Am. 1998;80:793–806. doi: 10.2106/00004623-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Oberc A, Sułko J. Fibular hemimelia—diagnostic management, principles, and results of treatment. J Pediatr Orthop B. 2013;22:450–456. doi: 10.1097/BPB.0b013e32836330dd. [DOI] [PubMed] [Google Scholar]
- 27.Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990;250:81–104. [PubMed] [Google Scholar]
- 28.Paley D, Herzenberg JE, Paremain G, Bhave A. Femoral lengthening over an intramedullary nail: a matched-case comparison with Ilizarov femoral lengthening. J Bone Joint Surg Am. 1997;79:1464–1480. doi: 10.2106/00004623-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Paley D, Standard SC. Lengthening reconstruction surgery for congenital femoral deficiency. In: Rozbruch SR, Ilizarov S, eds. Limb Lengthening and Reconstruction Surgery. New York, NY: Informa Healthcare USA, Inc; 2007:393–428.
- 30.Pappas AM. Congenital abnormalities of the femur and related lower extremity malformations: classification and treatment. J Pediatr Orthop. 1983;3:45–60. doi: 10.1097/01241398-198302000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Radler C, Antonietti G, Ganger R, Grill F. Recurrence of axial malalignment after surgical correction in congenital femoral deficiency and fibular hemimelia. Int Orthop. 2011;35:1683–1688. doi: 10.1007/s00264-011-1266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Ramirez A, Thacker MM, Becerra LC, Riddle EC, Mackenzie WG. Limb length discrepancy and congenital limb anomalies in fibular hemimelia. J Pediatr Orthop B. 2010;19:436–440. doi: 10.1097/BPB.0b013e32832d5d7d. [DOI] [PubMed] [Google Scholar]
- 33.Rogala EJ, Wynne-Davies R, Littlejohn A, Gormley J. Congenital limb anomalies: frequency and aetiological factors: data from the Edinburgh Register of the Newborn (1964–68) J Med Genet. 1974;11:221–233. doi: 10.1136/jmg.11.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rozbruch SR, Kleinman D, Fragomen AT, Ilizarov S. Limb lengthening and then insertion of an intramedullary nail: a case-matched comparison. Clin Orthop Relat Res. 2008;466:2923–2932. doi: 10.1007/s11999-008-0509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiedel FM, Pip S, Wacker S, Pöpping J, Tretow H, Leidinger B, Rödl R. Intramedullary limb lengthening with the Intramedullary Skeletal Kinetic Distractor in the lower limb. J Bone Joint Surg Br. 2011;93:788–792. doi: 10.1302/0301-620X.93B6.25581. [DOI] [PubMed] [Google Scholar]
- 36.Simpson AH, Shalaby H, Keenan G. Femoral lengthening with the Intramedullary Skeletal Kinetic Distractor. J Bone Joint Surg Br. 2009;91:955–961. doi: 10.1302/0301-620X.91B7.21466. [DOI] [PubMed] [Google Scholar]
- 37.Song HR, Oh CW, Mattoo R, Park BC, Kim SJ, Park IH, Jeon IH, Ihn JC. Femoral lengthening over an intramedullary nail using the external fixator: risk of infection and knee problems in 22 patients with a follow-up of 2 years or more. Acta Orthop. 2005;76:245–252. doi: 10.1080/00016470510030652. [DOI] [PubMed] [Google Scholar]
- 38.Stevens PM, Arms D. Postaxial hypoplasia of the lower extremity. J Pediatr Orthop. 2000;20:166–172. [PubMed] [Google Scholar]
- 39.Young N, Bell DF, Anthony A. Pediatric pain patterns during Ilizarov treatment of limb length discrepancy and angular deformity. J Pediatr Orthop. 1994;14:352–357. doi: 10.1097/01241398-199405000-00015. [DOI] [PubMed] [Google Scholar]


