Abstract
Background
Adverse tissue reactions are known to occur after total hip arthroplasty using both conventional and metal-on-metal (MoM) bearings and after MoM hip resurfacing arthroplasty (SRA). A variety of imaging tools, including ultrasound (US), CT, and MRI, have been used to diagnose problems associated with wear after MoM hip arthroplasty and corrosion at the head-trunnion junction; however, the relative advantages and disadvantages of each remain a source of controversy.
Questions/purposes
The purposes of this review were to evaluate the advantages and disadvantages of (1) US; (2) CT; and (3) MRI as diagnostic tools in the assessment of wear-related corrosion problems after hip arthroplasty.
Methods
A systematic literature review was performed through Medline, EMBASE, Scopus CINAHL, and the Cochrane Library without time restriction using search terms related to THA, SRA, US, CT, MRI, adverse tissue reactions, and corrosion. Inclusion criteria were Level I through IV studies in the English language, whereas expert opinions and case reports were excluded. The quality of included studies was judged by their level of evidence, method of intervention allocation, outcome assessments, and followup of patients. Four hundred ninety unique results were returned and 40 articles were reviewed.
Results
The prevalence of adverse local tissue reactions in both asymptomatic and symptomatic patients varies based on the method of evaluation (US, CT, MRI) and imaging protocols. US is accessible and relatively inexpensive, yet has not been used to report synovial thicknesses in the setting of wear-related corrosion. CT scans are highly sensitive and provide information regarding component positioning but are limited in providing enhanced soft tissue contrast and require ionizing radiation. MRI has shown promise in predicting both the presence and severity of adverse local tissue reactions but is more expensive.
Conclusions
All three imaging modalities have a role in the assessment of adverse local tissue reactions and tribocorrosion after total hip arthroplasty. Although US may serve as a screening technique for the detection of larger periprosthetic collections, only MRI has been shown to predict the severity of tissue destruction found at revision and correlate to the degree of tissue necrosis at histologic evaluation.
Introduction
Metal-on-metal (MoM) bearings were reintroduced in the 1990s and demonstrated promising early results that led to a rapid increase in their use [5, 7, 8, 10, 26]. Proposed advantages of MoM bearings in THA include decreased wear, lower frequencies of dislocation, greater ROM, and an improved ability to withstand high-impact activities [14]. However, concerns have arisen with the use of large MoM bearings as a result of reports of catastrophic aseptic reactions resulting in soft tissue destruction, periprosthetic osteolysis, and associated complications [2, 13, 15, 21, 22].
As reports of adverse local tissue reactions emerged, attention was drawn not only to the MoM bearing surface as the source of debris, but also the taper-trunnion junction between the head and the stem [3, 4, 17, 31]. Corrosion at the head-neck interface has resulted in concerns about the mechanical behavior of large-diameter modular heads on the trunnion [3, 4, 8, 15, 17]. However, issues of trunnion corrosion and wear are not isolated to large-diameter MoM THAs, as Cooper et al. [9] reported on 10 primary THAs with metal-on-polyethylene bearings that required revision as a result of corrosion at the head-neck taper junction.
A number of imaging surveillance mechanisms have been reported in the literature to detect adverse local tissue reactions, including ultrasonography (US), CT, and MRI with metal artifact reduction sequences (MARS). The reported prevalence of these lesions ranges from 4% to 71% based on the patient population, implant type, and imaging modality [8, 22, 32]. Although there are a variety of imaging tools in use, there has been no systematic evaluation of the strengths and weaknesses of each imaging modality in detecting the presence of adverse tissue reactions after hip arthroplasty.
The purposes of this review were to evaluate the advantages and disadvantages of (1) US; (2) CT; and (3) MRI as diagnostic tools in the assessment of wear-related corrosion problems after hip arthroplasty.
Search Strategy and Criteria
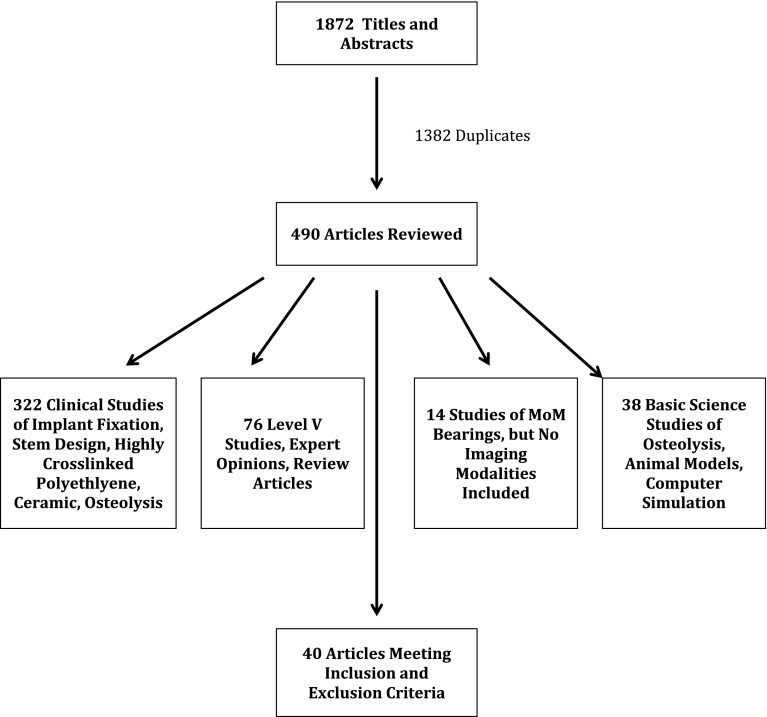
We performed a systematic review of the literature to compare reports of imaging surveillance mechanisms used to diagnose wear-related corrosion problems. Keywords used in the search were total hip arthroplasty, total hip replacement, hip resurfacing, surface replacement, ultrasound, computed tomography, magnetic resonance imaging, pseudotumor, aseptic lymphocyte-dominated vasculitis-associated lesion, adverse local tissue reaction, and corrosion. The search was run on the Medline, EMBASE, Scopus, CINAHL, and Cochrane library databases, respectively. Only studies qualifying as levels of evidence I through IV addressing the subject matter of our investigation were included for review. Inclusion criteria were: English language, human subjects, and level I through IV studies investigating imaging surveillance mechanisms to diagnose wear-related corrosion problems. Exclusion criteria were: non-English language studies, animal studies, level V studies, and nonindexed data. To exclude animal studies, the Human filter for Medline recommended in the Cochrane Handbook for Systematic Reviews of Interventions was used as a model to create similar filters for other databases searched [19]. Interventions studied in the review were the use of US, CT, and/or MRI to detect adverse tissue reactions in asymptomatic and symptomatic patients with various hip arthroplasty designs: MoM THA, conventional metal-on-polyethylene THA, and surface replacement arthroplasty (SRA). No information was obtained from any funding agencies, pharmaceutical companies, or personal files. Hand-searching of recent conference proceedings (annual meetings of the American Academy of Orthopaedic Surgeons, American Association of Hip and Knee Surgeons, Orthopaedic Research Society, Closed and Open Meeting of the Hip Society) for relevant abstracts was initially performed but did not yield additional information to our topic not previously published. Bibliographies of retrieved studies were also searched for relevant articles. We applied the inclusion and exclusion criteria to the titles and abstracts. The initial search yielded 1872 titles and abstracts. An independent medical librarian (KG) performed the initial screening of titles and abstracts. There were 1382 duplicates from our search, which were removed for a total of 490 unique citations. We obtained full articles for the eligible titles and abstracts. The inclusion and exclusion criteria were then reapplied to full articles. One of the authors (DN) independently screened the full-length articles and judged the quality of included studies by their level of evidence, method of intervention allocation, outcome assessments, and patient followup [23]. The majority of studies did not address our intervention of interest, but instead were studies of the survivorship of alternative implant designs and bearing surfaces (ie, highly crosslinked polyethylene, ceramic) in hip arthroplasty. Forty articles met the inclusion and exclusion criteria and were included in the review (Fig. 1).
Fig. 1.
Flowchart demonstrating reasons for article inclusion and exclusion from this systematic review.
Results
Ultrasound
Seven studies pertaining to US were reviewed. The main advantages of US in the surveillance of wear-related corrosion problems in hip arthroplasty are its relatively low cost, availability, and accessibility [1, 13, 22, 32]. However, disadvantages include that it is highly operator-dependent and studies regarding its ability to accurately report synovial thicknesses and its sensitivity in detecting smaller, deep tissue deposits have not been investigated. Williams et al. [32] used US to assess the prevalence of pseudotumor formation in asymptomatic patients with a MoM hip arthroplasty and to assess whether a correlation exists between elevated serum metal ion levels and pseudotumor formation. At a minimum followup of 2 years, 31 asymptomatic patients with a MoM THA, 24 asymptomatic patients with a metal-on-polyethylene THA, and 20 asymptomatic patients with a MoM SRA (Durom; Zimmer, Warsaw, IN, USA) were evaluated. Ten patients (32%) in the MoM THA, five patients (25%) in the SRA, and one patient (4%) in the metal-on-polyethylene THA cohort had a solid or cystic mass with no correlation present between serum metal ion levels and the size of the pseudotumor. The authors concluded the use of high-resolution US to be effective in the surveillance of MoM hip prostheses, even in asymptomatic patients, and recommended its use as a first-line surveillance mechanism [32].
In the only study comparing US with MRI in the assessment of pseudotumor detection and progression, Garbuz et al. [13] performed a prospective evaluation of 40 asymptomatic patients who received a large-head MoM THA. All patients received both an US and MRI on the same visit, and they defined the gold standard as the presence of a pseudotumor as a positive finding on both imaging modalities. If there was disagreement about the presence of pseudotumor between the MRI and US assessment, either repeat US and MRI or a dual-energy CT scan was performed at a minimum of 6 months later to confirm whether a pseudotumor was truly present. They found concordance between the two modalities in 93% (37 of 40) of patients with US demonstrating a sensitivity of 100% and specificity of 96%, whereas MRI had a sensitivity of 92% and specificity of 100%. Based on its sensitivity, availability, and low cost versus MRI, they concluded that US should be the initial screening modality for pseudotumor detection. However, this study had several limitations that the authors noted, including a lack of intraoperative, histologic specimens to confirm the presence of a pseudotumor. Thus, the study is more a concordance evaluation than a true assessment of diagnostic accuracy of the imaging tools [13].
The accessibility and low cost of US make it an attractive surveillance mechanism, and it has demonstrated a high degree of sensitivity in detecting the presence of adverse tissue reactions after MoM hip prostheses (Fig. 2). However, its ability to measure synovial thicknesses in this setting has not been reported. Furthermore, no studies have correlated the size and character of the lesions found on US to the severity of soft tissue destruction seen histologically. Studies assessing the use of US to detect adverse tissue reactions were of level III and IV quality, with no level I studies available for review.
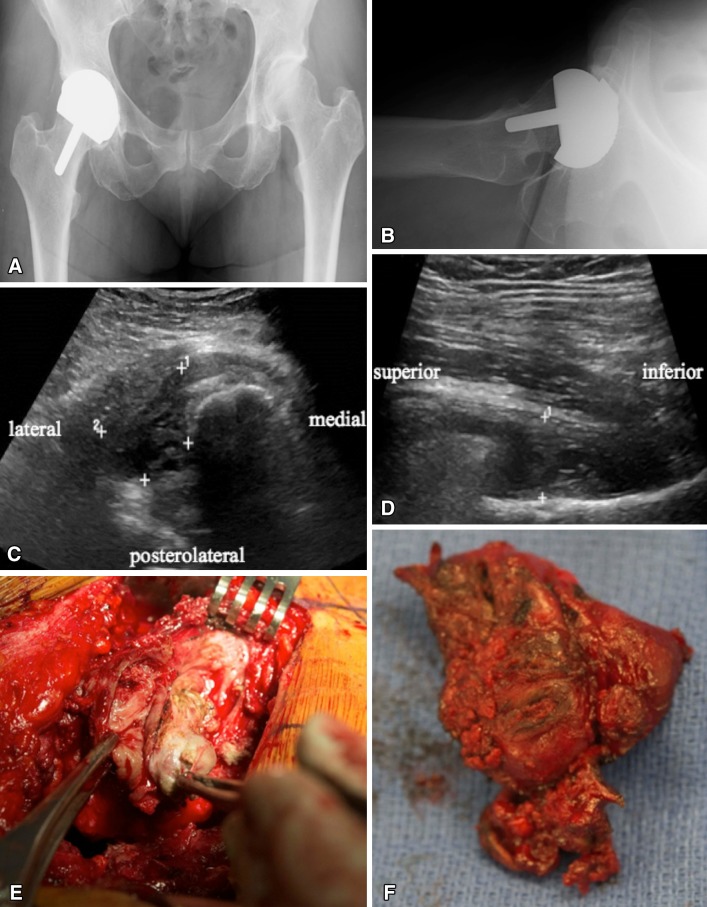
Fig. 2A–F.
The patient was a 55-year-old woman who presented 5 years after a SRA with mild swelling in her thigh and diffuse pain throughout the hip region. (A) AP and (B) cross-table lateral radiographs demonstrate a well-fixed SRA with acceptable inclination but slightly increased anteversion. (C) Transverse and (D) longitudinal US images at the level of the greater trochanter demonstrate the presence of a pseudotumor (demarcated by the + symbols). (E, F) These findings correlated with the intraoperative tissue specimens encountered during the time of revision.
Computed Tomography
Four studies pertaining to CT were reviewed. Advantages of CT scan in the detection of adverse tissue reactions include that it is widely available, and companies have developed metal artifact reduction protocols and/or software to reduce the beam-hardening artifact generated during the filtered back projection of CT reconstruction. Furthermore, CT scans provide additional information regarding component positioning. However, disadvantages of CT include the increased radiation burden to the patient, especially when using protocols to decrease metal artifact, and its limited ability to provide soft tissue detail.
Two studies from The Netherlands have used CT in the diagnosis of pseudotumors after both MoM THAs and SRAs [2, 4]. Bosker et al. [4] performed CT scans on 108 patients (109 hips) at a minimum followup of 2.5 years (mean, 3.6 years; range, 2.5–4.5 years) after a large-diameter femoral head MoM THA (ReCap; Biomet Inc, Warsaw, IN, USA). They reported a prevalence of pseudotumor of 39% (42 THAs; 95% confidence interval [CI], 30%–48%). Ten percent of patients with pseudotumors reported swelling, 45% reported nonspecific groin pain, and 31% reported clicking sensations. Multivariate analysis revealed that patients with serum cobalt levels > 5 μg/L had a fourfold increased risk of developing a pseudotumor (odds ratio [OR], 4.0; 95% CI, 1.6–10.1), although acetabular inclination and anteversion angles were not associated with the prevalence of pseudotumor [4].
Bisschop et al. [2] performed a single-center, cross-sectional prospective cohort study of 125 patients (143 hips) who received a SRA (Birmingham Hip Resurfacing; Smith & Nephew Inc, Memphis, TN, USA) [2]. A pseudotumor was found using CT in 39 patients (40 SRAs [28%]), of whom only 10 patients (11 SRAs [28%]) had groin pain and discomfort, a notable mass, or paresthesias. Symptomatic pseudotumors were significantly larger than asymptomatic pseudotumors (mean volume 53.3 cm3 versus 16.3 cm3, p = 0.05), and the chance of having a pseudotumor was significantly higher (OR, 4.9) in patients with an elevated serum cobalt level of > 85 ppb. Although aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL) scores based on histological analysis were reported using the score described by Campbell et al. [6] (histologic scoring system based on synovial lining, inflammatory infiltrate, and tissue organization), correlations were not made between the radiographic findings on CT scans and the ALVAL score appreciated intraoperatively.
Therefore, CT provides several potential advantages over US, including being less operator-dependent, while also providing information regarding component positioning. However, CT scans are limited in their ability to provide enhanced soft tissue contrast, they require ionizing radiation, and they have not been proven to predict the severity of tissue destruction appreciated intraoperatively (Fig. 3). Studies assessing the use of CT in the detection of adverse tissue reactions have been limited to cohort studies on consecutive patients, and its sensitivity and specificity have not been directly compared with other imaging modalities.
Fig. 3.
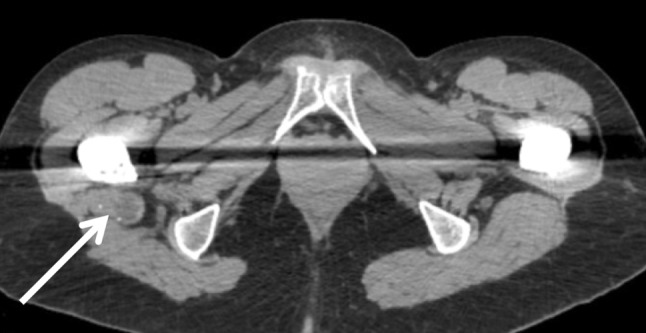
An axial CT image of an adverse local tissue reaction (ALTR) (arrow) posterior to the right femoral component. Note that the diminished soft tissue contrast makes the deposit appear more subtle.
Magnetic Resonance Imaging
Twenty-nine studies pertaining to MRI were reviewed. Advantages of MRI include the enhanced soft tissue detail it provides in addition to its potential promise in predicting not only the presence, but also severity of an adverse local tissue reaction [28, 29]. However, disadvantages include the cost associated with its use along with differences in the accessibility of specific sequencing protocols at different institutions. MRI with or without MARS has been the most commonly reported surveillance mechanism for the presence of wear-related corrosion problems in hip arthroplasty [8, 11, 12, 16, 17, 28–30]. The use of specific, metal artifact reduction sequences such as MAVRIC (multiple acquisition variable-resonance image combination) and SEMAC (slice encoding for metal artifact correction) has increased the ability to both identify and characterize these soft tissue lesions [18, 20, 25].
Chang et al. [8], in a review of 192 MoM THAs, demonstrated 69% of patients had pseudotumors present on MRI, but the presence or size of the pseudotumors was unrelated to the presence of symptoms (p = 0.4151–0.6648). However, Fisher’s exact test demonstrated the presence of a bone marrow edema pattern (p < 0.01) and tendon tearing (p < 0.05) to be significant predictors of pain, thus highlighting an advantage of MRI in its ability to identify concomitant pathologies that may cause patient symptoms [8].
The prevalence of tissue reactions around MoM implants is affected by the imaging techniques used, because smaller reactions in the inferomedial recess may be obscured without the use of newer pulse sequences. A recent cohort of 69 patients (74 hips) with SRA was studied using the MAVRIC technique, dividing patients into three groups: those with unexplained pain (32 hips), symptomatic patients whose pain could be attributed to mechanical causes (20 hips), and an asymptomatic cohort (22 hips). Synovitis was detected in 15 (68%) of the asymptomatic hips, 15 (75%) of the symptomatic hips with a mechanical cause, and 25 (78%) of the hips with unexplained pain. The mean volume of synovitis was 5.0 ± 6.9 cm3 (95% CI, 1.2–8.7 cm3) in the asymptomatic group, 10.2 ± 15.9 cm3 (95% CI, 1.4–19.4 cm3) in the mechanical cause group, and 31.0 ± 47.3 cm3 (95% CI, 11.5–50.5 cm3) in the unexplained pain group. Good repeatability was demonstrated between examiners for the assessment of synovial volume using MRI. Although synovitis was noted in a similar proportion of all groups, osteolysis was rare [28]. Further longitudinal assessment of these cohorts could potentially disclose the clinical relevance of smaller synovial volumes in these patients in a variety of bearing surfaces.
The importance of synovial thickness and synovial volume measured on MRI as predictive factors of pain and soft tissue destruction has been reported [27, 29]. Nawabi et al. [29] used MRI to compare patients who had undergone revision of either a SRA or large-head (> 36 mm) MoM THA for unexplained pain (35 hips) with a control group (59 hips) revised for other causes. They found the degree of synovial thickness on prerevision MRIs to be significantly higher in the unexplained pain group versus the control group (p = 0.04) with a synovial thickness of > 7 mm having a sensitivity of 88% and specificity of 90% in predicting the presence of an ALVAL lesion [29]. Similarly, in a statistical MRI analysis of 68 patients with failed MoM arthroplasties, both maximum synovial thickness and synovial volume correlated with intraoperative ALVAL scores of ≥ 5 and the extent of tissue damage at revision. The MRI predictive model showed a sensitivity and specificity of 94% and 87%, respectively, for detecting ALVAL and 90% and 86%, respectively, for quantifying intraoperative tissue damage [27]. Note should be made that these are higher than the reported predictive value of serum ion levels.
Therefore, although the cost and time associated with the use of MRI as a surveillance mechanism remains a concern, MRI is unique in its ability to predict the severity of tissue destruction found at revision and the degree of tissue necrosis at histologic evaluation. Furthermore, in predictive models, the maximum synovial thickness and the presence of solid synovial deposits on MRI have greater sensitivity and specificity in detecting ALVAL scores and quantifying intraoperative tissue damage than isolated serum ion levels. Studies evaluating the use of MRI to detect wear-related corrosion problems after hip arthroplasty were predominantly level II or III studies, with no level I studies available for review.
Discussion
Concerns regarding ALVALs, adverse local tissue reactions, and pseudotumors after the use of MoM hip arthroplasty, modular neck-stem junctions, and even conventional metal-on-polyethylene-bearing surfaces in THA have led to numerous, recently published reports regarding imaging mechanisms of surveillance for these lesions. However, although a variety of imaging tools have been described, there has been no systematic evaluation of the strengths and weaknesses of each imaging modality in detecting the presence of adverse tissue reactions after hip arthroplasty. The purposes of this systematic review were to elucidate the advantages and disadvantages of current surveillance mechanisms in diagnosing wear-related corrosion problems after hip arthroplasty.
This systematic literature review does have several limitations. First, the vast majority of studies reviewed were single-cohort studies describing the use of only one imaging modality. Thus, direct comparisons between the sensitivity and specificity of US, CT, and MRI in the diagnosis of tribocorrosion are limited. Furthermore, serial evaluations of patients with MoM devices are limited to small cohorts, single followup imaging, or relatively short-term followup intervals, and thus future directions must be focused on expanding both the size of the cohorts studied in longitudinal analysis along with defining imaging parameters that may be associated with disease progression and more rapid advancement to implant revision. In addition, longitudinal studies should include a comparison between “implants at risk” and highly crosslinked polyethylene constructs to ascertain the natural history of adverse local tissue reactions versus biologically irrelevant fluid collections that do not progress or resolve over time and, furthermore, do not serve as a harbinger for tissue destruction. Lastly, none of the studies reported have a true gold standard for comparing imaging study results with intraoperative, histologic specimens, because the presence or absence of false-negatives based on imaging studies cannot be confirmed.
Imaging surveillance mechanisms to detect wear-related corrosion problems after hip arthroplasty are crucial, because the majority of studies demonstrate that adverse local tissue reactions can present in asymptomatic patients with well-fixed, well-aligned components, and no consistent association has been established between serum metal ion content and the presence of a soft tissue reaction [2, 6, 8, 11, 12, 17, 24, 32, 33]. The accessibility and low cost of US make it an attractive surveillance mechanism after MoM THAs and SRAs. Ultrasound has demonstrated a high level of sensitivity in the detection of adverse tissue reactions and high concordance rates with MRI [13]. Thus, given its low cost, accessibility, and safety, it may be an appropriate screening tool for the presence of larger soft tissue lesions associated with wear-related corrosion. However, only level III and IV studies assessing the use of US have been reported, and no level I studies comparing its sensitivity and specificity with other imaging modalities were available for review. Potential disadvantages of US include a lack of reporting in the literature of synovial thicknesses, which may correlate with the degree of intraoperative soft tissue destruction as well as the potential to miss deeper, more solid (and biologically reactive) reactions. Future studies focusing on correlations and the ability of US to predict the severity of soft tissue destruction would be beneficial.
CT scans provide useful information regarding the presence and size of a soft tissue reaction and have the additional benefit of demonstrating component positioning. Studies assessing CT have been limited to cohort studies on consecutive series of patients [2, 4], but CT has been shown to be highly effective in detecting soft tissue lesions in both asymptomatic and symptomatic patients. However, concerns remain with the routine use of CT for surveillance because it exposes patients to ionizing radiation. Whereas increasing the kilovolt (kVp) and milliampere second (mAs) will decrease metal artifact, it will also increase the radiation burden to the patient, particularly if serial studies are indicated. Like with US, the sensitivity and specificity of CT in detecting wear-related corrosion after hip arthroplasty have not been directly compared with other imaging modalities and is an area that requires future investigation.
MRI has been the most widely reported surveillance mechanism for detecting adverse tissue reactions and provides the additional advantage (versus US and CT) of providing enhanced soft tissue detail and potentially identifying other concomitant sources of pain such as abductor tendon tears [8]. Recent studies pertaining to MRI have focused on potential predictive factors of soft tissue destruction seen intraoperatively at the time of revision surgery [27–29]. In predictive models, the maximum synovial thickness and the presence of solid synovial deposits on MRI have greater sensitivity and specificity in detecting ALVAL scores and quantifying intraoperative tissue damage than isolated serum ion levels (Fig. 4) [27]. However, concerns with MRI remain, including the cost, time, and reproducibility of the advanced protocols used at the investigating centers. In addition, future studies should address potential costs and benefits and the clinical feasibility of using MRI as a screening tool versus US or CT.
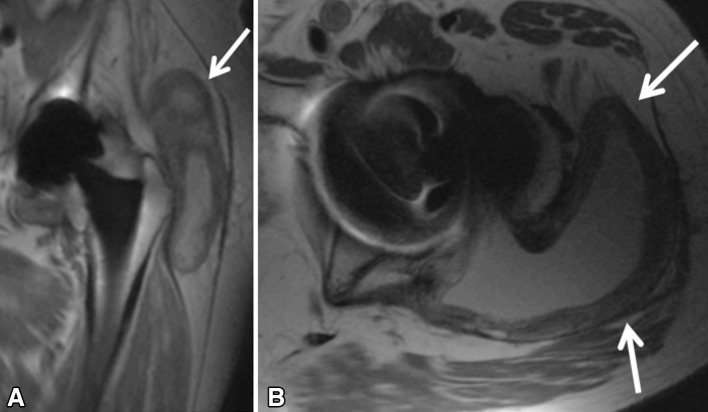
Fig. 4A–B.
Coronal MAVRIC moderate echo time (A) and axial moderate echo time (B) images of a symptomatic 80-year-old man 1.8 years after placement of a recalled dual-taper modular hip arthroplasty system (Stryker Rejuvenate, Mahwah, NJ, USA). Note the expanded pseudocapsule with a markedly thickened lining (arrows) with dehiscence of the lateral attachment of the posterior capsule allowing for the adverse local tissue reaction to extend into the greater trochanteric bursa. There is no evidence of osteolysis or implant loosening. Note that the artifact reduction on the coronal image is sufficient to visualize the head-stem junction.
In conclusion, US, CT, and MRI have all been shown to be effective in assessing the presence of adverse local tissue reactions and tribocorrosion after THA and SRA with all three modalities having specific advantages and disadvantages. Future studies should focus on directly comparing each of these modalities both for their effectiveness as a screening tool of patients and as a specific predictor of soft tissue destruction.
Acknowledgments
We thank Karen Gutzman for her contribution to developing search strategies and identifying citations for this systematic review.
Footnotes
One of the authors certifies that he (DN) has or may receive payments or benefits, during the study period, an amount of less than USD 10,000 from OrthAlign Inc (Aliso Viejo, CA, USA). One of the authors certifies that he (RLB) has or may receive benefits, during the study period, an amount of more than USD 1,000,001 from Smith and Nephew Inc (Memphis, TN, USA) and an amount of more than USD 1,000,001 from Stryker Orthopaedics Inc (Mahwah, NJ, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
This work was performed at the Washington University School of Medicine, St Louis, MO, USA, and the Hospital for Special Surgery, New York, NY, USA.
References
- 1.Almousa SA, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. The natural history of inflammatory pseudotumors in asymptomatic patients after metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2013;471:3414–3421. doi: 10.1007/s11999-013-2944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisschop R, Boomsma MF, Van Raay JJ, Tiebosch AT, Maas M, Gerritsma CL. High prevalence of pseudotumors in patients with a birmingham hip resurfacing prosthesis: a prospective cohort study of one hundred and twenty-nine patients. J Bone Joint Surg Am. 2013;17:1554–1560. doi: 10.2106/JBJS.L.00716. [DOI] [PubMed] [Google Scholar]
- 3.Bolland BJ, Culliford DJ, Langton DJ, Millington JP, Arden NK, Latham JM. High failure rates with a large-diameter hybrid metal-on-metal total hip replacement: clinical, radiological and retrieval analysis. J Bone Joint Surg Br. 2011;5:608–615. doi: 10.1302/0301-620X.93B5.26309. [DOI] [PubMed] [Google Scholar]
- 4.Bosker BH, Ettema HB, Boomsma MF, Kollen BJ, Maas M, Verheyen CC. High incidence of pseudotumour formation after large-diameter metal-on-metal total hip replacement: a prospective cohort study. J Bone Joint Surg Br. 2012;6:755–761. doi: 10.1302/0301-620X.94B6.28373. [DOI] [PubMed] [Google Scholar]
- 5.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the united states. J Bone Joint Surg Am. 2009;7:1614–1620. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 6.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;9:2321–2327. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell P, Urban RM, Catelas I, Skipor AK, Schmalzried TP. Autopsy analysis thirty years after metal-on-metal total hip replacement. A case report. J Bone Joint Surg Am. 2003;11:2218–2222. doi: 10.2106/00004623-200311000-00027. [DOI] [PubMed] [Google Scholar]
- 8.Chang EY, McAnally JL, Van Horne JR, Statum S, Wolfson T, Gamst A, Chung CB. Metal-on-metal total hip arthroplasty: do symptoms correlate with MR imaging findings? Radiology. 2012;3:848–857. doi: 10.1148/radiol.12120852. [DOI] [PubMed] [Google Scholar]
- 9.Cooper HJ, Della Valle CJ, Berger RA, Tetreault M, Paprosky WG, Sporer SM, Jacobs JJ. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;18:1655–1661. doi: 10.2106/JBJS.K.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunstan E, Sanghrajka AP, Tilley S, Unwin P, Blunn G, Cannon SR, Briggs TW. Metal ion levels after metal-on-metal proximal femoral replacements: a 30-year follow-up. J Bone Joint Surg Br. 2005;5:628–631. doi: 10.1302/0301-620X.87B5.15384. [DOI] [PubMed] [Google Scholar]
- 11.Ebreo D, Bell PJ, Arshad H, Donell ST, Toms A, Nolan JF. Serial magnetic resonance imaging of metal-on-metal total hip replacements. Follow-up of a cohort of 28 mm ultima TPS THRs. Bone Joint J. 2013;8:1035–1039. doi: 10.1302/0301-620X.95B8.31377. [DOI] [PubMed] [Google Scholar]
- 12.Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472:517–522. doi: 10.1007/s11999-013-3222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garbuz DS, Hargreaves BA, Duncan CP, Masri BA, Wilson DR, Forster BB. The John Charnley Award: Diagnostic accuracy of MRI versus ultrasound for detecting pseudotumors in asymptomatic metal-on-metal THA. Clin Orthop Relat Res. 2014;472:417–423. doi: 10.1007/s11999-013-3181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard J, Bocquet D, Autissier G, Fouilleron N, Fron D, Migaud H. Metal-on-metal hip arthroplasty in patients thirty years of age or younger. J Bone Joint Surg Am. 2010;14:2419–2426. doi: 10.2106/JBJS.I.01644. [DOI] [PubMed] [Google Scholar]
- 15.Haddad FS, Thakrar RR, Hart AJ, Skinner JA, Nargol AV, Nolan JF, Gill HS, Murray DW, Blom AW, Case CP. Metal-on-metal bearings: the evidence so far. J Bone Joint Surg Br. 2011;5:572–579. doi: 10.1302/0301-620X.93B4.26429. [DOI] [PubMed] [Google Scholar]
- 16.Hart AJ, Sabah S, Henckel J, Lewis A, Cobb J, Sampson B, Mitchell A, Skinner JA. The painful metal-on-metal hip resurfacing. J Bone Joint Surg Br. 2009;6:738–744. doi: 10.1302/0301-620X.91B6.21682. [DOI] [PubMed] [Google Scholar]
- 17.Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, Cobb JP, Skinner JA, Mitchell AW. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;4:317–325. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- 18.Hayter CL, Koff MF, Shah P, Koch KM, Miller TT, Potter HG. MRI after arthroplasty: comparison of MAVRIC and conventional fast spin-echo techniques. AJR Am J Roentgenol. 2011;3:W405–W411. doi: 10.2214/AJR.11.6659. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Inverventions Version 5.1.0. Baltimore, MD, USA: The Cochrane Collaboration; 2011.
- 20.Koch KM, Brau AC, Chen W, Gold GE, Hargreaves BA, Koff M, McKinnon GC, Potter HG, King KF. Imaging near metal with a MAVRIC-SEMAC hybrid. Magn Reson Med. 2011;1:71–82. doi: 10.1002/mrm.22523. [DOI] [PubMed] [Google Scholar]
- 21.Kwon YM, Jacobs JJ, MacDonald SJ, Potter HG, Fehring TK, Lombardi AV. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;8(Suppl):20–25. doi: 10.1016/j.arth.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Kwon YM, Ostlere SJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW. ‘Asymptomatic’ pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty. 2011;4:511–518. doi: 10.1016/j.arth.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 23.Levels of evidence for primary research question. Available at: www.editorialmanager.com/corr/account/LOE.doc. Accessed November 30, 2013.
- 24.Lohmann CH, Meyer H, Nuechtern JV, Singh G, Junk-Jantsch S, Schmotzer H, Morlock MM, Pfluger G. Periprosthetic tissue metal content but not serum metal content predicts the type of tissue response in failed small-diameter metal-on-metal total hip arthroplasties. J Bone Joint Surg Am. 2013;17:1561–1568. doi: 10.2106/JBJS.L.01273. [DOI] [PubMed] [Google Scholar]
- 25.Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. Slice encoding for metal artifact correction with noise reduction. Magn Reson Med. 2011;5:1352–1357. doi: 10.1002/mrm.22796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migaud H, Putman S, Krantz N, Vasseur L, Girard J. Cementless metal-on-metal versus ceramic-on-polyethylene hip arthroplasty in patients less than fifty years of age: a comparative study with twelve to fourteen-year follow-up. J Bone Joint Surg Am. 2011:137–142. [DOI] [PubMed]
- 27.Nawabi DH, Gold S, Lyman S, Fields K, Padgett DE, Potter HG. MRI predicts ALVAL and tissue damage in metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2014;472:471–481. doi: 10.1007/s11999-013-2788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nawabi DH, Hayter CL, Su EP, Koff MF, Perino G, Gold SL, Koch KM, Potter HG. Magnetic resonance imaging findings in symptomatic versus asymptomatic subjects following metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg Am. 2013;10:895–902. doi: 10.2106/JBJS.K.01476. [DOI] [PubMed] [Google Scholar]
- 29.Nawabi DH, Nassif NA, Do HT, Stoner K, Elpers M, Su EP, Wright T, Potter HG, Padgett DE. What causes unexplained pain in patients with metal-on metal hip devices? A retrieval, histologic, and imaging analysis. Clin Orthop Relat Res. 2014;472:543–554. doi: 10.1007/s11999-013-3199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Weegen W, Smolders JM, Sijbesma T, Hoekstra HJ, Brakel K, van Susante JL. High incidence of pseudotumours after hip resurfacing even in low risk patients; results from an intensified MRI screening protocol. Hip Int. 2013;3:243–249. doi: 10.5301/hipint.5000004. [DOI] [PubMed] [Google Scholar]
- 31.Wiley KF, Ding K, Stoner JA, Teague DC, Yousuf KM. Incidence of pseudotumor and acute lymphocytic vasculitis associated lesion (ALVAL) reactions in metal-on-metal hip articulations: a meta-analysis. J Arthroplasty. 2013;7:1238–1245. doi: 10.1016/j.arth.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Williams DH, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am. 2011;23:2164–2171. doi: 10.2106/JBJS.J.01884. [DOI] [PubMed] [Google Scholar]
- 33.Wynn-Jones H, Macnair R, Wimhurst J, Chirodian N, Derbyshire B, Toms A, Cahir J. Silent soft tissue pathology is common with a modern metal-on-metal hip arthroplasty. Acta Orthop. 2011;3:301–307. doi: 10.3109/17453674.2011.579518. [DOI] [PMC free article] [PubMed] [Google Scholar]