Abstract
Background
The biologic reactions to byproducts of wear or corrosion can involve innate and adaptive processes and are dependent on many factors, including the composition, size, surface properties, shape, and concentration of debris.
Questions/purposes
We used a systematic literature review to compare the reported patterns of inflammation in tissues around total hip implants with the goal of identifying whether there are unique or characteristic patterns associated with the newer bearing options or modular components.
Methods
A search of the Ovid Medline database between 1996 and early December 2013 identified articles that compared the histology around six implant groups: (1) metal-on-metal; (2) ceramic-on-ceramic; (3) metal-on-crosslinked polyethylene; (4) metal-on-conventional polyethylene with or (5) without modularity; and (6) tissue obtained at primary arthroplasty. Our initial search yielded 865 citations. After excluding articles that lacked a quantitative or semiquantitative description of histologic findings in periprosthetic tissue, we reviewed 34 articles.
Results
No pattern of inflammation is specific for any given bearing combination. Histologic features suggestive of an adaptive immune response appear to be more frequent and of greater magnitude in failed metal-on-metal implants, but tissues around many failed metal-on-metal implants show features of an “innate” foreign body reaction without lymphocytes. Occasional nonmetal-on-metal implants show features of an immune reaction, possibly associated with metal particles. Modular connections are one source of metal debris in nonmetal-on-metal implants. Features of an immune reaction appear rare in ceramic-on-ceramic implants that lack corrosion. Insufficient reports are available to characterize the biologic response to crosslinked polyethylene.
Conclusions
All total hip bearing combinations will wear in vivo, and modular interfaces are a likely source of metal that may be associated with a biological response regardless of the composition of the bearing surfaces. Surgeons must weigh the potential advantages of each articular combination and modular connection with the potential adverse tissue reactions in any given patient. Additional work is needed to clarify the implant and host-related factors associated with adverse tissue reactions and that seem to induce an immune reaction in some patients.
Introduction
The histologic appearance of tissues around failed total joint prostheses reflects the biologic reactions of the host to those devices and can often provide clues concerning the mechanisms of failure. Wear is an inevitable consequence of any joint arthroplasty, and for many years, particles of ultrahigh-molecular-weight polyethylene (UHMWPE) have been the most important type of debris particle around failed hips and knees. The inflammatory reaction to particles of UHMWPE is usually that of an “innate,” foreign body, granulomatous reaction characterized primarily by macrophages and foreign body-type giant cells [28]. Tissue around some failed metal-on-polyethylene implants, however, contains lymphocytes and/or plasma cells as well as a macrophage reaction to debris. The biological importance of this chronic inflammation has been unclear, but occasional reports have suggested the possibility of an adaptive immune reaction related to wear of the articular surfaces [16] or corrosion of modular connections [39].
New bearing surfaces and designs have been introduced, in part in the hope of reducing wear. However, the composition, size, surface properties, shape, and concentration of wear debris particles vary among these new designs, and the innate and adaptive processes that take place as part of the reaction to those wear particles likewise differ from one another and from conventional metal-on-UHMWPE bearings. A systematic synthesis of the large and diverse body of knowledge on this topic would be important.
The purpose of this review is to address the following question: “How have new bearing surfaces altered the biological reactions to byproducts of wear and modularity?” More specifically, we sought to address the following questions: (1) Are there patterns of inflammation around failed metal-on-metal hips that are unique when compared with ceramic-on-ceramic, metal-on-crosslinked polyethylene, or metal-on-“conventional” polyethylene? (2) Do all failed metal-on-metal hips show histologic features suggestive of an immune reaction? (3) Are some types of debris more or less likely to be associated with histologic features suggestive of an immune reaction? (4) Are there sources of metal debris in nonmetal-on-metal implants that might be implicated in an immune reaction? (5) Are there types of debris that induce acute inflammation (neutrophils), thereby resembling the histologic appearance of a periprosthetic infection?
Materials and Methods
We performed a systematic review of the Ovid Medline database between 1996 (the approximate date that crosslinked polyethylene [PE] became available) and early December 2013 to identify six comparative groups: (1) metal-on-metal; (2) ceramic-on-ceramic; (3) metal-on-crosslinked PE; (4) metal-on-PE without modularity; (5) metal-on-PE with modularity; and (6) tissue obtained at primary arthroplasty (Table 1). We attempted to formulate our literature review in a way that would allow us to directly compare the histologic findings that have been described in tissue samples among each of the six groups. We especially focused on the morphologic variables that reflect local biologic reactions as well as the variables related to the materials and designs of associated articular surfaces (Table 2). Our hope was that the literature review would help define which patterns of biologic reaction might independently correlate with material or design issues to help point toward cause and effect.
Table 1.
Search strategy*
| Group A search terms | Group B search terms | Group C search terms |
|---|---|---|
| [“biologic…” within 2 words of “react…”] Foreign Bodies “foreign bodies” “foreign body” Corrosion “corrosion” Foreign-Body Reaction Inflammation “inflam…” “patholog…” “osteolys…” “immunolog…” |
“alumina” Aluminum Oxide “aluminum oxide…” “chrome” Chromium “chromium” Cobalt “cobalt” Metals “metal” Ceramics “ceramic…” “XLPE”, “HXPE”, “HXLP”,” HXLPE”, “UHMWPE”, “SQXL”, “SXPE” [“polyethylene…” and “crosslink….”] [“polyethylene…” and “cross link…”] |
“hip” or “hips” |
* Capitalized terms = medical subject headings (MeSH index terms); “words” in quotes = words in article titles, abstracts, or indexing; “word…” = any ending accepted in place of the … (eg, singular/plural word forms, verb tenses).
Table 2.
Morphologic and material-related factors of special note in the review process
| Morphologic indices of local biologic reactions | Material and design factors |
|---|---|
| Necrosis and/or apoptosis | CoCr particles and/or ions |
| Diffuse lymphocytes | Corrosion products |
| Perivascular lymphocytes | Other metal particles (eg, titanium) |
| Plasma cells | PE particles |
| Actual vasculitis | Ceramic particles |
| Macrophages and giant cells | PMMA and/or contrast (barium, zirconium) |
| Eosinophils | Monoblock versus modular heads/sleeves |
| Acute inflammation (neutrophils) | Other modularity |
| Normal (not inflamed) |
CoCr = cobalt-chromium; PE = polyethylene; PMMA = polymethylmethacrylate.
Our initial search yielded 865 results. Based on manual review of the abstracts, we excluded reviews and clinical or imaging studies in which periarticular tissue samples were not described (n = 672) (Fig. 1). We also excluded in vitro incubation studies (n = 61) as well as those that only evaluated explanted devices without tissue (n = 70). We initially excluded studies that did not directly compare samples from multiple groups in a quantitative or semiquantitative way but that yielded so few articles that we ultimately retained studies that used some kind of quantification or semiquantitative grading scale for individual morphologic findings that reflect the biologic response, even if there was no control or comparison group, but we excluded articles for which there was no quantitative or semiquantitative description of the biologic reaction that would facilitate comparisons among groups (n = 97). Review of the publications identified by this search yielded several additional relevant publications that were also included in the final review. A total of 34 publications fit our inclusion criteria (Appendix 1).
Fig. 1.
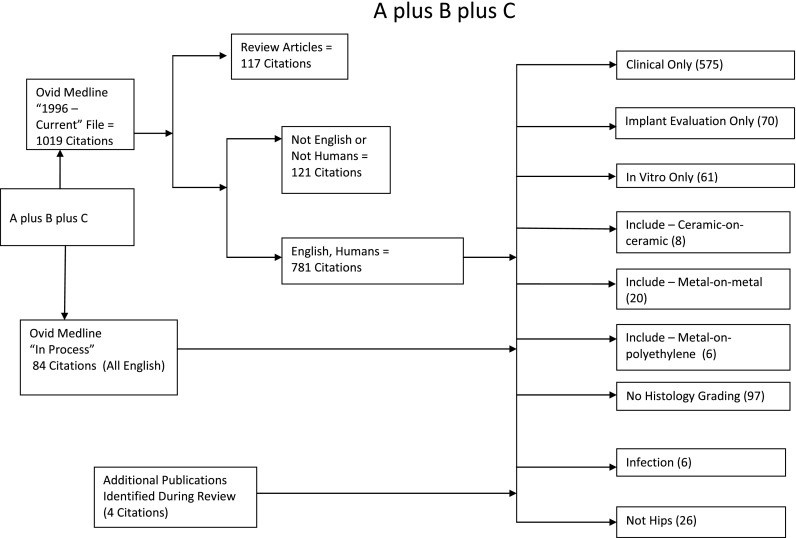
Flow diagram illustrates literature search results.
Results
Are the Patterns of Inflammation Around Failed Metal-on-metal Hips Unique When Compared With Other Articular Combinations?
Studies by Willert et al. [43], Davies et al. [10], Campbell et al. [7, 8, 14], and others [19, 26, 27, 29, 34, 40] have illustrated histologic features suggestive of an immune reaction in tissues around some failed metal-on-metal hip implants (see subsequently). These features are characteristic, but a failed metal-on-PE implant may demonstrate similar histologic findings [17, 41]. The recognition of modular connections as potential sources of metal particles, ions, and corrosion products raises the possibility that the histologic features suggestive of an immune reaction in failed nonmetal-on-metal implants could still be related to modular interfaces between metal components. Indeed, the study by Fujishiro and coauthors [17] found that increased extent of diffuse and perivascular chronic inflammation in failed nonmetal-on-metal implants correlated with visible debris particles that were morphologically suggestive of metal. The source of that metal debris was not identified in the Fujishiro et al. study, but all of the failed implants are thought to have had modular head/neck connections. Therefore, although histologic features suggestive of an immune reaction are not unique to failed metal-on-metal implants, that histologic pattern does seem to be associated with metal debris and appears to be more frequently seen in metal-on-metal implants than ceramic-on-ceramic and metal-on-PE.
Do All Failed Metal-on-metal Hips Show Histologic Features Suggestive of an Immune Reaction?
The histologic features thought to reflect an adaptive immune reaction include a laminated appearance to the periprosthetic tissue, disruption of the surface of that membrane, diffuse and perivascular chronic inflammation (lymphocytes), plasma cells, and sometimes eosinophils. Lymphoid aggregates, sometimes containing germinal centers, may also be present and implicate a B-lymphocyte response. The extent to which these individual features are present, however, varies considerably among implant groups and among different samples from the same patient. Indeed, some cases of failed metal-on-metal arthroplasty do not show any features suggestive of an adaptive immune response and instead show the macrophage and giant cell reaction more consistent with an “innate” foreign body reaction essentially identical to that seen in most cases of failed metal-on-PE arthroplasty [26]. Recognizing that tissues around failed metal-on-metal implants show a range of findings, some authors have attempted to develop grading or scoring systems intended to provide an index that might reflect the continuum from an innate, macrophage reaction to an adaptive immune response [7, 15, 19, 33]. Studies attempting to relate the extent of the histological reaction with the amount of wear of metal-on-metal implants have generally reported a low degree of correlation [19, 24].
Are Some Types of Debris More or Less Likely to Be Associated With Histologic Features Suggestive of an Immune Reaction?
The histologic features most closely associated with an adaptive immune response include diffuse and perivascular chronic inflammation characterized primarily by lymphocytes with variable numbers of plasma cells and sometimes eosinophils. The diffusely distributed chronic inflammation is often composed mainly of T-lymphocytes, whereas lymphoid aggregates are commonly combinations of B- and T-cells with occasional plasma cells [25, 29, 33]. These findings appear to be more common in either failed metal-on-metal hips [2, 10, 43] or in cases of failed nonmetal-on-metal hips in which metal particles can be identified [17]. Histologic features suggestive of an immune reaction appear to be very uncommon in cases of failed ceramic-on-ceramic implants unless there is coexisting evidence of wear and/or corrosion at a modular interface. Too few reports of failed metal-on-crosslinked PE or monoblock (nonmodular) metal-on-PE implants are available to confidently characterize patterns of inflammation.
Are There Sources of Metal Debris in Nonmetal-on-metal Implants That Might Be Implicated in an Immune Reaction?
As noted, wear and/or corrosion at modular interfaces can be sources of metal particles, ions, and corrosion products. Metal debris can also arise from the various fixation surfaces of metal implants. Although few studies have quantified evidence of tribocorrosion with different patterns of inflammation in surrounding tissues, Huber et al. [22] noted lymphocytic inflammation in association with chromium-containing solid corrosion products from modular tapers. Evidence is accumulating to suggest that modular interfaces are a likely source of metal that may be associated with an adaptive immune response regardless of the composition of the bearing surfaces.
Do the Products of Wear and Modularity of New Bearing Surfaces Induce Acute Inflammation (Neutrophils), Thereby Resembling the Histologic Appearance of a Periprosthetic Infection?
One study reported significantly more neutrophils in tissues around failed Mittelmeier alumina ceramic-on-ceramic implants than in failed metal-on-PE implants [20] and a relatively high concentration of neutrophils (“at least 10 per 10 high-power fields”) without other features of infection was reported in one of two cases of failed ceramic-on-ceramic implants in which impingement was associated with both ceramic and titanium debris [32]. However, neutrophils were not reported in several other studies of tissue around failed ceramic-on-ceramic implants [4, 15, 36, 44, 46], and in one other study, neutrophils were only associated with other features of infection [38]. Several reports have described the clinical difficulty distinguishing infection from other causes of failure of metal-on-metal implants [1, 18], and occasional metal-on-metal cases with slightly increased neutrophils but without other features of infection have been noted [37], but most cases of failed metal-on-metal implants lack significant neutrophils.
Discussion
All total hip bearing combinations will wear in vivo, but the biologic reactions to the products of wear of hard-hard bearings may be different from those associated with conventional metal-on-PE bearings and would be difficult to test in preclinical animal models. Small series of failed metal-on-metal hip implants have provided morphologic evidence suggestive of an immune reaction in a subset of patients, but few individual studies have attempted to compare the spectrum of morphologic findings associated with bearing surfaces of different types. The purpose of this review has been to address the following question: “How has the introduction of new bearing surfaces altered the biological reaction to byproducts of wear and modularity?” We attempted to identify publications that have used quantitative or semiquantitative methods to document morphologic features that reflect the biological reactions of periimplant tissues to byproducts of wear and modularity of new bearing surfaces and to compare those features with tissues around conventional metal-on-polyethylene or ceramic-on-polyethylene implants.
There are a number of limitations to this study. First, by confining our review to studies that included quantitative or semiquantitative grading of the histologic findings, we excluded many publications that have used only qualitative descriptions of periarticular findings [5, 12, 29, 35, 42, 44, 46] or that have focused on particle quantitation [13, 30, 45], cytokine detection [9, 21], or lymphocyte subsets [29]. Among the studies that have graded morphologic features of periprosthetic tissues, many have not included conventional metal-on-PE or ceramic-on-polyethylene cases as controls, and differences in grading criteria among studies preclude pooling the data into larger groups. The sources of the particles are also not clearly defined in most reports, in part because the articular surfaces and modular connections have not been simultaneously evaluated. Also complicating the interpretation of periprosthetic chronic inflammation is the recognition that subtle inflammation can occur in periprosthetic infections, especially those associated with low-virulent organisms. Our methods of literature review were not designed to compare all of the clinical features and diagnostic tests that might relate to periprosthetic infections (eg, serum C-reactive protein [CRP] and/or erythrocyte sedimentation rate [ESR]) among these groups.
Do Inflammation Patterns Differ Between Metal-on-metal THAs and Other Bearing Surfaces?
We found that for any given bearing combination, a wide range of periarticular histologic findings has been reported. In a small minority of failed metal-on-PE cases, and in a larger proportion of failed metal-on-metal cases, periimplant tissues show a laminated membrane with prominent diffuse and perivascular lymphocytes along with variable numbers of plasma cells and occasionally eosinophils. Some of the articles have identified subsets of lymphocytes associated with the inflammation (Table 1). Among those studies, Mital and coworkers [29] described morphological and lymphocyte phenotype proportions supporting the development of tertiary lymphoid organs with mixed B- and T-lymphocytes in 29% of 62 cases of failed metal-on-metal hip implants. Although no nonmetal-on-metal controls were evaluated, the lymphoid aggregates and B-lymphocytes shown in this study further support an adaptive immune response in these patients. However, additional studies are needed, especially with respect to the features of byproducts of wear and modularity and the host factors that influence the extent to which a given patient develops an innate versus an adaptive immune reaction. Such studies might include the identification of the antigens or haptens possibly associated with various wear or corrosion products; the pathways by which the observed responses are stimulated; the roles and interactions of macrophages, T-cells, B-cells, and plasma cells; and the mechanisms involved in soft tissue destruction leading to massive tissue necrosis. The results of such studies may clarify the nature of the immune response, which, to date, is largely based on histology.
Necrosis (or necrobiosis) has been reported around metal-on-metal hips but is also relatively common in patients with failed metal-on-PE implants [41]. That necrosis is not necessarily associated with morphologic evidence of vascular damage, and several investigators have speculated that it may represent a direct toxic effect of metals or ions rather than ischemia [25, 33]. Studies directed toward quantifying the amount and type of necrosis in periprosthetic tissues would be useful to clarify the etiology. Some patients with failed metal-on-metal implants as well as occasional patients with failed metal-on-PE or ceramic-on-ceramic implants have developed soft tissue masses (pseudotumors). The histologic findings of pseudotumors have also been variable. In some cases, the findings show primarily metal debris in macrophages; others have lymphocyte-dominated inflammation with or without necrosis.
Relatively few studies have described in a semiquantitative way the histologic features around failed contemporary ceramic-on-ceramic devices. The available literature suggests that in cases of ceramic wear, the debris can be associated with macrophages in a pattern similar to that seen in most failed metal-on-PE devices, but most cases of aseptically loose ceramic-on-ceramic implants have demonstrated relatively low concentrations of visible ceramic particles and little if any evidence of an immune reaction [6, 23].
We were able to identify only a single publication that attempted to compare the concentration and physical attributes of particles derived from highly crosslinked PE acetabular components compared with those of conventional polyethylene [3]. Although the authors reported a higher concentration of particles in tissues around the conventional implants compared with the highly crosslinked components, the conventional hips had been in situ for an average of 13.3 years, whereas the highly crosslinked hips had been in place an average of only 3.3 years. Polyethylene particles are difficult to isolate and quantify from tissue, but further studies with longer followup are needed to characterize the histologic response to particles and the various different formulations of crosslinked PE.
Do All Failed Metal-on-metal Hips Show Histology Consistent With Immune Reaction?
In most patients with failed metal-on-conventional PE, and in many patients with failed metal-on-metal implants, the tissues show primarily a macrophage and giant cell reaction to particles of debris with relatively few lymphocytes. Recognizing that the process of activating macrophages is complex and may involve a relatively small number of lymphocytes, these morphologic features are generally thought to reflect an “innate” inflammatory reaction. Therefore, only a subset of failed metal-on-metal hips show morphologic features suggestive of an immune reaction.
Are Some Types of Debris More Associated With Histology Suggestive of an Immune Reaction?
Early reports of failed metal-on-metal hip implants implicated metal particles and/or ions in the apparent immune reaction, especially ions or particles from the cobalt-chromium-based alloys commonly used in metal-on-metal articulations. Our literature review was not designed to identify articles that may have evaluated the reactions to titanium or titanium-alloy particles. Our literature review suggests that immune reactions are more commonly associated with metal-on-metal hips than other articular combinations, but one report [17] found that even in failed metal-on-PE implants, the increasing extent of chronic inflammation correlated with visible metal particles, again implicating a form of metallic wear or corrosion product as a potential antigen even in nonmetal-on-metal hips.
Are Some Kinds of Nonmetal-on-metal Debris Implicated in Immune Reactions?
Particles and ions can be generated from modular connections, including between a modular head and matching taper as well as at the junction of other modular pieces in an implant. The tribology of mechanically assisted crevice corrosion, or fretting corrosion, and the mechanical disruption of a passivated metal surface are beyond the scope of this study, but it seems likely that modular connections can be a source of wear and corrosion products that may be associated with an inflammatory reaction in some patients [22].
Do Some Kinds of Debris Induce Acute Inflammation Similar to Periprosthetic Infection?
Distinguishing periprosthetic infection from aseptic loosening can be difficult and often requires a combination of clinical observations and analytical tests. The presence or absence of acute inflammation in periprosthetic tissues is widely regarded as suggestive of infection. Although cutoff thresholds for morphologically suggesting infection have varied, a Practice Guidelines Committee of the American Academy of Orthopaedic Surgeons recently found adequate published data to support thresholds of either (1) five neutrophils in each of five high-power fields; or (2) five neutrophils in each of 10 high-power fields [11]. Another recent publication suggested that finding 23 neutrophils in a total of 10 high-power fields is strongly suggestive of infection [31]. Although several studies identified in this review reported neutrophils in apparently uninfected cases of failed ceramic-on-ceramic implants, most studies of periprosthetic tissue around failed ceramic-on-ceramic implants have not identified neutrophils. This observation requires further studies, but based on the available evidence, we do not recommend deviating from the morphologic criteria of infection as recommended by the American Academy of Orthopaedic Surgeons Practice Guidelines Committee [11] when reviewing periprosthetic tissue samples from different bearing couples.
This review has illustrated the variability of histologic findings in tissues adjacent to failed implants with several different types of bearing surfaces. Additional studies are needed to help correlate those findings with soft tissue masses (pseudotumors) and areas of bone resorption (osteolysis), evidence of wear from the articular surfaces and modular connections of the corresponding implants, local and systemic metal levels, evidence of potential systemic inflammatory responses (eg, elevated ESR and CRP levels) or coexisting proinflammatory conditions (eg, an underlying inflammatory arthropathy or periprosthetic infection), and immunological characterization of the lymphocytes and plasma cells. The wider use of a histological grading or scoring system to reflect the variable patterns of periimplant inflammation would be helpful when comparing findings from different groups of investigators. Studies of host factors that might influence biologic reactivity are also needed.
Appendix 1
Summary of articles included in the review
| Reference | Bearing combination | Comparative groups | Brief summary | Lymphocyte type |
|---|---|---|---|---|
| Aroukatos et al. [2] | Metal-on-metal (n = 20) | Ceramic-on-polyethylene (n = 13) and primary arthroplasty (n = 7) | Metal-on-metal cases had more necrosis and more diffuse and perivascular lymphocytic infiltration than other groups | T-cells and CD8+ B-cells |
| Baxter et al. [3] | Metal-on-crosslinked polyethylene (n = 9) | Metal-on-polyethylene (n = 9) | Differences in particles and macrophages were noted, but metal-on-crosslinked polyethylene had only been in situ for 3.3 years, whereas the metal-on-polyethylene group had been in situ 13.3 years | Unspecified |
| Bos and Willmann [6] | Ceramic-on-ceramic (n = 24) | Ceramic-on-polyethylene (n = 7) and metal-on-polyethylene (n = 17) | Ceramic-on-ceramic cases had thinner synovial layer, less necrosis, and fewer infiltrates of macrophages than either PE group | Unspecified |
| Campbell et al. [8] | Metal-on-metal (n = 4) | None | Periprosthetic tissue samples from four patients with groin pain after receiving metal-on-metal hip resurfacing all showed extensive infiltrates of lymphocytes suggestive of an immunologic reaction | Unspecified |
| Campbell et al. [7] | Metal-on-metal (n = 32) | None | Hips failed for suspected metal sensitivity had higher aseptic lymphocyte-dominated vasculitis associated lesion scores than hips thought to have failed as a result of wear | Unspecified |
| Davies et al. [10] | Metal-on-metal (n = 25) | Metal-on-polyethylene (n = 19) and primary arthroplasty (n = 9) | Emphasized laminated membrane, ulcerated surface, perivascular, and diffuse lymphocytes with plasma cells in metal-on-metal cases | No phenotyping was done, but plasma cells were identified in 10 of 25 metal-on-metal cases |
| Doorn et al. [14] | “Early-generation” metal-on-metal (n = 4) | “Contemporary design” metal-on-metal (n = 5) | Describe “slate blue histiocytes” as typical of metal-on-metal implants; emphasized macrophages and necrosis, but several cases also had “masses of lymphocytes” or “severe chronic inflammation” | Unspecified |
| Esposito et al. [15] | Ceramic-on-ceramic (n = 21) | None | Only mild synovial changes similar to osteoarthritis in 19 of 21 cases; none with features “of an adverse local tissue reaction characteristic of metal-on-metal” | Unspecified |
| Fujishiro et al. [17] | Nonmetal-on-metal (ie, metal-on-polyethylene and ceramic-on-polyethylene) (n = 130) | None | Low grades of perivascular and diffuse chronic inflammation were common, but approximately 4% of specimens had Grade 2+ or 3+ inflammation; increasing extent of inflammation correlated with visible metal particles | Unspecified; phenotyping not done, but plasma cells were identified in 7% of 107 patients with aseptically loose metal-on-polyethylene hips |
| Grammatopoulos et al. [19] | Metal-on-metal (n = 56) | None | Necrosis, macrophages, and immunologic changes were common in periprosthetic tissues and pseudotumors; most pseudotumors were associated with highly worn prostheses, necrosis, and both macrophagic and lymphocytic inflammation, but histological features did not strongly correlate with wear | Unspecified |
| Hatton et al. [20] | Ceramic-on-ceramic (n = 10) | Metal-on-polyethylene (n = 6) | Tissues around ceramic-on-ceramic (Mittelmeier) hips had variable findings; there were significantly more macrophages and giant cells around metal-on-polyethylene hips (Charnley), but more neutrophils in the ceramic-on-ceramic hips | Unspecified |
| Huber et al. [22] | Metal-on-metal (n = 9) | None | Tissue obtained at autopsy from 7 patients, 6 asymptomatic; metal particles were identified around all hips; the extent of inflammation was variable, but B- and T-lymphocytes were present in 8 of 9 hips | Diffuse and perivascular infiltrates both contained mixed populations of CD3+ T-cells and CD20+ B-lymphocytes |
| Huber et al. [22] | Metal-on-metal (n = 11 with particles suspicious of corrosion products) | Metal-on-metal (n = 57 without particles suspicious of corrosion products) | All cases with corrosion products and 31 of 57 without visible corrosion products had variable amounts of lymphocytes interpreted as hypersensitivity-associated. 26 of the 57 metal-on-metal cases without visible corrosion products lacked inflammatory response | Unspecified. |
| Lerouge et al. [23] | Ceramic-on-ceramic (n = 12) | Metal-on-polyethylene (n = 18) | Cellular reaction in metal-on-polyethylene hips appeared related to polyethylene, whereas in the ceramic-on-ceramic group, it was associated with bone cement and contrast media; neutrophils were not reported in either group | No mention of lymphocytic infiltrates; typical macrophage and giant cell reactions to both metal and PE debris |
| Lohmann et al. [24] | Metal-on-metal (n = 28) | None | 78% showed “lymphocyte-dominated histological profile”; total tissue metal content of cases with macrophage-dominated histology was lower than cases with primarily lymphocytic inflammation | Perivascular and intramural lymphocytic infiltrations including T- and B-cells and plasma cells |
| Mahendra et al. [25] | Metal-on-metal (n = 50) | None | Necrosis varied in amount, but exceeded 25% of a tissue section in 56% of cases; diffuse T-lymphocytes and perivascular T- and B-lymphocytes were present in some, but not in all cases; necrosis did not always correlate with lymphocytes, and there was no evidence of vasculitis, suggesting the possibility of direct cytotoxicity of Co-Cr | The diffusely distributed lymphocytes were mostly CD3+ T-cells, with “few or no” CD20+ B-cells Lymphoid aggregates contained both T- and B-lymphocytes; plasma cells were common around lymphoid aggregates |
| Meyer et al. [26] | Metal-on-metal (n = 114) | None | Tissue contained mostly Ti particles (apparently from the interface of ceramic-on-ceramic sleeves of large-diameter femoral heads on Ti femoral neck tapers); most tissue reflected a foreign body reaction; only 9 had lymphocytic infiltrates suggestive of hypersensitivity | Most cases had essentially no lymphocytes; only four of 114 hips had CD3+ T-lymphocytes as the dominant inflammatory patter; most contained primarily CD68+ macrophages |
| Milosev et al. [27] | Metal-on-metal (n = 17) | Ceramic-on-polyethylene (n = 7) and monoblock metal-on-polyethylene (n = 8) | Features of a “hypersensitivity-like reaction” in 13 of the 17 of metal-on-metal cases; smaller numbers of lymphocytes in control cases | Unspecified |
| Murali et al. [32] | Ceramic-on-ceramic (n = 2) | None | Ceramic and Ti debris reported in tissue approximately 2 cases in which impingement was associated with both ceramic and Ti debris; one case had increased neutrophils but lacked other features of infection | Unspecified |
| Natu et al. [33] | Metal-on-metal (n = 120) | None | Extensive necrosis in 43% of cases, diffuse synovitis in 50%, plasma cells in 38%, lymphoid aggregates in 86%; germinal centers in 67%, suggestive of progression of a Type IV immune response; hyalinized vessels were also reported, but there did not appear to be a clear association between vascular wall damage and extent of necrosis | Diffuse chronic inflammation composed of both B- and T-lymphocytes in 70 of 120 cases; lymphoid aggregates with germinal centers in 40 cases with central B-cells surrounded by T-cells |
| Ng et al. [34] | Metal-on-metal (n = 32) | Metal-on-polyethylene hips (n = 166), ceramic-on-polyethylene hips (n = 8), metal-on-polyethylene knees (n = 242) | Perivascular lymphocytes were common in both failed metal-on-polyethylene knees (40%) and metal-on-polyethylene hips (24%); perivascular lymphocytes were more extensive in metal-on-metal cases than metal-on-polyethylene | Unspecified |
| Nygaard et al. [36] | Ceramic-on-ceramic (n = 13), ceramic-on-polyethylene (N = 15), Metal-on-metal (N = 9) |
Primary arthroplasty (n = 8) | Periprosthetic biopsies obtained 1 year after arthroplasty from 37 asymptomatic volunteers; macrophages and particles were found in all implanted groups, but there were no significant differences; macrophages were also found in patients at primary arthroplasty | Unspecified (no mention of lymphocytic response) |
| Pandit et al. [37] | Metal-on-metal (n = 4) | None | Described periprosthetic pseudotumors, characterized by necrosis and variable, but focally heavy macrophage and lymphocyte infiltrates | Pseudotumors contained numerous CD14/CD68+ macrophages with CD3+/CD4+ T-cells and scattered CD20+ B-cells and a few CD11c+, S100+, DC SIGN+ dendritic cells |
| Savarino et al. [38] | Ceramic-on-ceramic (n = 30) | None | Tissues around some ceramic-on-ceramic hips were nearly normal; others contained macrophages and focal necrosis; correlation between increasing ceramic wear and grade of macrophage infiltrate; acute inflammation correlated with other clinical features of infection (presence of neutrophils) | Unspecified |
| Thomas et al. [40] | Metal-on-metal (n = 16) | None | Increasing histologic features of “delayed type hypersensitivity” correlated with systemic indices of metal sensitivity as defined by either patch testing or lymphocyte transformation tests | Unspecified |
| von Domarus et al. [41] | Metal-on-polyethylene (n = 28) | None | Necrobiosis and T-lymphocytes are common findings in tissues around failed metal-on-polyethylene implants; did not find a significant correlation between lymphocytes and visible metallosis | All 24 cases had some CD3+ T-lymphocytes, and 4 cases more than 300 CD3+ lymphocytes per high-power field; T-lymphocytic infiltration involved both CD4+ and CD8+ cells |
| Willert et al. [43] | Metal-on-metal (n = 24) | Ceramic-on-polyethylene (n = 18), monoblock metal-on-polyethylene (n = 11), and “classic” metal-on-metal (n = 15) |
Metal-on-metal cases showed “distinct lymphocytic infiltration” characterized as “aseptic lymphocyte-dominated vasculitis associated lesion (ALVAL)”; other groups lacked features of ALVAL | Diffusely distributed or perivascularly aggregated lymphocytic infiltrates: CD20+ B-lymphocytes, CD3+ T-lymphocytes |
PE = polyethylene; Co-Cr = cobalt-chromium; Ti = titanium.
Footnotes
The Biological Working Group consisted of: Ed Greenfield PhD, Case Western Reserve University, Cleveland, OH, USA; Joshua J. Jacobs MD, Midwest Orthopaedics at Rush, Chicago, IL, USA; Michael A. Mont MD, Sinai Hospital of Baltimore, Baltimore, MD, USA; Ed Purdue PhD, Hospital for Special Surgery, New York, NY, USA; Edward M. Schwarz PhD, University of Rochester Medical Center, Rochester, NY, USA; R. Lane Smith PhD, Stanford University School of Medicine, Redwood City, CA, USA; D. Rick Sumner PhD, Rush University Medical Center, Chicago, IL, USA; and, Paul H. Wooley PhD, Wichita State University, Wichita, KS, USA.
One of the authors certifies that he (TWB) has received or may receive payments or benefits during the study period an amount less than USD 10,000 each from Stryker Orthopaedics (Kalamazoo, MI, USA), Smith & Nephew Orthopaedics (Fort Washington, PA, USA), and Biomet (Warsaw, IN, USA). Another of the authors certifies that she (PAC) has received an amount of USD 10,000 to USD 100,000 from DePuy (Warsaw, IN, USA). The institution of one of the authors (PAC) has received, during the study period, funding from DePuy.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
This work was performed at the Cleveland Clinic, Cleveland, OH, USA.
References
- 1.Abdul N, Fountain J, Stockley I. Infection versus ALVAL: acute presentation with abdominal pain. BMJ Case Rep. 2013 Jun 10;2013. pii: bcr2013009976. [DOI] [PMC free article] [PubMed]
- 2.Aroukatos P, Repanti M, Repantis T, Bravou V, Korovessis P. Immunologic adverse reaction associated with low-carbide metal-on-metal bearings in total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2135–2142. doi: 10.1007/s11999-009-1187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter RM, Ianuzzi A, Freeman TA, Kurtz SM, Steinbeck MJ. Distinct immunohistomorphologic changes in periprosthetic hip tissues from historical and highly crosslinked UHMWPE implant retrievals. J Biomed Mater Res A. 2010;95:68–78. doi: 10.1002/jbm.a.32813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohler M, Mochida Y, Bauer TW, Plenk H, Jr, Salzer M. Wear debris from two different alumina-on-alumina total hip arthroplasties. J Bone Joint Surg Br. 2000;82:901–909. doi: 10.1302/0301-620X.82B6.9722. [DOI] [PubMed] [Google Scholar]
- 5.Bonnaig NS, Freiberg RA, Freiberg AA. Total hip arthroplasty with ceramic-on-ceramic bearing failure from third-body wear. Orthopedics. 2011;34:132. doi: 10.3928/01477447-20101221-36. [DOI] [PubMed] [Google Scholar]
- 6.Bos I, Willmann G. Morphologic characteristics of periprosthetic tissues from hip prostheses with ceramic-ceramic couples: a comparative histologic investigation of 18 revision and 30 autopsy cases. Acta Orthop Scand. 2001;72:335–342. doi: 10.1080/000164701753541970. [DOI] [PubMed] [Google Scholar]
- 7.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–2327. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell P, Shimmin A, Walter L, Solomon M. Metal sensitivity as a cause of groin pain in metal-on-metal hip resurfacing. J Arthroplasty. 2008;23:1080–1085. doi: 10.1016/j.arth.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Catelas I, Campbell PA, Dorey F, Frausto A, Mills BG, Amstutz HC. Semi-quantitative analysis of cytokines in MM THR tissues and their relationship to metal particles. Biomaterials. 2003;24:4785–4797. doi: 10.1016/S0142-9612(03)00378-8. [DOI] [PubMed] [Google Scholar]
- 10.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 11.Della Valle C, Parvizi J, Bauer TW, Dicesare PE, Evans RP, Segreti J, Spangehl M, Watters WC, 3rd, Keith M, Turkelson CM, Wies JL, Sluka P, Hitchcock K, American Academy of Orthopaedic Surgeons Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18:760–770. doi: 10.5435/00124635-201012000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Donell ST, Darrah C, Nolan JF, Wimhurst J, Toms A, Barker TH, Case CP, Tucker JK. Norwich Metal-on-Metal Study Group. Early failure of the Ultima metal-on-metal total hip replacement in the presence of normal plain radiographs. J Bone Joint Surg Br. 2010;92:1501–1508. doi: 10.1302/0301-620X.92B11.24504. [DOI] [PubMed] [Google Scholar]
- 13.Doorn PF, Campbell PA, Worrall J, Benya PD, McKellop HA, Amstutz HC. Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles. J Biomed Mater Res. 1998;42:103–111. doi: 10.1002/(SICI)1097-4636(199810)42:1<103::AID-JBM13>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 14.Doorn PF, Mirra JM, Campbell PA, Amstutz HC. Tissue reaction to metal on metal total hip prostheses. Clin Orthop Relat Res. 1996;429(Suppl):187–205. doi: 10.1097/00003086-199608001-00017. [DOI] [PubMed] [Google Scholar]
- 15.Esposito C, Maclean F, Campbell P, Walter WL, Walter WK, Bonar SF. Periprosthetic tissues from third generation alumina-on-alumina total hip arthroplasties. J Arthroplasty. 2013;28:860–866. doi: 10.1016/j.arth.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Evans EM, Freeman MA, Miller AJ, Vernon-Roberts B. Metal sensitivity as a cause of bone necrosis and loosening of the prosthesis in total joint replacement. J Bone Joint Surg Br. 1974;56:626–642. doi: 10.1302/0301-620X.56B4.626. [DOI] [PubMed] [Google Scholar]
- 17.Fujishiro T, Moojen DJ, Kobayashi N, Dhert WJ, Bauer TW. Perivascular and diffuse lymphocytic inflammation are not specific for failed metal-on-metal hip implants. Clin Orthop Relat Res. 2011;469:1127–1133. doi: 10.1007/s11999-010-1649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galbraith JG, Butler JS, Browne TJ, Mulcahy D, Harty JA. Infection or metal hypersensitivity? The diagnostic challenge of failure in metal-on-metal bearings. Acta Orthop Belg. 2011;77:145–151. [PubMed] [Google Scholar]
- 19.Grammatopoulos G, Pandit H, Kamali A, Maggiani F, Glyn-Jones S, Gill HS, Murray DW, Athanasou N. The correlation of wear with histological features after failed hip resurfacing arthroplasty. J Bone Joint Surg Am. 2013;95:e81. doi: 10.2106/JBJS.L.00775. [DOI] [PubMed] [Google Scholar]
- 20.Hatton A, Nevelos JE, Nevelos AA, Banks RE, Fisher J, Ingham E. Alumina-alumina artificial hip joints. Part I: a histological analysis and characterisation of wear debris by laser capture microdissection of tissues retrieved at revision. Biomaterials. 2002;23:3429–3440. doi: 10.1016/S0142-9612(02)00047-9. [DOI] [PubMed] [Google Scholar]
- 21.Holding CA, Findlay DM, Stamenkov R, Neale SD, Lucas H, Dharmapatni AS, Callary SA, Shrestha KR, Atkins GJ, Howie DW, Haynes DR. The correlation of RANK, RANKL and TNFalpha expression with bone loss volume and polyethylene wear debris around hip implants. Biomaterials. 2006;27:5212–5219. doi: 10.1016/j.biomaterials.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 22.Huber M, Reinisch G, Trettenhahn G, Zweymuller K, Lintner F. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009;5:172–180. doi: 10.1016/j.actbio.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 23.Lerouge S, Huk O, Yahia L, Witvoet J, Sedel L. Ceramic-ceramic and metal-polyethylene total hip replacements: comparison of pseudomembranes after loosening. J Bone Joint Surg Br. 1997;79:135–139. doi: 10.1302/0301-620X.79B1.6621. [DOI] [PubMed] [Google Scholar]
- 24.Lohmann CH, Meyer H, Nuechtern JV, Singh G, Junk-Jantsch S, Schmotzer H, Morlock MM, Pfluger G. Periprosthetic tissue metal content but not serum metal content predicts the type of tissue response in failed small-diameter metal-on-metal total hip arthroplasties. J Bone Joint Surg Am. 2013;95:1561–1568. doi: 10.2106/JBJS.L.01273. [DOI] [PubMed] [Google Scholar]
- 25.Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80:653–659. doi: 10.3109/17453670903473016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer H, Mueller T, Goldau G, Chamaon K, Ruetschi M, Lohmann CH. Corrosion at the cone/taper interface leads to failure of large-diameter metal-on-metal total hip arthroplasties. Clin Orthop Relat Res. 2012;470:3101–3108. doi: 10.1007/s11999-012-2502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milosev I, Trebse R, Kovac S, Cor A, Pisot V. Survivorship and retrieval analysis of Sikomet metal-on-metal total hip replacements at a mean of seven years. J Bone Joint Surg Am. 2006;88:1173–1182. doi: 10.2106/JBJS.E.00604. [DOI] [PubMed] [Google Scholar]
- 28.Mirra JM, Amstutz HC, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop Relat Res. 1976;117:221–240. [PubMed] [Google Scholar]
- 29.Mittal S, Revell M, Barone F, Hardie DL, Matharu GS, Davenport AJ, Martin RA, Grant M, Mosselmans F, Pynsent P, Sumathi VP, Addison O, Revell PA, Buckley CD. Lymphoid aggregates that resemble tertiary lymphoid organs define a specific pathological subset in metal-on-metal hip replacements. PLoS One. 2013;8:e63470. doi: 10.1371/journal.pone.0063470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mochida Y, Boehler M, Salzer M, Bauer TW. Debris from failed ceramic-on-ceramic and ceramic-on-polyethylene hip prostheses. Clin Orthop Relat Res. 2001;389:113–125. doi: 10.1097/00003086-200108000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Morawietz L, Tiddens O, Mueller M, Tohtz S, Gansukh T, Schroeder JH, Perka C, Krenn V. Twenty-three neutrophil granulocytes in 10 high-power fields is the best histopathological threshold to differentiate between aseptic and septic endoprosthesis loosening. Histopathology. 2009;54:847–853. doi: 10.1111/j.1365-2559.2009.03313.x. [DOI] [PubMed] [Google Scholar]
- 32.Murali R, Bonar SF, Kirsh G, Walter WK, Walter WL. Osteolysis in third-generation alumina ceramic-on-ceramic hip bearings with severe impingement and titanium metallosis. J Arthroplasty. 2008;23:1240.e1213–1249. [DOI] [PubMed]
- 33.Natu S, Sidaginamale RP, Gandhi J, Langton DJ, Nargol AV. Adverse reactions to metal debris: histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. J Clin Pathol. 2012;65:409–418. doi: 10.1136/jclinpath-2011-200398. [DOI] [PubMed] [Google Scholar]
- 34.Ng VY, Lombardi AV, Jr, Berend KR, Skeels MD, Adams JB. Perivascular lymphocytic infiltration is not limited to metal-on-metal bearings. Clin Orthop Relat Res. 2011;469:523–529. doi: 10.1007/s11999-010-1570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nygaard M, Bastholm L, Elling F, Soballe K, Borgwardt A. Can ultrastructural particle location predict aseptic loosening? A biopsy study of nonloose hip implants one year postoperative using three bearing material combinations. J Long Term Eff Med Implants. 2007;17:321–334. doi: 10.1615/JLongTermEffMedImplants.v17.i4.60. [DOI] [PubMed] [Google Scholar]
- 36.Nygaard M, Elling F, Bastholm L, Soballe K, Borgwardt A. No difference in early cellular response of the pseudo-synovial membrane after total hip arthroplasty: comparison of 3 combinations of bearing materials. Acta Orthop. 2006;77:402–412. doi: 10.1080/17453670610046325. [DOI] [PubMed] [Google Scholar]
- 37.Pandit H, Vlychou M, Whitwell D, Crook D, Luqmani R, Ostlere S, Murray DW, Athanasou NA. Necrotic granulomatous pseudotumours in bilateral resurfacing hip arthoplasties: evidence for a type IV immune response. Virchows Arch. 2008;453:529–534. doi: 10.1007/s00428-008-0659-9. [DOI] [PubMed] [Google Scholar]
- 38.Savarino L, Baldini N, Ciapetti G, Pellacani A, Giunti A. Is wear debris responsible for failure in alumina-on-alumina implants? Acta Orthop. 2009;80:162–167. doi: 10.3109/17453670902876730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svensson O, Mathiesen EB, Reinholt FP, Blomgren G. Formation of a fulminant soft-tissue pseudotumor after uncemented hip arthroplasty. A case report. J Bone Joint Surg Am. 1988;70:1238–1242. [PubMed] [Google Scholar]
- 40.Thomas P, Braathen LR, Dorig M, Aubock J, Nestle F, Werfel T, Willert HG. Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy. 2009;64:1157–1165. doi: 10.1111/j.1398-9995.2009.01966.x. [DOI] [PubMed] [Google Scholar]
- 41.von Domarus C, Rosenberg JP, Ruther W, Zustin J. Necrobiosis and T-lymphocyte infiltration in retrieved aseptically loosened metal-on-polyethylene arthroplasties. Acta Orthop. 2011;82:596–601. doi: 10.3109/17453674.2011.625534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitehouse MR, Endo M, Masri BA. Adverse local tissue reaction associated with a modular hip hemiarthroplasty. Clin Orthop Relat Res. 2013;471:4082–4086. doi: 10.1007/s11999-013-3133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 44.Wirganowicz PZ, Thomas BJ. Massive osteolysis after ceramic on ceramic total hip arthroplasty. A case report. Clin Orthop Relat Res. 1997;338:100–104. doi: 10.1097/00003086-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Xia Z, Kwon YM, Mehmood S, Downing C, Jurkschat K, Murray DW. Characterization of metal-wear nanoparticles in pseudotumor following metal-on-metal hip resurfacing. Nanomedicine. 2011;7:674–681. doi: 10.1016/j.nano.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Yoon TR, Rowe SM, Jung ST, Seon KJ, Maloney WJ. Osteolysis in association with a total hip arthroplasty with ceramic bearing surfaces. J Bone Joint Surg Am. 1998;80:1459–1468. doi: 10.2106/00004623-199810000-00007. [DOI] [PubMed] [Google Scholar]


