Abstract
Background
The survivorship of total elbow arthroplasties is lower than surgeons and patients would like it to be, especially in patients with posttraumatic arthritis of the elbow. To improve durability, it is important to understand the failure modes of existing implants. Total elbow arthroplasties were designed primarily for low-demand rheumatoid patients. As surgical indications have extended to more active patient populations, the mechanical performance of current designs must meet an increased mechanical burden. Evaluating the degree to which they do this will guide conclusions about which contemporary devices might still meet the need and, as importantly, what design and material changes might be needed to improve performance.
Where Are We Now?
The reasons for failures of total elbow arthroplasties include infection, loosening, polyethylene wear, locking mechanism failure, periprosthetic fracture, implant fracture, and instability. Implant design factors that have influenced wear include implant constraint, material, coatings, and metal backing. Surgical factors associated with increased wear and subsequent total elbow arthroplasty failure include soft tissue balancing and restoration of alignment and implant positioning.
Where Do We Need to Go?
A clear need exists for improving the performance of total elbow arthroplasty. Many of the failures that have limited the survivorship of elbow arthroplasties thus far are mechanical in nature with wear-related problems a dominating influence. Much of what we know about the results of total elbow arthroplasty is from small studies frequently involving the designer of the implant. The establishment of total elbow arthroplasty registries coupled with the increasing regulatory burden of postmarket surveillance would lead to a better understanding of the complications and survivorship of elbow arthroplasties. Another primary goal must be to achieve a better understanding of the biomechanics of the normal elbow and how the mechanics are altered after the insertion of elbow arthroplasty components.
How Do We Get There?
Improving the performance and survivorship of total elbow arthroplasty will require the integration of clinical and implant performance data gained through the establishment of registries with a concerted basic science effort to better understand the functional loads across the joint and to incorporate these loads into experimental and computational models to allow assessment of design and material changes intended to improve durability.
Introduction
Considerable effort has been expended in enhancing the performance of hip and knee arthroplasty over the past 40 years. Steady improvements in implant designs and bearing materials, fixation methods, surgical techniques, and patient education and rehabilitation have all contributed to the success of these treatments. Much of the effort has gone into combating the problems of wear and tribocorrosion as reviewed in the publications from predecessors to the current Association of Bone and Joint Surgeons® Brighton Workshop [13, 42, 43] published in this issue of Clinical Orthopaedics and Related Research®.
However, much less is known about the impact of wear, tribocorrosion, and other mechanically related failure mechanisms in total elbow arthroplasty (TEA). Thus, we set out to answer two key questions. First, what is the survivorship of TEA, especially at long-term followup exceeding 10 years? Second, what implant, patient, and surgical factors are associated with lower survivorship values? The answers form the basis for describing the current status of elbow arthroplasty (eg, Where are we now?) and suggest where we need to go and the steps necessary to get there in our efforts to provide patients requiring elbow arthroplasty with better durability.
Where Are We Now?
TEAs fail as a result of infection, loosening, polyethylene wear, locking mechanism failure, periprosthetic fracture, implant fracture, and instability. Failures are often catastrophic; loss of bone stock and insufficient ligamentous structures make revision surgery a challenge (Fig. 1). Revision is further hampered by poor bone quality in the presence of inflammatory arthritis and immunosuppressive medications.
Fig. 1.
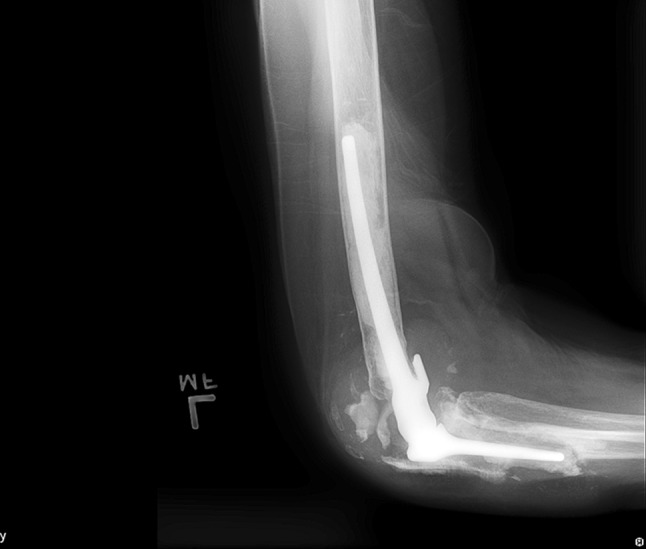
This figure demonstrates ulnar loosening with significant bone loss with a semiconstrained implant.
TEA survivorship in the Scandinavian registry was 83% at 10 years in patients with rheumatoid arthritis; the Norwegian registry showed a similar 10-year survivorship of 85% [6, 35]. As expected, more active posttraumatic arthritis patients have a higher failure rate [6, 23, 30, 35, 38]. Besides diagnosis, other patient factors attributed to high failure rates include excessive deformity, instability, age, and activity level [3, 23, 30, 34]. In 41 patients with posttraumatic arthritis who underwent TEA with the Coonrad-Morrey implant (Zimmer, Warsaw, IN, USA), the revision rate at 7.5 years was 22% [30]. Five patients sustained fracture of the metallic ulnar component; two other patients required polyethylene bushing exchanges to correct excessive wear. The patients who had bushing exchanges all later experienced recurrent deformities.
Implant design factors that influence wear include implant constraint, material, coatings, and metal backing. Nonconstrained designs, including the Capitellocondylar (J&J, New Brunswick, NJ, USA), Kudo (Styker-Howmedica, Mahwah, NJ, USA), Souter-Strathclyde (Stryker-Howmedica, Limerick, UK), and Pritchard ERS (DePuy, Warsaw, IN, USA), depend on soft tissue support to stabilize the implant and reduce load transfer through the implant components to the fixation interfaces. Survivorship of nonconstrained implants vary considerably from 54% at 10 years to 90% at 16 years [16, 18–20, 22, 28, 37, 40] with instability and dislocation as major complications. Wear of the polyethylene components suggests inadequate soft tissue constraint. For example, Landor et al. [20] and Robinson et al. [27] found their revised Souter-Strathclyde components to be heavily damaged. van Riet et al. [40] noted similar damage in all cases of failed Pritchard ERS implants revised for instability, some with dramatic asymmetric polyethylene wear through to the underlying metal. Similar catastrophic wear was reported on the ulnar components of the Kudo implant [22]. Early nonconstrained designs used all-polyethylene ulnar components, which often failed as a result of polyethylene fracture; later versions incorporated metal-backed components with improved clinical results [5, 16, 37].
Semiconstrained devices, eg, the Coonrad-Morrey, Triaxial (DePuy), and Solar (Stryker-Howmedica), also suffer from failure secondary to polyethylene wear (Fig. 2A–B; Table 1). The Triaxial, which had a snap-fit articulation, had late dislocations secondary to wear [8]. Patil et al. [24] reported two of 13 Solar TEAs revised for bushing wear. Bushing wear of the Coonrad-Morrey has been widely reported; as bushings wear, varus-valgus ROM increases until eventually revision surgery is required. In 78 arthroplasties performed for rheumatoid arthritis, 11 bushings (15%) were worn at an average 8.4-year followup [11]. In a much larger review of 919 Coonrad-Morrey TEAs, the revision rate for bushing wear was 1.3% at an average 8 years followup [21].
Fig. 2A–B.
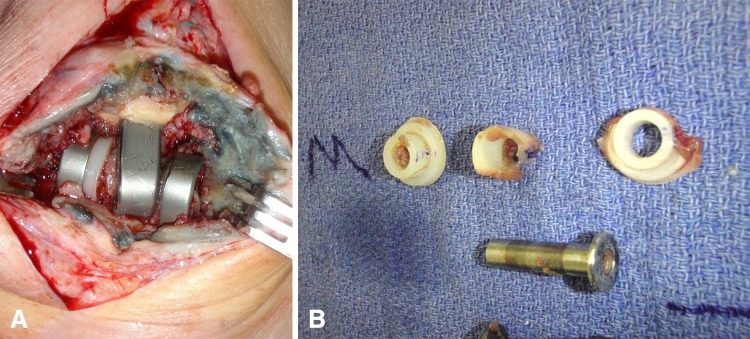
(A) This figure illustrates marked bushing wear in a Coonrad-Morrey implant with resulting metallosis. (B) Significant polyethylene wear is evident in this figure.
Table 1.
Bushing revisions in semiconstrained implants
| Implant | Diagnosis | Number of elbows | Revisions | Followup (years) | Reference |
|---|---|---|---|---|---|
| Coonrad-Morrey (Zimmer, Warsaw, IN, USA) | RA | 78 | 5 (7%) fully worn; 6 (8%) partial wear | 8.4 | 11 |
| Coonrad-Morrey | RA/TA | 919 | 12 (1.3%) | 8 | 21 |
| Coonrad-Morrey | TA | 41 | 2 (5%) | 5.7 | 30 |
| Coonrad-Morrey | TA | 85 | 7 (8.2%) | 9 | 38 |
| Solar (Stryker-Howmedica, Limerick, UK) | RA/TA | 13 | 2 (15.4%) | 8.4 | 24 |
RA = rheumatoid arthritis; TA = traumatic arthritis.
Goldberg et al. [12] examined 16 retrieved Coonrad-Morrey elbows; all but one of the humeral bushings exhibited asymmetrical thinning, whereas all but one of the ulnar bushings showed elliptical plastic deformation. Unintended wear between the bearing surface and a nonbearing surface occurred in 54% of the cases, and metal-on-metal wear was observed in 62%. Third-body bone cement or metal debris usually contributed to bearing surface wear.
Alterations to fixation surfaces were made to enhance fixation. When the original sintered metallic bead-coated Coonrad-Morrey components were compared with an alternate design with cement precoating, survival at 7 years dropped from 93% to 83% [17]. The cause of the premature failures remains elusive, but like with precoated THAs, precoat debonding and the generation of a large burden of PMMA and metallic debris are likely factors. When the coating was altered again to a plasma-sprayed metallic coating, the survival remained at 100% at 7 years.
Fractured stems in TEAs have been reported at an alarming rate (Fig. 3A–B; Table 2). The Kudo Type 4 design had six fractures in 44 implants (14%) in one study [26], and five fractures in 32 implants (16%) were reported by the designer himself [18]. Failure rates in other designs are lower; for example, a study of the Solar design reported one fracture in 13 implants [24]. The occurrence of a fracture is affected by stem size and shape, patient factors (higher rates are reported in posttraumatic compared with rheumatoid patients [1, 11, 30, 38]), notch sensitivity of titanium alloy, and the large cyclic loads and large numbers of load cycles applied to the elbow.
Fig. 3A–B.
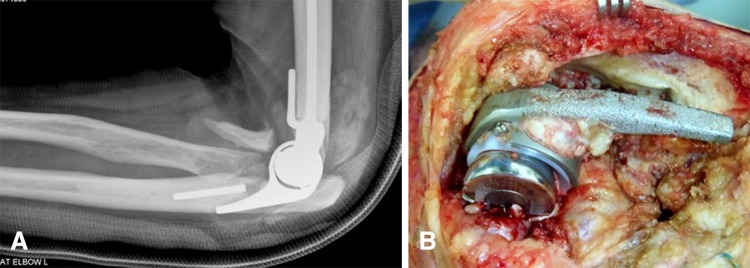
(A) A fracture of an ulnar stem is seen in this radiograph. (B) This is an intraoperative photograph of a fractured stem.
Table 2.
Implant fractures
| Implant | Diagnosis | Number of elbows | Fractures | Percent | Followup (years) | Reference |
|---|---|---|---|---|---|---|
| Coonrad-Morrey (Zimmer, Warsaw, IN, USA) | TA | 41 | 5 ulnar | 12.2 | 5.7 | 30 |
| Coonrad-Morrey | RA | 78 | 1 ulnar | 1.3 | 8.4 | 11 |
| Coonrad-Morrey | TA | 85 | 3 ulnar | 3.5 | 9 | 38 |
| Kudo (Styker-Howmedica, Mahwah, NJ, USA) | RA | 44 | 1 ulnar; 5 humeral | 13.6 | 7 | 26 |
| Kudo | RA | 32 | 5 humeral | 15.6 | 3 | 18 |
| Solar (Stryker-Howmedica, Limerick, UK) | RA/TA | 13 | 1 humeral | 7.7 | 8.4 | 24 |
TA = traumatic arthritis; RA = rheumatoid arthritis.
Failures of the locking mechanisms that link ulnar and humeral components in semiconstrained TEAs have also been reported (Fig. 4) with rates as high as 5.3% by 5.5 years [9] and 9.8% at 13.5 years [14]. These failures have been attributed to posttraumatic arthritis, young age, males, high physical demand, and deficient humeral condyles [44].
Fig. 4.
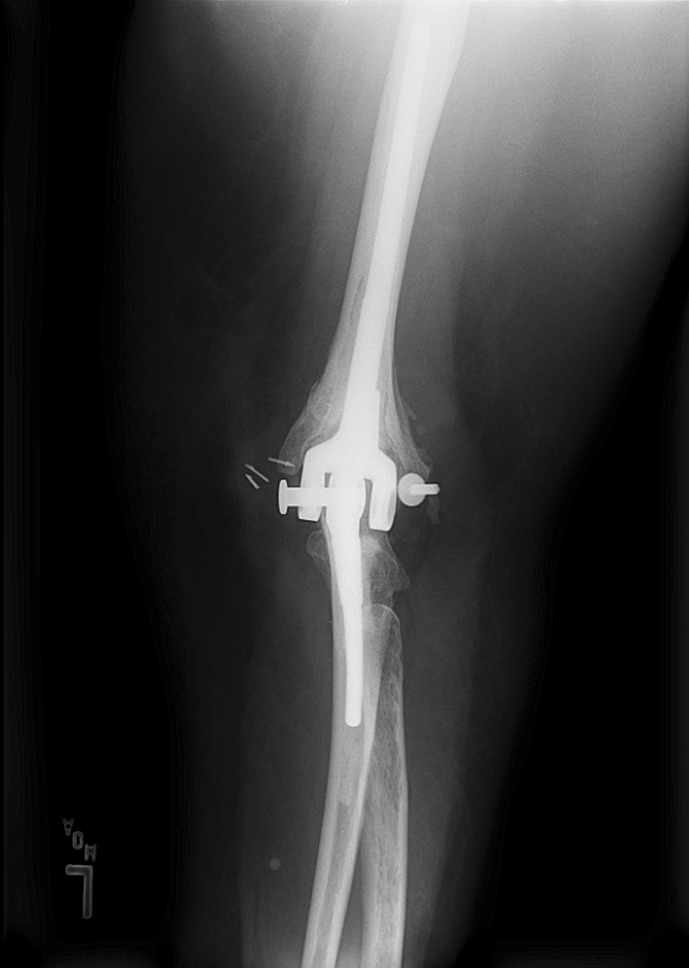
Axle failure and disassociation of a Coonrad-Morrey implant is evident in this radiograph.
Metallosis secondary to metallic debris generated from implant fracture surfaces or unintended metal-on-metal wear has been reported across several designs [4, 9, 18, 21, 26, 29, 32]. In the report by Kudo et al. [19] on the five cases revised for stem fracture, all had accompanying metallosis. In a study of metallosis in revision TEA, 10 of 11 nonconstrained implants had unintended metal-on-metal wear [4]. In the Coonrad-Morrey implant, metallosis was noted in 10 of 12 revised for bushing failure and three of five revised for locking mechanism failure [21, 32]. In the study by Goldberg et al. [12], 10 of 16 retrieved Coonrad-Morrey implants had metallosis including four cases with fractured fixation stems.
Surgical factors associated with TEA failure include poor soft tissue balancing and failure to restore alignment [2, 7, 31]. Inadequate humeral positioning was directly related to implant loosening in a study of the Souter-Strathclyde implant [33], and the most important reasons for failure were related to incongruity and poor alignment that led to early wear and instability in Pritchard ERS implants [40].
Where Do We Need to Go?
A clear need exists to improve the performance of TEA. Many of the failures that have limited the survivorship of elbow arthroplasties thus far are mechanical in nature with wear-related problems a dominating influence. Even the patient and surgical factors that influence treatment outcome such as diagnosis and component alignment appear to do so by their influence on the mechanical burdens and hence the susceptibility for polyethylene and metal wear. Much of what we know about the results of TEA is from small studies frequently involving the designer of the implant. The establishment of TEA registries coupled with the increasing regulatory burden of postmarket surveillance would lead to a better understanding of the complications and survivorship of elbow arthroplasties.
National and institutional registries provide important information, but most (like that of the United Kingdom) are in their infancy, and the more longstanding registries thus far encompass only small numbers of cases [25]. The primary outcome of most joint arthroplasty registries is revision surgery; this is usually necessitated by cost because collecting more information on such large numbers of surgeries as are included in hip and knee arthroplasty becomes prohibitive. However, for smaller patient populations such as in TEA, the emphasis should be in collecting not just implant information (eg, what device was implanted and when, if ever, was it revised?), but also information on perioperative and longer-term complications and patient-reported outcomes (eg, pain, satisfaction, function) so that these important measures of clinical success or failure can be related to implant design and surgical technique.
Another primary goal must be to achieve a better understanding of the biomechanics of the normal elbow and how the mechanics are altered after the insertion of elbow arthroplasty components. Reliable data on loads across the elbow are sparse and cover few daily activities. Previous studies often have severe limitations. For example, a biomechanical study by Brownhill et al. [2] demonstrated that implant malpositioning influenced loading and potentially contributed to wear and loosening; however, collateral ligaments were sacrificed in this study, so the important contributions from the soft tissues were ignored. Similarly, sparse finite element analysis and in vivo kinematic data exist for elbow activities [10, 36, 39, 41]. More information might help us better understand implant performance and provide input data for computational models of the elbow through which muscle and joint loads could be calculated. Stokdijk et al. [36] described a method for determining the optimal elbow axis using an electromagnetic tracking device. van der Lugt [39] reported on migration patterns of Souter-Strathclyde humeral components using radiostereometric analysis. Fluoroscopic imaging was used to evaluate in vivo three-dimensional elbow motion in 12 patients who had received 15 Osaka (Finsbury Orthopaedics, Surrey, UK) nonconstrained elbow arthroplasties [10]. Relative positions between humeral and ulnar components showed extremely wide variation among patients throughout elbow flexion. Excessive valgus angle and internal rotation of the components led to edge loading that could lead to increased wear. Furthermore, in such a nonconstrained device, these angulations would decrease the contact area across the articular surfaces, thus reducing the ability of these surfaces to provide constraint. Thus, the elbow would be at higher risk for instability and, with the accompanying increase in contact stress, predisposed to suffer polyethylene wear. These types of studies point to the importance of establishing and maintaining levels of constraint. However, competing goals exist: namely, to provide constraint while not leading to excessive wear and deformation of the bearing surfaces that in turn creates the dual problems of excessive wear debris as well as instability. These competing goals have not been reached as yet for elbow arthroplasty and are further hampered by both simple limitations (the size of the elbow and hence the size of the bearing surfaces are much smaller than in knee, hip, and shoulder arthroplasties) and by more complicated limitations like the lack of functional activity data in the form of kinematics and load data for the elbow.
The question as to how much constraint a device should provide must be tailored to the patient’s needs and the surgeon’s ability to assess the state of the joint and to preserve important soft tissue structures that provide secondary stabilizing loads during functional activities. Although semiconstrained devices may be unnecessary in the presence of adequate and balanced soft tissues across the joint, little objective data exist to guide surgeon choice, either in terms of comparative data among available devices as to their stability under realistic load scenarios or clear ways for interpreting how such data can best be used in making the correct choice for the patient.
How Do We Get There?
Improving the performance of TEA with the goal of limiting wear and instability problems secondary to polyethylene damage and unintended metal-on-metal wear will require an integrated approach combining clinical and translational research. Clinical research might be best focused on multicenter studies. Although difficulties can arise in pooling data, such efforts would allow more thorough assessment of key factors that have emerged from smaller, low level of evidence studies. Such an approach was recently taken for TKA in patients with juvenile idiopathic arthritis, for which a better measure of survivability and functional outcomes emerged than would have been possible using the data from one center alone [15]. Longitudinal clinical studies performed this way are also necessary. For example, one might expect increased rates of instability over time with polyethylene wear in nonconstrained implants, but to our knowledge, no such evaluation has been done. The results of such a study would not only inform surgeons of the expected behavior of nonconstrained implants over time, but also provide important data that could be used in improving the design of these types of implants.
Translational research studies should aim to measure loads across the elbow and elbow kinematics for a spectrum of daily activities and in both normal individuals and patients with elbow arthroplasties. Instrumented prostheses to provide in vivo joint load data combined with computational models to determine muscle and joint reaction loads can serve as valuable inputs to stress analyses aimed at understanding how wear and fixation are influenced by implant design and materials. More kinematics studies of the type performed by Futai et al. [10] using fluoroscopy to understand kinematics would provide additional complementary data for this research.
Performance of elbow arthroplasty can be improved by understanding which factors, biomechanical, patient, and surgical, most influence failure mechanisms in contemporary elbow arthroplasty devices. This approach has proven successful in providing the basis for rational solutions to similar problems in knee and hip arthroplasty by combining biomechanical analyses with clinical evidence of failure modes. A similar approach should work in elbow arthroplasty, because the most prevalent failure modes—aseptic loosening of fixation stems, excessive polyethylene wear, and periprosthetic osteolysis—all suggest that biomechanical factors play a dominant role. In the case of elbow arthroplasty, however, a comprehensive failure analysis is hampered by the lack of meaningful observations of clinical failures and limited information as to the loads occurring across the elbow as patients undergoing elbow arthroplasty go about their daily activities. These limitations must be overcome by determining how biomechanical, patient, and surgical factors affect implant performance in TEA.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
References
- 1.Athwal GS, Morrey BF. Revision total elbow arthroplasty for prosthetic fractures. J Bone Joint Surg Am. 2006;88:2017–2026. doi: 10.2106/JBJS.E.00878. [DOI] [PubMed] [Google Scholar]
- 2.Brownhill JR, Pollock JW, Ferreira LM, Johnson JA, King GJ. The effect of implant malalignment on joint loading in total elbow arthroplasty: an in vitro study. J Shoulder Elbow Surg. 2012;21:1032–1038. doi: 10.1016/j.jse.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Celli A, Morrey BF. Total elbow arthroplasty in patients forty years of age or less. J Bone Joint Surg Am. 2009;91:1414–1418. doi: 10.2106/JBJS.G.00329. [DOI] [PubMed] [Google Scholar]
- 4.Degreef I, Sciot R, De Smet L. Metallosis in revision total elbow arthroplasty. Complications and staging method. Acta Orthop Belg. 2008;74:753–760. [PubMed] [Google Scholar]
- 5.Ewald FC, Simmons ED, Jr, Sullivan JA, Thomas WH, Scott RD, Poss R, Thornhill TS, Sledge CB. Capitellocondylar total elbow replacement in rheumatoid arthritis. Long-term results. J Bone Joint Surg Am. 1993;75:498–507. doi: 10.2106/00004623-199304000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Fevang BT, Lie SA, Havelin LI, Skredderstuen A, Furnes O. Results after 562 total elbow replacements: a report from the Norwegian Arthroplasty Register. J Shoulder Elbow Surg. 2009;18:449–456. doi: 10.1016/j.jse.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Figgie HE, 3rd, Inglis AE, Mow C. A critical analysis of alignment factors affecting functional outcome in total elbow arthroplasty. J Arthroplasty. 1986;1:169–173. doi: 10.1016/S0883-5403(86)80027-4. [DOI] [PubMed] [Google Scholar]
- 8.Figgie MP, Gerwin M, Weiland AJ. Revision total elbow replacement. Hand Clin. 1994;10:507–520. [PubMed] [Google Scholar]
- 9.Figgie MP, Su EP, Kahn B, Lipman J. Locking mechanism failure in semiconstrained total elbow arthroplasty. J Shoulder Elbow Surg. 2006;15:88–93. doi: 10.1016/j.jse.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Futai K, Tomita T, Yamazaki T, Murase T, Yoshikawa H, Sugamoto K. In vivo three-dimensional kinematics of total elbow arthroplasty using fluoroscopic imaging. Int Orthop. 2010;34:847–854. doi: 10.1007/s00264-010-0972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill DR, Morrey BF. The Coonrad-Morrey total elbow arthroplasty in patients who have rheumatoid arthritis. A ten to fifteen-year follow-up study. J Bone Joint Surg Am. 1998;80:1327–1335. doi: 10.1302/0301-620X.80B6.8658. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg SH, Urban RM, Jacobs JJ, King GJ, O’Driscoll SW, Cohen MS. Modes of wear after semi-constrained total elbow arthroplasty. J Bone Joint Surg Am. 2008;90:609–619. doi: 10.2106/JBJS.F.01286. [DOI] [PubMed] [Google Scholar]
- 13.Goodman SB, Wright TM. Osteolysis and implant wear: biological, biomedical engineering, and surgical principles. J Am Acad Orthop Surg. 2008;16(Suppl):x–xi. [PubMed]
- 14.Gschwend N, Scheier NH, Baehler AR. Long-term results of the GSB III elbow arthroplasty. J Bone Joint Surg Br. 1999;81:1005–1012. doi: 10.1302/0301-620X.81B6.9495. [DOI] [PubMed] [Google Scholar]
- 15.Heyse TJ, Ries MD, Bellemans J, Goodman SB, Scott RD, Wright TM, Lipman JD, Schwarzkopf R, Figgie MP. Total knee arthroplasty in patients with juvenile idiopathic arthritis. Clin Orthop Relat Res. 2014;472:147–154. doi: 10.1007/s11999-013-3095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikävalko M, Tiihonen R, Skyttä ET, Belt EA. Long-term survival of the Souter-Strathclyde total elbow replacement in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2010;92:656–660. doi: 10.1302/0301-620X.92B5.22613. [DOI] [PubMed] [Google Scholar]
- 17.Jeon IH, Morrey BF, Sanchez-Sotelo J. Ulnar component surface finish influenced the outcome of primary Coonrad-Morrey total elbow arthroplasty. J Shoulder Elbow Surg. 2012;21:1229–1235. doi: 10.1016/j.jse.2011.08.062. [DOI] [PubMed] [Google Scholar]
- 18.Kudo H, Iwano K, Nishino J. Cementless or hybrid total elbow arthroplasty with titanium-alloy implants. A study of interim clinical results and specific complications. J Arthroplasty. 1994;9:269–278. doi: 10.1016/0883-5403(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 19.Kudo H, Iwano K, Nishino J. Total elbow arthroplasty with use of a nonconstrained humeral component inserted without cement in patients who have rheumatoid arthritis. J Bone Joint Surg Am. 1999;81:1268–1280. doi: 10.2106/00004623-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Landor I, Vavrik P, Jahoda D, Guttler K, Sosna A. Total elbow replacement with the Souter-Strathclyde prosthesis in rheumatoid arthritis. Long-term follow-up. J Bone Joint Surg Br. 2006;88:1460–1463. doi: 10.1302/0301-620X.88B11.17807. [DOI] [PubMed] [Google Scholar]
- 21.Lee BP, Adams RA, Morrey BF. Polyethylene wear after total elbow arthroplasty. J Bone Joint Surg Am. 2005;87:1080–1087. doi: 10.2106/JBJS.D.02163. [DOI] [PubMed] [Google Scholar]
- 22.Little CP, Graham AJ, Karatzas G, Woods DA, Carr AJ. Outcomes of total elbow arthroplasty for rheumatoid arthritis: comparative study of three implants. J Bone Joint Surg Am. 2005;87:2439–2448. doi: 10.2106/JBJS.D.02927. [DOI] [PubMed] [Google Scholar]
- 23.Morrey BF, Adams RA, Bryan RS. Total replacement for post-traumatic arthritis of the elbow. J Bone Joint Surg Br. 1991;73:607–612. doi: 10.1302/0301-620X.73B4.2071644. [DOI] [PubMed] [Google Scholar]
- 24.Patil N, Cheung EV, Mow CS. High revision rate after total elbow arthroplasty with a linked semiconstrained device. Orthopedics. 2009;32:321. doi: 10.3928/01477447-20090501-11. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen JV, Olsen BS, Fevang BT, Furnes O, Skytta ET, Rahme H, Salomonsson B, Mohammed KD, Page RS, Carr AJ. A review of national shoulder and elbow joint replacement registries. J Shoulder Elbow Surg. 2012;21:1328–1335. doi: 10.1016/j.jse.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Reinhard R, van der Hoeven M, de Vos MJ, Eygendaal D. Total elbow arthroplasty with the Kudo prosthesis. Int Orthop. 2003;27:370–372. doi: 10.1007/s00264-003-0491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson E, Burke N, Douglas P, Orr J, Pooley J. Mechanism of loosening of the Souter-Strathclyde total elbow replacement evidence from revision surgery. Acta Orthop Belg. 2010;76:27–29. [PubMed] [Google Scholar]
- 28.Rozing P. Souter-Strathclyde total elbow arthroplasty. J Bone Joint Surg Br. 2000;82:1129–1134. doi: 10.1302/0301-620X.82B8.10148. [DOI] [PubMed] [Google Scholar]
- 29.Sayed-Noor AS, Sjödén GO. Severe metallosis after total elbow arthroplasty—a case report. Hand (NY). 2010;5:86–89. doi: 10.1007/s11552-009-9201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneeberger AG, Adams R, Morrey BF. Semiconstrained total elbow replacement for the treatment of post-traumatic osteoarthrosis. J Bone Joint Surg Am. 1997;79:1211–1222. doi: 10.2106/00004623-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Schuind F, O’Driscoll S, Korinek S, An KN, Morrey BF. Loose-hinge total elbow arthroplasty. An experimental study of the effects of implant alignment on three-dimensional elbow kinematics. J Arthroplasty. 1995;10:670–678. doi: 10.1016/S0883-5403(05)80214-1. [DOI] [PubMed] [Google Scholar]
- 32.Seitz WH, Jr, Bismar H, Evans PJ. Failure of the hinge mechanism in total elbow arthroplasty. J Shoulder Elbow Surg. 2010;19:368–375. doi: 10.1016/j.jse.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Shah BM, Trail IA, Nuttal D, Stanley JK. The effect of epidemiologic and intraoperative factors on survival of the standard Souter-Strathclyde total elbow arthroplasty. J Arthroplasty. 2000;15:994–998. doi: 10.1054/arth.2000.9839. [DOI] [PubMed] [Google Scholar]
- 34.Shi LL, Zurakowski D, Jones DG, Koris MJ, Thornhill TS. Semicontrained primary and revision total elbow arthroplasty with use of the Coonrad-Morrey prosthesis. J Bone Joint Surg Am. 2007;89:1467–1475. doi: 10.2106/JBJS.F.00715. [DOI] [PubMed] [Google Scholar]
- 35.Skyttä ET, Eskelinen A, Paavolainen P, Ikävalko M, Remes V. Total elbow arthroplasty in rheumatoid arthritis: a population-based study from the Finnish Arthroplasty Register. Acta Orthop. 2009;80:472–477. doi: 10.3109/17453670903110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stokdijk M, Meskers CGM, Veeger HEJ, deBoer YA, Rozing PM. Determination of the optimal elbow axis for evaluation of placement of prostheses. Clin Biomech. 1999;14:177–184. [DOI] [PubMed]
- 37.Tanaka N, Kudo H, Iwano K, Sakahashi H, Sato E, Ishii S. Kudo total elbow arthroplasty in patients with rheumatoid arthritis: a long-term follow-up study. J Bone Joint Surg Am. 2001;83:1506–1513. doi: 10.1302/0301-620X.83B5.11745. [DOI] [PubMed] [Google Scholar]
- 38.Throckmorton T, Zarkadas P, Sanchez-Sotelo J, Morrey B. Failure patterns after linked semiconstrained total elbow arthroplasty for posttraumatic arthritis. J Bone Joint Surg Am. 2010;92:1432–1441. doi: 10.2106/JBJS.I.00145. [DOI] [PubMed] [Google Scholar]
- 39.van der Lugt JCT, Valstar ER, Witvoet-Braam SW, Nelissen RGHH. Migration of the humeral component of the Souter-Strathclyde elbow prosthesis. J Bone Joint Surg-Br. 2010;92:235–241. doi: 10.1302/0301-620X.92B2.22636. [DOI] [PubMed] [Google Scholar]
- 40.van Riet RP, Morrey BF, O’Driscoll SW. The Pritchard ERS total elbow prosthesis: lessons to be learned from failure. J Shoulder Elbow Surg. 2009;18:791–795. doi: 10.1016/j.jse.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Willing R, King GJ, Johnson JA. The effect of implant design of linked total elbow arthroplasty on stability and stress: a finite element analysis. Comput Methods Biomech Biomed Eng. 2014;17:1165–1172. doi: 10.1080/10255842.2012.739161. [DOI] [PubMed] [Google Scholar]
- 42.Wright TM, Goodman SB. Implant Wear: The Future of Total Joint Replacement. Rosemont, IL, USA: American Academy of Orthopaedic Surgeons; 1996. [Google Scholar]
- 43.Wright TM, Goodman SB. Implant Wear in Total Joint Replacement: Clinical and Biologic Issues, Materials and Design Considerations. Rosemont, IL, USA: American Academy of Orthopaedic Surgeons; 2001. [Google Scholar]
- 44.Wright TW, Hastings H. Total elbow arthroplasty failure due to overuse, C-ring failure, and/or bushing wear. J Shoulder Elbow Surg. 2005;14:65–72. doi: 10.1016/j.jse.2004.04.015. [DOI] [PubMed] [Google Scholar]


