Abstract
Background
Limb salvage implants that rely on compliant compression osseointegration to achieve bone fixation may achieve longer survivorship rates compared with traditional cemented or press-fit stemmed implants; however, failures resulting from rotational instability have been reported. The effect of using antirotation pins on the rotational stability of the fixation has not been well studied.
Questions/purposes
We asked the following question: When tested in a cadaver model, does the use of antirotation pins increase the torque required to cause implant failure or rotation?
Methods
Thirty-two cadaver femurs were divided into four groups of eight femurs. We compared the torque to failure among groups containing zero, one, two, three, and four pins using a servohydraulic testing device.
Results
Adding antirotation pins increased the torque required to cause failure (R2 = 0.77; p < 0.001). This increase was most notable in groups comparing zero pins with one pin (14 N-m, [95% CI, 10.9–17.1] versus 23 N-m, [95% CI 22.5–23.48]; p = 0.01) and two compared with three pins (29 N-m, [95% CI, 21.7–36.3] versus 42 N-m, [95% CI, 37.8–46.2]; p = 0.35).
Conclusions
It appears that the use of antirotation pins improves rotational stability of the compliant compression endoprosthesis. Although these findings need to be verified in a clinical study, the addition of antirotation pins may improve osteointegration and we have changed our practice to use a minimum of three antirotation pins when implanting this device.
Clinical Relevance
Improvements in implant technology and surgical techniques may lead to improved clinical outcomes and patient quality of life. Addition of antirotation pins appears to improve implant stability and may decrease the need for revision surgery.
Introduction
Endoprosthetic joint reconstruction after bone sarcoma resection is a common limb salvage technique. Although these implants are durable in the short term, many patients eventually require revision surgery as a result of prosthesis failure [10, 16, 18, 21]. With cancer survival improving to as much as 80% at 5 years for individuals who present with localized extremity sarcoma, many patients will outlive their implants and may require complex revision surgery [2, 4–6]. Therefore, there is a critical need for reconstruction techniques that are durable enough to meet the demands of this expanding patient population and avoid the morbidity of additional surgery. Traditional endoprosthetic implants rely on intramedullary stems to achieve fixation at the bone-prosthesis interface. These reconstructions eventually fail owing to aseptic loosening, particle-induced osteolysis, infection, and stress shielding [12–14, 16].
Compliant prestress osseointegration (Compress®; Biomet, Warsaw, IN, USA) is a relatively new implant technology and may have improved survival compared with traditional implants [9]. This device achieves immediate compliant fixation onto host bone using a spindle that attaches directly onto the cut end of the bone. The spindle is secured by tightening a nut across a series of Belleville washers that act as springs and generate a compliant compression force across the bone-prosthesis interface. Loading forces are transmitted directly onto the host bone in an axial direction resulting in bone growth at the bone-prosthesis interface and osseointegration (Fig. 1) [1, 8, 15]. This device has short- and intermediate-term survivorship that is at least equivalent if not superior to stemmed implants, but there is concern that rotational forces at the bone-prosthesis interface can lead to loosening and failure of the implant (Fig. 2) [3, 8, 17]. A strategy to overcome this problem is to insert antirotation pins through the spindle into the host bone (Fig. 3). A theoretical disadvantage of using antirotation pins is that the presence of drill holes and pins may act as a stress riser and increase the risk of fracture. Another consideration is that drilling may cause damage to the periosteum and inhibit osseointegration. Therefore, some surgeons favor using no pins or the minimum number necessary to achieve rotational stability [8]. However, to our knowledge, there are no studies to indicate how the number of pins affects rotational stability, increases the risk of fracture, or interferes with osseointegration.
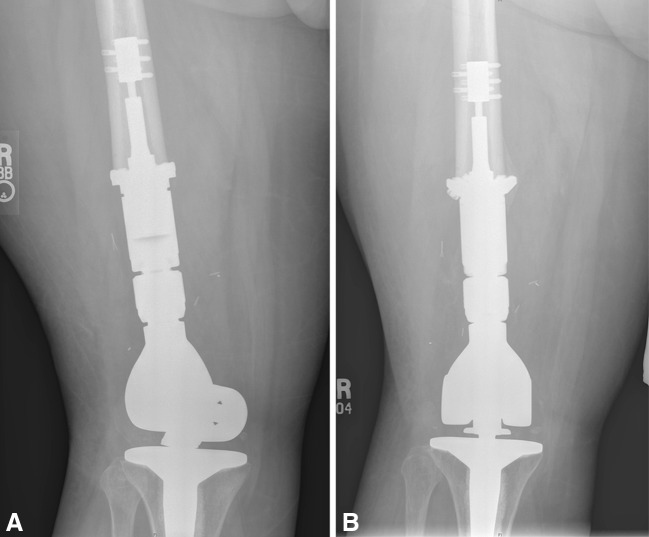
Fig. 1A–B.
The radiographs show the femur (A) immediately after implanting a Compress® device and (B) 15 months later where a bone growth implant interface is present.
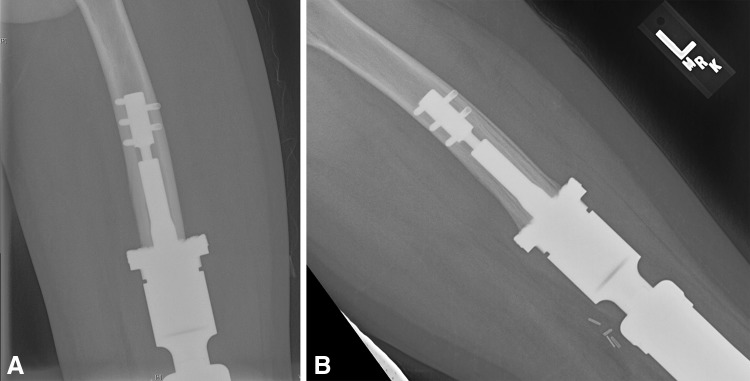
Fig. 2A–B.
(A) A true AP radiograph of the lower limb shows the distal femoral component rotated externally approximately 45°. (B) This AP radiograph shows a successful revision. The patient was a 22-year-old man who noticed sudden rotation of his knee when he crossed his leg to tie his shoe.
Fig. 3.

The photograph shows the implant with antirotation pins in place.
We therefore sought to evaluate the biomechanical effects of antirotation pins on rotational stability using this compression device. We specifically asked the following question: (1) When tested in a cadaver model, does the use of antirotation pins increase the torque required to cause implant failure or rotation?
Materials and Methods
To examine the effect of antirotation pins on torque to failure and risk of fracture, we conducted a biomechanical study using eight matched pairs of formalin-fixed adult human femurs (Stanford University School of Medicine). These specimens were divided into four groups of two pairs each. Because each femur underwent osteotomy at the middiaphysis to yield four testing specimens per matched femur pair, bone quality was controlled among the testing groups. Dual-energy x-ray absorptiometry was performed to ensure uniform bone mineral density among the specimens. To further control for bone quality we organized testing groups by matched pairs that were cut in half as described above. Group I was designed to be a comparison among four femurs that had no antirotation pins and four with one antirotation pin. Group II compared four femurs with one pin with four with two pins. Group III was made up of femurs with two pins and with three pins. Group IV was made up of femurs with three pins and with four pins. Each femur was potted in acrylic cement and an implant was attached to the cut end of the femur using standard surgical techniques and instruments. The exposed end of the compression implant device was mated to a servohydraulic device (Fig. 4) (MTS, Eden Prairie, MN, USA). An axial force of 600 pounds was applied at the bone-prosthesis interface to simulate the standard load applied in clinical practice. This force is determined by the number of washers set in the chamber in the factory. In clinical practice, the surgeon can request a 400-pound, 600-pound, or 800-pound implant. We elected to use 600 pounds as the force of attachment of the implant on the bone for this study since this force is commonly used in clinical practice.
Fig. 4.

The biomechanical testing setup is shown with the Compress® device mounted to a servohydraulic testing device and implanted in a cadaver femur.
A large spindle was used in all specimens. Testing was done by applying a rotational force at a rate of 1° per second until failure occurred, which was defined as rotation of the implant as measured by the servohydraulic device. Using data from preliminary testing a power analysis for superiority (α = 0.05; power 0.8) indicated four specimens were needed per group to detect a difference of 5 N-m in torque to failure.
Results
Insertion of antirotation pins increased the torque needed to cause failure. After accounting for bone mineral density, a linear regression analysis showed a significant correlation between adding pins and increasing torque required for failure (R2 = 0.77; p < 0.001). In Group I, the average loads to failure were 14 N-m (95% CI, 10.9–17.1) and 23 N-m (95% CI, 22.5–23.48) for the samples with no pins and one pin, respectively (p = 0.01). In Group II, the average loads to failure were 23 N-m (± 3) and 27 (± 5) for the samples with one and two pins, respectively (p = 0.33). In Group III, the average loads to failure were 29 N-m (95% CI, 21.7–36.3) and 42 N-m (95% CI, 37.8–46.2) for the samples with two and three pins, respectively (p = 0.035). In Group IV, the average loads to failure were 39 N-m (± 7) and 42 N-m (± 5) for the samples with three and four pins, respectively (p = 0.57). Four of the eight femurs tested with three pins and two of the four tested with four pins exceeded the torque capacity of 45 N-m of the testing machine and did not fail.
Discussion
Traditional limb salvage implants rely on stems to achieve fixation in the host bone. Compression osseointegration technology is an alternative form of implant fixation that attaches directly to the cut end of a given bone with compliant compressive force. This method of fixation may eliminate the problems of stress shielding, osteolysis, and aseptic loosening. Although the short-term clinical results of this device are encouraging, there are reports of loosening of the device at the bone attachment site that require revision surgery [8, 17]. These failures may be the result of rotational instability at the bone-prosthesis interface which may be mitigated by insertion of antirotation pins. However, there are no data regarding this issue to help guide clinical decision-making of physicians who care for patients with bone sarcoma. The purpose of the current study was to determine whether antirotation pins increased the stability of the bone-implant interface.
There are several limitations to this biomechanical study. First, this experiment was designed to evaluate torque to failure, which is an oversimplification of the forces experienced in vivo. There are other forces that may contribute to device failure such as bending and tension, however rotation is thought to be the major force contributing to failure [1, 7, 17]. In addition, although the Compress® device may be attached to host bone using 400, 600, and 800 pounds of force, we tested only the 600-pound attachment because this is what we use most frequently in practice. Because we kept force constant and measured relative torque to failure with different numbers of pins we think these results would be similar to those of the other commercially available force attachment options. Finally, we did not test if there is a difference in torque to failure between two pins inserted at 180° or 90° relative to each other. Because we are testing rotational rather than bending forces, it is unlikely that pin orientation affects the torsion required for failure; however, our study does not address this question and we cannot draw any conclusions regarding pin orientation.
The results of our study indicate that the torque needed to cause rotational failure of the device increases with each additional pin and the risk of fracture decreases. The stability of this compression osseointegration implant appears to be sufficient to withstand forces that would be seen at the bone-prosthesis interface with activities of daily living. In a study using instrumented implants, Taylor and Walker [19] measured forces and moments in the shafts of distal femur replacements in two human subjects. Data that were obtained using telemetry from the implants showed a peak torque in the stem of 9 N-m while jogging and 6 N-m in the stance phase of level walking. In an earlier study using the same telemetry technique, Taylor et al. [20] found axial forces in a distal femur stem to be 6 N-m, 7 N-m, and 8 N-m for ascending stairs, descending stairs, and rising from a chair, respectively. In the current study, the least amount of torque that led to failure of the implant was 12 N-m in the no-pin group and the average torque to failure was 14 N-m in that group. These data suggest that when no antirotation pins are used, the friction at the bone-prosthesis interface may be sufficient to withstand in vivo rotational forces and that insertion of antirotation pins adds a margin of safety to withstanding these forces. However, one must remember that in clinical practice, these patients are asked to limit their weightbearing on the affected limb and to use assistive devices such as crutches or a walker for prolonged times. In addition, for patients with cancer who are on strict chemotherapy regimens, even a low rate of implant failure is important because revision surgery poses risks of delays in cancer treatment, infections in patients who are immunocompromised, poor wound healing, and prolonged morbidity [10, 11]. Therefore, some surgeons may wish to use antirotation pins to increase implant stability to allow early weightbearing, mitigate risks associated with chemotherapy, and decrease the overall risk of rotational failures. In addition, antirotation pins may be beneficial in situations in which patients may not be able to adhere to activity and crutch precautions, such as after thoracic surgery for metastasis removal.
In our biomechanical study, antirotation pins inserted in the Compress® device increased the torque required to cause rotational failure of this implant at the bone-prosthesis interface. Advantages of using these pins in the clinical setting are that they may decrease the failure rate of this device, allow for earlier ambulation and full weightbearing after surgery, and may decrease the need for revision surgery. Disadvantages may include disruption of the periosteum and blood supply at the bone-prosthesis interface that may slow osseointegration and the theoretical increased risk of fracture at the pin insertion site. Based on the results of our biomechanical study, we now use at least three antirotation pins when inserting a compliant compression osseointegration device. Future trials designed to address whether use of antirotation pins can allow patients to immediately bear full weight after surgery without increasing risk of failure and whether the drill holes for antirotation pins act as stress risers and increase the risk of fractures would be helpful.
Acknowledgments
We thank Daniel Martin MD, for assistance in designing and implementing the biomechanical portion of this study.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved or waived approval for the cadaver protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This study was performed at Stanford University and Veterans’ Health Administration Palo Alto.
References
- 1.Avedian RS, Goldsby RE, Kramer MJ, O’Donnell RJ. Effect of chemotherapy on initial compressive osseointegration of tumor endoprostheses. Clin Orthop Relat Res. 2007;459:48–53. doi: 10.1097/BLO.0b013e3180514c66. [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Longhi A, Cesari M, Versari M, Bertoni F. Influence of local recurrence on survival in patients with extremity osteosarcoma treated with neoadjuvant chemotherapy: the experience of a single institution with 44 patients. Cancer. 2006;106:2701–2706. doi: 10.1002/cncr.21937. [DOI] [PubMed] [Google Scholar]
- 3.Bhangu AA, Kramer MJ, Grimer RJ, O’Donnell RJ. Early distal femoral endoprosthetic survival: cemented stems versus the Compress implant. Int Orthop. 2006;30:465–472. doi: 10.1007/s00264-006-0186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickels J, Wittig JC, Kollender Y, Henshaw RM, Kellar-Graney KL, Meller I, Malawer MM. Distal femur resection with endoprosthetic reconstruction: a long-term followup study. Clin Orthop Relat Res. 2002;400:225–235. doi: 10.1097/00003086-200207000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.20.3.776. [DOI] [PubMed] [Google Scholar]
- 6.Bielack SS, Kempf-Bielack B, Winkler K. Osteosarcoma: relationship of response to preoperative chemotherapy and type of surgery to local recurrence. J Clin Oncol. 1996;14:683–684. doi: 10.1200/JCO.1996.14.2.683. [DOI] [PubMed] [Google Scholar]
- 7.Calvert GT, Cummings JE, Bowles AJ, Jones KB, Wurtz LD, Randall RL. A dual-center review of compressive osseointegration for fixation of massive endoprosthetics: 2- to 9-year followup. Clin Orthop Relat Res. 2014;472:822–829. doi: 10.1007/s11999-013-2885-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farfalli GL, Boland PJ, Morris CD, Athanasian EA, Healey JH. Early equivalence of uncemented press-fit and Compress femoral fixation. Clin Orthop Relat Res. 2009;467:2792–2799. doi: 10.1007/s11999-009-0912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Healey JH, Morris CD, Athanasian EA, Boland PJ. Compress knee arthroplasty has 80% 10-year survivorship and novel forms of bone failure. Clin Orthop Relat Res. 2013;471:774–783. doi: 10.1007/s11999-012-2635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–429. doi: 10.2106/JBJS.J.00834. [DOI] [PubMed] [Google Scholar]
- 11.Imran H, Enders F, Krailo M, Sim F, Okuno S, Hawkins D, Neglia J, Randall RL, Womer R, Mascarenhas L, Arndt CA. Effect of time to resumption of chemotherapy after definitive surgery on prognosis for non-metastatic osteosarcoma. J Bone Joint Surg Am. 2009;91:604–612. doi: 10.2106/JBJS.H.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeys LM, Kulkarni A, Grimer RJ, Carter SR, Tillman RM, Abudu A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am. 2008;90:1265–1271. doi: 10.2106/JBJS.F.01324. [DOI] [PubMed] [Google Scholar]
- 13.Kawai A, Lin PP, Boland PJ, Athanasian EA, Healey JH. Relationship between magnitude of resection, complication, and prosthetic survival after prosthetic knee reconstructions for distal femoral tumors. J Surg Oncol. 1999;70:109–115. doi: 10.1002/(SICI)1096-9098(199902)70:2<109::AID-JSO9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Kawai A, Muschler GF, Lane JM, Otis JC, Healey JH. Prosthetic knee replacement after resection of a malignant tumor of the distal part of the femur: medium to long-term results. J Bone Joint Surg Am. 1998;80:636–647. doi: 10.1302/0301-620X.80B4.8216. [DOI] [PubMed] [Google Scholar]
- 15.Kramer MJ, Tanner BJ, Horvai AE, O’Donnell RJ. Compressive osseointegration promotes viable bone at the endoprosthetic interface: retrieval study of Compress implants. Int Orthop. 2008;32:567–571. doi: 10.1007/s00264-007-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittermayer F, Krepler P, Dominkus M, Schwameis E, Sluga M, Heinzl H, Kotz R. Long-term followup of uncemented tumor endoprostheses for the lower extremity. Clin Orthop Relat Res. 2001;388:167–177. doi: 10.1097/00003086-200107000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Pedtke AC, Wustrack RL, Fang AS, Grimer RJ, O’Donnell RJ. Aseptic failure: how does the Compress® implant compare to cemented stems? Clin Orthop Relat Res. 2012;470:735–742. doi: 10.1007/s11999-011-2159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz AJ, Kabo JM, Eilber FC, Eilber FR, Eckardt JJ. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clin Orthop Relat Res. 2010;468:2198–2210. doi: 10.1007/s11999-009-1197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor SJ, Walker PS. Forces and moments telemetered from two distal femoral replacements during various activities. J Biomech. 2001;34:839–848. doi: 10.1016/S0021-9290(01)00042-2. [DOI] [PubMed] [Google Scholar]
- 20.Taylor SJ, Walker PS, Perry JS, Cannon SR, Woledge R. The forces in the distal femur and the knee during walking and other activities measured by telemetry. J Arthroplasty. 1998;13:428–437. doi: 10.1016/S0883-5403(98)90009-2. [DOI] [PubMed] [Google Scholar]
- 21.Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13. [PubMed] [Google Scholar]