Abstract
A fully functioning immune system is essential in order to maintain good health. However, the immune system deteriorates with advancing age, and this contributes to increased susceptibility to infection, autoimmunity, and cancer in the older population. Progress has been made in identifying age-related defects in the adaptive immune system. In contrast, relatively little research has been carried out on the impact of ageing on the innate immune response. This area requires further research as the innate immune system plays a crucial role in protection against infection and represents a first line of defence. Macrophages are central effector cells of the innate immune system and have many diverse functions. As a result, age-related impairments in macrophage function are likely to have important consequences for the health of the older population. It has been reported that ageing in macrophages impacts on many processes including toll-like receptor signalling, polarisation, phagocytosis, and wound repair. A detailed understanding of the impact of ageing on macrophages is required in order to develop therapeutics that will boost immune responses in the older population.
Keywords: ageing, immune system, macrophage, phagocytosis, polarisation
Introduction
The global population is currently undergoing unprecedented demographic changes. For example, in the year 2000, individuals over 60 years of age represented 10% of the global population, but this is projected to increase to 22% by 2050 [1]. This demographic shift is in part due to an increase in life span. In 150 years, average global life span has increased from 40 years to approximately 80 years [2]. However, ageing is inevitably accompanied by deterioration in health. Chronic diseases and increased susceptibility to infection negatively impact on the quality of life of the older population. In addition to this, there are rising health care costs and major economic consequences associated with this demographic shift. For all of these reasons, maintaining the health of the older population has become an important research and therapeutic goal.
The biological consequences of ageing are similar across mammalian species and include curvature of the spine, impairments in eyesight and hearing, frailty, graying, cognitive impairments, and immune decline [3]. This review will focus on the impact of ageing on the immune system with particular focus on macrophages.
The immune system is essential for survival and has evolved to defend against dangerous pathogens and maintain homeostasis within an organism. The immune system combats invaders such as bacteria and viruses but also altered endogenous factors including cancerous cells. The immune system can be broadly divided into two components known as the innate and adaptive systems [4]. The innate immune system represents the rapid first line of defence and is present in all multicellular organisms. Pathogen entry is initially prevented by the barrier function of the skin and epithelial linings of the respiratory, urogenital and gastrointestinal, and other mucosal systems. Additionally, antimicrobial factors in secretions including β-defensins impede infection at these sites. However, if these barriers are breached, invading microbes come into contact with cellular components of the innate immune system such as tissue macrophages. Upon encountering pathogens, tissue macrophages initiate inflammation via secretion of cytokines and chemokines, resulting in the recruitment and activation of other cells including neutrophils and monocytes. These cells attempt to prevent pathogen invasion via phagocytosis, secretion of lytic enzymes, and activation of complement. During this process, dendritic cells take up antigen and migrate to the lymph nodes where they interact with cells of the adaptive immune response [5].
The adaptive immune response is antigen specific, takes days to initially develop, and is only present in vertebrates [6]. T cells and B cells interact with antigen presenting cells (APC) presenting antigen and become activated, proliferate and migrate to the site of inflammation. B cells produce antibodies that target extracellular pathogens. T cell populations include CD8+ cytotoxic T cells that lyse infected cells and CD4+ T helper cells that secrete a wide range of cytokines and activate other components of the immune response. However, unlike the innate system, after an antigen has been encountered by the adaptive immune system, a potent lasting memory response is generated. Subsequent exposure leads to fast responses from the adaptive immune system. Although the innate and adaptive systems are frequently described as separate entities, in reality, components of the innate and adaptive responses overlap and, in most cases, both systems are required to work in conjunction to produce an effective immune response.
Immunosenescence
Age-related impairments in the immune system, referred to as immunosenescence, contribute to increased susceptibility to infection in the older population. This is evident in the particularly high incidence of urinary tract, skin, and respiratory system infections with increasing age. In addition, the severity and morbidity associated with these infections also increase with age. For example, each year, more than 90% of deaths due to influenza occur in individuals over 65 years of age [7]. Protecting this older population from infectious diseases is made even more challenging by age-related decline in responses to vaccination. Notably, influenza vaccination is only effective in 30–40% of this older population [8]. As such, the World Health Organisation has identified the development of new vaccines, targeted specifically at older adults, as an important part of their future research agenda [9].
However, the health implications of immunosenescence are not confined to infectious diseases. Age-related decline in immune function is also associated with increased incidence of cancer, potentially due to decreased immunosurveillance and antitumor effector function [10]. Interestingly, ageing is also associated with increased risk of autoimmunity suggesting that ageing impacts immunoregulatory and tolerogenic mechanisms of the immune system [11].
Increased levels of proinflammatory cytokines such as IL-6 and TNF-α are often observed in the serum of older individuals. This chronic low-grade inflammation is referred to as “Inflamm-ageing” [12]. It has been hypothesised that this long-term low-grade inflammation may contribute to susceptibility to many chronic diseases such as Alzheimer’s disease and cardiovascular disease [13].
Impact of ageing on the adaptive immune response
T cells
The majority of ageing research has focused on the adaptive immune response. There are some well-established changes that occur with age, particularly in the T cell compartment. Involution of the thymus had been observed in both humans and mice and involves reduction in size of the organ and increase in adipocyte content. This is associated with decreased output of naive T cells from the thymus [14]. However, it is likely that other factors also impact on production of naive T cells. For example, ageing also affects hematopoietic stem cells (HSCs). It has been reported that, with increasing age, HSCs have a decreased capacity to produce lymphoid progenitors and are biased toward myeloid progenitors [15].
Decreased naive T cells in the periphery leads to a decreased response to novel antigen and contributes to the reduced efficiency of vaccination in the older population. This reduction in naive T cells in the periphery is accompanied by an increase in memory T cells. As a result, the peripheral T cell repertoire is dominated by terminally differentiated memory cells. Notably, there is accumulation of CD8+ memory T cells and many of these CD8+ T cells lack expression of the costimulatory molecule CD28 [16]. It has been hypothesised that repeated exposure to antigen over a lifetime shapes the T cell repertoire in ageing. For example, it has been demonstrated that CD8+CD28− T cells in ageing are part of oligoclonal expansions that are often specific for viruses such as cytomegalovirus [17].
B cells
The B cell compartment is also affected by ageing, impacting on the efficiency of the antibody response. There is an age-related decrease in the percentage and number of B cells in the blood in humans [18]. Impaired class-switch recombination has been reported in human and murine B cells, and this has been associated with decreased induction of activation-induced cytidine deaminase (AID) enzyme [19]. B cell production in the bone marrow declines with age in mice; however, it remains unclear whether this occurs in humans [20]. In mice, it has been established that the frequency of pro-B cells declines with age, and the number of B cells migrating from the bone marrow to the spleen is also reduced. Similar to T cells, this results in reduced numbers of naive B cells in the periphery. Accumulation of antigen-experienced memory B cells also occurs [21], leading to reduced diversity in the peripheral B cell repertoire and limited ability to respond to new antigenic challenges.
Although the emphasis in ageing research has been focused on the adaptive immune response, the innate immune system is also impacted [22]. Age-associated changes in the innate immune system are not well-defined, and there is considerable controversy and conflicting reports in the literature. However, it is now beginning to be recognised that we must also understand the impact of ageing on cells of the innate immune system in order to develop strategies to maintain health in the older population. In particular, age-related defects in macrophages have the potential to impact on many different processes in the body.
Functions of macrophages
Macrophages have many diverse functions within the immune system as summarised in Table 1. These potent immune effectors play an important role in elimination of invading pathogens via phagocytosis and production of reactive oxygen and nitrogen species. Macrophages release a vast range of inflammatory mediators, including cytokines and chemokines that are central to initiation and propagation of the inflammatory process [23]. Macrophages also play an important role in instruction of the adaptive immune response, capable of antigen presentation and activation of T and B cells. Importantly and often overlooked, macrophages play a vital role in maintenance of tissue homeostasis by scavenging apoptotic cells and production of growth factors. There is also evidence to suggest that macrophages play an important role in the development of many tissues [24]. In addition, macrophages aid efficient wound healing and tissue repair by clearance of debris and secretion of angiogenic and fibrogenic factors [25].
Table 1.
Summary of macrophage functions
| Functions of macrophages |
|---|
| – Elimination of pathogens |
| – Initiation of inflammatory responses |
| – Stimulation of adaptive immune response |
| – Tissue homeostasis |
| – Development of tissues |
| – Repair of damaged tissue |
Tissue macrophages are one of the first responders to pathogens once epithelial barriers have been breached. In order to detect pathogens/danger and subsequently initiate inflammation, macrophages express a range of germline-encoded pathogen recognition receptors (PRRs) [26, 27]. These receptors enable macrophages to recognise conserved and invariant microbial products referred to as pathogen associated molecular patterns (PAMPs). There are several classes of PRRs, including toll-like receptors (TLRs), Nod-like receptors (NLRs), and RIG-I like receptors (RLRs) [28]. Ligand-binding to PRRs results in production of proinflammatory cytokines, initiating the inflammatory response. For example, TLRs are transmembrane receptors on the cell surface and endosomal compartments that recognise viral nucleic acids and bacterial products including lipopolysaccharide (LPS) and lipoteichoic acid (LTA), as well as endogenous ligands [29]. Activation of TLRs in macrophages results in production of inflammatory cytokines including TNF-α, IL-1β, and IL-6. TNF-α induces endothelial activation which facilitates recruitment of leukocytes and serum proteins to the site of infection. In addition, IL-1β and IL-6 induce hepatocytes to secrete acute phase proteins such as collectins and pentraxins. These acute phase proteins opsonise microbial products and also activate the complement system. The complement system provides further opsonisation of pathogens and recruitment of other immune cells and directs killing of pathogens via membrane attack complexes [4].
It is important to note that macrophages are extremely heterogeneous. Tissue-resident macrophages are distributed widely throughout the body and functional capabilities and morphology varies between sites [24]. For example, Kupffer cells in the liver are involved in the clearance of pathogens and toxins from the circulation. Alveolar macrophages in the lungs eliminate dust, allergens, and microorganisms from the airway, and osteoclasts in the skeletal system play a role in bone resorption [30].
Macrophage phagocytosis
Macrophages are among the most potent phagocytic cells which also include neutrophils and immature dendritic cells and are collectively referred to as “professional phagocytes”. The phagocytic process is initiated by ligand binding to phagocytic receptors and activation of numerous intracellular signalling pathways. This leads to rearrangement of the actin cytoskeleton and internalisation of the target into a vacuole known as a phagosome. The phagosome acidifies and fuses with the lysosome leading to degradation of the internalised material [31]. This breakdown process involves reactive oxygen species generated by NADPH oxidase on phagosomal membranes and proteolytic enzymes such as lysozyme released from granules [32].
The main classes of phagocytic receptors are detailed in Table 2. Phagocytic receptors can be divided into two categories, opsonin-dependent and opsonin-indepenent. Opsonin-dependent receptors rely on antibody or complement-coating of ligand to facilitate phagocytosis. These include Fc receptors and complement receptors [33]. In addition, fibronectin and vitronectin can nonspecifically opsonise particles, and these are primarily taken up by integrins such as α5β1 [32]. Opsonin-independent receptors include scavenger receptors which bind to a variety of substances, including modified lipoproteins. Macrophage receptor with a collagenous structure (MARCO), a class A scavenger receptor, can bind gram-negative and gram-positive bacteria and has been suggested to be involved in the uptake of polystyrene particles [34]. Many opsonin-independent receptors bind to conserved microbial structures. For example, mannose receptor recognises α-mannans and dectin-1 binds to β-glucan, both of which are structures present in fungal species [35]. However, it must be noted that most particles are recognised by more than one receptor. As well as this, it has been suggested that there are many receptors with roles in phagocytosis that have yet to be identified.
Table 2.
Main classes of phagocytic receptors
| Phagocytic receptors | Family | Examples |
|---|---|---|
| Opsonin-dependent | Fc receptors (FcR) | FcγRI, FcγRIII |
| Complement receptors (CR) | CR1, CR3, CR4 | |
| Intergrins | α5β1 | |
| Opsonin-independent | C-type lectins | Dectin-1, Mannose receptor |
| Scavenger receptors (SR) | SR-AI/II, MARCO, CD36 | |
The process of phagocytosis involves complex signalling networks which vary depending on the receptors that have been ligated and the particles that are being processed. Many of these signalling pathways have yet to be defined. The most well-characterised signalling pathway is initiated by binding of IgG opsonised particles to FcγR. These receptors have intracellular immunoreceptor tyrosine-based activation (ITAM) motifs in their cytoplasmic tail or in associated subunits. Receptor clustering leads to phosphorylation of ITAMs, which in turn recruits Syk kinase. This activates a phosphorylation cascade including activation of PI3K [36]. Downstream signalling leads to actin reorganisation, activation of NADPH oxidase, and production of proinflammatory mediators. However, this model only applies to FcγR-mediated phagocytosis. Further elucidation of other phagocytic signalling pathways is required. This is a challenging task considering multiple receptors involved in uptake of particles and the potential crosstalk between these different signalling pathways.
The vital role of phagocytosis in inflammation and defence against infection has been recognised since the pioneering work of Elie Metchnikoff in the 19th century. Metchnikoff proposed that mobile phagocytic cells survey tissues for foreign particles and was awarded the Nobel prize for his work in 1908 [37]. In addition to pathogen elimination, phagocytosis also plays a critical role in clearance of apoptotic cells from tissues. More than 109 dead cells must be efficiently cleared from the body every day [38, 39]. It is vital that this process is carried out efficiently as remaining apoptotic debris can become necrotic and promote inflammation. In fact, it has been suggested that defects in apoptotic cell clearance and degradation can result in autoimmune disease [40]. As such, phagocytosis of apoptotic debris is essential in order to maintain health, immune tolerance, and tissue homeostasis. Similarly, in order for efficient wound healing to occur, debris and apoptotic cells must be cleared by macrophages. For example, following demyelination in the central nervous system (CNS), phagocytosis of myelin debris is essential in order for efficient myelin regeneration to occur [41].
Origin and development of macrophages
Macrophages were originally designated as part of the reticuloendothelial system (RES) in the early 20th century. However, this concept was later rejected and the mononuclear phagocyte system (MPS) was established by Van Furth. This system proposes that all macrophages are derived from monocytes that differentiate from monoblasts in the bone marrow. Monocytes in the bloodstream are recruited into tissues and differentiate into macrophages in the steady state and during inflammation [42]. The MPS is composed of monocytes, macrophages, dendritic cells (DCs), and progenitors. This framework is still in use today, although recent findings have revealed more complex differentiation pathways and cell origins than originally proposed [43].
Specific lineage relationships between different macrophage and DC populations are now being further dissected. In 2006, an important breakthrough was made with the identification of a specific progenitor, named the macrophage DC precursor (MDP) [44]. This precursor is defined as CX3CR1+CD117+ Lin− and can give rise to both dendritic cells and monocytes but cannot differentiate into granulocytes.
Within the bone marrow, hematopoietic stem cells (HSCs) give rise to myeloid progenitors (MP). MPs produce MDPs, which give rise to monocytes and common DC precursor (CDPs). During inflammation, monocytes enter tissues via the bloodstream and give rise to macrophages and also certain subsets of DCs. CDPs give rise to preclassical DCs and plasmacytoid DCs [43].
Monocytes in the bone marrow and bloodstream can be divided into two populations based on expression of Gr-1/Ly6C [45]. It has been suggested that Ly6C+ monocytes are in fact precursors for Ly6− monocytes [46, 47]. Surprisingly, it has also been shown that Ly6C+ monocytes can shuttle back to the bone marrow in absence of inflammation [46].
Debate continues over the exact fate and functions of Ly6C+ and Ly6C− monocytes. It has been reported that Ly6C+ monocyte can give rise to inflammatory macrophages in infectious models [48]. Studies have shown that Ly6C− monocytes patrol the vascular endothelium [49] and could potentially play a role in tissue repair [50]. Further work is required to fully elucidate the contributions of Ly6C+ and Ly6C− monocytes to macrophage populations in steady state and in disease.
The proposed developmental pathway is further complicated by the suggestion that certain macrophage populations are maintained independently of monocytes or bone marrow progenitors. This concept has been debated for many years [51]. Some early studies concluded that tissue macrophages were maintained by a slow influx of monocytes [42]. It is now thought that high doses of irradiation or corticosteroids could have interfered with results from initial studies. Other researchers have reported that tissue-resident macrophages are not replaced by monocytes in the steady state [52, 53]. This has been verified for microglia, CNS-resident macrophages, which originate from yolk sac-derived primitive myeloid progenitors and are maintained by self-renewal [54]. In addition to this, it is now well-established that a subset of skin DCs known as Langerhans cells arise from yolk sac and foetal liver progenitors and are maintained independently of monocytes [55]. Schulz et al. confirmed that development of Langerhans cells and microglia, as well as Kupffer cells in the liver, is independent of bone marrow hematopoiesis [56]. This concept has since been further extended to include other tissue-resident populations. Yona et al. demonstrated that peritoneal, splenic and alveolar macrophages are established prior to birth and there is no on-going contribution of monocytes in steady state [56]. In agreement with this, Guilliams et al. recently reported that alveolar macrophages are derived from foetal monocytes and are self-maintained throughout life [57]. It remains to be determined whether all tissue macrophages are seeded and maintained in this manner in the absence of inflammation.
Macrophage polarisation
Macrophages are highly plastic cells and functionally adapt to signals produced in the local microenvironment [58]. These signals include cytokines such as interferon-γ (IFN-γ). It is well-established that IFN-γ enhances the inflammatory and cytotoxic activities of macrophages [59]. However, in 1992, it was demonstrated that treatment of macrophages with IL-4 resulted in a functional phenotype that was distinct from that induced by IFN-γ [60]. This phenotype was characterised by upregulation of the mannose receptor. This work introduced the concept of alternative activation of macrophages, a topic that has since received considerable interest [61].
Activated macrophages are now broadly classified into two subsets, termed M1 and M2 cells. Classical activation is induced by IFN-γ and LPS and produces cells with a M1 phenotype. IFN-γ is predominantly produced by natural killer (NK) cells and Th1 cells. LPS is a component of the cell wall of gram-negative bacteria. M1 cells function as inducer and effector cells in Th1 responses. These cells are characterised by a high microbicidal activity, antitumor activity, and high capacity to present antigen. M1 cells are efficient producers of effector molecules (reactive oxygen and nitrogen intermediates) and inflammatory cytokines such as IL-1β, TNF-α, and IL-6.
Alternative activation is induced by IL-4 and IL-13 [62] and produces M2 cells. IL-4 and IL-13 signal via STAT-6, and these cytokines are produced by many cell types including Th2 cells, basophils, mast cells, eosinophils, and nuocytes. M2 cells participate in Th2 responses and tissue repair, promote tumor growth, are involved in elimination of parasites, and have immunoregulatory functions [61].
M1 and M2 cells differentially express a range of chemokines [63]. M1 cells express Th1-attracting chemokines such as CXCL9 and CXCL10, and M2 cells express CCL17, CCL22, and CCL24, which attract Th2, Treg, eosinophils, and basophils. M1 and M2 cells also have distinct metabolic programmes. l-Arginine is predominantly processed by inducible nitric oxide synthase (iNOS) to produce nitric oxide in M1 cells, which plays a role in the antimicrobial activity of M1 cells. However, in M2 cells, l-arginine is processed by arginase and results in the production of ornithine, proline, and polyamines which promote tissue repair [64].
In addition to IL-4 and IL-13, it has been shown that other stimuli can also produce an M2-like phenotype. There have been many different classification schemes proposed to describe these variations of M2 phenotype. For example, macrophages activated by IL-4 and IL-13 can be described as M2a cells [65]. M2b cells are induced by immune complexes in combination with IL-1β or LPS. These cells are also referred to by others as type II activated macrophages [66]. M2c cells are induced by IL-10, transforming growth factor-β1 (TGF-β1) or glucocorticoid hormones. It has been proposed that M2c cells participate in immune suppression [65]. Alternatively, Mosser et al. classified activated macrophages based in their effector functions. In this case, wound healing macrophages are induced by IL-4 and regulatory macrophages are induced by a variety of stimuli including IL-10, immune complexes, prostaglandins, glucocorticoids, and apoptotic cells [67]. However, in practice, M2 is often used as a generic term for various forms of activation other than classically activated M1 cells.
Whether M1 and M2 populations are fixed lineages or reversible adaptations to microenvironmental signals has been debated. Stout at el. showed that sequential treatment of macrophages with multiple cytokines results in progression through multiple functional phenotypes [68]. This suggests that M1 and M2 populations do not represent stable terminally differentiated subsets and are instead highly plastic populations that have the ability to adapt to a changing microenvironment. Therefore, when drawing conclusions from simplified in vitro polarisation experiments, it should be noted that M1 and M2 cells are two extremes of a spectrum of possible forms of macrophage activation that could occur in vivo. It must be considered that there is a vast number of modulating signals that could potentially impact macrophage phenotype in vivo. This includes cytokines, chemokines, hormones, TLR ligands, leptin, complement, immunoglobulin, and apoptotic cells.
Impact of ageing on macrophage function
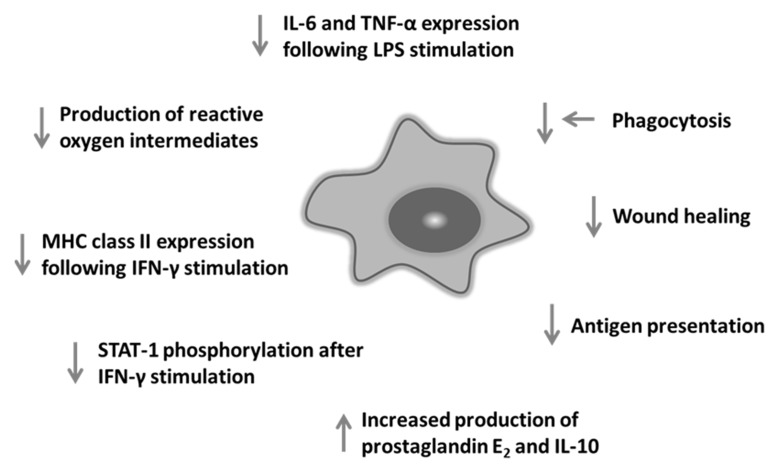
It has been reported that ageing impairs many aspects of macrophage function as summarised in Fig. 1 [22, 69, 70].
Fig. 1.
Summary of impact of ageing on macrophage function in mice. Ageing impacts on many aspects of macrophage function including response to TLR activation and expression of MHC class II
TLR activation
Ageing alters cytokine secretion by macrophages in response to TLR stimulation. It has been shown that splenic and thioglycollate-elicited peritoneal macrophages from old mice secreted substantially lower amounts of TNF-α and IL-6 compared to macrophages from young mice in response to a range of TLR ligands [71]. Indeed, similar findings have been reported by others. Boehmer et al. reported decreased secretion of IL-6 and TNF-α in response to LPS in thioglycollate-elicited peritoneal macrophages from old mice [72]. Chelvarajan et al. also demonstrated that splenic macrophages from old mice secreted reduced IL-6, TNF-α, IL-1β, and IL-12 in response to LPS, and this was accompanied by an increase in IL-10 production [73]. The molecular basis of this age-associated cytokine dysregulation in LPS-stimulated macrophages was further investigated by microarray analysis [74]. LPS stimulation regulated 853 genes in macrophages (169 in only the young, 184 in only the aged, and 500 in both). Immune response and signal transduction genes were specifically reduced in macrophages from old mice. In agreement with studies mentioned above, this study also found that expression of IL-1β and IL-6 was reduced and IL-10 was increased in macrophages from old mice. It has also been shown that LPS-stimulated macrophages from old mice secreted increased amounts of prostaglandin E2 [75].
Decreased TLR4 expression has been suggested as a reason for the observed age-related alterations in TLR signalling [71]. However, other studies have indicated that surface TLR4 expression on macrophages is unchanged with age [72]. It has been suggested that the underlying mechanism is instead impaired intracellular signalling, specifically a reduction in LPS-induced phosphorylation of the p38 and JNK mitogen-activated protein kinases (MAPK) [72]. Others have suggested reduced expression of CD14, a coreceptor for TLR4, could be involved [73].
Studies investigating the impact of ageing on production of cytokines by human monocytes have yielded some conflicting results. In fact, early studies reported increased, unchanged, and decreased LPS-induced cytokine secretion [69]. However, more recently, an age-related reduction in IL-6 production by monocytes in response to TLR1/2 ligand has been demonstrated [76]. In addition, van Duin et al. also reported reduced TLR1/2-induced TNF-α and IL-6 production by monocytes from older individuals using flow cytometry [77]. Interestingly, this study also observed decreased surface expression of TLR1 and also TLR4 in human monocytes from older individuals. In contrast to murine studies, no difference was observed in production of TNF-α, IL-6, or IL-1β in response to TLR4 activation in monocyte-derived macrophages from young and old individuals [78].
IFN-γ stimulation
As previously mentioned, IFN-γ plays an important role in macrophage activation. It has been shown that macrophages from old mice expressed 50% less MHC class II molecules on cell surface compared to macrophages from young mice following stimulation with IFN-γ [79]. This has been suggested to contribute to impaired antigen presentation by macrophages from old mice. Studies in rats have demonstrated a 75% decrease in the ability of macrophages from old rats to produce superoxide anion following incubation with IFN-γ [80]. Yoon et al. reported reduced STAT-1α phosphorylation in response to IFN-γ in macrophages from old mice compared to young mice [81], indicating that ageing alters intracellular signalling in macrophages.
Macrophage polarisation
Mahbub et al. investigated the impact of ageing on response to polarising stimuli in adherent splenocytes. Expression of both M1 and M2 markers was reduced in adherent splenocytes from old mice, indicating that ageing did not cause a skew towards M1 or M2 phenotype [82]. However, other groups have reported that ageing results in polarisation of macrophages toward an M2-like phenotype. In a mouse model of age-related macular degeneration, macrophages from old mice polarised towards a proangiogenic phenotype. IL-10 was upregulated, and IL-12 and TNF-α were downregulated in ocular macrophages in old mice; such macrophages were unable to inhibit angiogenesis following laser injury to the retina [83]. Interestingly, it has also been reported that there is an increase in M2 macrophages in spleen, lymph nodes, and bone marrow of old mice [84].
Macrophage differentiation
It is unclear whether generation and differentiation of macrophages from precursors are impaired with age. A decrease in CD68+ cells in bone marrow with age has been observed in humans [85]. In contrast, it has been reported that Mac1+ cells are increased in bone marrow in old mice [86]. Others have reported no difference in number, size, or Mac1 expression during macrophage maturation in old mice [79]. Further investigation of the impact of ageing on macrophage differentiation is needed in light of the recent advances in understanding of macrophage differentiation.
Macrophage phagocytosis
Literature regarding the impact of ageing on macrophage phagocytic function is conflicted, and apparently, opposing results have been reported. Early studies described reduced clearance of parasitic infection and salmonella infection with age [87, 88]. These papers have been subsequently referenced as demonstrating reduced phagocytosis, but the process of phagocytosis was not investigated in either study. De La Fuente demonstrated a decrease in macrophage phagocytosis in old mice compared to young mice [89]. However, while an age-related decline in phagocytosis by neutrophils was observed in rats, phagocytosis by alveolar macrophages was not impaired by age in the same model [90]. Aprahamian et al. demonstrated an age-related reduction in phagocytosis of apoptotic cell debris in mice in vivo but not in vitro [91]. Microglia isolated from old mice internalised less amyloid beta peptide compared to microglia isolated from young mice in vitro [92]. Others have observed an age-related increase in phagocytic activity in microglia from old rats [93]. Furthermore, recent human studies have reported impaired phagocytosis by CD14+ monocytes from older individuals [94], while earlier studies reported no effect of ageing on phagocytic function in humans [95].
We investigated phagocytosis of fluorescent particles in young and old mice and found that ageing significantly impaired phagocytosis by peritoneal macrophages, both in vitro and in vivo. We demonstrated that this impairment was due to age-related changes in the microenvironment in the peritoneum and not intrinsic defects in macrophages. As such, we suggest that defects may be reversible and macrophages could be targeted therapeutically in order to boost immune function in the elderly [96]. One potential area of controversy in murine studies is the age of young and old mice. In our studies, young mice were 8–12 weeks representing early adulthood and full immunological maturity. It has previously been reported that the median lifespan of C57BL/6 mice can be up to 28.5 months in a specific pathogen free unit [97]. However, we observed an increase in the incidence of tumors, skin conditions, and other morbidities that would skew immune responses in mice over 20 months of age. In order to reduce confounding factors in our experiments, such as undetected pathology in old mice, we chose 20 months as a maximum age. It is important that studies reporting effects of ageing clearly define the age ranges chosen and the rationale behind the choice.
Wound repair and tissue regeneration
Ageing impairs wound repair and tissue regeneration in both humans and rodents. In an experimentally induced cutaneous wound model, Swift et al. demonstrated that reepithelialisation, collagen synthesis, and angiogenesis were substantially delayed in old mice compared to young mice. The authors hypothesised that age-related alterations in macrophage function might be in part responsible for the delay in wound healing. Production of vascular endothelial growth factor (VEGF), an angiogenic factor, was significantly reduced in macrophages from old mice compared to macrophages from young mice [98]. Application of peritoneal macrophages from young mice onto cutaneous wounds in old mice accelerated the rate of repair [99]. Swift et al. later demonstrated that wound macrophages from old mice exhibited impaired phagocytic activity, resulting in delayed removal of debris at the site of injury [100]. This has implications not only for cutaneous wound repair but other tissue repair and regeneration processes. For example, macrophages also play an important role in repair in the central nervous system. We have found that ageing impairs phagocytosis of myelin debris by macrophages (unpublished data), a key prerequisite step for efficient myelin regeneration (remyelination) and Ruckh et al. elegantly demonstrated that monocytes from young mice have the potential to boost remyelination in old mice [101].
Concluding remarks
As a result of current global demographic changes, there is an increased urgency to drive advances in ageing research. The complex nature of the ageing process means that it is a highly challenging area of study, and progress can often be hampered by seemingly conflicting data. Such controversial findings arise in part due to the inherent difficulty of studying the ageing process within the complex phenotypes that arise with age. Rodent studies may vary between labs due to factors such as life-long environmental exposures including commensal and pathogenic microbes, diet, housing conditions, and environmental enrichment. While investigation of tissue macrophage biology is accessible in rodent models in vivo and ex vivo, such studies are challenging in human experimental models due to much less accessibility to tissue. Consequentially, efforts to translate findings from rodent tissue systems to human biology often rely on the use of peripheral blood monocytes with or without in vitro differentiation to macrophage subsets. Our recent work emphasised the importance of the aged tissue microenvironment in inhibiting macrophage phagocytosis with age as bone marrow-derived progenitors and monocytes from aged mice were not impaired compared to young controls. Such studies underscore the need to research immune ageing in complex, albeit challenging models that allow dissection of such nuances. It is also important that uniform cell purification, characterisation markers, activation stimuli, and readouts are adopted across the field as such variables may well explain apparently contradicting findings in previous studies.
Despite the challenges of ageing research, it is crucial to understand the mechanisms responsible for age-related changes in the immune system in order to develop strategies to maintain good health in the elderly population.
Contributor Information
E. Linehan, Centre for Infection and Immunity, Queen’s University Belfast, 97 Lisburn Road, Belfast BT9 7AE, Northern Ireland, United Kingdom
D. C. Fitzgerald, Centre for Infection and Immunity, Queen’s University Belfast, 97 Lisburn Road, Belfast BT9 7AE, Northern Ireland, United Kingdom.
References
- 1.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 2.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newgard CB, Sharpless NE. Coming of age: molecular drivers of aging and therapeutic opportunities. J Clin Invest. 2013;123:946–950. doi: 10.1172/JCI68833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medzhitov R. Recognition of microorgani sms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A, Medzhitov R. Regulation of a daptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper MD, Alder MN. The evolution of a daptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Katz JM, Plowden J, Renshaw-Hoelscher M, Lu X, Tumpey TM, Sambhara S. Immunity to influenza: the challenges of protecting an aging population. Immunol Res. 2004;29:113–124. doi: 10.1385/IR:29:1-3:113. [DOI] [PubMed] [Google Scholar]
- 8.Vu T, Farish S, Jenkins M, Kelly H. A m eta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–1836. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 9.Thomas-Crusells J, McElhaney JE, Aguado MT. Report of the ad-hoc consultation on aging and immunization for a future WHO research agenda on life-course immunization. Vaccine. 2012;30:6007–6012. doi: 10.1016/j.vaccine.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Fulop T, Kotb R, Fortin CF, Pawelec G, de Angelis F, Larbi A. Potential role of immunosenescence in cancer development. Ann N Y Acad Sci. 2010;1197:158–165. doi: 10.1111/j.1749-6632.2009.05370.x. [DOI] [PubMed] [Google Scholar]
- 11.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5:136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 13.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 14.Linton PJ, Dorshkind K. Age-related ch anges in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 15.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 17.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011;10:330–335. doi: 10.1016/j.arr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, Blomberg BB. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- 20.Scholz JL, Diaz A, Riley RL, Cancro MP, Frasca D. A comparative review of aging and B cell function in mice and humans. Curr Opin Immunol. 2013;25:504–510. doi: 10.1016/j.coi.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson SA, Rozzo SJ, Cambier JC. Aging-dependent exclusion of antigen-inexperienced cells from the peripheral B cell repertoire. J Immunol. 2002;168:5014–5023. doi: 10.4049/jimmunol.168.10.5014. [DOI] [PubMed] [Google Scholar]
- 22.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37(1):S9–17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 24.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 26.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 27.Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 30.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinchen JM, Ravichandran KS. Phagosome maturation: going through the acid test. Nat Rev Mol Cell Biol. 2008;9:781–795. doi: 10.1038/nrm2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 33.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol. 2012;12:492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanno S, Furuyama A, Hirano S. A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicol Sci. 2007;97:398–406. doi: 10.1093/toxsci/kfm050. [DOI] [PubMed] [Google Scholar]
- 35.Kerrigan AM, Brown GD. C-type lectins and phagocytosis. Immunobiology. 2009;214:562–575. doi: 10.1016/j.imbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanson JA, Hoppe AD. The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol. 2004;76:1093–1103. doi: 10.1189/jlb.0804439. [DOI] [PubMed] [Google Scholar]
- 37.Gordon S. Elie Metchnikoff: father of natural immunity. Eur J Immunol. 2008;38:3257–3264. doi: 10.1002/eji.200838855. [DOI] [PubMed] [Google Scholar]
- 38.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 41.Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia. 2001;35:204–212. doi: 10.1002/glia.1085. [DOI] [PubMed] [Google Scholar]
- 42.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 45.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 46.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 50.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–627. [PubMed] [Google Scholar]
- 52.Melnicoff MJ, Horan PK, Breslin EW, Morahan PS. Maintenance of peritoneal macrophages in the steady state. J Leukoc Biol. 1988;44:367–375. doi: 10.1002/jlb.44.5.367. [DOI] [PubMed] [Google Scholar]
- 53.Sawyer RT, Strausbauch PH, Volkman A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab Invest. 1982;46:165–170. [PubMed] [Google Scholar]
- 54.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M, Ginhoux F. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 57.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Doyle AG, Herbein G, Montaner LJ, Minty AJ, Caput D, Ferrara P, Gordon S. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-gamma. Eur J Immunol. 1994;24:1441–1445. doi: 10.1002/eji.1830240630. [DOI] [PubMed] [Google Scholar]
- 63.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Varin A, Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiology. 2009;214:630–641. doi: 10.1016/j.imbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 66.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 69.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 70.Sebastian C, Espia M, Serra M, Celada A, Lloberas J. MacrophAging: a cellular and molecular review. Immunobiology. 2005;210:121–126. doi: 10.1016/j.imbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 71.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 72.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 73.Chelvarajan RL, Collins SM, Van Willigen JM, Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol. 2005;77:503–512. doi: 10.1189/jlb.0804449. [DOI] [PubMed] [Google Scholar]
- 74.Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79:1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- 75.Wu D, Mura C, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Age-associated increase in PGE2 synthesis and COX activity in murine macrophages is reversed by vitamin E. Am J Physiol. 1998;275:C661–668. doi: 10.1152/ajpcell.1998.275.3.C661. [DOI] [PubMed] [Google Scholar]
- 76.Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol. 2010;30:806–813. doi: 10.1007/s10875-010-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Duin D, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E, Allore HG, Medzhitov R, Shaw AC. Ageassociated defect in human TLR-1/2 function. J Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 78.Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010;11:30. doi: 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herrero C, Marques L, Lloberas J, Celada A. IFN-gammadependent transcription of MHC class II IA is impaired in macrophages from aged mice. J Clin Invest. 2001;107:485–493. doi: 10.1172/JCI11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davila DR, Edwards CK, 3rd, Arkins S, Simon J, Kelley KW. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB J. 1990;4:2906–2911. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- 81.Yoon P, Keylock KT, Hartman ME, Freund GG, Woods JA. Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mech Ageing Dev. 2004;125:137–143. doi: 10.1016/j.mad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 82.Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 2012;32:18–26. doi: 10.1089/jir.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jackaman C, Radley-Crabb HG, Soffe Z, Shavlakadze T, Grounds MD, Nelson DJ. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging Cell. 2013;12:345–357. doi: 10.1111/acel.12062. [DOI] [PubMed] [Google Scholar]
- 85.Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev. 2000;117:57–68. doi: 10.1016/s0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 86.Wang CQ, Udupa KB, Xiao H, Lipschitz DA. Effect of age on marrow macrophage number and function. Aging (Milano) 1995;7:379–384. doi: 10.1007/BF03324349. [DOI] [PubMed] [Google Scholar]
- 87.Albright JW, Albright JF. Ageing alters the competence of the immune system to control parasitic infection. Immunol Lett. 1994;40:279–285. doi: 10.1016/0165-2478(94)00066-2. [DOI] [PubMed] [Google Scholar]
- 88.Bradley SF, Kauffman CA. Aging and the response to Salmonella infection. Exp Gerontol. 1990;25:75–80. doi: 10.1016/0531-5565(90)90012-q. [DOI] [PubMed] [Google Scholar]
- 89.De La Fuente M. Changes in the macrophage function with aging. Comp Biochem Physiol A Comp Physiol. 1985;81:935–938. doi: 10.1016/0300-9629(85)90933-8. [DOI] [PubMed] [Google Scholar]
- 90.Mancuso P, McNish RW, Peters-Golden M, Brock TG. Evaluation of phagocytosis and arachidonate metabolism by alveolar macrophages and recruited neutrophils from F344xBN rats of different ages. Mech Ageing Dev. 2001;122:1899–1913. doi: 10.1016/s0047-6374(01)00322-0. [DOI] [PubMed] [Google Scholar]
- 91.Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin Exp Immunol. 2008;152:448–455. doi: 10.1111/j.1365-2249.2008.03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2012;33:195.e1–195.e12. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lynch AM, Murphy KJ, Deighan BF, O’Reilly JA, Gun’ko YK, Cowley TR, Gonzalez-Reyes RE, Lynch MA. The impact of glial activation in the aging brain. Aging Dis. 2010;1:262–278. [PMC free article] [PubMed] [Google Scholar]
- 94.Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, Crowe SM. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11:867–875. doi: 10.1111/j.1474-9726.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 95.Fietta A, Merlini C, Dos Santos C, Rovida S, Grassi C. Influence of aging on some specific and nonspecific mechanisms of the host defense system in 146 healthy subjects. Gerontology. 1994;40:237–245. doi: 10.1159/000213591. [DOI] [PubMed] [Google Scholar]
- 96.Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell. 2014;13:699–708. doi: 10.1111/acel.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79:1479–1487. [PubMed] [Google Scholar]
- 99.Danon D, Kowatch MA, Roth GS. Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci U S A. 1989;86:2018–2020. doi: 10.1073/pnas.86.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–1035. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 101.Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]