Abstract
Chemokine and antibody response profiles were investigated in children and adults with severe or uncomplicated Plasmodium falciparum malaria; the aim was to reveal which profiles are associated with severe disease, as often seen in nonimmune children, or with mild and uncomplicated disease, as seen in semi-immune adults. Blood samples were obtained from children under 5 years of age as well as adults with falciparum malaria. Classification of malaria was performed according to parasite densities and hemoglobin concentrations. Plasma levels of chemokines (IL-8, IP-10, MCP-4, TARC, PARC, MIP-1δ, eotaxins) were quantified, and antibody responses (IgE, IgG1, and IgG4) to P. falciparum, Entamoeba histolytica-specific antigen, and mite allergen extracts were determined. In children with severe malaria proinflammatory, IL-8, IP10, MIP-1δ, and LARC were at highly elevated levels, suggesting an association with severe disease. In contrast, the Th2-type chemokines TARC, PARC, and eotaxin-2 attained in children the same levels as in adults suggesting the evolution of immune regulatory components. In children with severe malaria, an elevated IgG1 and IgE reactivity to mite allergens and intestinal protozoan parasites was observed. In conclusion, exacerbated proinflammatory chemokines together with IgE responses to mite allergens or E. histolytica-specific antigen extract were observed in children with severe falciparum malaria.
Keywords: antibody, chemokine, children, malaria, Plasmodium falciparum
Introduction
Induction, development, and maintenance of protective immunity against Plasmodium falciparum infection will start with the first parasite encounter; immune sensitization to Plasmodium-derived antigens may already occur prenatally and will, in any event, continue in children shortly after birth [1–3]. During the first months of life, maternal antibodies may mediate partial protection against P. falciparum infection [4, 5], while naturally acquired protective immunity against malaria is slow to develop, remains incomplete, and requires repeated exposure to infection which will generate memory B cells, which produce specific IgG subclasses able to neutralize the blood developmental stages of the parasite [6–10]. The secretion of cytokines and chemokines by T cells, monocytes, and NK cells is another essential prerequisite for the regulation of cellular effector mechanisms against P. falciparum blood-stage parasites [11], but inflammatory cytokines and chemokines may also exacerbate disease manifestation and organ-specific pathogenesis [12].
Activation of Th1 cytokines, including IL-12 and the early production of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) by T lymphocytes and natural killer (NK) are known to support resistance to infection and host survival [13]. Prominent Th2 response profiles have been reported in patients suffering from severe malaria, with an early IL-10 response being associated with higher susceptibility to infection; however, IL-10 will also exert anti-inflammatory effects, thus, limiting pathogenic and excessive Th1 cytokine responses [14, 15]. With repeated exposure to Plasmodium spp. and immune maturation, adequate and regulatory cytokine and chemokine responses may set off cellular effector mechanisms which will not induce severe inflammation and which will avoid excessive host tissue and organ damage [16]. Recent studies have shown that, in children with P. falciparum malaria, the profile of cytokine and chemokine levels varied with disease severity; moreover, the proinflammatory chemokines MIP-1α/CCL3, MIP-1β/CCL4, and MIG/CXCL9 and cytokines IL-31 and IL-33 were elevated, while RANTES/CCL5 and IL-27 appeared suppressed in children with severe falciparum malaria [17, 18]. Heightened chemokine levels may contribute to neuropathology and, potentially, predict mortality in children with cerebral malaria [19]; however, with repeated exposure to P. falciparum, an immune response repertoire should evolve enabling better parasite control [11, 12]. Investigation and comparison of the chemokine responses in children and adults with severe or uncomplicated falciparum malaria have the potential to elucidate which response profiles are associated with progression to severe disease (as seen in nonimmune children) or with the capability to control infection (as seen in semi-immune adults).
In addition, with repeated exposure to Plasmodium spp., an immune response repertoire, chiefly consisting of cytophilic IgG1 and IgG3 subclasses, but also IgE, will progressively evolve in children, having the capacity to control and reduce parasite multiplication [9, 20–22]. Such responses may gradually approach, and come to resemble, the immune reactivity profiles observed in adults, where antibodies specific against multiple P. falciparum antigens mediate partial parasite control [23].
In human P. falciparum malaria, elevated IgE may be instrumental in the pathogenesis of this disease. The initial finding indicated that IgE elevation was most pronounced in sera from patients with cerebral malaria [22]. Later observations suggested that IgE may be considered a pathogenic factor in severe disease without cerebral involvement and, probably, in P. falciparum malaria in general [24]. In addition, allergic-type inflammatory mechanisms were suggested as enhancing the pathogenesis of severe forms of malaria disease [25]. Inflammatory responses may arise with the production of IgE against environmental allergens and parasitic worms; effector cells such as mast cells and granulocytes which express Fcε receptors may mediate this hyper-reactivity, but parasite-specific IgE antibodies, by cooperating with Fcε-receptor-bearing cells, may also protect against malaria infection [26]. Furthermore, malaria infection is a strong driver of IgE production irrespective of helminth coinfection, parasite density, and helminth egg output [27].
The present work revealed that, in children with severe falciparum malaria, proinflammatory chemokines, which activate monocytes and neutrophil granulocytes, were at highly elevated levels and such responses were not observed in children or adults with uncomplicated falciparum malaria, while Th2-type chemokines in children attained the same levels as in adults. Furthermore, in children with severe malaria, an elevated IgG1 and IgE reactivity to mite allergens and Entamoeba histolytica-specific antigen was observed, which was most likely induced following immune sensitization and infection during early postnatal life.
Materials and methods
Study population
This work was conducted at the Centre Hospitalier Regional (CHR) in Sokodé in the Central Region of Togo. The study was approved by the National Committee de Bioethique pour la Recherche en Santé in Togo and by the Ethics Committee of the University Clinics of Tübingen, Germany. Informed written consent was obtained from all adults and from all parents for the participation of their children in this study; to ensure complete understanding of the study’s aims, investigations, risks, and procedures, explanations were given in the local languages by the medical personnel of the CHR. Children under 5 years of age as well as adults with falciparum malaria were recruited. Classification of malaria type was performed according to previously published criteria for severe malaria characterized by parasitemias of higher than 250,000 parasites/µl blood and/or the presence of severe anemia with hemoglobin concentrations of less than 5 g/dl. Matched uncomplicated malaria patients were defined by parasite counts of less than 250,000 parasites/μl blood and hemoglobin concentrations of higher than 5 g/dl plus the absence of any signs or symptoms of severe malaria. P. falciparum-exposed children testing negative for parasites in thick blood film and negative in rapid detection test kits for P. falciparum (Malaria P.f HRP-II Antigen Rapid Test, Standard Diagnostics Inc., Korea) were defined as those participants with previous malaria episode(s) and an absence of illness due to malaria within the last 2 weeks. Blood samples were obtained prior to treatment with antimalarials and/or antipyretics, and all children with malaria were given antimalaria and the appropriate supportive therapy, such as is required and recommended by the guidelines for malaria treatment issued by the Ministry of Health in Togo.
Synthetic P. falciparum peptides
Five synthetic peptides corresponding to highly conserved B-cell epitopes were used. These were the following: 1) the epitope (EENV)4 of the C-terminal part of the P. falciparum ring-infected erythrocyte surface antigen (RESA), which is immune-dominant, and antibodies against which are associated with resistance to clinical malaria [28]; 2) the epitope KLYQAQYDLSF representing amino acids 277 to 287 of the N-terminal conserved part of the P. falciparum merozoit surface protein 1 (MSP-1-Nt) [29], antibodies associated with protection against malaria disease [30]; 3) the epitope KAASNTFINNA representing amino acids 27 to 34 of the N-terminal conserved region of the P. falciparum merozoit surface protein 2 (MSP-2-Nt); 4) the epitope AAAQHGHMHGS representing amino acids 199 to 206 of MSP-2 (MSP-2-Ct1); and 5) the epitope AAANTSDSQKE representing amino acids 213 to 220 of MSP-2 (MSP-2-Ct2) [31].
Preparation of E. histolytica-specific antigen, and Dermatophagoides farinae allergen extracts
E. histolytica trophozoites (strain HM-1 axenic culture) were a gift from Dr. Brigitte Walderich (Institute for Tropical Medicine, University Clinics of Tübingen, Germany). E. histolytica trophozoites were transferred into a Ten-Broek tissue grinder and homogenized extensively on ice. The homogenate was then sonicated twice (30% intensity) for 3 min on ice and centrifuged at 16,000g for 30 min at 4 °C. The supernatants were collected and then sterile-filtered (0.22 mm), and the protein concentration was determined with a BCA protein assay (Pierce, Rockford, USA). D. farinae extracts were purchased from Allergopharma (Rheinbeck, Germany).
P. falciparum-, E. histolytica-, and D. farinae-specific antibody ELISA
Microtitre 96 well EIA/RIA plates (Corning Costar, NY, USA) were coated with P. falciparum peptides (final concentration 5 µg/ml), E. histolytica antigen (final concentration 1 µg/ml), or D. farinae antigen (final concentration 1 µg/ml) in PBS (Sigma Aldrich, St. Louis, USA) overnight at room temperature (RT). Plates were then blocked with 5% PBS-BSA (Sigma Aldrich, St. Louis, USA) for 1.5 h. Patients’ sera were diluted to 1:20 in PBS, and 50 µl was added to each well. Patients’ samples were applied in duplicates. After 1 h of incubation at RT, plates were washed three times with PBS. For subclass-specific antibody detection, HRP-conjugated antibodies against human IgG1, IgG4 (Invitrogen, CA, USA), and IgE (AbDSeroTec, Oxford, UK) (1:500) were applied and incubated for 1 h at RT. Following washing of the plates as described above, 50 µl of tetramethylbenzidine (TMB) substrate solution (Thermo Fisher Scientific, Waltham, USA) was added to each well. Plates were read at 405 nm in a BioTek (Winooski, VT, USA) microplate autoreader.
Chemokine and cytokine ELISA
Quantitative enzyme-linked immunosorbent assay (ELISA) was performed using commercially available assays (Duo-Set: R&D Minneapolis, MN, USA) to determine plasma levels of the chemokines NAP-1/CXCL8/IL-8 (assay detection limit [DL] as indicated by the manufacturer: 30 pg/ml), IP-10/CXCL10 (DL: 30 pg/ml), eotaxin-1/CCL11 (DL: 15 pg/ml), MCP-4/CCL13 (DL: 8 pg/ml), MIP-1δ/CCL15 (DL: 15 pg/ml), TARC/CCL17 (DL: 8 pg/ml), PARC/CCL18 (DL: 8 pg/ml), LARC/CCL20 (DL: 15 pg/ml), eotaxin-2/CCL24 (DL: 20 pg/ml), and eotaxin-3/CCL26 (DL: 60 pg/ml). Sample concentrations of each cytokine and chemokine were quantified from standard curves generated with recombinant chemokines/cytokines included in the DuoSet ELISA kit.
Statistical data analysis
The statistical package JMP version 5.0.1.2 was used for data analysis. For cytokine and for chemokine analysis, differences between groups were determined after logarithmic transformation to stabilize the variance of data [log (pg/ml + 1)]. The level of significance was adjusted according to Bonferroni–Holm (α = 0.0025).
Results
Study subject characteristics
A total of 234 children ranging from 0 to 5 years of age were included in this investigation. Table 1 shows the characteristics of the children patient groups; the endemic control group (NEG) was children in whom P. falciparum was not detectable by thick blood smear and rapid antigen detection kits. The uncomplicated malaria cases (uM) were children positive for P. falciparum infection, with parasite densities not exceeding 250.000 parasites/µl blood and with hemoglobin levels equal and superior to 5 g/dl. The children group with severe malaria (sM: >250.000 parasite/µl; <5 g/dl hemoglobin) presented with significant lower age and leucocytes counts than did NEGs and sM children (Table 1), and in both malaria groups, hemoglobin levels were significantly lower as compared to the NEG children (p < 0.0001).
Table 1.
Age, hemoglobin levels, leukocyte count, and Plasmodium falciparum parasite density in children with uncomplicated (uM) or severe (sM) malaria, in children negative (NEG) for P. falciparum and in adults with uncomplicated falciparum malaria (uM). Differences between study groups are indicated as **p < 0.0001 when compared to all other groups; *p < 0.05 when compared to Child-uM, and &p < 0.0001 when compared to Child-uM. The level of significance was adjusted according to Bonferroni–Holm (α = 0.0025)
| Patient groups | N | Age
(years) (mean; range) |
Leucocytes/µl (mean; range) |
Pf/µl (mean; range) |
Hb (mean; range) |
|---|---|---|---|---|---|
| Child-NEG | 79 | 2.6 | 11,175 | 0 | 10.1 |
| (0.1–5) | (2800–4700) | (3–17.7) | |||
| Child-uM | 78 | 2.3 | 11,361 | 23,887 | 8.8 |
| (0.02–5) | (2900–6400) | (77–223,500) | (5.3–16.7) | ||
| Child-sM | 77 | 2.1 | 13,930 | 44,536* | 4.1** |
| (0–5) | (4100–6900) | (130–453,333) | (1.7–11.4) | ||
| Adult-uM | 74 | 32.9 | 7608** | 9255** | 11.7& |
| (18–65) | (1800–35,400) | (122–87,000) | (6.5–18.2) |
Adult patients were n = 74, between 18 and 65 years of age and with uncomplicated falciparum malaria; i.e., they tested positive for P. falciparum infection with parasite densities not exceeding 250.000 parasites/µl blood and with hemoglobin levels equal or superior to 5 g/dl. Immediately following primary diagnosis, all P. falciparum-positive children and adult patients received treatment according to the recommended guidelines of the Togolese malaria control program. All hospitalized malaria cases, both uncomplicated and severe, were followed until release from the hospital’s paediatric ward.
Chemokine plasma levels in uncomplicated or severe malaria
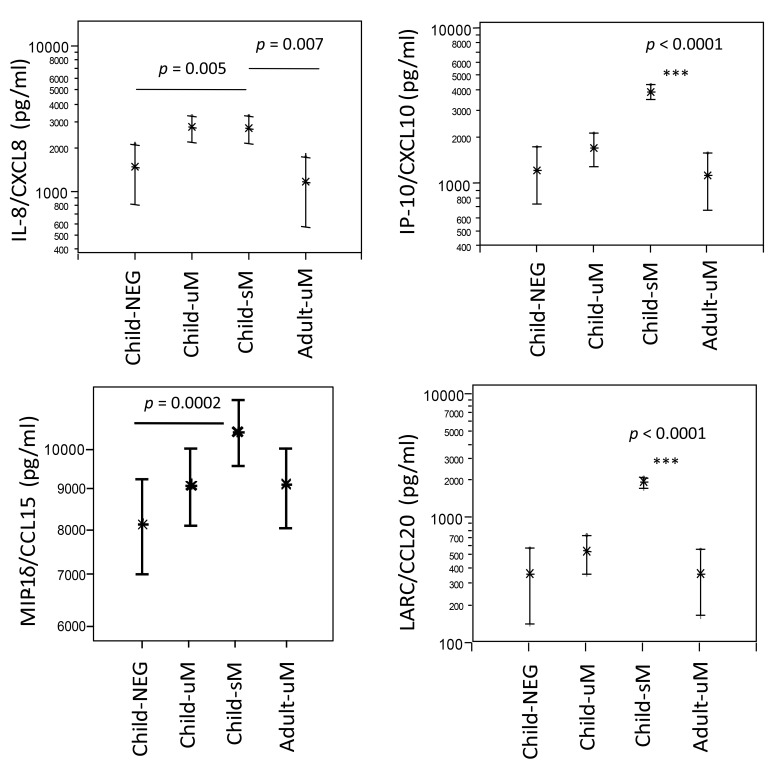
Plasma levels of the neutrophil granulocyte-activating protein NAP-1/CXCL8/IL-8, interferon-induced protein IP-10/CXCL10, MIP-1δ/CCL15, and the liver and activation-regulated chemokine LARC/CCL20 were quantified by specific ELISA in NEG, uM, and sM children, but also in adults with uncomplicated malaria (Adult-uM) (Fig. 1). IL-8, IP-10, and LARC were highly elevated in children with uncomplicated and severe malaria, and IP-10 and LARC progressively heightened in cases of enhanced malaria disease severity. In adults with uncomplicated malaria, levels of IL-8, IP10, and LARC were as low as in NEG children. LARC levels were similar in the children and adults with uncomplicated malaria, but LARC/CCL20 presented highest in children with severe malaria. MIP-1δ/CCL15 plasma levels were highest in children with severe falciparum malaria (p = 0.0002 compared to NEG children) and slightly above those measured in children and adults with uncomplicated malaria.
Fig. 1.
CXCL and CC chemokines in children with uncomplicated (uM) or severe (sM) malaria in children negative (NEG) for Plasmodium falciparum and in adults with uncomplicated falciparum malaria (uM). Differences between study groups are indicated as **p < 0.0001 when compared to all other groups; otherwise, differences between groups are given as p values. The level of significance was adjusted according to Bonferroni–Holm (α = 0.0025)
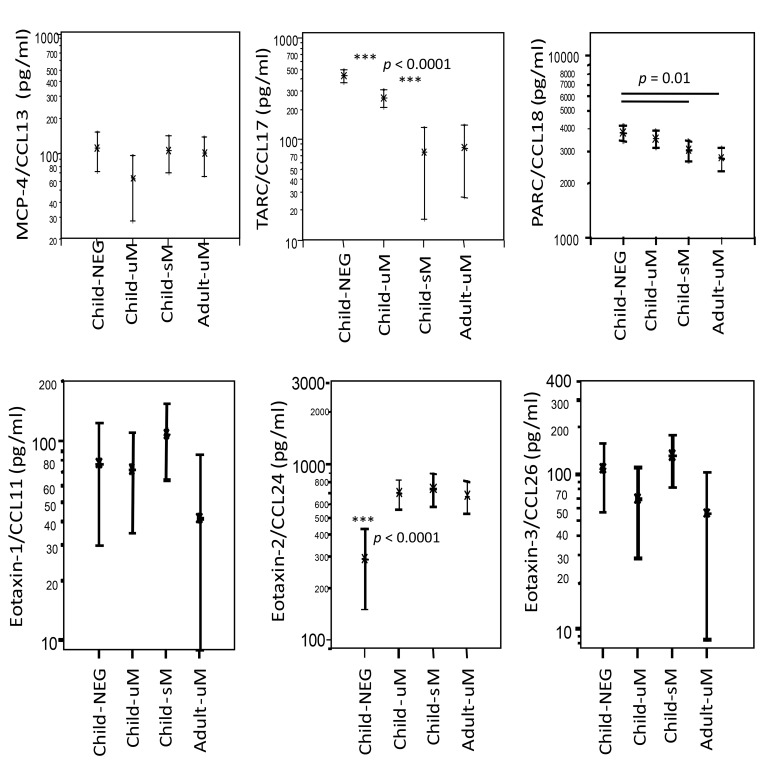
In contrast, the plasma concentrations of the tissue and pulmonary chemokines TARC/CCL17 and PARC/CCL18, both regulated by activation, were highest in NEG children and progressively decreased with enhanced malaria severity, being similarly low in children with severe and in adults with uncomplicated disease (Fig. 2). The levels of the monocyte inflammatory chemokine MCP-4/CCL13 were similar in all study groups. The eosinophil granulocyte-attracting chemokines eotaxin-1/CCL11 and eotaxin-3/CCL26 exhibited similar plasma concentrations in the children and adult study groups. The eotaxin-2/CCL24 was enhanced in all P. falciparum-infected groups to similar levels but was lowest in children negative for P. falciparum infection.
Fig. 2.
CC chemokines in children with uncomplicated (uM) or severe (sM) malaria, in children negative (NEG) for Plasmodium falciparum, and in adults with uncomplicated falciparum malaria (uM). Differences between study groups are indicated as **p < 0.0001 when compared to all other groups; otherwise, differences between groups are given as p values. The level of significance was adjusted according to Bonferroni–Holm (α = 0.0025)
Chemokine level analysis
Chemokine–chemokine correlations and their plasma levels were analyzed for their associations with age, hemoglobin concentration, and parasite densities (Table 2). With increasing age, the chemokine plasma concentrations of IL-8/CXCL8, IP10/CXCL9, LARC/CCL20, and TARC/CCL17 decreased (for all p < 0.001). Levels of the interferon gamma-induced chemokine IP10/CXCL9 and the liver- and activation-regulated chemokine LARC/CCL20 correlated positively with parasite densities, while TARC/CCL17 (tissue- and activation-regulated chemokine) diminished with heightening parasite loads (for all p<0.0001). With lowering hemoglobin concentration the levels of IL-8, IP10, and LARC enhanced (for all p < 0.0001), and in opposite, TARC levels heightened with increasing hemoglobin values (p = 0.0002). IL-8/CXCL8 associated positively with IP10 and LARC (p < 0.0001), CXCL10/IP10 levels enhanced together with LARC/CCL20, and the monocyte chemoattractant chemokine MCP4 correlated positively with IP10 (p < 0.0001), LARC (p < 0.0001), and TARC (p = 0.0006).
Table 2.
Chemokine–chemokine correlations and their association with age, parasite densities, and hemoglobin concentrations in children with uncomplicated (uM) or severe (sM) falciparum malaria, in children negative (NEG) for P. falciparum, and in adults with uncomplicated falciparum malaria (uM)
| Variable | Variable | n | R 2 | p value |
|---|---|---|---|---|
| Hb (g/dl) | Age (months) | 291 | 0.4610 | <0.0001 |
| Hb (g/dl) | Leucocytes (n/µl) | 283 | −0.3523 | <0.0001 |
| Leucocytes (n/µl) | Age (months) | 282 | −0.2205 | 0.0002 |
|
| ||||
| Parasite density (n/µl) | Hb (g/dl) | 292 | −0.2150 | 0.0002 |
| Parasite density (n/µl) | IP10 (pg/ml) | 292 | 0.3033 | <0.0001 |
| Parasite density (n/µl) | LARC (pg/ml) | 290 | 0.2802 | <0.0001 |
| Parasite density (n/µl) | TARC (pg/ml) | 288 | −0.3787 | <0.0001 |
|
| ||||
| IL8 (pg/ml) | Age (months) | 291 | −0.1931 | 0.0009 |
| IL8 (pg/ml) | Hb (g/dl) | 292 | −0.2377 | <0.0001 |
| IL8 (pg/ml) | IP10 (pg/ml) | 292 | 0.2536 | <0.0001 |
| IL8 (pg/ml) | LARC (pg/ml) | 290 | 0.3143 | <0.0001 |
| IP10 (pg/ml) | Age (months) | 291 | −0.2628 | <0.0001 |
| IP10 (pg/ml) | Hb (g/dl) | 292 | −0.3374 | <0.0001 |
| IP10 (pg/ml) | MCP4 (pg/ml) | 292 | 0.2778 | <0.0001 |
| IP10 (pg/ml) | LARC (pg/ml) | 290 | 0.4869 | <0.0001 |
|
| ||||
| LARC (pg/ml) | Age (months) | 289 | −0.2696 | <0.0001 |
| LARC (pg/ml) | Leucocytes (n/µl) | 281 | 0.2499 | <0.0001 |
| LARC (pg/ml) | Hb (g/dl) | 290 | −0.4883 | <0.0001 |
| LARC (pg/ml) | MCP4 (pg/ml) | 290 | 0.2321 | <0.0001 |
| TARC (pg/ml) | Age (months) | 287 | −0.2217 | 0.0002 |
| TARC (pg/ml) | Hb (g/dl) | 288 | 0.2184 | 0.0002 |
| TARC (pg/ml) | MCP4 (pg/ml) | 288 | 0.2014 | 0.0006 |
Antibody responses to mite allergen and parasitic antigens in children and adults
P. falciparum peptide-specific antibody responses in children and adults
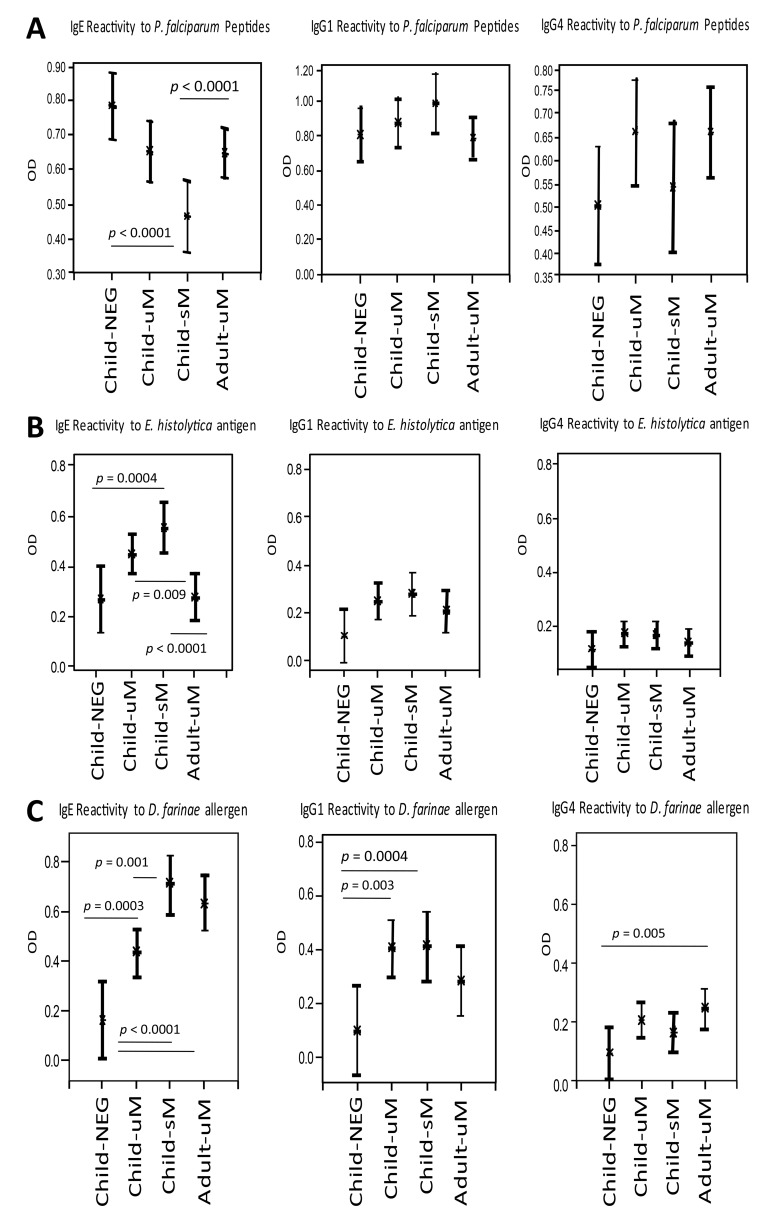
Figure 3A shows the subclass-specific antibody reactivity to P. falciparum peptides in children and adults with falciparum malaria as well as in children without P. falciparum infection. Highest IgE antibody responses against P. falciparum peptides were detected in infection-free controls. Antibody reactivity decreased in children with progression of disease severity. Adults with mild malaria presented with similar antibody responses as those in children with mild malaria. IgG1 specific for P. falciparum peptides was lowest in P. falciparum-negative children and slightly more elevated in children with mild malaria, while IgG1 responses were highest in children with severe malaria. In adults with mild malaria, P. falciparum peptide-specific IgG1 attained similar levels as observed in infection-free children. IgG4 reactivity to P. falciparum peptides was highest in adults and children with mild malaria, without any difference between the two groups, while both children with severe malaria and children without P. falciparum infection presented with reduced P. falciparum specific IgG4 responses.
E. histolytica antigen-specific antibody responses in children and adults
In Fig. 3B, the subclass-specific antibody reactivity to an antigen extract of the intestinal protozoan parasite E. histolytica antigen is shown for children and adults with falciparum malaria and for children without P. falciparum infection. IgE reactivity to E. histolytica antigen was lowest in P. falciparum negative children and enhanced in uncomplicated cases of children, with the highest responses observed in children with severe malaria. IgE levels in adults with mild malaria did not differ from the P. falciparum negative children group. IgG1-mediated recognition of E. histolytica antigens was lowest in children without P. falciparum infection, and enhanced in children with mild and with severe malaria. Adults and children with mild malaria presented with similar E. histolytica-specific IgG1 responses. No major differences in E. histolytica specific IgG4 levels were observed between the malaria and P. falciparum negative groups.
Fig. 3.
Antibody subclass reactivity to Plasmodium falciparum peptides (Part A), Entamoeba histolytica antigen (HM1 axenic strain) (Part B), and D. farinae allergen extract (Part C) in children with uncomplicated (uM) or severe (sM) malaria, in children negative (NEG) for P. falciparum, and in adults with uncomplicated falciparum malaria (uM). Differences between study groups are indicated as p values. The level of significance was adjusted according to Bonferroni–Holm (α = 0.0025)
D. farinae allergen-specific antibody responses
Figure 3C shows the subclass specific antibody reactivity to mite allergens (D. farinae) in children and adults with falciparum malaria as well as in children without P. falciparum infection. Lowest D. farinae specific IgE responses were observed in children without P. falciparum infection. In children, antibody levels heightened with increasing malaria severity, being highest in children with severe malaria. Adults with mild malaria presented with a slightly reduced IgE reactivity to mite allergens as compared to children with severe malaria. D. farinae-specific IgG1 responses were lowest in infection-free controls. Both in children with mild and with severe malaria, these D. farinae-specific IgG1 levels were clearly enhanced, without any difference between the groups. D. farinae-specific IgG1 levels were reduced in adults, as compared to both mild and severe children malaria groups, but adult responses were higher than those in infection-free controls. Both adults and children with P. falciparum infection presented with higher IgG4 responses to D. farinae than did children without infection.
Discussion
Cytokines and chemokines can act as central contributors to severe and life-threatening illness, and parallels exist between falciparum malaria and other illnesses such as sepsis, influenza [9, 32, 33] and allergic inflammatory responses [25, 34, 35]. Our present work showed that in children with severe malaria, chemokines activating granulocytes and monocytes (IL-8, IP10, MIP-1δ/CCL15, and LARC/CCL20) were at high levels disclosing pronounced innate inflammatory responses, which may predispose to malaria aggravation. In parallel, these children had chemokine plasma levels at magnitudes observed to an equal extent in adults with uncomplicated malaria, notably TARC/CCL17, PARC/CCL18, and eotaxin-2/CCL24, which may signify a gradual evolution of regulated immune responses during early postnatal life.
Resolution of P. falciparum infection will require immune responses which are of both proinflammatory and regulatory type; an early Th1-type gamma interferon (IFN-γ) cytokine response may support resistance [13]; and while high regulatory IL-10 levels were reported in children with severe malaria [18, 36, 37], IL-10 also exerts anti-inflammatory effects, thus, limiting pathogenic and excessive Th1 cytokine responses. In the present work, the neutrophil granulocyte-activating and proinflammatory chemokine NAP-1/CXCL8/IL-8 was at highest levels in children with severe malaria, while CXCL8/IL-8 was low in adults with uncomplicated disease. Neutrophil granulocytes inducing allergic-type reactions were shown to play a central role in the development of experimental cerebral malaria [25]. Similarly, the chemokines IP-10/CXCL10, MIP-1δ/CCL15, and LARC/CCL20, which attract and activate granulocytes and monocytes, were enhanced in children but not in adults. Notably high IP-10 levels were considered as biomarker and risk indicators for cerebral malaria and progression to lethal disease [19]. The neutrophil-activating CXCL8/IL-8 was previously found to be weakly elevated in children with severe malaria [38, 39] but present at higher concentrations in adults [40], and elevated IL-8 and also IP-10 in cerebral spinal fluid were predictive markers for cerebral malaria and lethality [19]. Expression of IL-8 [41] and also IP-10 [42] can be triggered following exposure to hemin or hemozoin. Released during rupture of P. falciparum-infected erythrocytes, hemozoin is phagocytized and will activate several proinflammatory chemokines; intravenous hemozoin injection correlates with malaria-associated acute respiratory distress syndrome and pulmonary inflammation in mice [42].
In the present work, plasma levels of IL-8 were elevated in children with uncomplicated or severe malaria, while interferon-induced protein IP-10 and liver- and activation-regulated chemokine LARC/CCL20 were highest in children with severe malaria, with no such amounts being measured in adults. This divergence may be due to unbalanced inflammatory innate responses in children, which only may become adequately regulated after repeated P. falciparum infections. This is supported by the negative correlation of IL-8/CXCL8 and IP10/CXCL10 with age, i.e., both will decrease with higher age (Table 2). In children, several chemokines approached the magnitudes observed in infected adults, with TARC/CCL17 and PARC/CCL18 lessening and eotaxin-2/CCL24 enhancing, and both CC-chemokines being involved in effector cell activation and immune regulation. PARC and LARC were negatively correlated with age, disclosing that, during aging and with repeated exposure to P. falciparum infections, pulmonary (PARC) and tissue (TARC) activation regulated chemokines will diminish. The alternative macrophage activation-associated chemokine PARC/CCL18 is mainly produced by antigen-presenting cells induced by Th2 type cytokines and may exhibit dual functions, with pro- and anti-inflammatory properties [43]. PARC/CCL18 was found to enhance protective cell-mediated immunity in murine malaria suggesting this chemokine as a potential adjuvant to enhance adaptive immune responses against Plasmodium yoelii infection [44]. In our study, plasma levels of TARC/CCL17 and PARC/CCL18 were similarly high in children with severe malaria and in adults with uncomplicated malaria, suggesting that even though being diminished in children to adult levels, PARC and TARC may not be the critical components which mediate an immune response preventive against severe malaria. In regard to the molecules able to generate such a disease-preventive immune responsiveness and to balance the proinflammatory IP-10, IL-8, and LARC chemokines, these regulators remain to be identified.
The eosinophil granulocyte-activating eotaxin-2/CCL24 was markedly elevated in all P. falciparum-infected cases and was low in negative children. Eotaxin-1/CCL11 and eotaxin-3/CCL26 were below 100 pg/ml in all study groups. This finding suggested a P. falciparum-induced eotaxin-2/CCL24 production, which may attract granulocytes and mast cells along CCL24 gradients into parasite-infested sites; after cross-linking of Fcε receptors with IgE, the subsequent degranulation of these cells may cause tissue damage, e.g., endothelial apoptosis. Such allergic-type inflammation was suggested as interacting with the outcome of malaria disease; moreover, the activation of effector mechanisms involving IgE and basophil [39] and neutrophil granulocytes with unconventional Fcε receptor expression [25, 45] was identified as components in the experimental induction of cerebral malaria in mice. Furthermore, the eotaxins CCL24 and CCL26 are mediators of persistent allergen-induced bronchial eosinophilic inflammation [46, 47]. We further observed in malaria children an IgE and IgG1 reactivity progressively enhancing with disease severity to mite allergens and E. histolytica-specific antigen; such antibody production was probably generated following allergen sensitization and E. histolytica infection during early postnatal life. The evolution of immunity to malaria results from broad and prominent antibodies responses to multiple plasmodium merozoite antigens [48], but parasite coinfections, e.g., helminthes or intestinal protozoan infections, may influence the antibody repertoire and its magnitude [49–51]. In consequence, parasite coinfections may hamper or bias the immune regulatory capacity, which may, in turn, serve to protect against disease or make it worse. In children concomitantly infected with Schistosoma mansoni, an increased number of malaria attacks were observed [52]; moreover, infection modulated P. falciparum-specific immune responses, potentially increasing the risk of plasmodial infection or disease [53]. Strong inflammatory chemokine responses to P. falciparum and E. histolytica antigens in newborns and 10-year-old children suggested that adequately balanced immune regulatory mechanisms may not yet have developed [3].
Conclusion
In children with falciparum malaria, prominent proinflammatory chemokines together with IgE responses to allergens or E. histolytica-specific antigens were observed; moreover, children generated immune regulatory-type chemokines to similar high levels as in adults, which suggested the evolution of regulatory immune responses; however, those may not yet suffice to mediate an immune response preventive against severe malaria. Repeated exposure to P. falciparum antigens during infancy and childhood is required to generate cytokine and chemokine responses which balance, prevent, or stop the progress of malaria pathogenesis, and these immune regulators need to be identified.
Acknowledgements
We kindly thank all parents and their children for participation and cooperation throughout the entire study. The expert assistance by the laboratory technicians of the Centre Hospitalier Regional in Sokodé/Togo was a most valuable contribution to completion of this work.
This work was supported by the Bundesministerium für Bildung und Forschung (BMBF Grant No. 01KA1008). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the article. The study was supported by the Onchocerciasis Reference Laboratory at the National Institute of Hygiene, Lomé, Togo, and by the University Hospital of Lomé, Togo. We acknowledge the support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of Tübingen University.
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
Contributor Information
B. Wangala, 1Université de Lomé, Centre Hospitalier Universitaire, Lomé, Togo; 3Institut National d’Hygiène, Onchocerciasis Reference Laboratory, Sokodé, Togo.
A. Vovor, 1Université de Lomé, Centre Hospitalier Universitaire, Lomé, Togo.
R. G. Gantin, 3Institut National d’Hygiène, Onchocerciasis Reference Laboratory, Sokodé, Togo; 4Institute of Tropical Medicine, University Clinics of Tübingen, Tübingen, Germany.
Y. F. Agbeko, 2Centre Hospitalier Régional (CHR), Sokodé, Togo.
C. J. Lechner, 3Institut National d’Hygiène, Onchocerciasis Reference Laboratory, Sokodé, Togo; 4Institute of Tropical Medicine, University Clinics of Tübingen, Tübingen, Germany.
X. Huang, 4Institute of Tropical Medicine, University Clinics of Tübingen, Tübingen, Germany.
P. T. Soboslay, 3Institut National d’Hygiène, Onchocerciasis Reference Laboratory, Sokodé, Togo; 4Institute of Tropical Medicine, University Clinics of Tübingen, Tübingen, Germany.
C. Köhler, 4Institute of Tropical Medicine, University Clinics of Tübingen, Tübingen, Germany.
References
- 1.Gallagher M, Malhotra I, Mungai PL, Wamachi AN, Kioko JM, Ouma JH, Muchiri E, King CL. The effects of maternal helminth and malaria infections on mother-to-child HIV transmission. AIDS. 2005;19:1849–1855. doi: 10.1097/01.aids.0000189846.90946.5d. [DOI] [PubMed] [Google Scholar]
- 2.Kirch AK, Agossou A, Banla M, Hoffmann WH, Schulz-Key H, Soboslay PT. Parasite-specific antibody and cytokine profiles in newborns from Plasmodium falciparum and Entamoeba histolytica/dispar-infected mothers. Pediatr Allergy Immunol. 2004;15:133–1341. doi: 10.1046/j.1399-3038.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 3.Kocherscheidt L, Agossou A, Gantin RG, Hamm DM, Banla M, Soboslay PT. Cytokine and chemokine responses in adults, newborns and children exposed to Entamoeba histolytica/dispar, Onchocerca volvulus and Plasmodium falciparum. Pediatr Allergy Immunol. 2010;21:e756–763. doi: 10.1111/j.1399-3038.2010.01048.x. [DOI] [PubMed] [Google Scholar]
- 4.Deloron P, Dubois B, Le Hesran JY, Riche D, Fievet N, Cornet M, Ringwald P, Cot M. Isotypic analysis of maternally transmitted Plasmodium falciparum-specific antibodies in Cameroon, and relationship with risk of P. falciparum infection. Clin Exp Immunol. 1997;110:212–218. doi: 10.1111/j.1365-2249.1997.tb08319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley EM, Wagner GE, Ofori MF. Lack of association between maternal antibody and protection of African children from malaria infection. Infect Immun. 2000;68:5856–5563. doi: 10.1128/iai.68.10.5856-5863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Druilhe P, Pérignon JL. Mechanisms of defense against P. falciparum asexual blood stages in humans. Immunol Lett. 1994;41:115–120. doi: 10.1016/0165-2478(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 7.Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, Gilson PR, Murphy VJ, Anders RF, Mueller I, Beeson JG. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77:1165–1174. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roussilhon C, Oeuvray C, Müller-Graf C, Tall A, Rogier C, Trape JF, Theisen M, Balde A, Pérignon JL, Druilhe P. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein. PLoS Med. 2007;4:e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol. 2002;14:129–135. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 10.Iriemenam NC, Khirelsied AH, Nasr A, ElGhazali G, Giha HA, Elhassan A-Elgadir TM, Agab-Aldour AA, Montgomery SM, Anders RF, Theisen M, Troye-Blomberg M, Elbashir MI, Berzins K. Antibody responses to a panel of Plasmodium falciparum malaria blood-stage antigens in relation to clinical disease outcome in Sudan. Vaccine. 2009;27:62–71. doi: 10.1016/j.vaccine.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Good MF. Identification of early cellular immune factors regulating growth of malaria parasites in humans. Immunity. 2005;23:241–244. doi: 10.1016/j.immuni.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Schofield L. Intravascular infiltrates and organ-specific inflammation in malaria pathogenesis. Immunol Cell Biol. 2007;85:130–137. doi: 10.1038/sj.icb.7100040. [DOI] [PubMed] [Google Scholar]
- 13.Torre D, Giola M, Speranza F, Matteelli A, Basilico C, Biondi G. Serum levels of interleukin-18 in patients with uncomplicated Plasmodium falciparum malaria. Eur Cytokine Netw. 2001;12:361–364. [PubMed] [Google Scholar]
- 14.Freitas do Rosario AP, Langhorne J. T cell-derived IL-10 and its impact on the regulation of host responses during malaria. Int J Parasitol. 2012;42:549–555. doi: 10.1016/j.ijpara.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Freitas do Rosário AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, Muller W, O’Garra A, Langhorne J. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol. 2012;188:1178–1190. doi: 10.4049/jimmunol.1102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lechner CJ, Komander K, Hegewald J, Huang X, Gantin RG, Soboslay PT, Agossou A, Banla M, Köhler C. Cytokine and chemokine responses to helminth and protozoan parasites and to fungus and mite allergens in neonates, children, adults, and the elderly. Immun Ageing. 2013;10:29. doi: 10.1186/1742-4933-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochiel DO, Awandare GA, Keller CC, Hittner JB, Kremsner PG, Weinberg JB, Perkins DJ. Differential regulation of beta-chemokines in children with Plasmodium falciparum malaria. Infect Immun. 2005;73:4190–4197. doi: 10.1128/IAI.73.7.4190-4197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayimba E, Hegewald J, Ségbéna AY, Gantin RG, Lechner CJ, Agosssou A, Banla M, Soboslay PT. Proinflammatory and regulatory cytokines and chemokines in children with uncomplicated and severe Plasmodium falciparum malaria. Clin Exp Immunol. 2011;166:218–26. doi: 10.1111/j.1365-2249.2011.04474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armah HB, Wilson NO, Sarfo BY, Powell MD, Bond VC, Anderson W, Adjei AA, Gyasi RK, Tettey Y, Wiredu EK, Tongren JE, Udhayakumar V, Stiles JK. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J. 2007;6:147. doi: 10.1186/1475-2875-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Köhler C, Tebo AE, Dubois B, Deloron P, Kremsner PG, Luty AJ. Temporal variations in immune responses to conserved regions of Plasmodium falciparum merozoite surface proteins related to the severity of a prior malaria episode in Gabonese children. Trans R Soc Trop Med Hyg. 2003;97:455–461. doi: 10.1016/s0035-9203(03)90090-3. [DOI] [PubMed] [Google Scholar]
- 21.Courtin D, Oesterholt M, Huismans H, Kusi K, Milet J, Badaut C, Gaye O, Roeffen W, Remarque EJ, Sauerwein R, Garcia A, Luty AJ. The quantity and quality of African children’s IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PLoS One. 2009;4:e7590. doi: 10.1371/journal.pone.0007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlmann H, Helmby H, Hagstedt M, Carlson J, Larsson PH, Troye-Blomberg M, Perlmann P. IgE elevation and IgE anti-malarial antibodies in Plasmodium falciparum malaria: association of high IgE levels with cerebral malaria. Clin Exp Immunol. 1994;97:284–292. doi: 10.1111/j.1365-2249.1994.tb06082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.John CC, Moormann AM, Pregibon DC, Sumba PO, McHugh MM, Narum DL, Lanar DE, Schluchter MD, Kazura JW. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg. 2005;73:222–228. [PubMed] [Google Scholar]
- 24.Perlmann P, Perlmann H, Flyg BW, Hagstedt M, Elghazali G, Worku S, Fernandez V, Rutta AS, Troye-Blomberg M. Immunoglobulin E, a pathogenic factor in Plasmodium falciparum malaria. Infect Immun. 1997;65:116–121. doi: 10.1128/iai.65.1.116-121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blank U, Mécheri S. Duality and complexity of allergictype inflammatory mechanisms in determining the outcome of malaria disease. Front Immunol. 2011;2:78. doi: 10.3389/fimmu.2011.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duarte J, Deshpande P, Guiyedi V, Mécheri S, Fesel C, Cazenave PA, Mishra GC, Kombila M, Pied S. Total and functional parasite specific IgE responses in Plasmodium falciparum-infected patients exhibiting different clinical status. Malar J. 2007;6:1. doi: 10.1186/1475-2875-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulu A, Kassu A, Legesse M, Erko B, Nigussie D, Shimelis T, Belyhun Y, Moges B, Ota F, Elias D. Helminths and malaria co-infections are associated with elevated serum IgE. Parasit Vectors. 2014;7:240. doi: 10.1186/1756-3305-7-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley EM, Allen SJ, Troye-Blomberg M, Bennett S, Perlmann H, Andersson G, Smedman L, Perlmann P, Greenwood BM. Association between immune recognition of the malaria vaccine candidate antigen Pf155/RESA and resistance to clinical disease: a prospective study in a malariaendemic region of west Africa. Trans R Soc Trop Med Hyg. 1991;85:436–443. doi: 10.1016/0035-9203(91)90207-f. [DOI] [PubMed] [Google Scholar]
- 29.Patarroyo ME, Romero P, Torres ML, Clavijo P, Moreno A, Martínez A, Rodríguez R, Guzman F, Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987;328:629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- 30.Riley EM, Allen SJ, Wheeler JG, Blackman MJ, Bennett S, Takacs B, Schönfeld HJ, Holder AA, Greenwood BM. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 31.Jones GL, Edmundson HM, Lord R, Spencer L, Mollard R, Saul AJ. Immunological fine structure of the variable and constant regions of a polymorphic malarial surface antigen from Plasmodium falciparum. Mol Biochem Parasitol. 1991;48:1–9. doi: 10.1016/0166-6851(91)90158-3. [DOI] [PubMed] [Google Scholar]
- 32.Clark IA, Alleva LM, Mills AC, Cowden WB. Pathogenesis of malaria and clinically similar conditions. Clin Microbiol Rev. 2004;17:509–539. doi: 10.1128/CMR.17.3.509-539.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: a consequence of inflammatory cytokine release. Malar J. 2006;5:85–117. doi: 10.1186/1475-2875-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mécheri S. Contribution of allergic inflammatory response to the pathogenesis of malaria disease. Biochim Biophys Acta. 2012;1822:49–56. doi: 10.1016/j.bbadis.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Pelleau S, Diop S, Dia Badiane M, Vitte J, Beguin P, Nato F, Diop BM, Bongrand P, Parzy D, Jambou R. Enhanced basophil reactivities during severe malaria and their relationship with the Plasmodium falciparum histamine-releasing factor translationally controlled tumor protein. Infect Immun. 2012;80:2963–2970. doi: 10.1128/IAI.00072-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luty AJ, Perkins DJ, Lell B, Schmidt-Ott R, Lehman LG, Luckner D, Greve B, Matousek P, Herbich K, Schmid D, Weinberg JB, Kremsner PG. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect Immun. 2000;68:3909–3915. doi: 10.1128/iai.68.7.3909-3915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gbédandé K, Varani S, Ibitokou S, Houngbegnon P, Borgella S, Nouatin O, Ezinmegnon S, Adeothy AL, Cottrell G, Massougbodji A, Moutairou K, Troye-Blomberg M, Deloron P, Fievet N, Luty AJ. Malaria modifies neonatal and early-life toll-like receptor cytokine responses. Infect Immun. 2013;81:2686–2696. doi: 10.1128/IAI.00237-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ong’echa JM, Davenport GC, Vulule JM, Hittner JB, Perkins DJ. Identification of inflammatory biomarkers for pediatric malarial anemia severity using novel statistical methods. Infect Immun. 2011;79:4674–4680. doi: 10.1128/IAI.05161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedland JS, Ho M, Remick DG, Bunnag D, White NJ, Griffin GE. Interleukin-8 and Plasmodium falciparum malaria in Thailand. Trans R Soc Trop Med Hyg. 1993;87:54–55. doi: 10.1016/0035-9203(93)90417-o. [DOI] [PubMed] [Google Scholar]
- 41.Graça-Souza AV, Arruda MA, de Freitas MS, Barja-Fidalgo C, Oliveira PL. Neutrophil activation by heme: implications for inflammatory processes. Blood. 2002;99:4160–4165. doi: 10.1182/blood.v99.11.4160. [DOI] [PubMed] [Google Scholar]
- 42.Deroost K, Tyberghein A, Lays N, Noppen S, Schwarzer E, Vanstreels E, Komuta M, Prato M, Lin JW, Pamplona A, Janse CJ, Arese P, Roskams T, Daelemans D, Opdenakker G, Van den Steen PE. Hemozoin induces lung inflammation and correlates with malaria-associated acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2013;48:589–600. doi: 10.1165/rcmb.2012-0450OC. [DOI] [PubMed] [Google Scholar]
- 43.Schutyser E, Richmond A, Van Damme J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J Leukoc Biol. 2005;78:14–26. doi: 10.1189/jlb.1204712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruna-Romero O, Schmieg J, Del Val M, Buschle M, Tsuji M. The dendritic cell-specific chemokine, dendritic cellderived CC chemokine 1, enhances protective cell-mediated immunity to murine malaria. J Immunol. 2003;170:3195–203. doi: 10.4049/jimmunol.170.6.3195. [DOI] [PubMed] [Google Scholar]
- 45.Porcherie A, Mathieu C, Peronet R, Schneider E, Claver J, Commere PH, Kiefer-Biasizzo H, Karasuyama H, Milon G, Dy M, Kinet JP, Louis J, Blank U, Mécheri S. Critical role of the neutrophil-associated high-affinity receptor for IgE in the pathogenesis of experimental cerebral malaria. J Exp Med. 2011;208:2225–2236. doi: 10.1084/jem.20110845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ying S, Robinson DS, Meng Q, Barata LT, McEuen AR, Buckley MG, Walls AF, Askenase PW, Kay AB. C-C chemokines in allergen-induced late-phase cutaneous responses in atopic subjects: association of eotaxin with early 6-hour eosinophils, and of eotaxin-2 and monocyte chemoattractant protein-4 with the later 24-hour tissue eosinophilia, and relationship to basophils and other C-C chemokines (monocyte chemoattractant protein-3 and RANTES) J Immunol. 1999;163:3976–3984. [PubMed] [Google Scholar]
- 47.Ravensberg AJ, Ricciardolo FL, van Schadewijk A, Rabe KF, Sterk PJ, Hiemstra PS, Mauad T. Eotaxin-2 and eotaxin-3 expression is associated with persistent eosinophilic bronchial inflammation in patients with asthma after allergen challenge. J Allergy Clin Immunol. 2005;115:779–785. doi: 10.1016/j.jaci.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 48.Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, Lowe B, Mwangi T, Bull PC, Thomas AW, Cavanagh DR, McBride JS, Lanar DE, Mackinnon MJ, Conway DJ, Marsh K. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76:2240–2248. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Remoue F, Diallo TO, Angeli V, Hervé M, de Clercq D, Schacht AM, Charrier N, Capron M, Vercruysse J, Ly A, Capron A, Riveau G. Malaria co-infection in children influences antibody response to schistosome antigens and inflammatory markers associated with morbidity. Trans R Soc Trop Med Hyg. 2003;97:361–364. doi: 10.1016/s0035-9203(03)90170-2. [DOI] [PubMed] [Google Scholar]
- 50.Roussilhon C, Brasseur P, Agnamey P, Pérignon JL, Druilhe P. Understanding human-Plasmodium falciparum immune interactions uncovers the immunological role of worms. PLoS One. 2010;5:e9309. doi: 10.1371/journal.pone.0009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esen M, Mordmüller B, de Salazar PM, Adegnika AA, Agnandji ST, Schaumburg F, Hounkpatin AB, Brückner S, Theisen M, Bélard S, Ngoa UA, Issifou S, Yazdanbakhsh M, Kremsner PG. Reduced antibody responses against Plasmodium falciparum vaccine candidate antigens in the presence of Trichuris trichiura. Vaccine. 2012;30:7621–7624. doi: 10.1016/j.vaccine.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 52.Sokhna C, Le Hesran JY, Mbaye PA, Akiana J, Camara P, Diop M, Ly A, Druilhe P. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar J. 2004;3:43. doi: 10.1186/1475-2875-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Courtin D, Djilali-Saïah A, Milet J, Soulard V, Gaye O, Migot-Nabias F, Sauerwein R, Garcia A, Luty AJ. Schistosoma haematobium infection affects Plasmodium falciparum-specific IgG responses associated with protection against malaria. Parasite Immunol. 2011;33:124–131. doi: 10.1111/j.1365-3024.2010.01267.x. [DOI] [PubMed] [Google Scholar]