Abstract
Background
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid that acts through a family of five G-protein-coupled receptors (S1PR1–5) and plays a key role in regulating the inflammatory response. Our previous studies demonstrated that rat sensory neurons express the mRNAs for all five S1PRs and that S1P increases neuronal excitability primarily, but not exclusively, through S1PR1. This raises the question as to which other S1PRs mediate the enhanced excitability.
Methods
Isolated sensory neurons were treated with either short-interfering RNAs (siRNAs) or a variety of pharmacological agents targeted to S1PR1/R2/R3 to determine the role(s) of these receptors in regulating neuronal excitability. The excitability of isolated sensory neurons was assessed by using whole-cell patch-clamp recording to measure the capacity of these cells to fire action potentials (APs).
Results
After siRNA treatment, exposure to S1P failed to augment the excitability. Pooled siRNA targeted to S1PR1 and R3 also blocked the enhanced excitability produced by S1P. Consistent with the siRNA results, pretreatment with W146 and CAY10444, selective antagonists for S1PR1 and S1PR3, respectively, prevented the S1P-induced increase in neuronal excitability. Similarly, S1P failed to augment excitability after pretreatment with either VPC 23019, which is a S1PR1 and R3 antagonist, or VPC 44116, the phosphonate analog of VPC 23019. Acute exposure (10 to 15 min) to either of the well-established functional antagonists, FTY720 or CYM-5442, produced a significant increase in the excitability. Moreover, after a 1-h pretreatment with FTY720 (an agonist for S1PR1/R3/R4/R5), neither SEW2871 (S1PR1 selective agonist) nor S1P augmented the excitability. However, after pretreatment with CYM-5442 (selective for S1PR1), SEW2871 was ineffective, but S1P increased the excitability of some, but not all, sensory neurons.
Conclusions
These results demonstrate that the enhanced excitability produced by S1P is mediated by activation of S1PR1 and/or S1PR3.
Keywords: Excitability, Sensitization, Sphingosine 1-phosphate, Sensory neuron, Dorsal root ganglia
Background
Sphingosine-1-phosphate (S1P) is a bioactive lipid which has been shown to exert important biological functions in a variety of systems such as the immune and cardiovascular systems as well as in the regulation of cancer cells [1-4]. S1P can function as a primary messenger to act on a family of five G-protein-coupled receptors (S1P receptors, S1PR1–5) (reviewed by [5,6]). Several recent studies also demonstrate that S1P is involved in the sensation and modulation of pain (reviewed by [7,8]). Previous work from our laboratory demonstrated that extracellular delivery of S1P was capable of enhancing the excitability of sensory neurons in a GDP-β-S-dependent manner [9]. Additional studies demonstrated that S1P activation of S1PRs augmented both heat- and capsaicin-activated membrane currents in mouse sensory neurons [10]. Application of S1P increased the firing frequency of polymodal C fibers in response to a thermal stimulus in a skin-nerve preparation, suggesting that this sensitization was not a result of immune cell invasion [10]. Similarly, injection of S1P into the rat’s hindpaw produced edema, which is a hallmark of inflammation [11,12] as well as significant thermal and mechanical hyperalgesia [10,13]. Recent single-cell quantitative real-time PCR studies from our laboratory demonstrated that small-, medium-, and large-diameter sensory neurons can express the mRNAs for all five S1PRs wherein S1PR subtype 1 (S1PR1) was the highest expressor in greater than 50% of these isolated single neurons [14].
To establish which S1PR mediated the enhanced excitability produced by S1P, a study using short-interfering RNA (siRNA) to selectively knockdown expression and selective agonists demonstrated that S1PR1 plays a crucial, but not exclusive, role in mediating neuronal sensitization. Small-diameter sensory neurons treated with siRNA targeted to S1PR1 were unresponsive to the S1PR1 selective agonist SEW2871; however, treatment with the more global agonist, S1P, was still capable of increasing the excitability in approximately one third of the siRNA-treated neurons [15]. Thus, these observations indicated that S1PR1 plays a prominent role in the S1P-induced neuronal sensitization, but there must be other S1P receptors capable of mediating the S1P-induced enhancement of excitability. The studies described below show that, in addition to S1PR1, activation of S1PR3 can lead to the enhancement of excitability in sensory neurons.
Methods
Isolation and maintenance of sensory neurons
Sensory neurons were harvested from young adult Sprague–Dawley rats (80 to 150 g) and from young adult mice on a C57BL/6 J background (Harlan Laboratories, Indianapolis, IN, USA). Sensory neurons isolated from the mouse were only used in the examination of membrane currents activated by S1P. Briefly, male rats or mice were killed by placing them in a chamber that was then filled with CO2. Dorsal root ganglia (DRGs) were isolated and collected in a conical tube with sterilized Puck’s solution. The tube was centrifuged for 1 min at approximately 2000 × g, and the pellet was resuspended in 1 ml Puck’s solution containing 10 U of papain (Worthington, Lakewood, NJ, USA). After a 15-min incubation at 37°C, the tube was centrifuged at 2000 × g for 1 min, and the supernatant was replaced by 1 ml F-12 medium containing 1 mg collagenase IA and 2.5 mg dispase II (Roche Diagnostics, Indianapolis, IN, USA). The DRGs were resuspended and incubated at 37°C for 20 min. The suspension was centrifuged for 1 min at 2000 × g, and the supernatant was removed. The pellet was resuspended in F-12 medium supplemented with 10% heat-inactivated horse serum and 30 ng/ml nerve growth factor (NGF) (Harlan Bioproducts, Indianapolis, IN, USA) and mechanically dissociated with a fire-polished glass pipette until all visible chunks of tissue disappeared. Isolated cells were plated onto plastic coverslips previously coated with 100 μg/ml poly-d-lysine and 5 μg/ml laminin. Cells were maintained in culture at 37°C and 3% CO2 for 18 to 24 h before electrophysiological recording. All procedures have been approved by the Animal Use and Care Committee of the Indiana University School of Medicine.
Electrophysiology
Recordings were made using the whole-cell patch-clamp technique as previously described [16]. Briefly, a coverslip with sensory neurons was placed in a recording chamber filled with normal Ringer’s solution of the following composition (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), and 10 glucose, with pH adjusted to 7.4 using NaOH. Recording pipettes were pulled from borosilicate glass tubing (Model G85165T-4, Warner Instruments, Hamden, CT, USA). Recording pipettes had resistances of 2 to 5 MΩ when filled with the following solution (in mM): 140 KCl, 5 MgCl2, 4 ATP, 0.3 GTP, 0.25 CaCl2, 0.5 EGTA (calculated free Ca2+ concentration of 100 nM, MaxChelator), and 10 HEPES, at pH 7.2 adjusted with KOH. Whole-cell voltages or currents were recorded with an Axopatch 200 or Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA). Data were acquired and analyzed with pCLAMP 10 (Molecular Devices, Sunnyvale, CA, USA). All drugs were applied with a VC-8 bath perfusion system (Warner Instruments, Hamden, CT, USA) unless otherwise noted. In the current-clamp experiments, the neurons were held at their resting potentials (between −45 and −65 mV), and a depolarizing current ramp (1,000 ms in duration) was applied. The amplitude of the ramp was adjusted to produce between 2 and 4 action potentials (APs) under control conditions and then the same ramp was used throughout the recording period for each individual neuron. Voltages were filtered at 5 kHz and sampled at 2 kHz. In voltage-clamp recordings, neurons were held at −60 mV. Currents were filtered at 5 kHz and sampled at 500 Hz. Additionally, the voltage-clamp recordings were digitally filtered after acquisition using a low-pass 8-pole Bessel filter function (60 Hz −3 dB cutoff) in Clampfit. At the end of each recording, the neuron was exposed to 1 μM capsaicin. This neurotoxin was used to distinguish capsaicin-sensitive sensory neurons as these neurons are believed to transmit nociceptive information [17]. However, the correlation between capsaicin sensitivity and that a neuron is a nociceptor is not absolute. Some nociceptive neurons are insensitive to capsaicin and some capsaicin-sensitive neurons are not nociceptors [18]. Therefore, this agent was used to define a population of small-diameter sensory neurons that could serve a nociceptive function. All results presented in this report were obtained from capsaicin-sensitive neurons, unless otherwise stated. All experiments were performed at room temperature, approximately 23°C.
siRNA treatment
The gene sequences of S1PR2 and S1PR3 were obtained from NCBI with the accession numbers NM_017192 and XM_225216, respectively. siRNAs targeting S1PR2 and S1PR3 were designed by the online tool provided by the Whitehead Institute for Biomedical Research, Cambridge, MA (http://sirna.wi.mit.edu) [19] and synthesized by Thermo Scientific (Waltham, MA, USA). Both siRNAs were labeled with the fluorescent tag, fluorescein, with 3′-end modification. For the siRNA targeted to S1PR2, the sense strand was 5′-CCUUCUGGUGCUAAUCGCAUU-3′, and the antisense strand was 3′-UUGGAAGACCACGAUUAGCGU-5′. For the siRNA targeted to S1PR3, the sense strand was 5′-CAUUCUGAUGUCCGGUAGGUU-3′, and the antisense strand was 3′-UUGUAAGACUACAGGCCAUCC-5′. The siRNA targeted to S1PR1 was the same sequence as described in [15] and labeled with the fluorescent tag DY547. A universal Silencer Negative Control #1 siRNA (cat #4390843, Ambion, Grand Island, NY, USA) was used as the negative control.
Neurons isolated from the rat DRG were maintained in culture in F-12 medium with 30 ng/ml NGF at 37°C for 24 h. F-12 was replaced with Opti-MEM medium (Life Technologies, Grand Island, NY, USA), and the neurons were incubated at 37°C for about 5 h for lipid transfection. The transfection reagent, metafectene (Biontex-USA, San Diego, CA, USA) and siRNA complex (5 μl, 100 nM) were prepared in 2 ml Opti-MEM. Neurons were exposed to either siRNA, negative control siRNA, or metafectene alone and maintained at 37°C for 48 h. F-12 medium was used to wash out the metafectene and the siRNA; neurons were then maintained in F-12 medium with 10% heat-inactivated horse serum and 30 ng/ml NGF. Neurons were incubated for an additional 48 h before real-time quantitative PCR (qPCR) or patch-clamp experiments were performed.
cDNA generation from siRNA-treated cells
Sensory neurons that had undergone siRNA treatments were collected for real-time qPCR measurements. The F-12 medium was aspirated from the cell-culture dish, and neurons were washed with PBS solution. Total RNA from the cells was extracted by using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA), following the manufacturer’s instructions. The concentration of each individual RNA from different treatments was measured with a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA). To eliminate contamination by genomic DNA, 500 ng of RNA was treated with 1 μl DNase I (Invitrogen, cat. #18068-015) in a 10-μl reaction at room temperature for 15 min. The reaction was terminated by adding 1 μl 25 mM ethylenedinitrilo-tetraacetic acid (EDTA), and the reaction mixture was incubated at 65°C for 10 min. To generate cDNA from RNA, the DNase-I-treated RNA template was mixed with 1 μl iScript reverse transcriptase in a 20-μl reaction (iScript cDNA Synthesis Kit cat #170-8891, Bio-Rad, Hercules, CA, USA). The reaction protocol was as follows: 25°C for 5 min, 42°C for 30 min, and 85°C for 5 min.
Pre-amplification of cDNA from siRNA-treated cells
A 0.5X pooled assay mix was prepared by adding 2 μl of 20X TaqMan® Gene Expression Assay for each gene of interest (GOI) to Tris-EDTA (TE) buffer pH 8.0, final volume 80 μl. All Gene Expression Assays are labeled with the reporter dye FAM, except for hypoxanthine phosphoribosyltransferase 1 (HPRT) which was labeled with the reporter dye VIC. To each 1 μl (25 ng) of cDNA, 5 μl of 2X Pre-amp Master Mix (Life Technologies, Grand Island, NY, USA, cat #4391128), 1 μl of 0.5X pooled assay mix, and 3 μl nuclease-free H2O (Ambion, cat #9932) were added. After a 10-min incubation at 95°C, 14 cycles of 95°C/15 s and 60°C/4 min were run, followed by storage at −20°C.
TaqMan quantitative qPCR
The pre-amplified cDNA was diluted fivefold with nuclease-free H2O, and 2.5 μl of the dilution was used as the template in a 10-μl qPCR reaction also containing 5 μl 2X Taqman Gene Expression Master Mix (Applied Biosystems, Waltham, MA, USA, cat #4369514), 0.5 μl 20X TaqMan GOI Assay, and 2 μl nuclease-free water. A positive control template was 25 ng of pooled rat lung cDNA. Reactions were run in triplicate on a 7500 Fast Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). The thermal-cycling condition was 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The quantification cycle (Cq) values of various GOIs were obtained at the threshold where the value of normalized fluorescence emission generated by FAM or VIC (ΔRn) reached 0.3. The expression of different genes was calculated based on the number of copies of each gene where Number of Copies = (Primer Efficiency)−Cq. The relative expression of the GOI was determined by dividing the average copy number of the GOI by that of the reference genes, acidic ribosomal phosphoprotein P0 (Arbp) or HPRT. Efficiencies of each primer pair were determined from the slope of a seven-point standard curve (details described in [14]).
Data analysis
Data are presented as the means ± standard error of the mean (SEM). Statistical differences in the mRNA expression levels between the control groups and the treatment groups were determined by either Student’s t-test or an analysis of variance (ANOVA). Statistical differences between the control recordings and those obtained under various treatment conditions were determined by either an ANOVA or a repeated measures (RM) ANOVA whenever appropriate. When a significant difference was obtained with an ANOVA, post hoc analyses were performed using a Holm-Sidak all-pairs test. If the data set failed the normality test, a Kruskal-Wallis one-way ANOVA on ranks was performed, followed by a Tukey or Dunn’s all pairwise test. The results were considered statistically significant when the P value was <0.05 (SigmaStat 3.5 software).
Chemicals
F-12 Nutrient Mixture (Gibco Catalog # 21700–075) was supplemented with the following per liter: 1.18 g NaHCO3 (Sigma cat # S6014), 1X (2 mM) L-glutamine (Gibco cat # 25030–081), 50 units penicillin-50 mg/ml streptomycin (Gibco cat #15070-063), 10% heat-inactivated horse serum (Gibco cat #26050-088), 9 μg/ml 5-fluoro-2′-deoyuridine (Sigma cat # F-0503), and 21 μg/ml uridine (Sigma cat #U-3750). S1P and VPC 23019 were obtained from Avanti Polar Lipids (Alabaster, AL, USA); S1P was dissolved according to the manufacturer’s instructions (http://www.avantilipids.com/index.php?option=com_content&view=article&id=1114&Itemid=173&catnumber=860492). Prostaglandin E2 (PGE2), W146, FTY720, sphingosine kinase inhibitor II (SKI-II), SEW2871, and CAY10444 were purchased from Cayman Chemical (Ann Arbor, MI, USA). CYM-5442 was purchased from Tocris Bioscience (Bristol, UK). VPC 44116 was a generous gift from Dr. Kevin R. Lynch, University of Virginia. All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). PGE2, W146, FTY720, SKI-II, SEW2871, CAY10444, VPC 23019, and VPC 44116 were dissolved in 1-methyl-2-pyrrolidinone (MPL). The MPL stock solutions were then diluted with Ringer’s solution to yield the appropriate concentrations. The vehicle, MPL was typically used at 1,000- to 5,000-fold dilutions. Our earlier studies demonstrated that MPL does not affect the potassium or sodium currents in the DRG sensory neurons [9,20].
Results
siRNAs effectively and specifically knock down S1PR expression
Our previous studies demonstrated that S1PR1 played a predominate, but not exclusive, role in augmenting the excitability of rat sensory neurons [15]. These results raise the question as to which other S1PRs contribute to the S1P-mediated sensitization. The existing literature indicates that in other model systems as well as in the nervous system S1PR1, R2, and R3 play important although varied roles in modulating cellular function; however, the impact of S1PR4 and R5 are poorly understood. To explore the idea that S1PR1, R2, and R3 are key players in the S1P-mediated sensitization, siRNA targeted to these S1PRs were designed and their ability to reduce the expression of their respective receptor was measured by qPCR. Our previous results showed that siRNA targeted to S1PR1 reduced its expression by about 75% [15]; this siRNA was used in experiments described below. Treatment with siRNAs (100 and 200 nM) targeted to S1PR2 or R3 significantly reduced the levels of mRNA compared to naïve untreated neurons by approximately 80% and 70%, respectively (see Figure 1A,B). Treatment with the transfecting detergent, metafectene, or the negative control siRNA had no significant effect on the mRNA levels for either S1PR2 or R3. In addition, siRNA targeted to either S1PR2 or R3 did not have any off-target effects on the expression levels of S1PR1 (see Figure 1C). In order to determine the potential contributions of multiple S1PRs to neuronal sensitization, the siRNAs targeted to S1PR1, R2, and R3 were pooled (100 nM each) to assess their knockdown of the mRNA levels for these individual receptors as well as their possible off-target effects. The combination of S1PR1, R2, and R3 siRNAs reduced the mRNAs for S1PR1, R2, and R3 by 62%, 74%, and 76%, respectively, compared to untreated neurons (see Figure 2A,B,C, respectively) and had no off-target effects. The pooled siRNAs were as equally effective as the individual siRNAs for S1PR2 and R3 (panels B and C of Figure 2). For example, in Figure 2C, the pooled siRNAs (100 nM each) reduced the mRNA levels of S1PR3 by 76%, and the single siRNA to S1PR3 (200 nM) reduced S1PR3 mRNA by 72%. As shown in Figure 2D, neither the pooled siRNAs nor the individual siRNAs targeted to S1PR2 or R3 affected the mRNA levels of S1PR4 and R5. Similar results were obtained when the mRNA levels were assessed relative to the reference gene HPRT (data not shown). Taken together, these results indicate that the siRNAs targeting S1PR1, R2, or R3 specifically reduced the mRNA expression of their respective receptor and have no off-target actions.
Figure 1.
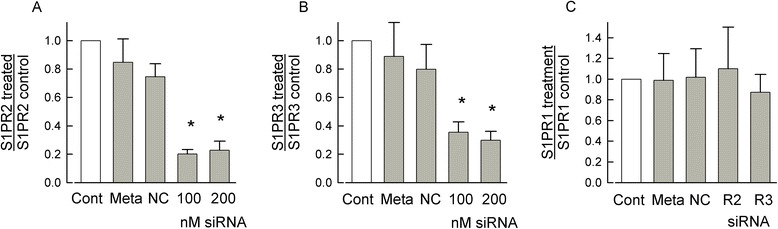
siRNAs targeted to S1PR2 or R3 specifically knockdown the mRNA levels for their respective receptors. (A) 100 and 200 nM siRNA targeted to S1PR2 significantly reduced the mRNA levels of S1PR2 by 79.8% ± 3.1% and 77.1% ± 6.4%, respectively, compared to the untreated control values. There was no difference between the knockdown values for 100 and 200 nM siRNA treatments. The different treatment groups (Cont - untreated control, Meta - metafectene alone, NC - negative control siRNA, and siRNA targeted to the S1PR) were normalized to their respective untreated control values. (B) 100 and 200 nM siRNA targeted to S1PR3 significantly reduced the mRNA levels of S1PR3 by 64.5% ± 7.3% and 70.2% ± 6.3%, respectively, compared to the untreated control values. There was no difference between the knockdown values for 100 and 200 nM siRNA treatments. (C) siRNA targeted to S1PR2 (200 nM) or S1PR3 (200 nM) does not alter the mRNA levels of S1PR1 (P = 0.88). (A-C) values were obtained from neurons isolated from five different tissue harvests; a Pfaffl analysis [70] was used to quantify the values of receptor mRNA relative to the reference gene, Arbp, for the different treatments; a Kruskal-Wallis ANOVA with a Tukey post hoc test was used to determine statistical differences between the different groups where the asterisks indicate P < 0.05. S1PR - sphingosine-1-phosphate receptor, siRNA - short-interfering RNA.
Figure 2.
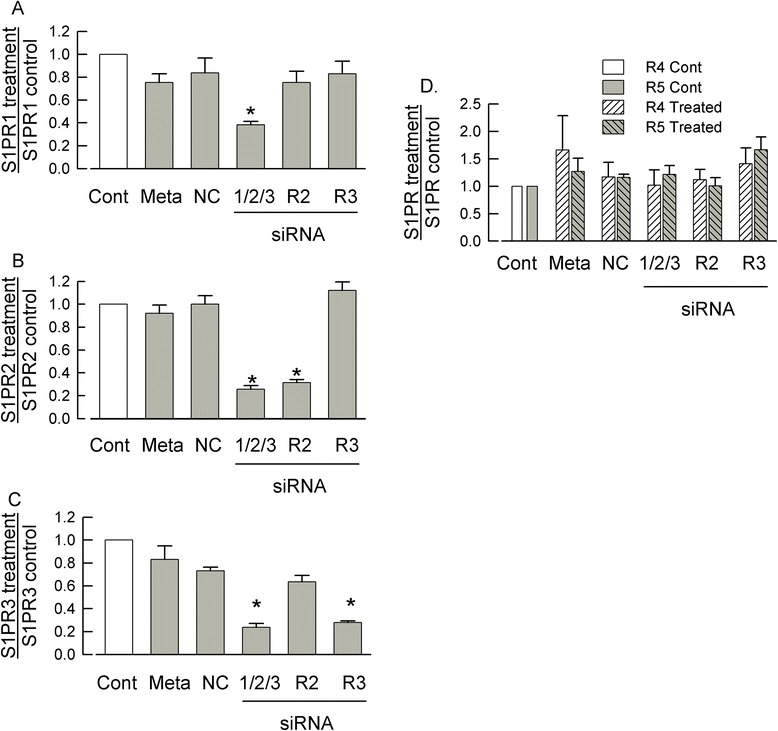
siRNAs targeted to S1PR1, R2, and R3 specifically knockdown receptor expression. (A) Combined short-interfering RNAs (siRNAs) targeted to S1PR1/R2/R3 (100 nM each) significantly reduced the mRNA expression of S1PR1 by 61.6% ± 2.9% compared to the untreated control (Cont). The levels of S1PR1 mRNA were not affected by treatment with metafectene (Meta), 200 nM of the negative control siRNA (NC), 200 nM siRNA targeted to S1PR2 (R2), or 200 nM siRNA targeted to S1PR3 (R3). The different treatment groups were normalized to their respective untreated controls. (B) Combined siRNAs targeted to S1PR1/R2/R3 (100 nM each) and siRNA targeted to S1PR2 (200 nM) alone significantly reduced the expression of S1PR2 mRNA by 74.3% ± 3.2% and 68.5% ± 2.6%, respectively. (C) Combined siRNAs targeted to S1PR1/R2/R3 (100 nM each) and siRNA targeted to S1PR3 (200 nM) alone significantly reduced the expression of S1PR3 mRNA by 76.3% ± 3.5% and 72.1% ± 1.5%, respectively. (D) The combined siRNAs targeted to S1PR1/R2/R3 did not significantly affect the mRNA levels of either S1PR4 or R5 (P = 0.88 and 0.20, respectively, ANOVA). (A-D) Values were obtained from neurons isolated from four different tissue harvests; a copy number analysis (see Kays et al. [14]) was used to quantify the values of receptor mRNA relative to the reference gene, Arbp, for the different treatments; a Kruskal-Wallis ANOVA with a Tukey post hoc test was used to determine statistical differences between the different groups where the asterisks indicate P < 0.05.
Pooled siRNAs targeted to S1PR1/2/3 or S1PR1/3 block the S1P-induced increase in excitability
Having validated the specificity of these siRNAs, the functional contributions of S1PRs to the S1P-induced enhancement of neuronal excitability were examined. As shown in the representative traces in Figure 3A, after treatment of sensory neurons with the pool of siRNAs targeted to S1PR1/2/3 (100 nM each), the ramp of current evoked only 4 APs after a 6-min exposure to 1 μM S1P (right panel) compared to 3 APs for the control conditions (left panel). In contrast, after treatment with 300 nM negative control (NC) siRNA, a 6-min exposure to 1 μM S1P increased the number of APs from a control value of 4 APs (Figure 3B, left panel) to 10 APs (right panel). The results obtained from a total of five neurons in each treatment group are summarized in Figure 3C. Exposure to 1 μM S1P significantly increased the number of APs in NC siRNA-treated neurons at both 6 and 10 min compared to the control values (P = 0.003, RM ANOVA Holm-Sidak all-pairs test) and is similar to our previous reports obtained from untreated sensory neurons [9,15]. However, S1P failed to augment AP firing in those neurons treated with the pooled siRNAs (P = 0.47). These results indicate that S1P can sensitize sensory neurons through the activation of S1PR1, R2, and/or R3 and that R4 and R5 are not sufficient to mediate the S1P-induced sensitization.
Figure 3.
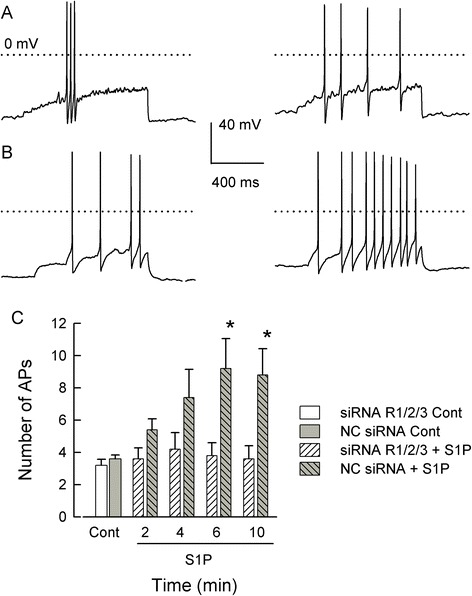
Combined siRNAs targeted to S1PR1/R2/R3 blocked the S1P-induced increase in excitability. (A) shows representative traces from a neuron treated with the combined short-interfering RNAs (siRNAs) targeted to sphingosine-1-phosphate (S1P)R1/R2/R3 (100 nM each); the trace shown in the left panel was obtained under control conditions whereas that in the right panel was after a 6-min exposure to 1 μM S1P. The dotted lines indicate the 0 mV level. (B) illustrates cells treated with 300 nM negative control siRNA. The left panel shows that under control conditions 4 action potentials (APs) were fired whereas, after a 6-min exposure to 1 μM S1P, the same ramp current elicited 10 APs. (C) summarizes the results obtained from five cells in each treatment group. S1P significantly increased the number of APs in neurons treated with negative control (NC) siRNA at 6 and 10 min. The asterisks (*) represent a significant difference compared to the control (P < 0.05 RM ANOVA with a Holm-Sidak all-pairs test).
Previously, we demonstrated that S1PR1 plays a prominent, but not exclusive, role in the sensitization mediated by S1P [15]. In addition, to examine the role of S1PR2, we used a putative S1PR2-specific antagonist JTE-013; surprisingly, JTE-013 itself increased the excitability of sensory neurons through an as-yet-to-be-defined G-protein-coupled receptor (GPCR) [21]. Thus, to distinguish a possible role for S1PR2 in the S1P-induced sensitization, sensory neurons were treated with a combination of siRNAs targeted to S1PR1 and R3 (100 nM each). As shown in Figure 4, under control conditions, a representative neuron generated 3 APs in response to the current ramp (Figure 4A), and after a 10-min exposure to 1 μM S1P, the ramp evoked 2 APs (Figure 4B). As a positive control, neurons were exposed to pro-inflammatory PGE2 to confirm that these neurons were capable of sensitization (via a Gs/cAMP/PKA pathway) [22-24]. After a 10-min exposure to 1 μM PGE2, the ramp evoked 13 APs (Figure 4C). As summarized in Figure 4D, S1P failed to enhance the excitability after treatment with siRNAs targeted to S1PR1 and R3, suggesting that S1PR1 and/or R3, but not R2, mediates the sensitization produced by S1P. In contrast, PGE2 significantly increased the AP firing at 14, 16, and 20 min compared to both the control and S1P treatment conditions (P < 0.001, ANOVA Holm-Sidak all-pairs test). Taken together, these results demonstrate that the S1P-induced sensitization is mediated through S1PR1 and/or R3 while R2, R4, and R5 appear to have no significant role.
Figure 4.
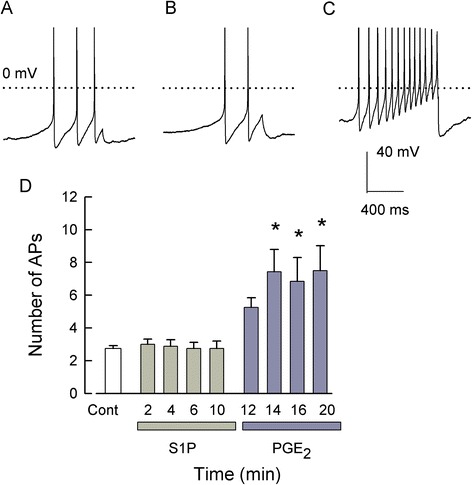
Neurons treated with siRNAs targeted to S1PR1 and R3 were not sensitized by S1P but did respond to PGE2. (A) demonstrates representative traces from a sensory neuron treated with combined siRNAs targeted to S1PR1 and R3 (100 nM each); under control conditions, the neuron generated 3 APs. (B) After a 10-min exposure to 1 μM S1P, this neuron fired only 2 APs. (C) A subsequent 10-min exposure to 1 μM PGE2 resulted in the generation of 13 APs. (D) summarizes the effects of S1P and PGE2 exposures after treatment with siRNAs targeted to S1PR1 and R3. S1P failed to augment AP firing, but PGE2 significantly increased the number of APs after 14-, 16-, and 20-min exposures. Results were obtained from eight neurons (control through 12 min), seven neurons at 14 min, and six neurons at 16 and 20 min. Asterisks (*) represent a significant difference between those treatments compared to control (P < 0.05, Kruskal-Wallis ANOVA on ranks with Dunn’s post hoc test). AP - action potential, Cont - control, PGE2 - prostaglandin E2, S1P - sphingosine-1-phosphate.
The selective S1PR3 agonist, CYM-5541, sensitizes sensory neurons
Our results suggest that S1PR3 can lead to the sensitization of sensory neurons. To test that idea directly, the recently discovered selective agonist of S1PR3, CYM-5541, was used [25]. In Chinese hamster ovary cells (CHOs) stably expressing S1PRs, the half-maximal effective concentration (EC50) values for CYM-5541 activation of S1PR3 was 105 nM and for S1PR1 it was approximately 33 μM, and there was no activity at S1PR2/4/5 for concentrations as high as 50 μM [25]. We found that CYM-5541 in a time- and concentration-dependent manner lead to the sensitization of AP firing in small-diameter sensory neurons. A representative recording (Figure 5A) shows that, under control conditions, the depolarizing ramp evoked 3 APs whereas, after a 10 min exposure to 10 μM CYM-5441, 11 APs were generated. The time- and concentration-dependence for the actions of CYM-5441 are summarized in Figure 5B. Exposure to 100 nM CYM-5541 failed to alter AP firing (n = 5, P = 0.80 RM ANOVA) or the resting membrane potential (see Table 1) over a 10-min recording period. Both 1 and 10 μM CYM-5541 significantly enhanced AP firing after 6- and 10-min exposures compared to their respective controls. However, neither of these concentrations of CYM-5541 depolarized the resting membrane potential (see Table 1). In addition, neither the resting membrane potential (−58.5 ± 1.7 mV control vs. −57.9 ± 3.0 mV CYM-5541 after 10 min, n = 7, P = 0.89 ANOVA, data not shown) nor the enhanced excitability produced by 10 μM CYM-5541 were affected by a 30-min pretreatment with 1 μM W146, a S1PR1 selective antagonist (inhibition constant (Ki) 70 to 80 nM) [26] (see Figure 5B). These results demonstrate that at 10 μM, CYM-5541 was capable of augmenting AP firing without changing the resting membrane potential through the activation of S1PR3. Figure 5C summarizes the concentration relation for the fold increase in APs generated at 10 min normalized to the number of APs obtained for their respective untreated control recordings; these results show that 10 μM CYM-5541 produces about a 2.5-fold increase in the number of evoked APs through the activation of S1PR3. To determine the maximal response, neurons were then exposed to 30 μM CYM-5541; surprisingly, this led to a rapid and large depolarization that was accompanied by a large number of APs (see Figure 5D). The left panel illustrates a 200-s recording of the resting membrane potential under normal control conditions (−51 mV) wherein there is a complete lack of any spontaneous AP activity; the right panel shows that, in this neuron, exposure to 30 μM CYM-5541 (duration 30 to 150 s) depolarized the membrane potential to −23 mV with a significant generation of spontaneous APs. In nine neurons, 30 μM CYM-5541 lead to an average depolarization of approximately 37 ± 3 mV (see Table 1). In four neurons, the recovery from the CYM-5541-induced depolarization was examined. After a 20-min washout with normal Ringers, the membrane potential had recovered by 40% ± 17% (range 4% to 82%). It is possible that this high concentration of CYM-5541 depolarizes the neuronal membrane through activation of S1PR1 rather than R3 as the EC50 for CYM-5541 at S1PR1 is approximately 33 μM [25]. To test this idea, sensory neurons were pretreated for 30 min with either 1 or 10 μM W146. In the presence of W146, 30 μM CYM-5541 did not significantly depolarize the resting membrane potential (see Table 1). In support of CYM-5541 activation of S1PR1, exposure to 30 μM SEW2871 (a selective S1PR1 agonist) produced a significant depolarization (control −56.8 ± 1.2 mV vs. −33.0 ± 4.8 mV after SEW2871, n = 5, P = 0.007 paired t-test, data not shown) that was associated with a large increase in spontaneous AP firing (see Figure 5E). These results are similar to those obtained with CYM-5541. Therefore, these results demonstrate that activation of S1PR3 can augment AP firing without directly altering the resting membrane potential; however, at the higher and likely unphysiological concentrations, activation of S1PR1 by either SEW2871 or CYM-5441 can produce a large depolarization accompanied by extensive AP firing.
Figure 5.
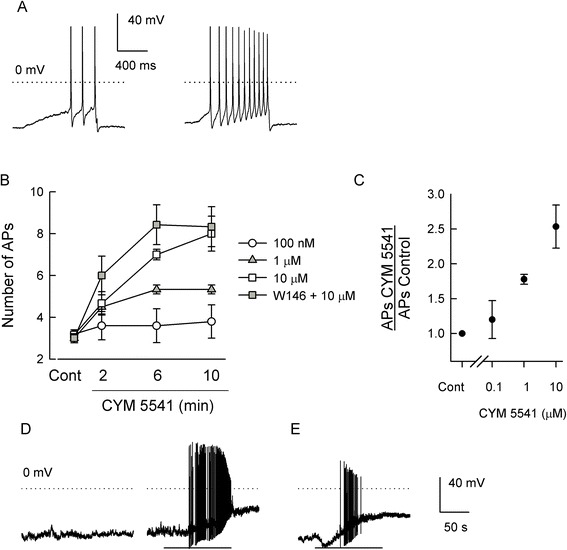
A selective agonist of S1PR3, CYM-5541, sensitizes AP firing. (A) Left panel illustrates a representative recording where the ramp evoked 3 APs under control conditions; right panel shows that after a 10-min exposure to 10 μM CYM-5441, 11 APs were generated. The dotted line indicates 0 mV. (B) the time- and concentration-dependence of CYM-5541 on the number of evoked APs over a 10-min recording period. For 100 nM, there was no effect on the number of evoked APs (n = 5, P = 0.80 RM ANOVA); for 1 μM, the increase in AP number at 6 and 10 min was significantly different than the control (n = 6, P = 0.002 RM ANOVA Friedman test on ranks); for 10 μM, the increase at 6 and 10 min was significantly different than the control (n = 6, P < 0.001 Kruskal-Wallis ANOVA on ranks Dunn’s test); for the 1 μM W146 + 10 μM CYM-5541, the increase at 6 and 10 min was significantly different than the control (n = 7 for control, 2 and 6 min, n = 6 for 10 min, P < 0.001 ANOVA Holm-Sidak all-pairs test). The AP values for 10 μM CYM-5541 and 1 μM W146 + 10 μM CYM-5541 at either 6 or 10 min were not different (P > 0.05 ANOVA). (C) Concentration dependence for the normalized fold increase in the number of APs measured after a 10-min exposure to CYM-5541. (D) Recording of membrane potential where the left panel shows the control condition; the right panel shows exposure to 30 μM CYM-5541 (30 to 150 s represented by the bar). (E) Depolarization produced by 30 μM SEW2871 (application 30 to 150 s). Recordings were acquired at 5 kHz; traces are reproduced at 0.5 kHz. Scale bars apply to all three panels. AP - action potential, Cont - control.
Table 1.
Effects of CYM-5541 on membrane potential
| CYM-5541 concentration | Untreated control (mV) | Posttreatment (mV) | n |
|---|---|---|---|
| 100 nM | −55.5 ± 2.4 | −52.8 ± 1.9 | 5 |
| 1 μM | −53.9 ± 1.0 | −50.5 ± 1.8 | 6 |
| 10 μM | −51.6 ± 1.0 | −48.1 ± 1.8 | 6 |
| 30 μM | −55.5 ± 1.8 | −18.6 ± 2.9* | 9 |
| 1 μM W146 + 30 μM | −61.7 ± 2.5 | −52.0 ± 5.6 | 4 |
| 10 μM W146 + 30 μM | −52.9 ± 1.2 | −47.8 ± 4.0 | 3 |
*P < 0.05 paired t-test; n = number of neurons.
Together, selective antagonists to S1PR1 and R3 abolish the S1P-induced sensitization
To corroborate the siRNA findings, specific antagonists were used to block receptor function: W146, a selective S1PR1 antagonist, and CAY10444 (BML-241), a selective S1PR3 antagonist. Although most studies indicate that CAY10444 is a low-potency antagonist of S1PR3 [27-29], a few studies have suggested that this compound lacks specificity [30-32]. CAY1044 (50 μM) has been reported to completely block the S1P-induced increase in intracellular Ca2+ in keratinocytes [28]; consistent with this finding, VPC 23019 (described below) also blocked this increase in Ca2+. Using a GPCR-β arrestin assay, CAY10444 suppressed the activation of S1PR3 with an IC50 value of approximately 5 μM [29]. In MCF-7 Neo cells, treatment with S1P produced a significant increase in the phosphorylation of ERK1/2; this increase was greatly suppressed by similar extents after exposure to either 10 μM CAY1044 or siRNA knockdown of S1PR3 [33]. In addition, S1P produced a relaxation of contracted coronary artery, which was significantly attenuated by treatment with 10 μM CAY10444 but was unaffected by W146 [34]. In contrast to the above, Jongsma et al. reported that in Flp-In-CHO cells, S1P increased intracellular Ca2+ and that treatment with 10 μM CAY10444 produced a rightward shift of about tenfold in the EC50 value for the mobilization of Ca2+ [30]. However, the EC50 values for the increases in intracellular Ca2+ produced by ATP activation of P2 receptors and phenylephrine activation of α1-adenoreceptors were also right-shifted by 10 μM CAY10444, although the shifts were smaller than that for S1P. Also, CAY10444 did not affect the S1P-mediated decrease in forskolin-elevated levels of cyclic AMP, suggesting that CAY10444 lacked specificity for S1PR3. The differences in these results have yet to be resolved.
To examine the role of S1PR1 and S1PR3, we found that a 30-min pretreatment with 1 μM W146 and 10 μM CAY10444 blocked the increase in the number of APs after exposure to 1 μM S1P (see Figure 6A, n = 10). In a separate series of experiments, treatment with W146 and CAY1044 did not alter the capacity of 1 μM PGE2 to significantly increase AP firing (see Figure 6B, n = 4 to 7). These results are consistent with our observations obtained with siRNAs targeted to S1PR1 and R3 and indicate that S1PR1 and/or R3, but not S1PR2, R4 or R5, is necessary for S1P-induced sensitization.
Figure 6.
W146, a selective S1PR1 antagonist, and CAY10444, a selective S1PR3 antagonist, together abolished the sensitizing effect of S1P, but not PGEx2. (A) Pretreatment with 1 μM W146 and 10 μM CAY10444 for 30 min blocked the sensitization produced by 1 μM S1P. Results were obtained from ten sensory neurons (P = 0.15 Friedman RM ANOVA on ranks). (B) In another series of experiments, pretreatment with W146 and CAY10444 for 30 min blocked the sensitization produced by 1 μM S1P; however, subsequent exposure to 1 μM PGE2 significantly increased the number of evoked APs (n = 7 control through 12 min, n = 6, 5, and 4 for 14, 16, and 20 min, respectively). The asterisks (*) represent a statistical difference compared to the 10-min S1P results (P < 0.05 Kruskal-Wallis ANOVA on ranks followed by Dunn’s post hoc test). The 10-min S1P results were not different from the control values. AP - action potential, Cont - control, PGE2 - prostaglandin E2, S1P - sphingosine-1-phosphate.
VPC 23019 and VPC 44116, antagonists at both S1PR1 and R3, block S1P-induced sensitization
The S1PR1/R3 antagonist, VPC 23019, was used to further examine the role of S1PR1 and R3 in the S1P-induced sensitization. VPC 23019 has Ki values of approximately 25 and 300 nM for S1PR1 and R3, respectively, and is also a partial agonist for S1PR4 and R5 (EC50 values of 120 and 480 nM, respectively) [35-37]. Control recordings indicated that exposure to 1 μM VPC 23019 did not change the number of APs in sensory neurons over a 15-min recording period (control 3.8 ± 0.3 APs vs. 15-min VPC 23019 4.3 ± 0.9 APs, n = 4, P = 0.59 RM ANOVA, data not shown). As shown in Figure 7A, a 30-min pretreatment with 1 μM VPC 23019 completely blocked the sensitizing actions of 100 nM SEW2871 (a selective S1PR1 agonist) and 1 μM S1P (the more global receptor agonist). In a separate group of sensory neurons, pretreatment with 1 μM VPC 23019 suppressed the enhanced excitability produced by 1 μM S1P but had no effect on the sensitization produced by 1 μM PGE2 (see Figure 7B). To corroborate the role of S1PR1 and R3 in augmenting neuronal excitability, the phosphonate analog of VPC 23019, VPC 44116, was also used. VPC 44116 is a potent antagonist at both S1PR1 and R3 (Ki values of 30 and 300 nM, respectively) and also a partial agonist for S1PR4 and R5 (EC50 values of 6100 and 33 nM, respectively) [36]. Neither VPC 23019 nor VPC 44116 have any effects at S1PR2 [32-34]. Similar to VPC 23019, exposure to 1 μM VPC 44116 did not alter the number of evoked APs over a 15-min recording period (control 3.0 ± 0.4 APs vs. 15-min VPC 44116 3.8 ± 0.6 APs, n = 4, P = 0.28 RM ANOVA, data not shown). A 30-min pretreatment with 1 μM VPC 44116 blocked the increase in excitability caused by 1 μM S1P but had no effect on the sensitization produced by 1 μM PGE2 (see Figure 7C). Thus, these results demonstrate that the enhanced excitability produced by S1P is mediated by activation of S1PR1 and/or R3 and that S1PR2, R4, or R5 do not contribute to the enhanced excitability produced by S1P in sensory neurons.
Figure 7.
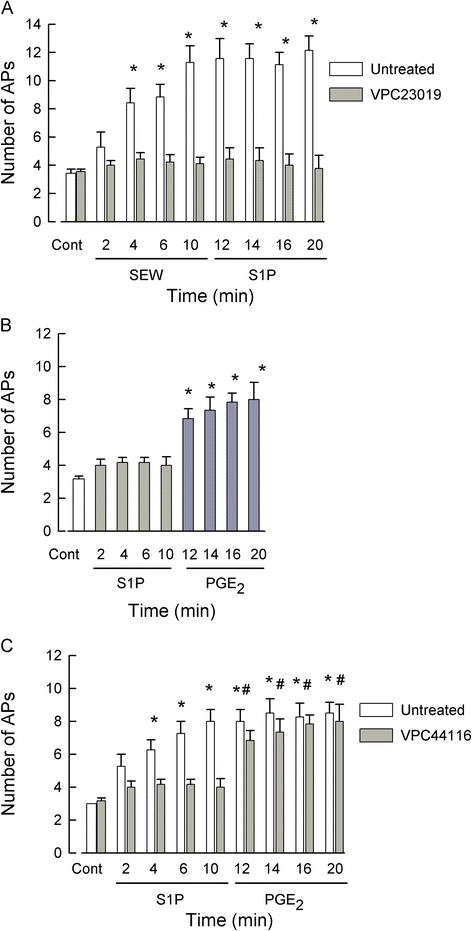
VPC 23019 and VPC 44116, S1PR1/R3 antagonists and S1PR4/R5 agonists, block S1P-induced sensitization. (A) A 30-min pretreatment with 1 μM VPC 23019 blocked the capacity of 100 nM SEW2871 (SEW), a selective sphingosine-1-phosphate (S1P)R1 agonist, and 1 μM S1P, the more global agonist, to augment action potential (AP) firing in sensory neurons (P = 0.87, RM ANOVA). In contrast, in untreated neurons, SEW2871 significantly increased the number of evoked APs after only a 4-min exposure (n = 7, P < 0.001, Friedman RM ANOVA with Tukey post hoc test) although the number of evoked APs after S1P was not different from that with SEW2871. (B) In a different set of experiments, a 30-min pretreatment with 1 μM VPC 23019 blocked the sensitization produced by 1 μM S1P; however, exposure to 1 μM PGE2 significantly increased the AP firing (P < 0.05 compared to control, ANOVA followed by Holm-Sidak all-pairs test). (C) A 30-min pretreatment with 1 μM VPC 44116 blocked the sensitization produced by 1 μM S1P; however, subsequent expose to 1 μM PGE2 significantly increased the number of evoked APs compared to control (represented by asterisks (*)) as well as the number of APs measured at 10-min S1P (represented by #)(n = 5, P < 0.001, RM ANOVA with Holm-Sidak all-pairs test). Cont - control.
FTY720, a functional antagonist at S1PR1/3/4/5, acutely increases excitability, but prolonged exposure blocks the S1P-mediated sensitization
FTY720 (fingolimod) is a structural analog of sphingosine that upon phosphorylation by sphingosine kinase 2 [38-42] has high affinities for all S1PRs except S1PR2 [38,43,44]. Receptor binding and functional assays indicated that FTY720-P has EC50 values of approximately 0.3 to 0.6 nM for S1PR1, R4, and R5 and approximately 3 nM for R3 but has no activity at S1PR2. Thus, FTY720 can act as a potent agonist for specific S1PRs. However, additional studies demonstrated that prolonged incubation with FTY720 resulted in the internalization and degradation of both S1PR1 [45-49] and R3 [50-53], which in effect removes these receptors from further activation/signaling (but see [49]). Therefore, such pharmacological agents capable of receptor activation with their consequent internalization and degradation have been termed functional antagonists. In this capacity, FTY720-P acts as a suppressor of neuroinflammation and has been approved for the treatment of relapsing-remitting multiple sclerosis [44,54,55]. To further explore the roles of S1PRs in neuronal sensitization, the capacity of FTY720 to act as a functional antagonist was utilized.
As expected of a functional antagonist, acute treatment with 100 nM FTY720 produced a significant increase in the excitability of sensory neurons. A representative recording is shown in Figure 8A where 4 APs were evoked by the current ramp under control conditions (left panel); however, after a 15-min exposure to 100 nM FTY720, the ramp now evoked 12 APs (right panel) and depolarized the resting membrane potential from −52 to −28 mV. Similar to our previous findings obtained for SEW2871 [15], a selective S1PR1 agonist, sensory neurons were either sensitive or insensitive to FTY720; these results are summarized in Figure 8B. In recordings from 13 neurons, 8 were significantly sensitized after exposure to 100 nM FTY720, exhibiting about a threefold increase in the number of evoked APs after 15- and 20-min exposures. Associated with the increased AP firing, the resting membrane potential was also significantly depolarized from a control value of −54.2 ± 1.2 to −39.3 ± 4.3 mV after a 15-min exposure (data not shown, P < 0.001 RM ANOVA, Holm-Sidak all-pairs test). With FTY720-P, the time to reach a significant increase in the number of APs was 15 min which is in contrast to SEW2871 or S1P wherein the time to reach a significant increase in the number of APs typically occurred between 4 and 6 min [9,15]. This delay may well reflect the fact that FTY720 must be phosphorylated by sphingosine kinase 2 and then transported extracellularly (see [56]) where it can function as an agonist at S1PRs (but not S1PR2). In contrast, 5 of the 13 neurons were insensitive to FTY720 wherein the number of APs evoked after a 15-min exposure was not different than the control values (control 2.6 ± 0.4 APs vs. 15-min exposure 3.0 ± 0.7 APs, P = 0.80 Kruskal-Wallis ANOVA). In these FTY720-insensitive neurons, the resting membrane potential was not changed after exposure to FTY720 (data not shown, control −58.8 ± 4.4 mV vs. 15-min exposure −58.5 ± 4.6 mV, P = 0.98 Kruskal-Wallis ANOVA). Exposure to either 10 or 30 nM FTY720 failed to increase the number of evoked APs in sensory neurons during a 20-min recording period. For example, under control conditions 3.6 ± 0.2 APs were evoked by the current ramp, and after a 20-min exposure to 30 nM FTY720, 4.4 ± 0.5 APs were generated (data not shown, n = 5, P = 0.92 RM ANOVA). Previous studies indicated that the actions of FTY720 were dependent upon phosphorylation by sphingosine kinase 2 [38-42]. To confirm that phosphorylation was required for the sensitizing actions of FTY720, sensory neurons were pretreated for 30 min with 5 μM SKI-II, a specific inhibitor of sphingosine kinases [57-59], and then exposed to 100 nM FTY720. Inhibition of sphingosine kinases completely blocked the ability of FTY720 to sensitize sensory neurons (see Figure 8C). This result demonstrates that the FTY720-induced increase in neuronal excitability depends on the activity of sphingosine kinases.
Figure 8.
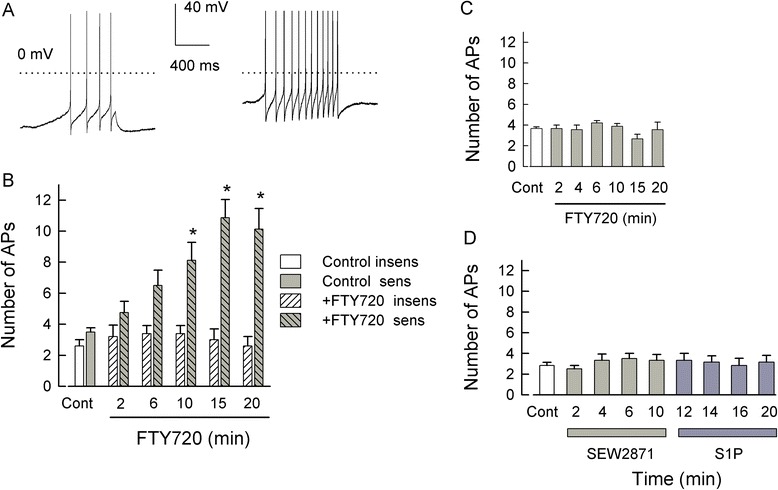
Acute FTY720 exposure increases neuronal excitability in a concentration- and time-dependent manner, but prolonged treatment blocks sensitization. (A) shows a representative recording wherein 4 APs were evoked under control conditions (left) whereas, after a 15-min exposure to 100 nM FTY720, the number of evoked APs were increased to 12. The dotted lines indicate the 0 mV level. (B) summarizes the effects of 100 nM FTY720 on sensory neurons. In one population, FTY720 significantly increased the number of APs after a 10-min exposure (n = 8), whereas another population appeared to be insensitive to FTY720 (n = 5). (C) A 30-min pretreatment with 5 μM SKI-II, a selective inhibitor of sphingosine kinases, blocked the capacity of 100 nM FTY720 to sensitize sensory neurons (n = 9 for the Cont-15 min and n = 7 for the 20-min time point, P = 0.16 Kruskal-Wallis ANOVA). (D) A 1-h pretreatment with 1 μM FTY720 blocks the ability of 100 nM SEW2871 and subsequent 1 μM S1P to sensitize sensory neurons (n = 6, P = 0.39 Friedman RM ANOVA on ranks). AP - action potential, Cont - control, S1P - sphingosine-1-phosphate.
The above results demonstrate that FTY720-P acutely augments the excitability of sensory neurons; this finding raises the question as to whether prolonged treatment with this agonist can result in the internalization/degradation of S1PRs (except S1PR2) as expected of a functional antagonist. Sensory neurons were pretreated with 1 μM FTY720 for 1 h. As shown in Figure 8D, under control conditions, the ramp evoked an average of 2.8 ± 0.3 APs (n = 6). To specifically test whether the sensitization was mediated by activation of S1PR1, these sensory neurons were exposed to 100 nM SEW2871, a selective S1PR1 agonist. After prolonged treatment with FTY720, SEW2871 failed to augment AP firing (3.3 ± 0.6 APs after a 10-min exposure, n = 6). These same neurons were then exposed to the more global agonist, S1P (1 μM), which also did not enhance AP firing (3.2 ± 0.7 APs after a 10-min exposure). There was no significant difference between the control values and any of the treatment time points (P = 0.39, RM ANOVA on ranks). Similarly, after pretreatment with FTY720, neither SEW2871 nor S1P had any effect on the resting membrane potential (control −61.8 ± 4.9 mV, 10-min SEW2871 − 63.2 ± 6.4 mV, 10-min S1P −60.6 ± 6.6 mV, n = 6, P = 0.96 RM ANOVA). In contrast, two untreated sensory neurons isolated from the same tissue harvests were sensitized after exposures to SEW2871 and S1P (control both cells evoked 3 APs, 10-min SEW2871 13 and 8 APs, and 10-min S1P 8 and 10 APs, respectively, data not shown). A 1-h pretreatment with 30 nM FTY720 did not block the capacity of 1 μM S1P to sensitize sensory neurons; in these two neurons, 3 APs were evoked under control conditions whereas, after a 10-min exposure to 1 μM S1P, the ramp evoked 14 and 9 APs. In a separate series of experiments, a 1-h pretreatment with 1 μM FTY720 blocked the capacity of 1 μM S1P to sensitize sensory neurons (data not shown; control 3.6 ± 0.3 APs vs. S1P at 10 min 5.0 ± 0.7 APs, n = 7); however, this FTY720 pretreatment had no effect on the subsequent enhancement of excitability produced by the exposure to 1 μM PGE2 (after 2 min 7.4 ± 0.7 APs and after 10 min 8.2 ± 0.7 APs, P < 0.05 vs. the control or the 10-min S1P, ANOVA on ranks). Therefore, these results demonstrate that FTY720-P acutely functions as an agonist to increase neuronal excitability and that prolonged treatment with this agonist leads to suppression of sensitization produced by either SEW2871 or S1P. A corollary to this result is that because FTY720-P does not affect S1PR2, and S1P failed to enhance the excitability after FTY720 treatment, this would clearly indicate that activation of S1PR2 is not sufficient to sensitize sensory neurons.
Pretreatment with the S1PR1 agonist, CYM-5442, prevents SEW2871, but not S1P, from increasing excitability; CYM-5442 also serves as a functional antagonist
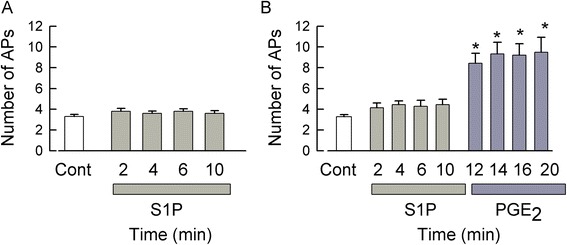
CYM-5442 is a selective agonist for S1PR1, and treatment with this compound, like FTY720, leads to the internalization and ubiquitination S1PR1 [60,61]. Using a similar approach as described above for FTY720, acute treatment with 100 nM CYM-5442 produced a significant increase in the excitability of small-diameter sensory neurons (see Figure 9A). As with FTY720, in a total of eight neurons, four neurons were sensitized by CYM-5442 within a 4-min exposure; however, four neurons remained insensitive to CYM-5442 even after 15 min. To test the idea that a prolonged treatment with CYM-5442 could lead to the down-regulation of S1PR1, we found that after a 1-h pretreatment with 100 nM CYM-5442, exposure to the selective agonist for S1PR1, SEW2871 (100 nM), failed to augment the excitability (Figure 9B). For example, after a 10-min application of SEW2871, the average number of APs (3.5 ± 0.5, n = 10) was not different than that obtained under control conditions (3.1 ± 0.1, n = 10, P = 0.44, Kruskal-Wallis ANOVA). These results suggest that CYM-5442 led to the selective down-regulation of S1PR1. If that is the case, then after prolonged treatment with CYM-5442, exposure to S1P should reveal the contributions of other S1PRs, namely S1PR3, to the S1P-mediated sensitization. As shown in Figure 9C, in a total of 15 neurons, exposure to 1 μM S1P did not alter the number of evoked APs in 10 neurons, although there was a small but significant increase in the average number of evoked APs measured at 10 min (control 3.7 ± 0.2 APs vs. 10 min 5.3 ± 0.21 APs, P = 0.002 Kruskal-Wallis ANOVA). However, in five neurons, the number of APs was significantly increased to 9.4 ± 1.2 from a control value of 3.4 ± 0.3 APs (P < 0.001, RM ANOVA Holm-Sidak all-pairs test). To reduce the variability in those sensory neurons pretreated with CYM-5442 and then exposed to these different agonists, the number of APs obtained for the different treatments was normalized to their respective values obtained for the control condition. As summarized in Figure 9D, there was no difference in the normalized number of APs obtained after exposure to SEW2871 compared to those neurons that were insensitive to S1P whereas there was a significant increase in the number of evoked APs in those sensory neurons that were sensitive to S1P. Thus, these results indicate that approximately one third of these CYM-5442-treated sensory neurons exhibited an increased excitability after exposure to S1P and, based on the results described above, suggest that S1PR3 likely mediates this effect. In addition, these results are similar to our previous findings wherein treatment with siRNA targeted to S1PR1 blocked the sensitization produced by SEW2871, yet in one third of these neurons (three of nine total), S1P produced a significant, twofold increase in the excitability [15].
Figure 9.
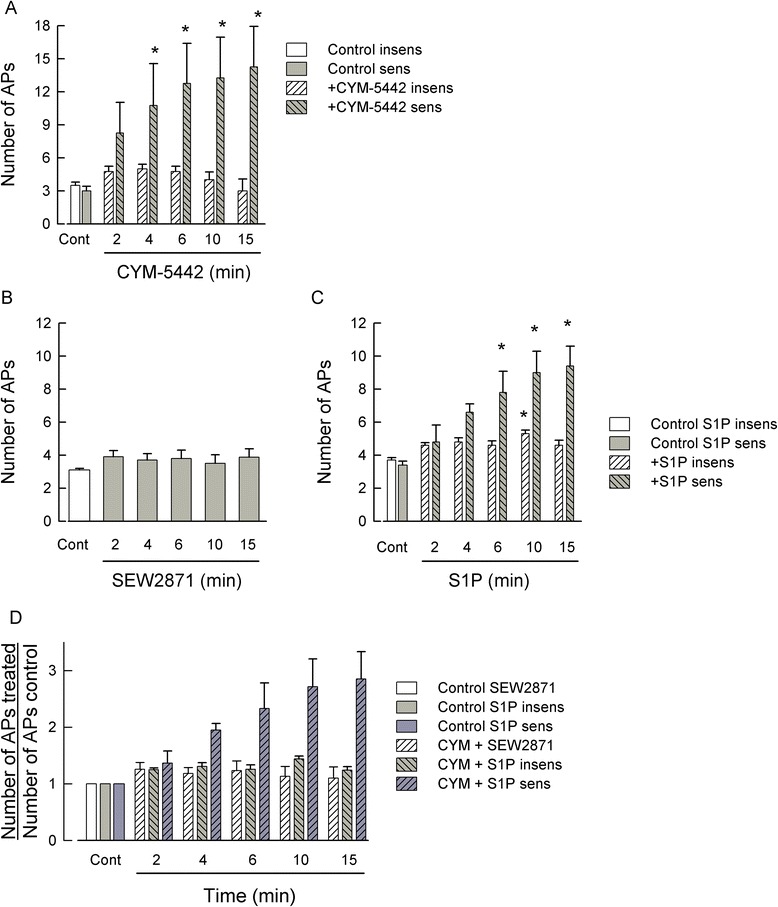
Acute CYM-5442 exposure increases neuronal excitability, but prolonged treatment blocks sensitization to SEW2871, but not to S1P. (A) summarizes the acute effects of 100 nM CYM-5442 on sensory neurons. In one group, CYM-5442 significantly increased the number of APs after only a 4-min exposure (n = 4), whereas the other group appeared to be insensitive to CYM-5442 (n = 4). (B) demonstrates that after a 1-h pretreatment with 100 nM CYM-5442, the S1PR1 selective agonist SEW2871 (100 nM) fails to increase the neuronal excitability (n = 10 control-10 min, n = 8 15 min). (C) shows that in a total of 15 sensory neurons, 10 were insensitive to 1 μM S1P although there was a small but significant increase in the number of APs measured only at the 10-min point. In contrast, five sensory neurons exhibited increased excitability in response to S1P. (D) demonstrates that after normalization of the number of APs to their respective control values, there was no difference in the average number of APs after exposure to 100 nM SEW2871 or in those neurons that appeared to be insensitive to 1 μM S1P. However, there was a significant increase in the number of APs in those sensory neurons that were sensitive to 1 μM S1P. For the 6-min point, the increase measured in the S1P-sensitive neurons was significant compared to all the SEW2871 time points, all the S1P-insensitive times except for the 10-min point, and the S1P-sensitive control. For the 10- and 15-min points, the increase measured in the S1P-sensitive neurons was significant compared to all the SEW2871 and S1P-insensitive time points, as well as the S1P-sensitive control and the 2-min point (P < 0.001 ANOVA Holm-Sidak all-pairs test). AP - action potential, Cont - control, S1P - sphingosine-1-phosphate.
S1P does not activate a membrane current in sensory neurons
A previous study [62] indicated that S1P, via S1PR3, was capable of directly mediating a membrane current conducted by chloride and is believed to result in the direct activation of nociceptive sensory neurons. As our work described above indicates that both S1PR1 and R3 mediate the sensitization of sensory neurons, the potential role of a S1PR3-induced current in regulating the excitability of sensory neurons was examined. As shown in a representative current-clamp recording obtained from a small-diameter (<25 μm) sensory neuron isolated from the rat DRG, a ramp of depolarizing current evoked 3 APs (left panel Figure 10A). However, in a voltage-clamp recording from this same neuron (holding potential of −60 mV, see the ‘Methods’ section for details), a 60-s exposure to 1 μM S1P via bath superfusion failed to produce a measureable change in membrane current (right panel). In a total of nine small-diameter capsaicin-sensitive sensory neurons (23.9 ± 0.6 μm), 1 μM S1P did not evoke any change in the existing membrane current. The studies performed by Camprubi-Robles et al. [62] used sensory neurons isolated from the mouse DRG. However, in small-diameter capsaicin-sensitive sensory neurons isolated from the mouse DRG, we found that 1 μM S1P did not evoke a change in membrane current (representative recording shown in Figure 10B, n = 7, average diameter 23.6 ± 0.7 μm). Furthermore, both 10 and 100 μM S1P failed to evoke any change in membrane current. In five mouse sensory neurons (average diameter 23.8 ± 0.4 μm, two capsaicin-sensitive, two capsaicin-insensitive, one lost before capsaicin application), 10 μM S1P was ineffective. In another three mouse sensory neurons (see representative recording in Figure 10C, average diameter 21.0 ± 1.0 μm, all three capsaicin-sensitive), 100 μM S1P evoked no change in the current. These results demonstrate that S1P, even at a high concentration, failed to elicit a change in membrane current in either rat or mouse small-diameter sensory neurons under our normal recording conditions. Although S1P can enhance the excitability of small-diameter sensory neurons, it appears to do so without evoking a direct change in membrane current. Based on this, the ability of S1P to enhance the excitability of medium- to large-diameter (>40 μm) sensory neurons isolated from rat DRG was determined. In these larger sensory neurons (average diameter 41.6 ± 0.4 μm), a 20-min exposure to 1 μM S1P did not increase the number of APs evoked by a ramp of depolarizing current (see Figure 10D, control 2.7 ± 0.2 APs, n = 15 vs. 20-min S1P 2.9 ± 0.6 APs, n = 13, P = 0.85 ANOVA on ranks). In addition, exposure to S1P did not alter the resting membrane potential in these neurons (data not shown, control −57.0 ± 0.9 mV vs. S1P 20 min −57.5 ± 1.6 mV, P = 0.87 ANOVA). S1P was applied by two different approaches, bath superfusion (n = 7) and micro-pipetting into the bath from a 100 μM stock solution (n = 8); neither of these delivery methods increased the number of evoked APs; the results obtained with these two methods were not statistically different, so they have been combined in Figure 10D. Taken together, these results suggest that small-, but not medium-large, diameter sensory neurons can be sensitized by S1P and that S1P cannot directly alter the membrane current in small-diameter sensory neurons isolated from either the rat or mouse DRG.
Figure 10.
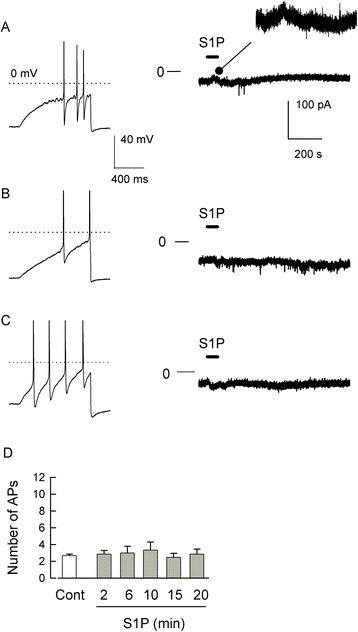
S1P does not evoke a change in membrane current in small-diameter sensory neurons. (A) Left panel shows a representative current-clamp recording where the ramp of depolarizing current evoked 3 APs in a small-diameter capsaicin-sensitive rat sensory neuron. The right panel shows the membrane current (holding voltage −60 mV) in response to a 60-s bath application of 1 μM S1P; the trace shows points at 20-ms intervals in order to reduce the dataset size; the inset represents an expanded portion of the current (35 to 235 s). (B) Left panel demonstrates a representative current-clamp recording where the ramp of depolarizing current evoked 2 APs in a small-diameter capsaicin-sensitive mouse sensory neuron. The right panel shows the membrane current (holding voltage −60 mV, 20-ms interval) in response to a 60-s bath application of 1 μM S1P. (C) Left panel shows that the ramp evokes 4 APs in this small-diameter capsaicin-sensitive mouse sensory neuron. The right panel shows the membrane current (holding voltage −60 mV, 20-ms interval) in response to a 60-s bath application of 100 μM S1P. In the left panels, the dotted line represents the 0 mV level; in the right panels, the line noted with 0 marks the zero-current level. (D) Medium- to large-diameter sensory neurons are not sensitized by S1P. Exposure of medium- to large-diameter sensory neurons isolated from rat DRG to 1 μM S1P by either bath superfusion (n = 7) or micro-injection of a stock into the bath (n = 8) does not alter the excitability. AP - action potential, Cont - control, S1P - sphingosine-1-phosphate.
Discussion
In this report, we demonstrate that S1P enhances the excitability of sensory neurons through the activation of S1PR1 and/or R3. A variety of approaches were used to isolate the contributions of specific receptors to the neuronal sensitization mediated by S1P. siRNAs targeted to individual S1PRs demonstrated that specific knockdown of the mRNA levels for S1PR1 and R3 were sufficient to prevent the sensitization produced by S1P. The results obtained with the specific agonist of S1PR3, CYM-5541, as well as pharmacological antagonists (W146, CAY10444, and the VPC compounds) are consistent with the idea that activation of S1PR1 and/or R3 augment excitability. Lastly, both FTY720 and CYM-5442 acutely increased the excitability of these sensory neurons. However, prolonged treatment with FTY720, which targets S1PR1, R3, R4, and R5, blocked the sensitization produced by either SEW2871 or S1P. In contrast, CYM-5442, which is selective for S1PR1, suppressed the effects of SEW2871 in all neurons, whereas the sensitizing actions of S1P still remained in approximately one third of the CYM-5442-treated sensory neurons. Therefore, these findings establish that the enhanced excitability produced by S1P results from the activation of S1PR1 and/or R3 but that R2, R4, and R5 are insufficient.
Our previous work indicated that S1PR1 played a prominent, although not exclusive, role in enhancing the excitability of small-diameter sensory neurons where treatment with siRNA targeted to S1PR1 completely blocked the SEW2871-induced sensitization, but in about one third of these siRNA treated-neurons, exposure to S1P was capable of producing significant increases in AP firing [15]. In a real-time single-cell qPCR study of the mRNA levels of the different S1PRs in isolated sensory neurons, we found that in small- (<25 μm, n = 18), medium- (25 to 40 μm, n = 17), and large-diameter (>50 μm, n = 17) neurons, S1PR1 was the highest expressing subtype in more than half (>10) of the total individual cells in each group [14]. In those neurons with S1PR1 as the highest expressor, five of the ten small- and five of the ten medium-diameter neurons expressed S1PR3 as the second highest subtype. In addition, there was a strong correlation between the expression of S1PR1 and R3 in both small- and medium-diameter sensory neurons (Pearson’s correlation coefficients of 0.89 and 0.92, respectively) [14]. Thus, after S1PR1, S1PR3 was the second highest expressor in approximately 50% of these identified neurons. These results are consistent with our previous siRNA studies examining the functional response of S1PR1 as well as those described above where the down-regulation of S1PR1 by CYM-5442 yields a group of neurons that were responsive to S1P, but not SEW2871. Based on the capacities of FTY720 and CYM-5442 to act as functional antagonists, these results suggest that after CYM-5442-induced down-regulation of S1PR1, S1PR3 remains capable of activation. The potential differences in cellular responses mediated by S1PR1 compared to S1PR3 may result from coupling to different G proteins and their respective downstream effectors. S1PR1 is believed to couple with only Gi/o whereas S1PR3 can couple with Gi/o, Gq/11, or G12/13, thus leading to the activation of a variety of effector systems; see reviews [5,63-65]. However, the specific roles of S1PR1 and R3 in the regulation of neuronal excitability remain to be defined and will be the focus of future investigations.
In addition, other studies support a role for S1P-S1PR1 in regulating the sensitivity of nociceptive sensory neurons. Opioid-induced hyperalgesia significantly decreased the latency of paw withdrawal to a thermal stimulus; this enhanced sensitivity was associated with a fourfold increase in the levels of S1P measured in the dorsal horn of the spinal cord [66]. Both the heightened sensitivity and the increase in S1P were blocked by pretreatment with either n-n-dimethylsphingosine or SK-I, inhibitors of sphingosine kinases. Injection of either S1P or SEW2871 into the rat or mouse paw produced thermal hyperalgesia ([13,10], respectively), which was blocked by treatment with W146, a selective antagonist for S1PR1 [13]. Localized perfusion of the L5 DRG with S1P increased the sensitivity of the rat’s paw to mechanical stimulation (by von Frey hairs) [67]. These authors also showed that localized injection of the inflammatory agent, zymosan, at the L5 DRG resulted in mechanical hypersensitivity of the hindpaw. However, a prior localized injection of siRNA targeted to S1PR1 at the L4/L5 DRG significantly reduced this hypersensitivity, suggesting that S1PR1 played a key role in the onset of this inflammatory-induced hypersensitivity [67].
An earlier study demonstrated that both intraperitonal and intrathecal delivery of FTY720 could reduce the nociceptive behaviors associated with either the inflammatory formalin model (number of flinches) or the neuropathic spared-nerve injury model (mechanical thresholds) [68]. Interestingly, effective doses of FTY720 did not have significant effects on the numbers of circulating white blood cells or lymphocytes, suggesting that the anti-nociceptive effects were not mediated by the immunosuppressive actions of FTY720. In contrast, the selective S1PR1 agonist, SEW2871, had no analgesic effect on the formalin-induced hypersensitivity.
Recently, it was shown that the intrathecal injection of SEW2871 produced a hypersensitivity (both allodynia and hyperalgesia) to mechanical stimulation of the rat’s hindpaw; this hypersensitivity was blocked by the S1PR1 selective antagonist, W146 [69]. Interestingly, the mechanical hypersensitivity resulting from the repeated injection of the chemotherapeutic agent, paclitaxel, was also blocked by W146 in a dose-dependent manner, suggesting that S1P-S1PR1 may play a role in the chemotherapy-induced peripheral neuropathy caused by paclitaxel. The peak of the increased sensitivity resulting from paclitaxel was associated with increased activity in the enzymes regulating the ceramide-sphingosine-S1P pathway, notably sphingosine kinase. Consistent with the idea that the paclitaxel-induced hypersensitivity was associated with S1P-S1PR1, prior intrathecal treatment with either FTY720 or CYM-5442 blocked, in a dose-dependent manner, the increased sensitivity caused by either SEW2871 or paclitaxel. Of significance, established paclitaxel-induced hypersensitivity could be reversed by exposure to either W146, FTY720, or CYM-5442, but not SEW2871. No effect on circulating white blood cells was observed. These results in combination with those results obtained by Coste et al. [68] strongly support the idea that antagonism rather than activation of S1PR1 is a key target in the suppression of this neuronal hypersensitivity. In future studies, it will be important to establish the signaling cascades that are activated by S1P-S1PR1 and determine the specific effectors that mediate the increased sensitivity as possible therapeutic targets.
A number of studies have established that the S1P-S1PR1 pathway plays a significant role in regulating the sensitivity of nociceptive sensory neurons through both cellular and behavioral approaches. However, in addition to our results described above, only one other study has explored the possible role of S1PR3 in regulating the sensitivity of sensory neurons. Camprubi-Robles et al. [62] demonstrated that S1P, presumably through activation of S1PR3, was capable of directly mediating a membrane current in nearly all sensory neurons isolated from the mouse DRG. Although neither the recordings of the reversal potential nor the concentration dependence were shown, application of 100 μM niflumic acid hastened the recovery phase of the S1P-induced current, suggesting that it was conducted by chloride. In current-clamp recordings, these authors report that 1 μM S1P, on average, depolarized the resting membrane potential from −54 to −36 mV with an increase in spontaneous AP firing. Our results demonstrate that FTY720 also depolarized the resting membrane potential by a similar amount (−54 to −39 mV); however, there was no enhancement of spontaneous activity, only AP firing evoked by the current ramp. We did observe a large depolarization in response to high concentrations of either CYM-5541 or SEW2871, and based on the suppressive effects of W146, this depolarization is thought to result from activation of S1PR1. In S1PR3−/− knockout mice, Camprubi-Robles et al. found that S1P depolarized the neuron by only approximately 5 mV; however, no membrane current recordings from the S1PR3−/− mice were shown. Using a fura-2-based assay, Camprubi-Robles et al. indicate that, in normal wildtype mice, approximately 60% of the neurons were responsive to 1 μM S1P and that niflumic acid reduced this to approximately 14%. However, in the S1PR3−/− mice, 40% of the neurons responded to S1P; if S1PR3 specifically mediates this response, it is curious why the knockout is not more similar to the actions of niflumic acid. Although the authors claim that this S1P-mediated current was exhibited by nearly all neurons, our experiments in both rat and mouse small-diameter sensory neurons failed to detect any measurable change in membrane current after exposure to even high concentrations of S1P (10 and 100 μM). In addition, S1P failed to augment the excitability in medium- to large-diameter rat sensory neurons. The basis for this difference remains an open question. One possibility could be differences in the culture media. In the Camprubi-Robles et al. study, sensory neurons were maintained in a synthetic serum-free medium supplemented with high levels of NGF (100 ng/ml) whereas, in our experiments, sensory neurons were maintained in an F-12 medium supplemented with 10% heat-inactivated horse serum and a lower concentration of NGF (30 ng/ml).
Conclusions
Our results demonstrate that although S1PR1 plays a prominent role in enhancing the excitability of small-diameter sensory neurons, activation of S1PR3 can lead to the augmentation of current-evoked AP firing. Clearly, additional studies will be required to fully elucidate the mechanistic role of S1PR3 in regulating neuronal excitability and sensitivity to nociceptive stimulation. Important future work could establish whether there are significant functional interactions between S1PR1 and R3 or potential interplay between their downstream signaling pathways that mediate the sensitization of sensory neurons.
Acknowledgements
We are grateful to Professor Kevin Lynch for providing us with VPC 44116. These studies were conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR015481-01 from the National Center for Research Resources, NIH. These studies were supported by the Ralph W. and Grace M. Showalter Trust.
Abbreviations
- ANOVA
analysis of variance
- APs
action potentials
- Arbp
acidic ribosomal phosphoprotein P0
- CHOs
Chinese hamster ovary cells
- Cq
quantification cycle
- dB
decibel
- DRG
dorsal root ganglion
- EC50
half-maximal effective concentration
- EDTA
ethylenedinitrilo-tetraacetic acid
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- GOI
gene of interest
- HEPES
4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid
- HPRT
hypoxanthine phosphoribosyltransferase 1
- IC50
half-maximal inhibitory concentration
- kHz
kilohertz
- Ki
inhibition constant
- Meta
metafectene
- MPL
1-methyl-2-pyrrolidinone
- ms
millisecond
- mV
millivolt
- MΩ
megaohm
- NC
negative control
- NGF
nerve growth factor
- PGE2
prostaglandin E2
- qPCR
real-time quantitative PCR
- RM ANOVA
repeated measures ANOVA
- S1P
sphingosine-1-phosphate
- S1PR
sphingosine-1-phosphate receptor
- siRNA
short-interfering RNA
- SKI-II
sphingosine kinase inhibitor II
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CL, JK, and GDN designed the study. CL, JL, and JK performed the experiments. CL, JL, JK, and GDN analyzed the data. MG provided reagents. CL, JK, and GDN wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Chao Li, Email: cdlichao@gmail.com.
Jun-nan Li, Email: junnli@iupui.edu.
Joanne Kays, Email: kaysj@iupui.edu.
Miguel Guerrero, Email: miguelg@scripps.edu.
Grant D Nicol, Email: gnicol@iupui.edu.
References
- 1.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 2.Pyne S, Pyne NJ. New perspectives on the role of sphingosine 1-phosphate in cancer. Handb Exp Pharmacol. 2013;216:55–71. doi: 10.1007/978-3-7091-1511-4_3. [DOI] [PubMed] [Google Scholar]
- 3.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Echten-Deckert G, Hagen-Euteneuer N, Karaca I, Walter J. Sphingosine-1-phosphate: boon and bane for the brain. Cell Physiol Biochem. 2014;34:148–57. doi: 10.1159/000362991. [DOI] [PubMed] [Google Scholar]
- 5.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem. 2009;78:743–68. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- 6.Rosen H, Germana Sanna M, Gonzalez-Cabrera PJ, Roberts E. The organization of the sphingosine 1-phosphate signaling system. Curr Top Microbiol Immunol. 2014;378:1–21. doi: 10.1007/978-3-319-05879-5_1. [DOI] [PubMed] [Google Scholar]
- 7.Welch SP, Sim-Selley LJ, Selley DE. Sphingosine-1-phosphate receptors as emerging targets for treatment of pain. Biochem Pharmacol. 2012;84:1551–62. doi: 10.1016/j.bcp.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Salvemini D, Doyle T, Kress M, Nicol G. Therapeutic targeting of the ceramide-to-sphingosine 1-phosphate pathway in pain. Trends Pharmacol Sci. 2013;34:110–8. doi: 10.1016/j.tips.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhang YH, Fehrenbacher JC, Vasko MR, Nicol GD. Sphingosine-1-phosphate via activation of a G-protein-coupled receptor(s) enhances the excitability of rat sensory neurons. J Neurophysiol. 2006;96:1042–52. doi: 10.1152/jn.00120.2006. [DOI] [PubMed] [Google Scholar]
- 10.Mair N, Benetti C, Andratsch M, Leitner MG, Constantin CE, Camprubí-Robles M, et al. Genetic evidence for involvement of neuronally expressed S1P1 receptor in nociceptor sensitization and inflammatory pain. PLoS One. 2011;6:e17268. doi: 10.1371/journal.pone.0017268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roviezzo F, Del Galdo F, Abbate G, Bucci M, D’Agostino B, Antunes E, et al. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci U S A. 2004;101:11170–5. doi: 10.1073/pnas.0401439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roviezzo F, Brancaleone V, De Gruttola L, Vellecco V, Bucci M, D’Agostino B, et al. Sphingosine-1-phosphate modulates vascular permeability and cell recruitment in acute inflammation in vivo. J Pharmacol Exp Ther. 2011;337:830–7. doi: 10.1124/jpet.111.179168. [DOI] [PubMed] [Google Scholar]
- 13.Doyle T, Finley A, Chen Z, Salvemini D. Role for peroxynitrite in sphingosine-1-phosphate-induced hyperalgesia in rats. Pain. 2011;152:643–8. doi: 10.1016/j.pain.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kays JS, Li C, Nicol GD. Expression of sphingosine 1-phosphate receptors in the rat dorsal root ganglia and defined single isolated sensory neurons. Physiol Genomics. 2012;44:889–901. doi: 10.1152/physiolgenomics.00053.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi XX, Nicol GD. The sphingosine 1-phosphate receptor, S1PR1, plays a prominent but not exclusive role in enhancing the excitability of sensory neurons. J Neurophysiol. 2010;104:2741–8. doi: 10.1152/jn.00709.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang YH, Kays J, Hodgdon KE, Sacktor TC, Nicol GD. Nerve growth factor enhances the excitability of rat sensory neurons through activation of the atypical protein kinase C isoform. PKMζ. J Neurophysiol. 2012;107:315–35. doi: 10.1152/jn.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 18.Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol. 2000;84:2365–79. doi: 10.1152/jn.2000.84.5.2365. [DOI] [PubMed] [Google Scholar]
- 19.Yuan B, Latek R, Hossbach M, Tuschl T, Lewitter F. siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucleic Acids Res. 2004;32(Web Server issue):W130–4. doi: 10.1093/nar/gkh366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na+ current and delayed rectifier K+ current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Chi XX, Xie W, Strong JA, Zhang JM, Nicol GD. Sphingosine 1-phosphate receptor 2 antagonist JTE-013 increases the excitability of sensory neurons independently of the receptor. J Neurophysiol. 2012;108:1473–83. doi: 10.1152/jn.00825.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32:577–80. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- 23.Cui M, Nicol GD. Cyclic AMP mediates the prostaglandin E2-induced potentiation of bradykinin excitation in rat sensory neurons. Neuroscience. 1995;66:459–66. doi: 10.1016/0306-4522(94)00567-O. [DOI] [PubMed] [Google Scholar]
- 24.Hingtgen CM, Waite KJ, Vasko MR. Prostaglandins facilitate peptide release from rat sensory neurons by activating the adenosine 3′,5′-cyclic monophosphate transduction cascade. J Neurosci. 1995;15:5411–9. doi: 10.1523/JNEUROSCI.15-07-05411.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerrero M, Poddutoori R, Urbano M, Peng X, Spicer TP, Chase PS, et al. Discovery, design and synthesis of a selective S1P3 receptor allosteric agonist. Bioorg Med Chem Lett. 2013;23:6346–9. doi: 10.1016/j.bmcl.2013.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:434–41. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 27.Koide Y, Hasegawa T, Takahashi A, Endo A, Mochizuki N, Nakagawa M, et al. Development of novel EDG3 antagonists using a 3D database search and their structure-activity relationships. J Med Chem. 2002;45:4629–38. doi: 10.1021/jm020080c. [DOI] [PubMed] [Google Scholar]
- 28.Lichte K, Rossi R, Danneberg K, ter Braak M, Kürschner U, Jakobs KH, et al. Lysophospholipid receptor-mediated calcium signaling in human keratinocytes. J Invest Dermatol. 2008;128:1487–98. doi: 10.1038/sj.jid.5701207. [DOI] [PubMed] [Google Scholar]
- 29.Wetter JA, Revankar C, Hanson BJ. Utilization of the Tango beta-arrestin recruitment technology for cell-based EDG receptor assay development and interrogation. J Biomol Screen. 2009;14:1134–41. doi: 10.1177/1087057109343809. [DOI] [PubMed] [Google Scholar]
- 30.Jongsma M, Hendriks-Balk MC, Michel MC, Peters SL, Alewijnse AE. BML-241 fails to display selective antagonism at the sphingosine-1-phosphate receptor, S1P(3) Br J Pharmacol. 2006;149:277–82. doi: 10.1038/sj.bjp.0706872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salomone S, Waeber C. Selectivity and specificity of sphingosine-1-phosphate receptor ligands: caveats and critical thinking in characterizing receptor-mediated effects. Front Pharmacol. 2011;2:9. doi: 10.3389/fphar.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pyne NJ, Pyne S. Selectivity and specificity of sphingosine 1-phosphate receptor ligands: “off-targets” or complex pharmacology? Front Pharmacol. 2011;2:26. doi: 10.3389/fphar.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long JS, Edwards J, Watson C, Tovey S, Mair KM, Schiff R, et al. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor-positive breast cancer cells. Mol Cell Biol. 2010;30:3827–41. doi: 10.1128/MCB.01133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mair KM, Robinson E, Kane KA, Pyne S, Brett RR, Pyne NJ, et al. Interaction between anandamide and sphingosine-1-phosphate in mediating vasorelaxation in rat coronary artery. Br J Pharmacol. 2010;161:176–92. doi: 10.1111/j.1476-5381.2010.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–41. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 36.Foss FW, Jr, Snyder AH, Davis MD, Rouse M, Okusa MD, Lynch KR, et al. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem. 2007;15:663–77. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynch KR, Macdonald TL. Sphingosine 1-phosphate chemical biology. Biochim Biophys Acta. 2008;1781:508–12. doi: 10.1016/j.bbalip.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 39.Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–15. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 40.Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–93. doi: 10.1016/S0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–90. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 42.Kharel Y, Lee S, Snyder AH, Sheasley-O’neill SL, Morris MA, Setiady Y, et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem. 2005;280:36865–72. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- 43.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–9. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 44.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–97. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 45.Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–3. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 46.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 47.LaMontagne K, Littlewood-Evans A, Schnell C, O’Reilly T, Wyder L, Sanchez T, et al. Antagonism of sphingosine-1 phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006;66:221–31. doi: 10.1158/0008-5472.CAN-05-2001. [DOI] [PubMed] [Google Scholar]
- 48.Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 49.Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–34. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 50.Jongsma M, van Unen J, van Loenen PB, Michel MC, Peters SL, Alewijnse AE. Different response patterns of several ligands at the sphingosine-1-phosphate receptor subtype 3 (S1P3) Br J Pharmacol. 2009;156:1305–11. doi: 10.1111/j.1476-5381.2009.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sykes DA, Riddy D, Stamp C, Bradley M, McGuiness N, Sattikar A, et al. Investigating the molecular mechanisms through which FTY720-P causes persistent S1P1 receptor internalisation. Br J Pharmacol. 2014;171:4797–807. doi: 10.1111/bph.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sensken SC, Stäubert C, Keul P, Levkau B, Schöneberg T, Gräler MH. Selective activation of G alpha i mediated signalling of S1P3 by FTY720-phosphate. Cell Signal. 2008;20:1125–33. doi: 10.1016/j.cellsig.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 53.Riddy DM, Stamp C, Sykes DA, Charlton SJ, Dowling MR. Reassessment of the pharmacology of Sphingosine-1-phosphate S1P3 receptor ligands using the DiscoveRx PathHunter™ and Ca2+ release functional assays. Br J Pharmacol. 2012;167:868–80. doi: 10.1111/j.1476-5381.2012.02032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groves A, Kihara Y, Chun J. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci. 2013;328:9–18. doi: 10.1016/j.jns.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halmer R, Walter S, Faßbender K. Sphingolipids: important players in multiple sclerosis. Cell Physiol Biochem. 2014;34:111–8. doi: 10.1159/000362988. [DOI] [PubMed] [Google Scholar]
- 56.Hisano Y, Kobayashi N, Kawahara A, Yamaguchi A, Nishi T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J Biol Chem. 2011;286:1758–66. doi: 10.1074/jbc.M110.171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–9. [PubMed] [Google Scholar]
- 58.French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- 59.Orr Gandy KA, Obeid LM. Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochim Biophys Acta. 2013;1831:157–66. doi: 10.1016/j.bbalip.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzalez-Cabrera PJ, Jo E, Sanna MG, Brown S, Leaf N, Marsolais D, et al. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like headgroup interactions. Mol Pharmacol. 2008;74:1308–18. doi: 10.1124/mol.108.049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez-Cabrera PJ, Cahalan SM, Nguyen N, Sarkisyan G, Leaf NB, Cameron MD, et al. S1P1 receptor modulation with cyclical recovery from lymphopenia ameliorates mouse model of multiple sclerosis. Mol Pharmacol. 2012;81:166–74. doi: 10.1124/mol.111.076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camprubí-Robles M, Mair N, Andratsch M, Benetti C, Beroukas D, Rukwied R, et al. Sphingosine-1-phosphate-induced nociceptor excitation and ongoing pain behavior in mice and humans is largely mediated by S1P3 receptor. J Neurosci. 2013;33:2582–892. doi: 10.1523/JNEUROSCI.4479-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pyne S, Pyne N. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol Ther. 2000;88:115–31. doi: 10.1016/S0163-7258(00)00084-X. [DOI] [PubMed] [Google Scholar]
- 64.Siehler S, Manning DR. Pathways of transduction engaged by sphingosine 1-phosphate through G protein-coupled receptors. Biochim Biophys Acta. 2002;1582:94–9. doi: 10.1016/S1388-1981(02)00142-7. [DOI] [PubMed] [Google Scholar]
- 65.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–22. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 66.Muscoli C, Doyle T, Dagostino C, Bryant L, Chen Z, Watkins LR, et al. Counter-regulation of opioid analgesia by glial-derived bioactive sphingolipids. J Neurosci. 2010;30:15400–8. doi: 10.1523/JNEUROSCI.2391-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie W, Strong JA, Kays J, Nicol GD, Zhang JM. Knockdown of the sphingosine-1-phosphate receptor S1PR1 reduces pain behaviors induced by local inflammation of the rat sensory ganglion. Neurosci Lett. 2012;515:61–5. doi: 10.1016/j.neulet.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coste O, Pierre S, Marian C, Brenneis C, Angioni C, Schmidt H, et al. Antinociceptive activity of the S1P-receptor agonist FTY720. J Cell Mol Med. 2008;12:995–1004. doi: 10.1111/j.1582-4934.2008.00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janes K, Little JW, Li C, Bryant L, Chen C, Chen Z, et al. The development and maintenance of paclitaxel-induced neuropathic pain requires activation of the sphingosine 1-phosphate receptor subtype 1. J Biol Chem. 2014;289:21082–97. doi: 10.1074/jbc.M114.569574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]


