Abstract
γ-Herpesviral immune evasion mechanisms are optimized to support the acute, lytic and the longterm, latent phase of infection. During acute infection, specific immune modulatory proteins limit, but also exploit, the antiviral activities of cell intrinsic innate immune responses as well as those of innate and adaptive immune cells. During latent infection, a restricted gene expression program limits immune targeting and cis-acting mechanisms to reduce the antigen presentation as well as antigenicity of latency-associated proteins. Here, we will review recent progress in our understanding of γ-herpesviral immune evasion strategies.
γ-Herpesviruses evolved elaborate strategies to evade and exploit host immune defense mechanisms to persist within their hosts. Innate immunity serves as the first line of defense against viral infection. The cellular intrinsic innate immune response is achieved through signal transduction that initiates pathways to alert neighboring cells (inflammatory response), to digest virus (autophagy), or to induce suicide of the infected cells (apoptosis or necrosis). The adaptive immune response, conveyed by T cells and B cells, directly targets virion particles or virus-infected cells for elimination by phagocytosis and cytotoxic cell-mediated lysis. As reviewed here, every aspect of the immune response is modulated by γ-herpesviruses.
Evasion of adaptive cellular immune responses
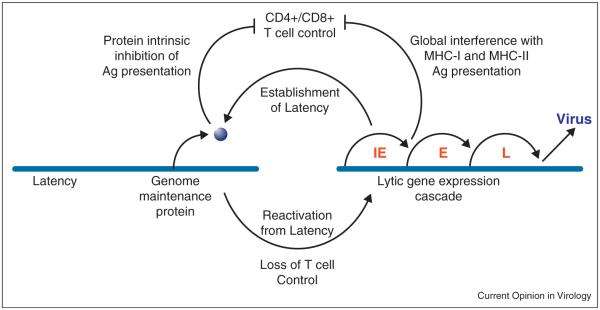
The fact that T cell depletion by HIV or by iatrogenic treatment leads to reactivation and proliferation of γ-herpesviruses, eventually leading to cancers such as KS, strongly suggests a key role of T cell control of γ-herpesvirus latency. The switch from acute to latent infection is reflected in the T cell response, which initially responds broadly to antigens highly expressed during lytic infection, but this short-lived response is then replaced with a long-term response to latency-associated antigens [1∙,2]. Limiting antigen expression is thus the first and foremost long-term T cell evasion mechanism (Figure 1). Moreover, latency levels are controlled by T cells since introduction of immunodominant epitopes into latent antigens reduces latent viral load [3,4].
Figure 1.
Evasion of T cell responses by g herpesviruses.
Limiting T cell recognition of latently-infected cells seems paramount to maintaining γ-herpesvirus latency. In the complete absence of gene expression, T cells would not notice infected cells since they depend on MHC-I display of viral peptides. However, at least one genome maintenance protein is present in all latently infected cells and γ-herpesviruses have evolved ingenious methods to protect this Achilles heel from immune exposure [5]. The main strategy is to limit the availability of antigenic peptides from latency-associated proteins. On a population level, this is achieved by selecting against MHC-I binding motifs [6]. Within an infected organism, each virus evolved unique strategies to avoid alerting T-cells. EBNA-1 of EBV controls its own transcription, translation and degradation to achieve minimal protein turnover [5]. Central Gly-Ala repeats encoded by purine-rich codons are central for biosynthetic control [5]. In mature EBNA-1 these repeats reduce the rate of proteasomal degradation [7] in a position-dependent manner [8]. Gly-Ala repeats additionally inhibit EBNA-1 translation initiation thus inhibiting production of defective ribosomal products (DRiPs) considered the main source of MHC-I bound peptides [9,10∙,11]. EBNA-1 mRNA translation might be further restricted by purine-biased codon usage [12].
Similar to EBNA-1, the major latency protein LANA-1 of KSHV contains central repeat regions that enhance LANA-1 stability and retard protein translation [13]. When fused to a heterologous antigen, the central repeat region of LANA-1 prevents antigen presentation [14]. Interestingly, inhibition of antigen presentation maps to the CR1 region whereas protein levels are regulated by CR2 and CR3 regions [15]. How LANA-1 regulates antigen processing in cis is thus still unclear. Even less is known about how non-human γ-herpesviruses escape T cell clearance during latency, although there is evidence that presentation of their respective ORF73 orthologs is limited [4,16].
During lytic infection, global stealth mechanisms limit T cell recognition of virus infected cells (Figure 1) Most γ-herpesviruses studied to date (with the notable exception of RRV) globally prevent MHC-I antigen presentation as part of the lytic gene expression program (for a recent review see [17]). EBV interferes with MHC-I antigen presentation at several levels: the protein BNLF2A inhibits peptide transport across the ER membrane by TAP [18,19,20∙∙] and thus inhibits antigen presentation of EBV-infected B cells to CD8+ T cells [21]. BNLF2A is a small 60AA protein that is post-translationally tail-anchored to the ER-membrane [22,23]. In addition, MHC-I at the cell surface is endocytosed by BILF1, a constitutively signaling G protein coupled receptor [24,25].
KSHV, MHV68 and rhesus fibromatosis herpesvirus (RFHV) hijack members of the MARCH transmembrane ubiquitin-ligase family found from yeast to man (for recent reviews see: [26,27]). MARCH proteins are transmembrane-spanning and locate to subcellular vesicular compartments, including the ER, Golgi, TGN and the plasma-membrane. They contain a RING-CH-domain structurally and functionally related to the RING-domain of E3 ubiquitin-ligases that catalyze the formation of poly-ubiquitin chains in the presence of the ubiquitin-activating and ubiquitin-conjugating enzymes. KSHV encodes two MARCH-homologs, K3 and K5, whereas MHV68 and RFHV encode a single homolog [28]. All of these proteins share the ability to downregulate MHC-I molecules suggesting that inhibition of antigen presentation to CD8+ T cells is the central function of this protein family. In addition, co-stimulatory molecules such as ICAM-1 and B7-2 as well as NK-cell ligands and cell adhesion molecules are targeted, particularly by K5 (reviewed in Ref. [29]). More recently, it was also shown that KSHV-K5 downregulates receptor tyrosine kinases and the antiviral protein BST2/Tetherin [30,31]. In vivo, MHC-I-downregulation by the MARCH-homolog MK3 of MHV68 did not alter the course of primary infection whereas, in the absence of MK3, latent virus levels were reduced in the spleen, which could be rescued by CD8+ T cell depletion [32]. Thus, MHC-I inhibition promotes optimal seeding of latency sites but does not prevent the induction of a robust and broad T cell response during lytic infection [1∙,2]. Moreover, under extreme T cell pressure, the virus is able to mutate immunodominant epitopes [33∙].
In addition to CD8+ T cells, MHC-II restricted CD4+ T cells are a major factor in controlling the establishment and maintenance of γ-herpesvirus latency [34,35∙,36]. In mixed cultures of tonsillar B and T cells, lytic replication of KSHV was suppressed by CD4+ T cells, which were essentially driving the virus into latency [35∙]. The finding that CD4+ T cells lyse latently MHV68-infected B cells [36], suggests that CD4+ T cells directly control latency rather than through helper T cell function. CD4+ T cells seem to be continuously stimulated by infected B cells and dendritic cells [34]. However, γ-herpesviruses also counteract CD4+ T cell activation. The KSHV vIRF3 protein was shown to inhibit the class II transactivator (CIITA) which is essential for transcriptional induction of MHC-II and auxiliary proteins [37]. Consequently, PEL cells expressing vIRF3 are not recognized by LANA-1 specific MHC-II-restricted CD4+ cells [38]. Adding CIITA restored some recognition. In EBV-infected B-cells, MHC-II-dependent antigen presentation is limited by BZLF1-mediated downregulation of invariant chain (CD74) [39∙∙]. Similarly, KSHV-infected endothelial cells were not optimally recognized by MHC-II restricted human CD4+ T cells due to inhibition of IFNγ-induced MHC-II transcription [40]. In latently infected monocytes, KSHV additionally down-regulates co-stimulatory molecules, which could affect T cell priming [41]. In addition, KSHV-infected endothelial cells were shown to secrete a factor that inhibits MHC-II expression on neighboring cells [40]. This might aid KSHV to prevent T cell stimulation by non-infected APC cross-presenting viral antigens.
Evasion of cell-intrinsic innate immune responses
Evasion of apoptosis
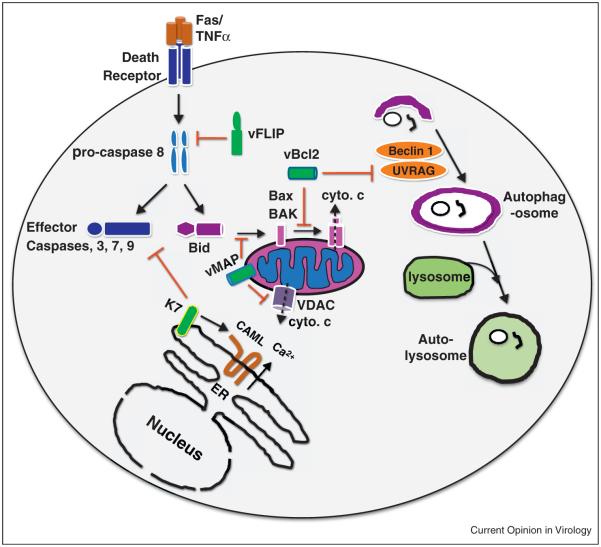
Extracellular apoptosis stimuli induce the oligomerization of death receptors whereas intracellular ‘danger’ signals perturb mitochondrial homeostasis. The death-inducing signal complex (DISC) and the permeabilization of mitochondrion membrane are key checkpoints of extrinsic and intrinsic pathway, respectively. To circumvent apoptosis, γ-herpesviruses express viral FLIP (vFLIP) and Bcl-2 (vBcl-2) homologs during the latent and lytic phase [42] (Figure 2). Death receptor-mediated apoptosis (triggered by CD95 and TNFa) delivered by cytotoxic T cells is counteracted by vFLIP via interfering with the assembly of DISC. Counterintuitively, vFLIP potently activates NFkB, which effectively enables the survival of KSHV-infected lymphoma cells [43 47]. Studies using cultured cells and transgenic mouse models demonstrated that vFLIP activated the IKK complex and was implicated in KSHV-associated lymphoproliferative diseases [47 51]. Surprisingly, vFLIP of herpesvirus saimiri was shown to be dispensable for viral replication, transformation, and pathogenesis in a primate model [52]. It would be interesting to examine the roles of vFLIP in the infection and pathogenesis of RRV using nonhuman primate models.
Figure 2.
Inhibition of the classical apoptosis and autophagy pathway by γ-herpesvirus proteins. KSHV vFLIP and K7 target pro-caspase 8 and activated caspase 3 for inhibition, whereas vBcl2 and MHV-68 vMAP antagonize the oligomerization of Bax/Bak and VDAC that release cytochrome c (cyto. c). Additionally, KSHV K7 activates CAML in releasing calcium from the ER to attenuate stress induced apoptosis. Of the classic autophagy pathway, vBcl2 suppresses UVRAG in autophagosome formation. Viral proteins are colored in green.
During productive lytic infection, processes of viral replication frequently induce stress responses that perturb cellular homeostasis. Cell death or survival is dictated by opposing activities between pro-apoptotic and anti-apoptotic factors, known as Bcl-2 homology proteins, which reside within the outer membrane of mitochondria (Figure 2). All γ-herpesviruses express anti-apoptotic Bcl-2 homologs during various stages of their infection cycle [53]. Viral Bcl-2 has lost the inhibitory loop that, when cleaved by an activated caspase, converts cellular antiapoptotic Bcl-2 into a proapoptotic product via removing the N-terminal BH4 domain [54,55]. Thus, viral Bcl-2 proteins either are not cleaved or, even if cleaved by caspase, do not behave as apoptosis-inducers. Our understanding of the in vivo functions of the antiapoptotic activity of vBcl-2 is largely derived from studies using MHV68. Recombinant MHV68 expressing a vBcl-2 mutant that fails to suppress apoptosis establishes normal latent infection in splenocytes, but is crippled to reactivate ex vivo [56∙]. Thus, viral antagonism of apoptosis is crucial to promote cell survival during early stages of lytic infection. Similarly, vBcl-2 expression immediately after infection by EBV promotes cell survival by enabling latent infection and transformation of lymphoma cells ex vivo [57∙]. The viral mitochondrial antiapoptotic protein (vMAP) of MHV68 displays a two-pronged mechanism to counteract intrinsic apoptosis [58]. vMAP binds to Bcl-2 to enhance its recruitment to mitochondria and subsequent inhibition of the BH3-only molecules, and impedes cytochrome c release through physical association with VDAC, effectively negating the mitochondrion apoptotic pathway. Viral infection with recombinant MHV68 demonstrated that this antiapoptotic activity was important for the lytic phase of infection.
It is also necessary to inactivate p53 during active viral replication and the growing list of such proteins includes the KSHV latent nuclear antigen (LANA), the EBV nuclear antigen 3C (EBNA3C), and the KSHV K7 and vIRF3. KSHV LANA and EBV EBNA3C are major latent gene products and both inactivate p53-mediated apoptosis. While KSHV LANA induces p53 degradation [59], EBNA3C stabilizes a cellular factor that prevents p53 to access DNA and sequestrates a co-activator key for p53-mediated transcription [60,61]. The KSHV K7 protein is a lytic gene product that antagonizes apoptosis by multiple mechanisms (Figure 2). Anchored in the membrane, K7 interfaces with the mitochondrion-dependent apoptotic pathway, the ER stress responses, and the proteasome/ ubiqutin system [62 64]. K7 expression protects cells from apoptosis induced by intrinsic stresses, including DNA damage and ER stress. Similar to LANA, vIRF4 targets p53 for degradation via two distinct mechanisms governing ubiquitination [65]. These findings highlight the importance of inhibiting intrinsic apoptosis triggered by viral replication events, danger signals within the infected cells.
Evasion of autophagy
Autophagy is a homeostatic process that engulfs and, upon fusion with lysosomes, digests bulk cytoplasm under nutrient-deprived conditions. Recent studies indicate that host cells deploy autophagy to defeat pathogen infection. vBcl-2 and vFLIP can inhibit autophagy, implying an inherent crosstalk between autophagy and apoptosis. vBcl-2 targets Beclin 1, a key regulator of autophagy, to negate autophagy [66]. Subsequently, vBcl-2 was found to target UVRAG, an integral component of the Beclin-1-PI3K complex, which arrests the PI3K complex and suppresses autophagy [67]. The anti-autophagic activity of viral Bcl-2 is conserved within γ-2-herpes-viruses (Figure 2). Using recombinant MHV68 carrying mutations that differentially ablate the anti-autophagic or anti-apoptotic activity of vBcl-2, Xiaofei et al. showed that inhibition of autophagy by MHV68 Bcl-2 was essential for the establishment of latent infection in splenocytes [56]. Viral and cellular Bcl-2 proteins show a difference in affinity for Beclin 1. Structural studies illustrated that this difference likely stems from the specific sequence of the hydrophobic groove of Bcl-2 that directly contacts the BH3 of Beclin 1 [68,69]. Recently, the KSHV vFLIP protein was discovered to bind to the LC3-processing enzyme, ATG3 [70]. As such, vFLIP and cellular FLIP molecules potently inhibit LC3 processing and autophagosome membrane elongation. Interestingly, vCyclin, another latent protein, induces autophagy and senescence, both of which are diminished by vFLIP [71]. These data suggest a complex stimulation and inhibition of autophagy during the lytic phase of γ-herpesviruses.
Evasion of interferon production
Type 1 interferon (IFN) and IFN-stimulated genes (ISGs) represent the first line of defense against virus infection. γ-Herpesviruses interfere with the IFN pathway at several levels as reviewed recently [72,73] (Table 1). Virus-infected cells secrete IFN upon recognition of specific viral signatures by pattern-recognition receptors (PRR). These PRRs include the Toll-like receptors (TLRs), nucleotide-binding and oligomerization, leucine-rich repeat (NLR) proteins, retinoic acid inducible gene (RIG)-I-like receptors (RLRs) and C-type lectin receptors (CLRs). Via adaptor proteins, these innate sensors in turn activate a family of transcription factors, the IFN regulatory factors (IRFs), which translocate to the nucleus to initiate transcription of IFN α/β genes. Secreted IFN binds to the type 1 IFN receptor (IFNAR) to trigger a signaling cascade that culminates in the expression of ISGs. γ-Herpesviruses antagonize both the expression and function of different components of the IRF pathway.
Table 1.
Antagonism of IFN signaling by human γ-herpesvirsues
| Virus | ORF | Gene product | Functional mechanism(s) |
|---|---|---|---|
| K9 | vIRF-1 | Suppresses IRF-1 and IRF-3 transactivational activity; inhibits IRF3 nuclear translocation; inhibits TLR3-driven IFN-β promoter activation and IFNβ production; downregulates TLR4 |
|
| K11.1/K11 | vIRF-2 | Suppresses IRF-1 and IRF-3 transactivational activity; inhibits TLR3-driven IFN-β promoter activation and IFNβ production; inhibits PKR function |
|
| K10.5/K10.6 | vIRF-3 | Inhibits IRF7 DNA binding and function; Inhibits IRF5-mediated promoter activation | |
| ORF45 | ORF45 | Prevents phosphorylation and nuclear translocation of IRF7 | |
| KSHV | ORF50 | RTA | Promotes IRF7 ubiquitination and degradation; degrades the TLR3/4 adaptor TRIF |
| ORF63 | Disrupts formation and activity of the NLRP1 inflammasome | ||
| ORF64 | Suppresses RIG-I-mediated signaling | ||
| ORF73 | LANA1 | Prevents IRF3-mediated induction of IFN-β during latency | |
| ORF74 | vGPCR | Downregulates TLR4 | |
| ORFK8 | K-bZIP | Prevents IRF3-mediated induction of IFN-β during virus reactivation | |
| miR-K9 | Downregulates MyD88 and IRAK1 | ||
| BGLF4 | PK | Binds and suppresses IRF3 transactivational activity; inhibits of IFN-β production | |
| BGLF5 | DNase | Degrades TLR9 transcript | |
| BZLF1 | Zta | Binds and suppresses IRF7 transactivational activity | |
| EBV | BRLF1 | Rta | Inhibits IRF3 and IRF7 transcription; inhibits IFN-β production during lytic infection. |
| LF2 | LF2 | Prevents IRF7 dimerization and IFN-α production | |
| LMP1 | LMP1 | Negatively regulates IFN response via induction of miR-146a; downregulates TLR9 | |
| LMP2A/2B | LMP2A/2B | Enhances turnover of type I/II IFN receptors |
Interferon regulatory factors
KSHV encodes four homologs of cellular IRFs, vIRF1 through 4, which interfere with the transactivating activity of specific IRFs. vIRF-1 binds to host IRF-1 or the coactivator protein p300, the latter action inhibiting formation of the transcriptionally active IRF3-CBP/300 complex [74]. vIRF-2 interferes with the transactivational activity of IRF-1 and IRF-3 [75,76] by direct binding to IRF1 and degradation of IRF3 via a caspase 3-dependent mechanism [75,77]. vIRF-2 also interacts with protein kinase R (PKR), inhibiting its autoactivation and antiviral function [78]. IRF7, together with IRF3, constitute the master regulators of type 1 IFN production, and KSHV vIRF3 antagonizes IRF 5 [79] and IRF7 [80] via direct inhibitory interactions. vIRF-4 does not appear to antagonize IFN-mediated signaling; instead, vIRF4 enhances the degradation of p53 (which is also targeted by vIRF1 and 3) via a stabilizing interaction with the E3 ubiquitin ligase MDM2 [70,81]. vIRF4 also interacts with CSL/CBF1, potentially antagonizing Notch/CBF1 signal transduction, and cooperates with RTA to facilitate KSHV reactivation [82,83]. Despite similar functional activities, the vIRFs display differential expression patterns, indicating that they may act at different times, and/ or in different cells [81]. Interestingly, a recent study identified mechanistic differences between the ability of the KSHV vIRFs to inhibit TLR3-dependent IFN signaling [84].
KSHV encodes additional gene products to antagonize host IRFs while EBV and MHV68 target host IRFs with non-homologous viral proteins. This common strategy underscores the importance of the IRF family in antiviral defense. Two KSHV proteins, LANA1 (ORF73) and K-bZIP (K8), bind competitively to the IFN-β promoter, preventing IFN-β induction during latency and virus reactivation [85,86]. The conserved viral kinase ORF36 inhibits IFN production. MHV68 ORF36 binds to nuclear IRF-3 to prevent activation of the IFN-β promoter [87]. Importantly, the growth and spread of an ORF36-null MHV68 in immunocompetent mice was attenuated, exemplifying the need to antagonize the IFN pathway for successful infection. The EBV ORF36 homolog, BGLF4, also binds to IRF3 to suppress IRF3-mediated signaling [88]. KSHV ORF45, a conserved tegument and IE protein, binds to the inhibitory domain of IRF7 to prevent its phosphorylation and nuclear translocation by acting as an alternate substrate for the virus-activated kinases IKKε and TBK1 [89 91]. As a virion component, ORF45 could inhibit IFN production at the earliest stages of de novo infection, as suggested by studies with an ORF45-null recombinant KSHV [92]. MHV68 ORF45 is also essential for an early stage of viral replication, suggesting a similar role in evasion of the early IFN response [93]. KSHV RTA (ORF50) also targets IRF7, promoting its ubiquitination and degradation [94].
Like KSHV, RRV expresses a set of viral IRFs [95]. As such, the RRV model provides insight into the role of the vIRFs in regulating the host response to infection. Accordingly, a vIRF deletion clone of RRV that inhibits IFN release from PMC in vitro exhibits attenuated replication in vivo and induces a more robust anti-inflammatory and anti-RRV T cell response [96,97]. Macaques infected with the vIRF knockout also display diminished B cell hyperplasia, a pathology characteristic of acute RRV infection.
While EBV does not express IRF homologs, it encodes several proteins that target host IRFs. The EBV IE transactivator protein BZLF1 (Zta) binds to IRF7 and inhibits its transactivational activity [98], while BRLF1 (Rta) inhibits transcription of IRF3 and IRF7, thus inhibiting IFN-β production during lytic infection [99]. In addition, binding of the tegument protein LF2 to IRF7 prevents IRF7 dimerization and IFN-α production [100]. Interestingly, a positive regulatory circuit exists between IRF7 and the EBV oncoprotein LMP1 [101]. LMP1 possesses potent immune escape functions, including induction of immunomodulatory cytokines, receptors and exosomes [102] and induction of miR-146a, a cellular miRNA that inhibits IFN response pathways [103,104], and inhibition of TLR9 expression [105,106] (discussed below). LMP2A and 2B enhance IFN receptor turnover in epithelial cells, inciting a broad effect on ISG expression [107]. These LMP functions likely contribute to the immune escape of EBV-positive tumors.
Recent work with MHV68 illustrates a novel mechanism of immunomodulation whereby a host IRF is co-opted to establish viral latency [108]. Specifically, transcription of the M2 protein, which plays a critical role in virus reactivation, is repressed by IRF2, which is in turn upregulated by the IFN produced in response to initial virus infection. The authors speculate that this IRF-sensing mechanism allows MHV68 to time virus reactivation to periods of localized immune quiescence. It is likely that other examples of ‘cooperative subversion’ between γ-herpes-viruses and host immune effectors will be identified.
Pattern recognition receptors and adaptor proteins
KSHV modulates TLR signaling by influencing the expression of TLRs or their adaptor molecules. Infection of monocytes leads to an upregulation of TLR3 and downstream innate immune effectors (CXCL10, IRF-1, CCL2, and IFN-β), an event that may facilitate establishment of latency [109]. In contrast, infection of endothelial cells results in downregulation of TLR4 mediated by the lytic proteins vIRF1 and ORF74 [110]. Interestingly, stimulation of PEL cells with agonists to TLR7 and 8 reactivates virus, suggesting a viral strategy to escape from cells targeted for immune attack [111]. Recent studies indicate that KSHV targets other PRRs in addition to TLRs. Inn et al. show that the tegument protein, ORF64, specifically targets and suppresses RIG-I-mediated signaling via its deubiquitinase activity [112]. Gregory et al. report that another tegument protein, ORF63, is a functional homolog of NLRP1 that disrupts the formation and activity of the NLRP1 inflammasome [113∙∙]. While NLRP1 is a cytoplasmic inflammasome, a study by Keruer et al. shows that, during KSHV infection of endothelial cells, IFN-γ-inducible protein 16 (IFI16) interacts with a caspase-1 activating complex to form a functional nuclear inflammasome, suggesting that KSHV may manipulate this pathway to promote latency after nuclear delivery of the viral genome [114,115]. These recent studies indicate a broader role for PRRs in sensing γ-herpesvirus infection and suggest that additional viral antagonists and strategies remain to be identified.
Similar to KSHV, MHV68 can be reactivated from latently-infected B cells with ligands for TLR3, 4, 5 and 9, and reactivation in vivo is accomplished by administration of LPS (TLR4) or CpG DNA (TLR9) [116]. Interestingly, LPS/CpG-induced reactivation led to an increase in the number of latently-infected splenocytes, suggesting that TLR sensitivity contributes to homeostatic maintenance of chronic infection. This phenomenon could also underlie the sensitivity of KSHV to TLR7/8 agonists. Alternatively, innate immune signaling pathways are hijacked by MHV68 to enable lytic replication that replenishes the latent pool. Indeed, it was shown that MHV68 exploits the MAVS-IKKbeta pathway to promote viral transcriptional activation and disable antiviral cytokine production [117 119]. It remains unclear how the MAVS-IKKbeta pathway is activated during MHV68 infection.
TLR signaling is mediated through one of two adapter proteins, myeloid differentiation primary-response protein 88 (MyD88) or Toll-interleukin-1 receptor (TIR) domain-containing adaptor-inducing b-interferon protein (TRIF) [120]. KSHV targets both adaptors. TRIF, an adaptor for TLR 3 and 4, is degraded in the presence of RTA, via either its E3 ligase activity or an unidentified mediator [121]. MyD88, which transmits signals from TLRs 7, 8 and 9, is downregulated by the viral microRNA miR-K9, which also targets a critical kinase, interleukin-1 receptor-associated kinase 1 (IRAK1), in the same pathway [122∙]. These data indicate that KSHV may block TLR7/9-induced IFN-α production via miRNA targeting.
In B cells, EBV infection exerts opposite effects on expression of TLR7 and TLR9. In naïve B cells, exposure to EBV downregulates TLR9 while inducing expression of TLR7 and key downstream adaptor (MyD88) and effector (IRF5) molecules, a scenario that promotes the initial phase of B cell proliferation; IRF5 activity is subsequently negatively regulated to allow for establishment of latency [123]. EBV proteins responsible for TLR9 downregulation have been identified: LMP1 downregulates TLR9 through NF-kB-dependent inhibition of TLR9 transcription [105], while BGLF5 degrades TLR9 mRNA [106]. EBV-infected B cells are also unresponsive to TLR7/8 and 9 agonists [124]. In plasmacytoid dendritic cells (pDC), EBV infection increases IFNa production by activating the TLR9 signaling pathway, but the simultaneous production of IL-10 from the pDC serves to blunt the net antiviral effect [125].
Evasion of inflammatory signaling
Inflammatory chemokines and cytokines produced in response to viral infection play an important role in the outcome of the immune response. Accordingly, the γ-herpesviruses have developed different strategies to imitate or neutralize these inflammatory mediators, including production of homologs, receptors and binding proteins.
Virus-encoded chemokines
KSHV encodes three viral chemokines with agonist or antagonist function against host chemokine receptors; vCCL1 (ORF K6), vCCL2 (ORF K4), vCCL3 (ORF K4.1). This family possess agonist function against CCR8 (vCCL1 and 2), CCR3 (vCCL2) and CCR4 (vCCL3), indicating a role in chemoattraction of Th2 T cells, which typically downmodulate immune responses [126]. Interestingly, vCCL2 also has broad-spectrum antagonist activity, binding promiscuously to several CC and CXC chemokine receptors and inhibiting the chemotactic responses of monocytes and Th1 T cells [126]. In combination with Th2 T cell recruitment, this would provide an effective means of blunting an inflammatory cytotoxic antiviral response.
As an alternate strategy for chemokine modulation, the EBV microRNA BHRF1, which is expressed in several EBV+ lymphomas, suppresses expression of CXCL11/I-TAC, an IFN-inducible T cell chemoattractant [127].
Other inflammatory modulators (vCD200 and vIL-6)
KSHV encodes a homolog of CD200, a molecule that negatively regulates myeloid-lineage cells [128], and a homolog of IL-6, a multifunctional inflammatory cytokine [129]. KSHV vCD200 is encoded by ORF K14 and binds with high affinity to the host receptor CD200R with immunosuppressive consequences [130]. KSHV vIL-6 is encoded by ORF K6 and, unlike human IL-6, can bind and signal exclusively through gp130 without the need for CD126 (gp80) [131,132]. In the presence of gp80 however, vIL-6 signaling is qualitatively different from that induced by human IL-6 [133]. These properties have relevance for immune evasion as well as oncogenesis, since IFN-modulation of the IL-6R complex would differentially impact virus and host IL-6 activity.
Like KSHV, RRV encodes a CD200 homolog, R15, which inhibits macrophage production of TNF [134] and a vIL-6, which signals through gp130 and is expressed in RRV-infected rhesus macaques [135,136].
The IL-10 homolog encoded by the EBV BCRF1 gene encodes a functional homolog of IL-10 that exhibits diverse immunosuppressive properties, including inhibition of T cell function, macrophage activation and synthesis of IRN-γ [137 140]. BCRF1 is functionally expressed during the earliest phase of de novo infection of primary B cells, a consequence of translation of virion-delivered mRNA, or transduced viral RNA (tvRNA) [21]. Additional immunomodulatory EBV tvRNAs were recently identified, including those encoding BGLF5, BNLF2a and LMP1; immediate translation of tvRNAs likely contributes to immune evasion in a crucial period before de novo gene expression [21]. EBV BARF1 could also contribute to immunomodulation via binding and neutralization of the pleiotropic cytokine colony-stimulating factor-1 (CSF-1) [141].
The MHV68 M3 protein is a secreted viral protein that binds selected CC and CXC chemokines with antiviral activity [142]. While an M3-deficient virus is not impaired in its capacity to establish latency in experimental hosts (C57BL/6 or BALB/c mice), when tested in a natural host (wood mice) it did attenuate infection and modulate the host inflammatory response in a manner consistent with its chemokine-binding properties [143,144∙], indicating that specific immunoevasins may only function effectively in the appropriate host.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
∙ of special interest
∙∙ of outstanding interest
- 1.Freeman ML, Lanzer KG, Cookenham T, Peters B, Sidney J, et al. Two kinetic patterns of epitope-specific CD8 T-cell responses following murine gammaherpesvirus 68 infection. J Virol. 2010;84:2881–2892. doi: 10.1128/JVI.02229-09. The authors identified a range of novel H-2(b) restricted epitopes and showed that the T cell response to these epitopes falls into two kinetic classes based on the speed of decline. The slowly declining response corresponded to the latent phase since decline was accelerated when mice were infected with a virus unable to establish latency. This suggests that T cell responses mirror the viral life cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gredmark-Russ S, Cheung EJ, Isaacson MK, Ploegh HL, Grotenbreg GM. The CD8 T-cell response against murine gammaherpesvirus 68 is directed toward a broad repertoire of epitopes from both early and late antigens. J Virol. 2008;82:12205–12212. doi: 10.1128/JVI.01463-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marques S, Alenquer M, Stevenson PG, Simas JP. A single CD8+ T cell epitope sets the long-term latent load of a murid herpesvirus. PLoS Pathog. 2008;4:1000177e. doi: 10.1371/journal.ppat.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett NJ, May JS, Stevenson PG. Gamma-herpesvirus latency requires T cell evasion during episome maintenance. PLoS Biol. 2005;3:120e. doi: 10.1371/journal.pbio.0030120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake N. Immune evasion by gammaherpesvirus genome maintenance proteins. J Gen Virol. 2010;91:829–846. doi: 10.1099/vir.0.018242-0. [DOI] [PubMed] [Google Scholar]
- 6.Vider-Shalit T, Fishbain V, Raffaeli S, Louzoun Y. Phasedependent immune evasion of herpesviruses. J Virol. 2007;81:9536–9545. doi: 10.1128/JVI.02636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci MG. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc Natl Acad Sci USA. 1997;94:12616–12621. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daskalogianni C, Apcher S, Candeias MM, Naski N, Calvo F, et al. Gly-Ala repeats induce position- and substrate-specific regulation of 26 S proteasome-dependent partial processing. J Biol Chem. 2008;283:30090–30100. doi: 10.1074/jbc.M803290200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apcher S, Komarova A, Daskalogianni C, Yin Y, Malbert-Colas L, et al. mRNA translation regulation by the Gly-Ala repeat of Epstein-Barr virus nuclear antigen 1. J Virol. 2009;83:1289–1298. doi: 10.1128/JVI.01369-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apcher S, Daskalogianni C, Manoury B, Fahraeus R. Epstein Barr virus-encoded EBNA1 interference with MHC class I antigen presentation reveals a close correlation between mRNA translation initiation and antigen presentation. PLoS Pathog. 2010;6:1001151e. doi: 10.1371/journal.ppat.1001151. It is shown that suppression of mRNA translation explains the inhibition of antigen presentaion by Gly-Ala repeats in cis. These results strongly support the notion that MHC-I derives most of its peptides from nascent polypeptide chains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin Y, Manoury B, Fahraeus R. Self-inhibition of synthesis and antigen presentation by Epstein-Barr virus-encoded EBNA1. Science. 2003;301:1371–1374. doi: 10.1126/science.1088902. [DOI] [PubMed] [Google Scholar]
- 12.Tellam J, Smith C, Rist M, Webb N, Cooper L, et al. Regulation of protein translation through mRNA structure influences MHC class I loading and T cell recognition. Proc Natl Acad Sci USA. 2008;105:9319–9324. doi: 10.1073/pnas.0801968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwun HJ, da Silva SR, Shah IM, Blake N, Moore PS, et al. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mimics Epstein-Barr virus EBNA1 immune evasion through central repeat domain effects on protein processing. J Virol. 2007;81:8225–8235. doi: 10.1128/JVI.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaldumbide A, Ossevoort M, Wiertz EJ, Hoeben RC. In cis inhibition of antigen processing by the latency-associated nuclear antigen I of Kaposi sarcoma herpes virus. Mol Immunol. 2007;44:1352–1360. doi: 10.1016/j.molimm.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Kwun HJ, da Silva SR, Qin H, Ferris RL, Tan R, et al. The central repeat domain 1 of Kaposi’s sarcoma-associated herpesvirus (KSHV) latency associated-nuclear antigen 1 (LANA1) prevents cis MHC class I peptide presentation. Virology. 2011;412:357–365. doi: 10.1016/j.virol.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Coulson JM, Whitehouse A, Blake N. Reduction in RNA levels rather than retardation of translation is responsible for the inhibition of major histocompatibility complex class I antigen presentation by the glutamic acid-rich repeat of herpesvirus saimiri open reading frame 73. J Virol. 2009;83:273–282. doi: 10.1128/JVI.01532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ressing ME, Horst D, Griffin BD, Tellam J, Zuo J, et al. Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products. Semin Cancer Biol. 2008;18:397–408. doi: 10.1016/j.semcancer.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Croft NP, Shannon-Lowe C, Bell AI, Horst D, Kremmer E, et al. Stage-specific inhibition of MHC class I presentation by the Epstein-Barr virus BNLF2a protein during virus lytic cycle. PLoS Pathog. 2009;5:1000490e. doi: 10.1371/journal.ppat.1000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horst D, van Leeuwen D, Croft NP, Garstka MA, Hislop AD, et al. Specific targeting of the EBV lytic phase protein BNLF2a to the transporter associated with antigen processing results in impairment of HLA class I-restricted antigen presentation. J Immunol. 2009;182:2313–2324. doi: 10.4049/jimmunol.0803218. [DOI] [PubMed] [Google Scholar]
- 20.Hislop AD, Ressing ME, van Leeuwen D, Pudney VA, Horst D, et al. A CD8+ T cell immune evasion protein specific to Epstein-Barr virus and its close relatives in Old World primates. J Exp Med. 2007;204:1863–1873. doi: 10.1084/jem.20070256. This paper demonstrates that BNLF2a is a TAP inhibitor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jochum S, Moosmann A, Lang S, Hammerschmidt W, Zeidler R. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog. 2012;8:1002704e. doi: 10.1371/journal.ppat.1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wycisk AI, Lin J, Loch S, Hobohm K, Funke J, et al. Epstein-Barr viral BNLF2a protein hijacks the tail-anchored protein insertion machinery to block antigen processing by the transport complex TAP. J Biol Chem. 2011;286:41402–41412. doi: 10.1074/jbc.M111.237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horst D, Favaloro V, Vilardi F, van Leeuwen HC, Garstka MA, et al. EBV protein BNLF2a exploits host tail-anchored protein integration machinery to inhibit TAP. J Immunol. 2011;186:3594–3605. doi: 10.4049/jimmunol.1002656. [DOI] [PubMed] [Google Scholar]
- 24.Zuo J, Quinn LL, Tamblyn J, Thomas WA, Feederle R, et al. The Epstein-Barr virus-encoded BILF1 protein modulates immune recognition of endogenously processed antigen by targeting major histocompatibility complex class I molecules trafficking on both the exocytic and endocytic pathways. J Virol. 2011;85:1604–1614. doi: 10.1128/JVI.01608-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuo J, Currin A, Griffin BD, Shannon-Lowe C, Thomas WA, et al. The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. PLoS Pathog. 2009;5:1000255e. doi: 10.1371/journal.ppat.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boname JM, Lehner PJ. What has the study of the K3 and K5 viral ubiquitin E3 ligases taught us about ubiquitin-mediated receptor regulation? Viruses. 2011;3:118–131. doi: 10.3390/v3020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan JA, Lehner PJ. The trafficking and regulation of membrane receptors by the RING-CH ubiquitin E3 ligases. Exp Cell Res. 2009;315:1593–1600. doi: 10.1016/j.yexcr.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Harris S, Lang SM, Means RE. Characterization of the rhesus fibromatosis herpesvirus MARCH family member rfK3. Virology. 2010;398:214–223. doi: 10.1016/j.virol.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Thomas M, Wills M, Lehner PJ. Natural killer cell evasion by an E3 ubiquitin ligase from Kaposi’s sarcoma-associated herpesvirus. Biochem Soc Trans. 2008;36:459–463. doi: 10.1042/BST0360459. [DOI] [PubMed] [Google Scholar]
- 30.Karki R, Lang SM, Means RE. The MARCH family E3 ubiquitin ligase K5 alters monocyte metabolism and proliferation through receptor tyrosine kinase modulation. PLoS Pathog. 2011;7:1001331e. doi: 10.1371/journal.ppat.1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansouri M, Viswanathan K, Douglas JL, Hines J, Gustin J, et al. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2009;83:9672–9681. doi: 10.1128/JVI.00597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson PG, May JS, Smith XG, Marques S, Adler H, et al. K3-mediated evasion of CD8(+) T cells aids amplification of a latent gamma-herpesvirus. Nat Immunol. 2002;3:733–740. doi: 10.1038/ni818. [DOI] [PubMed] [Google Scholar]
- 33.Loh J, Popkin DL, Droit L, Braaten DC, Zhao G, et al. Specific mutation of a gammaherpesvirus-expressed antigen in response to CD8 T cell selection in vivo. J Virol. 2012;86:2887–2893. doi: 10.1128/JVI.06101-11. The first demonstration of immune evasion by epitope mutation in a herpesvirus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman ML, Burkum CE, Lanzer KG, Jensen MK, Ahmed M, et al. Cutting edge: activation of virus-specific CD4 T cells throughout gamma-herpesvirus latency. J Immunol. 2011;187:6180–6184. doi: 10.4049/jimmunol.1102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myoung J, Ganem D. Active lytic infection of human primary tonsillar B cells by KSHV and its noncytolytic control by activated CD4+ T cells. J Clin Invest. 2011;121:1130–1140. doi: 10.1172/JCI43755. Using tonsillar B and T cells it is demonstrated that CD4+ T cells are capable of suppressing reactivation of latent KSHV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuller KA, Flano E. CD4 T cells mediate killing during persistent gammaherpesvirus 68 infection. J Virol. 2009;83:4700–4703. doi: 10.1128/JVI.02240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt K, Wies E, Neipel F. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 3 inhibits gamma interferon and major histocompatibility complex class II expression. J Virol. 2011;85:4530–4537. doi: 10.1128/JVI.02123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabbah S, Jagne YJ, Zuo J, de Silva T, Ahasan MM, et al. T-cell immunity to Kaposi sarcoma-associated herpesvirus: recognition of primary effusion lymphoma by LANA-specific CD4+ T cells. Blood. 2012;119:2083–2092. doi: 10.1182/blood-2011-07-366476. [DOI] [PubMed] [Google Scholar]
- 39.Zuo J, Thomas WA, Haigh TA, Fitzsimmons L, Long HM, et al. Epstein-Barr virus evades CD4+ T cell responses in lytic cycle through BZLF1-mediated downregulation of CD74 and the cooperation of vBcl-2. PLoS Pathog. 2011;7:1002455e. doi: 10.1371/journal.ppat.1002455. It is demonstrated that BZLF1 downregulates invariant chain thus inhibiting MHC-II antigen presentation. This is the first reported viral protein targeting invariant chain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butler LM, Jeffery HC, Wheat RL, Long HM, Rae PC, et al. Kaposi’s sarcoma-associated herpesvirus inhibits expression and function of endothelial cell major histocompatibility complex class II via suppressor of cytokine signaling 3. J Virol. 2012;86:7158–7166. doi: 10.1128/JVI.06908-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregory SM, Wang L, West JA, Dittmer DP, Damania B. Latent Kaposi’s sarcoma-associated herpesvirus infection of monocytes downregulates expression of adaptive immune response costimulatory receptors and proinflammatory cytokines. J Virol. 2012;86:3916–3923. doi: 10.1128/JVI.06437-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang C, Jung JU EX. Downregulation of autophagy by herpesvirus Bcl-2 homologs. Autophagy. 2008;4:268–272. doi: 10.4161/auto.5210. [DOI] [PubMed] [Google Scholar]
- 43.Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P, et al. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med. 1999;190:1025–1032. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999;18:5738–5746. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- 45.Guasparri I, Keller SA, Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med. 2004;199:993–1003. doi: 10.1084/jem.20031467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Keller SA, Schattner EJ, Cesarman E. Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood. 2000;96:2537–2542. [PubMed] [Google Scholar]
- 47.Matta H, Chaudhary PM. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP) Proc Natl Acad Sci USA. 2004;101:9399–9404. doi: 10.1073/pnas.0308016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chugh P, Matta H, Schamus S, Zachariah S, Kumar A, et al. Constitutive NF-kappaB activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc Natl Acad Sci USA. 2005;102:12885–12890. doi: 10.1073/pnas.0408577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Q, Matta H, Chaudhary PM. The human herpes virus 8-encoded viral FLICE inhibitory protein protects against growth factor withdrawal-induced apoptosis via NF-kappa B activation. Blood. 2003;101:1956–1961. doi: 10.1182/blood-2002-07-2072. [DOI] [PubMed] [Google Scholar]
- 50.Ballon G, Chen K, Perez R, Tam W, Cesarman E. Kaposi sarcoma herpesvirus (KSHV) vFLIP oncoprotein induces B cell transdifferentiation and tumorigenesis in mice. J Clin Invest. 2011;121:1141–1153. doi: 10.1172/JCI44417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grossmann C, Podgrabinska S, Skobe M, Ganem D. Activation of NF-kappaB by the latent vFLIP gene of Kaposi’s sarcomaassociated herpesvirus is required for the spindle shape of virus-infected endothelial cells and contributes to their proinflammatory phenotype. J Virol. 2006;80:7179–7185. doi: 10.1128/JVI.01603-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glykofrydes D, Niphuis H, Kuhn EM, Rosenwirth B, Heeney JL, et al. Herpesvirus saimiri vFLIP provides an antiapoptotic function but is not essential for viral replication, transformation, or pathogenicity. J Virol. 2000;74:11919–11927. doi: 10.1128/jvi.74.24.11919-11927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hardwick JM, Bellows DS. Viral versus cellular BCL-2 proteins. Cell Death Differ. 2003;10(suppl. 1):S68–S76. doi: 10.1038/sj.cdd.4401133. [DOI] [PubMed] [Google Scholar]
- 54.Cheng EH, Nicholas J, Bellows DS, Hayward GS, Guo HG, et al. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellows DS, Chau BN, Lee P, Lazebnik Y, Burns WH, et al. Antiapoptotic herpesvirus Bcl-2 homologs escape caspasemediated conversion to proapoptotic proteins. J Virol. 2000;74:5024–5031. doi: 10.1128/jvi.74.11.5024-5031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hwang EX, Oh SS, Lee JS, Jeong JH, et al. Viral Bcl-2-mediated evasion of autophagy aids chronic infection of gammaherpesvirus 68. PLoS Pathog. 2009;5:1000609e. doi: 10.1371/journal.ppat.1000609. This work provides in vivo evidence that viral Bcl-2 is important to evade autophagy during latent infection of gamma herpesvirus 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altmann M, Hammerschmidt W. Epstein-Barr virus provides a new paradigm: a requirement for the immediate inhibition of apoptosis. PLoS Biol. 2005;3:404e. doi: 10.1371/journal.pbio.0030404. This study shows that inhibition of apoptosis is an integral component of the transformation triggered by human gamma herpesviruses in B lymphocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feng P, Liang C, Shin YC, Xiaofei E, Zhang W, et al. A novel inhibitory mechanism of mitochondrion-dependent apoptosis by a herpesviral protein. PLoS Pathog. 2007;3:174e. doi: 10.1371/journal.ppat.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friborg J, Jr, Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 60.Cai Q, Guo Y, Xiao B, Banerjee S, Saha A, et al. Epstein-Barr virus nuclear antigen 3C stabilizes Gemin3 to block p53-mediated apoptosis. PLoS Pathog. 2011;7:1002418e. doi: 10.1371/journal.ppat.1002418. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Saha A, Bamidele A, Murakami M, Robertson ES. EBNA3C attenuates the function of p53 through interaction with inhibitor of growth family proteins 4 and 5. J Virol. 2011;85:2079–2088. doi: 10.1128/JVI.02279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang HW, Sharp TV, Koumi A, Koentges G, Boshoff C. Characterization of an anti-apoptotic glycoprotein encoded by Kaposi’s sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J. 2002;21:2602–2615. doi: 10.1093/emboj/21.11.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng P, Park J, Lee BS, Lee SH, Bram RJ, et al. Kaposi’s sarcoma-associated herpesvirus mitochondrial K7 protein targets a cellular calcium-modulating cyclophilin ligand to modulate intracellular calcium concentration and inhibit apoptosis. J Virol. 2002;76:11491–11504. doi: 10.1128/JVI.76.22.11491-11504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng P, Scott CW, Cho NH, Nakamura H, Chung YH, et al. Kaposi’s sarcoma-associated herpesvirus K7 protein targets a ubiquitin-like/ubiquitin-associated domain-containing protein to promote protein degradation. Mol Cell Biol. 2004;24:3938–3948. doi: 10.1128/MCB.24.9.3938-3948.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee HR, Choi WC, Lee S, Hwang J, Hwang E, et al. Bilateral inhibition of HAUSP deubiquitinase by a viral interferon regulatory factor protein. Nat Struct Mol Biol. 2011;18:1336–1344. doi: 10.1038/nsmb.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 67.Liang C, Feng P, Ku B, Dotan I, Canaani D, et al. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 68.Ku B, Woo JS, Liang C, Lee KH, Hong HS, et al. Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68. PLoS Pathog. 2008;4:25e. doi: 10.1371/journal.ppat.0040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sinha S, Colbert CL, Becker N, Wei Y, Levine B. Molecular basis of the regulation of Beclin 1-dependent autophagy by the gamma-herpesvirus 68 Bcl-2 homolog M11. Autophagy. 2008;4:989–997. doi: 10.4161/auto.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee HR, Toth Z, Shin YC, Lee JS, Chang H, et al. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 4 targets MDM2 to deregulate the p53 tumor suppressor pathway. J Virol. 2009;83:6739–6747. doi: 10.1128/JVI.02353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leidal AM, Cyr DP, Hill RJ, Lee PW, McCormick C. Subversion of autophagy by Kaposi’s sarcoma-associated herpesvirus impairs oncogene-induced senescence. Cell Host Microbe. 2012;11:167–180. doi: 10.1016/j.chom.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 72.de Weerd NA, Nguyen T. The interferons and their receptors — distribution and regulation. Immunol Cell Biol. 2012;90:483–491. doi: 10.1038/icb.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 74.West JA, Damania B. Kaposi’s sarcoma-associated herpesvirus and innate immunity. Future Virol. 2010;5:185–196. doi: 10.2217/fvl.10.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burysek L, Yeow WS, Pitha PM. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2) J Hum Virol. 1999;2:19–32. [PubMed] [Google Scholar]
- 76.Fuld S, Cunningham C, Klucher K, Davison AJ, Blackbourn DJ. Inhibition of interferon signaling by the Kaposi’s sarcomaassociated herpesvirus full-length viral interferon regulatory factor 2 protein. J Virol. 2006;80:3092–3097. doi: 10.1128/JVI.80.6.3092-3097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Areste C, Mutocheluh M, Blackbourn DJ. Identification of caspase-mediated decay of interferon regulatory factor-3, exploited by a Kaposi sarcoma-associated herpesvirus immunoregulatory protein. J Biol Chem. 2009;284:23272–23285. doi: 10.1074/jbc.M109.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burysek L, Pitha PM. Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits doublestranded RNA-activated protein kinase. J Virol. 2001;75:2345–2352. doi: 10.1128/JVI.75.5.2345-2352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wies E, Hahn AS, Schmidt K, Viebahn C, Rohland N, et al. The Kaposi’s Sarcoma-associated herpesvirus-encoded vIRF-3 inhibits cellular IRF-5. J Biol Chem. 2009;284:8525–8538. doi: 10.1074/jbc.M809252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joo CH, Shin YC, Gack M, Wu L, Levy D, et al. Inhibition of interferon regulatory factor 7 (IRF7)-mediated interferon signal transduction by the Kaposi’s sarcoma-associated herpesvirus viral IRF homolog vIRF3. J Virol. 2007;81:8282–8292. doi: 10.1128/JVI.00235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jacobs SR, Damania B. The viral interferon regulatory factors of KSHV: immunosuppressors or oncogenes? Front Immunol. 2011;2:19. doi: 10.3389/fimmu.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heinzelmann K, Scholz BA, Nowak A, Fossum E, Kremmer E, et al. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor 4 (vIRF4/K10) is a novel interaction partner of CSL/CBF1, the major downstream effector of Notch signaling. J Virol. 2010;84:12255–12264. doi: 10.1128/JVI.01484-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xi X, Persson LM, O’Brien MW, Mohr I, Wilson AC. Cooperation between viral interferon regulatory factor 4 and RTA to activate a subset of Kaposi’s sarcoma-associated herpesvirus lytic promoters. J Virol. 2012;86:1021–1033. doi: 10.1128/JVI.00694-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jacobs SR, Gregory SM, West JA, Wollish AC, Blackbourn DJ, et al. The KSHV vIRFs differ in their inhibition of interferon activation mediated by TLR3. J Virol. 2012;87:798–806. doi: 10.1128/JVI.01851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cloutier N, Flamand L. Kaposi sarcoma-associated herpesvirus latency-associated nuclear antigen inhibits interferon (IFN) beta expression by competing with IFN regulatory factor-3 for binding to IFNB promoter. J Biol Chem. 2010;285:7208–7221. doi: 10.1074/jbc.M109.018838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lefort S, Soucy-Faulkner A, Grandvaux N, Flamand L. Binding of Kaposi’s sarcoma-associated herpesvirus K-bZIP to interferon-responsive factor 3 elements modulates antiviral gene expression. J Virol. 2007;81:10950–10960. doi: 10.1128/JVI.00183-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hwang S, Kim KS, Flano E, Wu TT, Tong LM, et al. Conserved herpesviral kinase promotes viral persistence by inhibiting the IRF-3-mediated type I interferon response. Cell Host Microbe. 2009;5:166–178. doi: 10.1016/j.chom.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang JT, Doong SL, Teng SC, Lee CP, Tsai CH, et al. Epstein-Barr virus BGLF4 kinase suppresses the interferon regulatory factor 3 signaling pathway. J Virol. 2009;83:1856–1869. doi: 10.1128/JVI.01099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liang Q, Fu B, Wu F, Li X, Yuan Y, et al. ORF45 of Kaposi’s sarcoma-associated herpesvirus inhibits phosphorylation of interferon regulatory factor 7 by IKKepsilon and TBK1 as an alternative substrate. J Virol. 2012;86:10162–10172. doi: 10.1128/JVI.05224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sathish N, Zhu FX, Golub EE, Liang Q, Yuan Y. Mechanisms of autoinhibition of IRF-7 and a probable model for inactivation of IRF-7 by Kaposi’s sarcoma-associated herpesvirus protein ORF45. J Biol Chem. 2011;286:746–756. doi: 10.1074/jbc.M110.150920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu FX, King SM, Smith EJ, Levy DE, Yuan Y. A Kaposi’s sarcoma-associated herpesviral protein inhibits virusmediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc Natl Acad Sci USA. 2002;99:5573–5578. doi: 10.1073/pnas.082420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu FX, Sathish N, Yuan Y. Antagonism of host antiviral responses by Kaposi’s sarcoma-associated herpesvirus tegument protein ORF45. PLoS ONE. 2010;5:10573e. doi: 10.1371/journal.pone.0010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jia Q, Chernishof V, Bortz E, McHardy I, Wu TT, et al. Murine gammaherpesvirus 68 open reading frame 45 plays an essential role during the immediate-early phase of viral replication. J Virol. 2005;79:5129–5141. doi: 10.1128/JVI.79.8.5129-5141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu Y, Wang SE, Hayward GS. The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity. 2005;22:59–70. doi: 10.1016/j.immuni.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 95.Searles RP, Bergquam EP, Axthelm MK, Wong SW. Sequence and genomic analysis of a Rhesus macaque rhadinovirus with similarity to Kaposi’s sarcoma-associated herpesvirus/ human herpesvirus 8. J Virol. 1999;73:3040–3053. doi: 10.1128/jvi.73.4.3040-3053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Robinson BA, Estep RD, Messaoudi I, Rogers KS, Wong SW. Viral interferon regulatory factors decrease the induction of type I and type II interferon during rhesus macaque rhadinovirus infection. J Virol. 2012;86:2197–2211. doi: 10.1128/JVI.05047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robinson BA, O’Connor MA, Li H, Engelmann F, Poland B, et al. Viral interferon regulatory factors are critical for delay of the host immune response against rhesus macaque rhadinovirus infection. J Virol. 2012;86:2769–2779. doi: 10.1128/JVI.05657-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hahn AM, Huye LE, Ning S, Webster-Cyriaque J, Pagano JS. Interferon regulatory factor 7 is negatively regulated by the Epstein-Barr virus immediate-early gene, BZLF-1. J Virol. 2005;79:10040–10052. doi: 10.1128/JVI.79.15.10040-10052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bentz GL, Liu R, Hahn AM, Shackelford J, Pagano JS. Epstein-Barr virus BRLF1 inhibits transcription of IRF3 and IRF7 and suppresses induction of interferon-beta. Virology. 2010;402:121–128. doi: 10.1016/j.virol.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu L, Fossum E, Joo CH, Inn KS, Shin YC, et al. Epstein-Barr virus LF2: an antagonist to type I interferon. J Virol. 2009;83:1140–1146. doi: 10.1128/JVI.00602-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ning S, Hahn AM, Huye LE, Pagano JS. Interferon regulatory factor 7 regulates expression of Epstein-Barr virus latent membrane protein 1: a regulatory circuit. J Virol. 2003;77:9359–9368. doi: 10.1128/JVI.77.17.9359-9368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Middeldorp JM, Pegtel DM. Multiple roles of LMP1 in Epstein-Barr virus induced immune escape. Semin Cancer Biol. 2008;18:388–396. doi: 10.1016/j.semcancer.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 103.Cameron JE, Yin Q, Fewell C, Lacey M, McBride J, et al. Epstein-Barr virus latent membrane protein 1 induces cellular microRNA miR-146a, a modulator of lymphocyte signaling pathways. J Virol. 2008;82:1946–1958. doi: 10.1128/JVI.02136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Motsch N, Pfuhl T, Mrazek J, Barth S, Grasser FA. Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) induces the expression of the cellular microRNA miR-146a. RNA Biol. 2007;4:131–137. doi: 10.4161/rna.4.3.5206. [DOI] [PubMed] [Google Scholar]
- 105.Fathallah I, Parroche P, Gruffat H, Zannetti C, Johansson H, et al. EBV latent membrane protein 1 is a negative regulator of TLR9. J Immunol. 2010;185:6439–6447. doi: 10.4049/jimmunol.0903459. [DOI] [PubMed] [Google Scholar]
- 106.van Gent M, Griffin BD, Berkhoff EG, van Leeuwen D, Boer IG, et al. EBV lytic-phase protein BGLF5 contributes to TLR9 downregulation during productive infection. J Immunol. 2011;186:1694–1702. doi: 10.4049/jimmunol.0903120. [DOI] [PubMed] [Google Scholar]
- 107.Shah KM, Stewart SE, Wei W, Woodman CB, O’Neil JD, et al. The EBV-encoded latent membrane proteins, LMP2A and LMP2B, limit the actions of interferon by targeting interferon receptors for degradation. Oncogene. 2009;28:3903–3914. doi: 10.1038/onc.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mandal P, Krueger BE, Oldenburg D, Andry KA, Beard RS, et al. A gammaherpesvirus cooperates with interferon-alpha/betainduced IRF2 to halt viral replication, control reactivation, and minimize host lethality. PLoS Pathog. 2011;7:1002371e. doi: 10.1371/journal.ppat.1002371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.West J, Damania B. Upregulation of the TLR3 pathway by Kaposi’s sarcoma-associated herpesvirus during primary infection. J Virol. 2008;82:5440–5449. doi: 10.1128/JVI.02590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lagos D, Vart RJ, Gratrix F, Westrop SJ, Emuss V, et al. Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma herpesvirus. Cell Host Microbe. 2008;4:470–483. doi: 10.1016/j.chom.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gregory SM, Damania B. KSHV and the toll of innate immune activation. Cell Cycle. 2009;8:3246–3247. doi: 10.4161/cc.8.20.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inn KS, Lee SH, Rathbun JY, Wong LY, Toth Z, et al. Inhibition of RIG-I-mediated signaling by Kaposi’s sarcoma-associated herpesvirus-encoded deubiquitinase ORF64. J Virol. 2011;85:10899–10904. doi: 10.1128/JVI.00690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa S, et al. Discovery of a viral NLR homolog that inhibits the inflammasome. Science. 2011;331:330–334. doi: 10.1126/science.1199478. This study broadens our understanding of the role of pattern recognition receptors (PRR) in g-herpesvirus defense. It shows for the first time that the NLR family of PRRs responds to both the primary and reactivation stages of KSHV infection, and that KSHV encodes a functional homolog of NLRP1 to counteract host defense. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singh VV, Kerur N, Bottero V, Dutta S, Chakraborty S, et al. Kaposi’s sarcoma-associated herpesvirus latency in endothelial and B cells activates interferon gamma-inducible protein 16 (IFI16) mediated inflammasomes. J Virol. 2013;87:4417–4431. doi: 10.1128/JVI.03282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gargano LM, Forrest JC, Speck SH. Signaling through toll-like receptors induces murine gammaherpesvirus 68 reactivation in vivo. J Virol. 2009;83:1474–1482. doi: 10.1128/JVI.01717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dong X, Feng P. Murine gamma herpesvirus 68 hijacks MAVS and IKKbeta to abrogate NFkappaB activation and antiviral cytokine production. PLoS Pathog. 2011;7:1002336e. doi: 10.1371/journal.ppat.1002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dong X, Feng H, Sun Q, Li H, Wu TT, et al. Murine gammaherpesvirus 68 hijacks MAVS and IKKbeta to initiate lytic replication. PLoS Pathog. 2010;6:1001001e. doi: 10.1371/journal.ppat.1001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dong X, He Z, Durakoglugil D, Arneson L, Shen Y, et al. Murine gammaherpesvirus 68 evades host cytokine production via replication transactivator-induced RelA degradation. J Virol. 2012;86:1930–1941. doi: 10.1128/JVI.06127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 121.Ahmad H, Gubbels R, Ehlers E, Meyer F, Waterbury T, et al. Kaposi sarcoma-associated herpesvirus degrades cellular Tollinterleukin-1 receptor domain-containing adaptor-inducing beta-interferon (TRIF) J Biol Chem. 2011;286:7865–7872. doi: 10.1074/jbc.M110.191452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abend JR, Ramalingam D, Kieffer-Kwon P, Uldrick TS, Yarchoan R, et al. Kaposi’s sarcoma-associated herpesvirus microRNAs target IRAK1 and MYD88, two components of the toll-like receptor/interleukin-1R signaling cascade, to reduce inflammatory-cytokine expression. J Virol. 2012;86:11663–11674. doi: 10.1128/JVI.01147-12. This study provides the first evidence that KSHV-encoded microRNAs target the TLR/IL-1R signaling cascade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martin HJ, Lee JM, Walls D, Hayward SD. Manipulation of the toll-like receptor 7 signaling pathway by Epstein-Barr virus. J Virol. 2007;81:9748–9758. doi: 10.1128/JVI.01122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Younesi V, Nikzamir H, Yousefi M, Khoshnoodi J, Arjmand M, et al. Epstein Barr virus inhibits the stimulatory effect of TLR7/8 and TLR9 agonists but not CD40 ligand in human B lymphocytes. Microbiol Immunol. 2010;54:534–541. doi: 10.1111/j.1348-0421.2010.00248.x. [DOI] [PubMed] [Google Scholar]
- 125.Lim WH, Kireta S, Russ GR, Coates PT. Human plasmacytoid dendritic cells regulate immune responses to Epstein-Barr virus (EBV) infection and delay EBV-related mortality in humanized NOD-SCID mice. Blood. 2007;109:1043–1050. doi: 10.1182/blood-2005-12-024802. [DOI] [PubMed] [Google Scholar]
- 126.Liang C, Lee JS, Jung JU. Immune evasion in Kaposi’s sarcoma-associated herpes virus associated oncogenesis. Semin Cancer Biol. 2008;18:423–436. doi: 10.1016/j.semcancer.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xia T, O’Hara A, Araujo I, Barreto J, Carvalho E, et al. EBV microRNAs in primary lymphomas and targeting of CXCL-11 by ebv-mir-BHRF1-3. Cancer Res. 2008;68:1436–1442. doi: 10.1158/0008-5472.CAN-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 129.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth factor Rev. 2011;22:83–89. doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 130.Lee HR, Lee S, Chaudhary PM, Gill P, Jung JU. Immune evasion by Kaposi’s sarcoma-associated herpesvirus. Future Microbiol. 2010;5:1349–1365. doi: 10.2217/fmb.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. A Kaposi’s sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J Biol Chem. 1997;272:19625–19631. doi: 10.1074/jbc.272.31.19625. [DOI] [PubMed] [Google Scholar]
- 132.Wan X, Wang H, Nicholas J. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptorbinding properties distinct from those of human IL-6. J Virol. 1999;73:8268–8278. doi: 10.1128/jvi.73.10.8268-8278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu F, Nicholas J. Signal transduction by human herpesvirus 8 viral interleukin-6 (vIL-6) is modulated by the nonsignaling gp80 subunit of the IL-6 receptor complex and is distinct from signaling induced by human IL-6. J Virol. 2006;80:10874–10878. doi: 10.1128/JVI.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Langlais CL, Jones JM, Estep RD, Wong SW. Rhesus rhadinovirus R15 encodes a functional homologue of human CD200. J Virol. 2006;80:3098–3103. doi: 10.1128/JVI.80.6.3098-3103.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Orzechowska BU, Manoharan M, Sprague J, Estep RD, Axthelm MK, et al. Viral interleukin-6 encoded by rhesus macaque rhadinovirus is associated with lymphoproliferative disorder (LPD) J Med Primatol. 2009;38(suppl. 1):2–7. doi: 10.1111/j.1600-0684.2009.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kaleeba JA, Bergquam EP, Wong SW. A rhesus macaque rhadinovirus related to Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 encodes a functional homologue of interleukin-6. J Virol. 1999;73:6177–6181. doi: 10.1128/jvi.73.7.6177-6181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Swaminathan S, Hesselton R, Sullivan J, Kieff E. Epstein-Barr virus recombinants with specifically mutated BCRF1 genes. J Virol. 1993;67:7406–7413. doi: 10.1128/jvi.67.12.7406-7413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bejarano MT, Masucci MG. Interleukin-10 abrogates the inhibition of Epstein-Barr virus-induced B-cell transformation by memory T-cell responses. Blood. 1998;92:4256–4262. [PubMed] [Google Scholar]
- 139.Salek-Ardakani S, Arrand JR, Mackett M. Epstein-Barr virus encoded interleukin-10 inhibits HLA-class I. ICAM-1, and B7 expression on human monocytes: implications for immune evasion by EBV. Virology. 2002;304:342–351. doi: 10.1006/viro.2002.1716. [DOI] [PubMed] [Google Scholar]
- 140.Hsu DH, de Waal Malefyt R, Fiorentino DF, Dang MN, Vieira P, et al. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990;250:830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- 141.Strockbine LD, Cohen JI, Farrah T, Lyman SD, Wagener F, et al. The Epstein-Barr virus BARF1 gene encodes a novel, soluble colony-stimulating factor-1 receptor. J Virol. 1998;72:4015–4021. doi: 10.1128/jvi.72.5.4015-4021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barton E, Mandal P, Speck SH. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol. 2011;29:351–397. doi: 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- 143.van Berkel V, Levine B, Kapadia SB, Goldman JE, Speck SH, et al. Critical role for a high-affinity chemokine-binding protein in gamma-herpesvirus-induced lethal meningitis. J Clin Invest. 2002;109:905–914. doi: 10.1172/JCI14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hughes DJ, Kipar A, Leeming GH, Bennett E, Howarth D, et al. Chemokine binding protein M3 of murine gammaherpesvirus 68 modulates the host response to infection in a natural host. PLoS Pathog. 2011;7:1001321e. doi: 10.1371/journal.ppat.1001321. This study demonstrates that the chemokine-binding protein M3 of MHV68 plays an important role in antagonizing the antiviral response, but that this influence is only revealed in the context of the natural host. [DOI] [PMC free article] [PubMed] [Google Scholar]